Abstract
For recurrent patellar dislocation, reconstruction of the medial patellofemoral ligament (MPFL) with replacement autografts has often been performed but with only little data on the tensile properties of the MPFL to guide graft selection. With its complex anatomy and geometry, these properties are difficult to obtain. In this study, we showed how the orientation of the femur-MPFL-patella complex (FMPC) during uniaxial tensile testing can have a significant effect on its structural properties. Twenty two FMPCs were isolated from porcine stifle joints and randomly assigned to two groups of 11 each. For the first group, the specimens were loaded to failure with the patella oriented 30 degrees away from the direction of the applied load to mimic its orientation in situ, called natural orientation. In the second group, the patella was aligned in the direction of the tensile load, called non-natural orientation. The stiffness for the natural orientation group was 65 ± 13 N/mm, 32% higher than that for the non-natural orientation group (50 ± 17 N/mm; p < 0.05). The ultimate loads were 438 ± 128 N and 386 ± 136 N, respectively (p > 0.05). Ten out of 11 specimens in the natural orientation group failed at the femoral attachment (the narrowest portion of the MPFL) compared to 6 out of 11 in the non-natural orientation group. Our findings suggest that the specimen orientation that mimics the in-situ loading conditions of the MPFL should be used to obtain more representative data for the structural properties of the FMPC.
Keywords: Medial patellofemoral ligament (MPFL), Biomechanics, Specimen orientation, Structural properties, Reconstruction
1. Introduction
Primary patellar dislocation has an annual incidence of 5.8 per 100,000 people in the US, and this number is five-fold higher for adolescents (Fithian et al., 2004). Follow-up studies of conservative treatment revealed high re-dislocation rates that ranged from 17% to 44%. Meanwhile, clinical outcomes following primary repair of the MPFL were also unsatisfactory (Buchner et al., 2005; Fithian et al., 2004; Palmu et al., 2008). As a result, surgical reconstruction with soft tissue autografts to replace the torn MPFL has gained popularity, especially for patients who had recurrent patellar dislocations (Avikainen et al., 1993; Gomes, 2008; Nomura and Inoue, 2006; Schottle et al., 2009, 2005).
In the literature, there are a plethora of surgical procedures for MPFL reconstruction (Ahmad et al., 2009; Koskinen et al., 1998; Noyes and Albright, 2006; Schottle et al., 2009; Wang et al., 2013). Yet, one of the key parameters, the biomechanical properties of the MPFL, has received little or no attention. A literature search found only one report on the ultimate load of the femur- MPFL-patella complex (FMPC) (Mountney et al., 2005). As such, there is a need to learn more about the biomechanical properties of the FMPC to serve as the basis for selection of replacement graft tissue.
The FMPC’s complex anatomy and geometry make the task of determining its tensile properties nontrivial. In particular, proper specimen orientation with respect to the applied load can be difficult to achieve experimentally, although it could have a significant effect on the outcome. Indeed, this was found to be the case for the human femur-anterior cruciate ligament (ACL)-tibia complex (Woo et al., 1991). The MPFL has a wide insertion at the patella spanning over its curved medial edge while its femoral insertion is relatively narrow in width, making it a fan-shaped ligament (Amis et al., 2003). Thus, we hypothesized that an orientation of the patella during a uniaxial tensile test that would allow the fibers of the MPFL to be loaded more uniformly would yield a higher stiffness and ultimate load while leading to a more uniform mode of failure. Thus, the objective of this study was to examine if a natural specimen orientation where the orientation of the MPFL with respect to the patella in situ is replicated during tensile testing could obtain more representative data for the structural properties of the FMPC that could serve as a guide for replacement graft selection for MPFL reconstruction.
2. Methods
A power analysis was performed using G*Power 3 (Erdfelder et al., 1996) based on preliminary data obtained with porcine stifle joints, and the sample size was determined to be 12 per group. Thus, a total of 24 fresh-frozen stifle joints of Yorkshire pigs (~250 lbs) were obtained from a local slaughterhouse. It has been shown in the literature that the porcine stifle joint anatomy is similar to the human knee (Fuss, 1991). At our research center, we have also shown that the porcine stifle joint is functionally similar to the human knee using a robotic/universal force-moment sensor (UFS) testing system (Xerogeanes et al., 1998). The ligament’s insertion at the patella spanned from the proximal medial corner to most of the curved medial edge of the patella. At the distal end, there was a band of loose connective tissue that attached to the patellar tendon, similar to the human MPFL (Mochizuki et al., 2013). Fibers at the proximal end attached directly to the patella underneath the attachment of the vastus medialis (pink tissue at the upper right corner in Fig. 1). On the other side, the femoral insertion of the ligament was relatively narrow.
