Hydrogels are being increasingly called upon to perform complex functions in biological applications[1] Their assistance in fulfilling these roles is frequently hypothesized to depend upon their playing a temporarily dynamic role via controlled degradation.[2] A variety of hydrogel systems prepared with degradable polymers have been previously developed (e.g., poly(lactide) and its derivatives,[3,4] hyaluronic acid[5] gelatin[6] or polymers modified to be labile to hydrolysis,[7] gels crosslinked with enzymatically labile molecules[8]), in which the degradation rate is mainly regulated by various intrinsic and extrinsic chemical factors. However, controlling material degradation via a simple physical dissociation of polymer molecules may provide advantages over chemical degradation. In this report, we introduce a new approach to regulate the degradation kinetics of ionically crosslinked gels via controlling the dissociation rate of the polymer chains. We also demonstrate the importance of controlling the degradation of these hydrogels to the formation of cartilage tissues that result from cell transplantation.
To investigate whether the degradation rate of hydrogels could be regulated by the dissociation of ionically crosslinked polymer chains, we hypothesized that controlling the size mismatch between polymer segments that control ionic crosslinking would modulate the dissociation rate of polymers. To test this hypothesis, calcium-crosslinked alginate hydrogels consisting of alginates having different molecular weights (MWs) were used, and the molecular weight of the guluronic acid (G) blocks (MWG) in the polymer chains was varied in order to alter the size of the polymer segments responsible for ionic cross linking.[9] Specifically, high-MW alginate rich in G blocks (MVG; MW ~ 269 100 g mol−1), high-MW alginate rich in mannuronic acid (M) blocks (MVM; MW ~280 000 g mol−1), and low-MW MVG (MW ~53 100 g mol−1) rich in G blocks were used in these studies in order to differentiate between the effects of the overall polymer chain MW and effects of MWG. Measurement of MWG following isolation of G blocks from the various alginates showed that G blocks in the MVG polymer (MWG~5540 g mol−1) were 1.5 × longer than those in MVM (MWG ~ 3640 g mol−1). The low MW MVG, prepared by irradiating MVG with γ-rays, maintained the MWG of MVG (MWG~4960 g mol−1).
Six different gels were prepared from the different alginates. The gels were comprised solely of each of the high and low-MW polymers (unary MVG, unary MVM, and unary low MW MVG gels), a gel formed from a combination of MVG and low-MW MVG (binary MVG gel)[10], a gel from MVM and low-MW MVG (binary MVM gel I), and a gel from MVM and MVG (binary MVM gel II). The latter two gels exhibited size mismatch between MWG, whilst the remaining gels exhibited a similar MWG between the chains forming the gels, even though the polymer chain MWs were varied.
The initial physical properties of the varying hydrogels were quantified with the compressive elastic moduli (E) and swelling ratios (Q) of the gels. The physical properties of these were greatly dependent upon MWG, but only weakly dependent on MW (Table 1). Thus, a lower E value was calculated for unary MVM gels and a higher Q value than both high-MW MVG and low-MW MVG gels. In contrast, the E and Q values of unary low-MW MVG gels and binary MVG gels were comparable to those of unary MVG gels. This was likely due to the comparable MWG of alginate chains. In the same context, binary MVM gel I had a significantly increased value of E, as compared to unary MVM gels.
Table 1.
Compressive elastic modulus (E) and swelling ratio (Q) of the 5 wt.-% gels used in studies. The weight fraction of high-MW (W(high MW)) and low-MW (W(low MW)) polymer, containing either a high (MVG) or low fraction of G blocks (MVM), are indicated for each gel type.f
| Hydrogels | W(High MW) | W(Low MW) | E [kPa] | Q |
|---|---|---|---|---|
| Unary MVG | 1.0 | 0 | 135±11 | 16±0.5 |
| Unary low MW MVG | 0 | 1.0 | 110±9 | 19±2 |
| Binary MVG | 0.5 | 0.5 | 130±10 | 20±0.3 |
| Unary MVM | 1.0 | 0 | 54±6 | 25±0.6 |
| Binary MVM I | 0.5 | 0.5 | 90±4 | 22±0.1 |
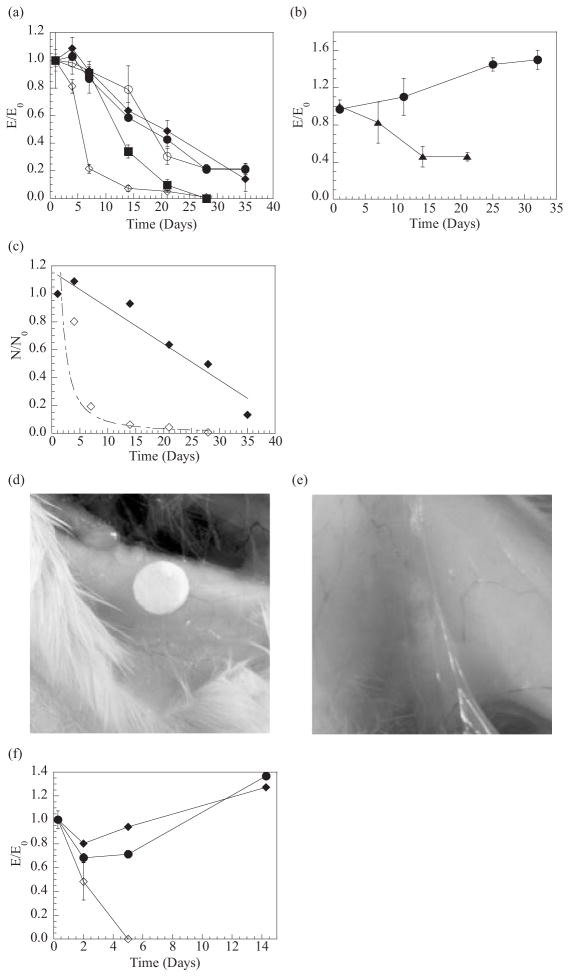
The in-vitro degradation of these hydrogels was examined by following the changes in E and Q values over time in order to determine the effects of the size mismatch of the