Fig. 1.
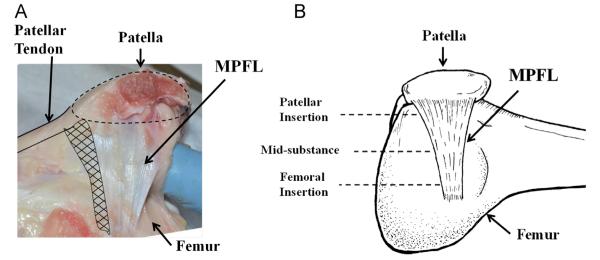
(A) A photograph showing the complex anatomy and geometry of the medial patellofemoral ligament. (B) A schematic diagram of the femur-MPFL-patella showing the fan-like shape of the MPFL with the cross-sectional area larger at the patellar attachment compared to the femoral attachment. The three designated locations where its CSA were measured are indicated.
The stifle joints were wrapped in saline-soaked gauze, stored in air-tight bags, and kept frozen at −20 °C until the day prior to testing (Woo et al., 1986a). After thawing at room temperature, all soft tissues around the joint were dissected away. At the distal end, the loose connective tissue that attached to the patellar tendon (crosshatched area in Fig. 1) was also removed, leaving only the femur, MPFL, and patella. A rectangular bone block was cut out from the femoral condyle around the femoral insertion of the MPFL to isolate the femur-MPFL-patella complex (FMPC). Cross-sectional areas (CSA) of the MPFL at the patellar insertion, mid-substance, and the femoral insertion were measured using a custom laser micrometer system (see Fig. 1) (Woo et al., 1990). The femoral bone block was then potted in a rectangular clamp using Ortho-Jet dental cement (Lang, Wheeling, IL) such that the MPFL was kept tangential to the surface of the femoral bone block during tensile loading. The patella was then fixed on a custom clamp that was designed to allow for rotation of the patella at 10 degree intervals, while keeping the loading axis aligned with the ligament to minimize moment. Two drill bits were placed through the patella without damaging the ligament and fixed on the patellar clamp to ensure that the patella did not rotate during tensile testing. The custom clamp was set up so that the patella was rotated 30 degrees externally to replicate the in-situ orientation of the patella for the natural orientation group (see Fig. 2A). For the non-natural orientation group, the MPFL was loaded with the patella aligned in the direction of the load (see Fig. 2B). For each group, 12 stifle joints were randomly assigned.
Fig. 2.
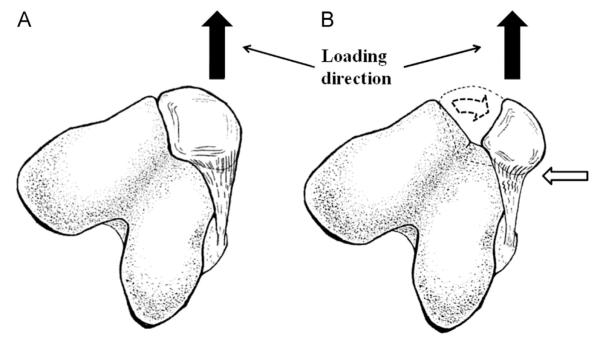
Orientations of the patella during uniaxial tensile testing: (A) Natural orientation. (B) Non-natural orientation. The white arrow highlights the non-uniform loading of those collagen fibers near the MPFL’s insertion at the patella.
Uniaxial tensile testing was performed following our well-established protocol for knee ligaments (Woo et al., 1986b). The specimen was mounted on a tensile testing machine (Instron, Norwood, MA) and kept in a saline bath at 32 °C throughout testing. After 30 min of equilibration in the saline bath, the tissue was preloaded to 2 N and pre-conditioned by subjecting it to cyclic tension between 0 mm and 2 mm at an extnesion rate of 10 mm/min for 10 cycles. After pre-conditioning, the specimen was preloaded to 2 N and subjected to a load-to-failure test at an extension rate of 10 mm/min. From the resulting load–elongation curves, structural properties of the FMPC obtained were stiffness (defined as the slope of the linear region of the load–elongation curve between 3 mm and 6 mm of extension), ultimate load, ultimate elongation, and energy absorbed. The mode of failure was also noted.
After testing the data sets for normality, independent t-tests were used to compare the CSA at the three different locations, stiffness, ultimate load, ultimate elongation, and energy absorbed between the two groups.
3. Results
Upon dissection of the 24 specimens, one had a CSA value 3 standard deviations higher than the mean and a second had visibly poor bone quality. Hence, they were not tested. For the remaining 22 specimens, the average CSA at the three locations, i.e. the patellar insertion, midsubstance, and the femoral insertion (see Fig. 1), were 105.6 ± 26.6 mm2, 69.4 ± 23.5 mm2, and 57.7 ± 19.6 mm2, respectively, for the natural orientation group; and 95.6 ± 23.1 mm2, 66.3 ± 13.6 mm2, and 54.9 ± 10.9 mm2, respectively, for the non-natural orientation group (see Table 1). There was no statistically significant difference in CSA between the two groups at all three respective locations (p > 0.05).
Table 1.
Cross-sectional area of the MPFL measured at the patellar insertion, mid-substance, and the femoral insertion.
| Location | Natural orientation group |
Non-natural orientation group |
|---|---|---|
| Patellar insertion (mm2) | 105.6 ± 26.6 | 95.6 ± 23.1 |
| Mid-substance (mm2) | 69.4 ± 23.5 | 66.3 ± 13.6 |
| Femoral insertion (mm2) | 57.7 ± 19.6 | 54.9 ± 10.9 |
p > 0.05 at all three respective locations.
During the uniaxial tensile testing, load–elongation curves of the FMPCs for both groups were obtained (see Fig. 3). The stiffness of the FMPC was measured to be 65 ± 13 N/mm and 50 ± 17 N/mm for the natural orientation group and the non-natural orientation group, respectively. The difference in stiffness was 32% (p < 0.05; see Table 2). The ultimate load was 438 ± 128 N for the natural orientation group and 386 ± 136 N for the non-natural orientation group, and the difference was not statistically significant (p > 0.05). The energy absorbed was 2141 ± 927 N-mm and 1828 ± 1078 N-mm, respectively, and again, not statistically significant (p > 0.05).
Fig. 3.
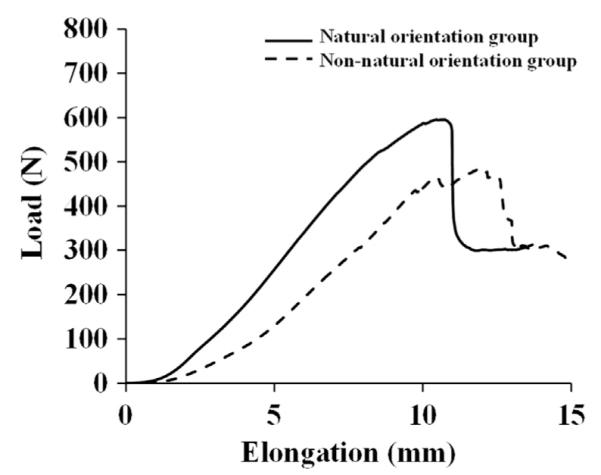
Typical load–elongation curves of the FMPC specimens tested in the natural orientation (solid line) and the non-natural orientation (dashed line).
Table 2.
The structural properties of the femur-MPFL-patella complexes under uniaxial tension.
| Properties | Natural orientation group |
Non-natural orientation group |
|---|---|---|
| Stiffness (N/mm)a | 65 ± 13a | 50 ± 17a |
| Ultimate load (N) | 438 ± 128 | 386 ± 136 |
| Ultimate elongation (mm) | 10.3 ± 2.2 | 10.5 ± 2.2 |
| Energy absorbed (N-mm) | 2141 ± 927 | 1828 ± 1078 |
Significant difference between the two groups (p < 0.05).
There were large differences in the failure modes. For the natural orientation group, all FMPCs failed at the femoral insertion either by ligament failure or through bony avulsion except for one specimen where failure occurred in the ligament substance (91%) (see Fig. 4A). Those from the non-natural orientation group had a large variation in the sites of failure as 6 out of 11 specimens failed at or near the femoral insertion, while the remaining 5 specimens failed at the patellar insertion: one through ligament failure and four by bony avulsion.
Fig. 4.
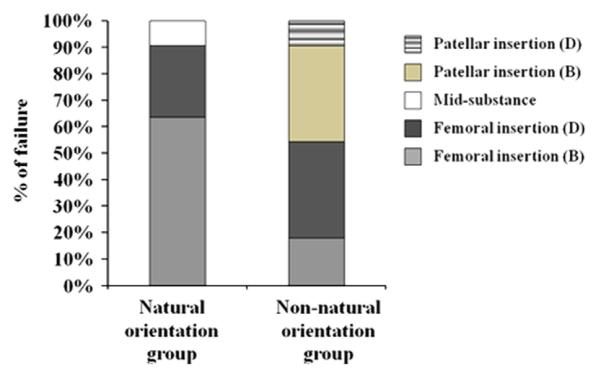
Distribution of the failure mode of the FMPC. D: Ligament detachment; B: bony avulsion.
4. Discussion
The results of this study showed that the orientation of the specimen during tensile testing has a significant effect on stiffness and failure modes of the FMPC. Stiffness is of significant importance, as this parameter represents how the MPFL behaves during normal function. With a natural orientation that closely reproduced the orientation of the FMPC in situ, its stiffness value was found to be about one-third higher than that for the non-natural orientation. This supported our hypothesis that keeping the FMPC specimens in the natural orientation would yield a higher stiffness value as this orientation retains proper fiber alignment. The current findings are also similar to the results of an earlier study of the femur-ACL-tibia complex, i.e. the structural properties of the FATC was higher when tested along its anatomical orientation (Woo et al., 1991).
The anatomy of the MPFL showed that its insertion to the patella spans widely over the curved edge of the medial surface of the patella. Due to this complexity, the patella is rotated away from its orientation in situ when the FMPC is tested in the non-natural orientation. As such, the fibers of the MPFL are no longer uniformly loaded during tensile testing, lowering the stiffness value. In fact, a longer toe region was observed in the load–elongation curves from the non-natural orientation group, suggesting that less fiber recruitment to resist tension at the beginning of loading had occurred. On the other hand, fibers of the ligament at the patellar insertion are aligned in its natural orientation, which resulted in more uniform loading and a higher stiffness value.
It was also found that specimens tested in the natural orientation failed predominantly at the femoral insertion where the cross-section was the smallest. This again confirmed that with the natural orientation, the fibers were loaded uniformly so that the FMPC would fail where the cross-section is the smallest. This finding was consistent with those observed in clinical studies (Ahmad et al., 2000; Sallay et al., 1996). On the other hand, with the non-natural orientation, failure locations varied as many FMPCs failed at or near the patellar insertion where the cross section was the largest. This clearly showed that the fibers were not uniformly loaded.
The results of the failure mode could also help to explain the absence of significant difference in ultimate load between the two groups. With the non-natural orientation, due to non-uniform loading of the fibers, only a portion of the cross-section was loaded. However, the mode of failure shifted toward the patellar insertion where the cross-section was the largest. The end result was that the cross-section at the failure point that was actually loaded with the non-natural orientation was not significantly lower than that of the natural orientation. Hence, the ultimate load did not significantly increase with the natural orientation.
A limitation of this study is that the results from the animal model may not be directly applicable to human FMPCs. Slight differences in anatomy between the porcine and human MPFL (Mochizuki et al., 2013) should be taken into account in future studies of the human MPFL. Nevertheless, our objective was to demonstrate that the specimen orientation of the FMPC is an important consideration when characterizing its structural properties. The large differences in the stiffness clearly call to attention the importance of natural orientation of the patella. Also, testing in the natural orientation ensures that the failure mode is consistent and representative. Obtaining correct data will be important in graft selection, as well as for modeling of the MPFL function. As such, experiments using a custom-designed device to properly orient the patella are recommended to obtain structural properties of the human FMPC that are more representative of the function of the MPFL.
Acknowledgements
Financial support from NIH T32 Biomechanics in Regenerative Medicine Predoctoral Training Fellowship (NIH/NIBIB EB003392-01) is gratefully acknowledged.
Footnotes
Conflict of interest statement All authors confirm they have no financial or other conflicts of interest relevant to this study.
References
- Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am. J. Sports Med. 2009;37:2021–2027. doi: 10.1177/0363546509336261. [DOI] [PubMed] [Google Scholar]
- Ahmad CS, Stein BE, Matuz D, Henry JH. Immediate surgical repair of the medial patellar stabilizers for acute patellar dislocation. A review of eight cases. Am. J. Sports Med. 2000;28:804–810. doi: 10.1177/03635465000280060701. [DOI] [PubMed] [Google Scholar]
- Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10:215–220. doi: 10.1016/s0968-0160(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Avikainen VJ, Nikku RK, Seppanen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin. Orthop. Relat. Res. 1993:12–16. [PubMed] [Google Scholar]
- Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin. J. Sports Med. 2005;15:62–66. doi: 10.1097/01.jsm.0000157315.10756.14. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996;28:1–11. [Google Scholar]
- Fithian DC, Paxton EW, Stone ML, Silva P, Davis DK, Elias DA, White LM. Epidemiology and natural history of acute patellar dislocation. Am. J. Sports Med. 2004;32:1114–1121. doi: 10.1177/0363546503260788. [DOI] [PubMed] [Google Scholar]
- Fuss FK. Anatomy and function of the cruciate ligaments of the domestic pig (Sus scrofa domestica): a comparison with human cruciates. J. Anat. 1991;178:11–20. [PMC free article] [PubMed] [Google Scholar]
- Gomes JL. Medial patellofemoral ligament reconstruction with half width (hemi tendon) semitendinosus graft. Orthopedics. 2008;31:322–326. doi: 10.3928/01477447-20080401-12. [DOI] [PubMed] [Google Scholar]
- Koskinen SK, Rantanen JP, Nelimarkka OI, Kujala UM. Effect of Elmslie-Trillat and Roux-Goldthwait procedures on patellofemoral relationships and symptoms in patients with patellar dislocations. Am. J. Knee Surg. 1998;11:167–173. [PubMed] [Google Scholar]
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg. Sports Traumatol. Arthrosc. 2013;21:305–310. doi: 10.1007/s00167-012-1993-7. [DOI] [PubMed] [Google Scholar]
- Mountney J, Senavongse W, Amis AA, Thomas NP. Tensile strength of the medial patellofemoral ligament before and after repair or reconstruction. J. Bone Joint Surg. Br. 2005;87:36–40. [PubMed] [Google Scholar]
- Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22:787–793. doi: 10.1016/j.arthro.2006.04.078. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22:e901–907. doi: 10.1016/j.arthro.2005.12.058. (904) [DOI] [PubMed] [Google Scholar]
- Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J. Bone Joint Surg. Am. 2008;90:463–470. doi: 10.2106/JBJS.G.00072. [DOI] [PubMed] [Google Scholar]
- Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am. J. Sports Med. 1996;24:52–60. doi: 10.1177/036354659602400110. [DOI] [PubMed] [Google Scholar]
- Schottle P, Schmeling A, Romero J, Weiler A. Anatomical reconstruction of the medial patellofemoral ligament using a free gracilis autograft. Arch. Orthop. Trauma Surg. 2009;129:305–309. doi: 10.1007/s00402-008-0712-9. [DOI] [PubMed] [Google Scholar]
- Schottle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus auto-graft for patella instability. Knee Surg. Sports Traumatol. Arthrosc. 2005;13:516–521. doi: 10.1007/s00167-005-0659-0. [DOI] [PubMed] [Google Scholar]
- Wang CH, Ma LF, Zhou JW, Ji G, Wang HY, Wang F, Wang J. Double-bundle anatomical versus single-bundle isometric medial patellofemoral ligament reconstruction for patellar dislocation. Int. Orthop. 2013;37:617–624. doi: 10.1007/s00264-013-1788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am. J. Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J. Biomech. 1986a;19:399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Orlando CA, Gomez MA, Frank CB, Akeson WH. Tensile properties of the medial collateral ligament as a function of age. J. Orthop. Res. 1986b;4:133–141. doi: 10.1002/jor.1100040201. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Danto MI, Ohland KJ, Lee TQ, Newton PO. The use of a laser micrometer system to determine the cross-sectional shape and area of ligaments: a comparative study with two existing methods. J. Biomech. Eng. 1990;112:426–431. doi: 10.1115/1.2891206. [DOI] [PubMed] [Google Scholar]
- Xerogeanes JW, Fox RJ, Takeda Y, Kim HS, Ishibashi Y, Carlin GJ, Woo SL-Y. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann. Biomed. Eng. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]


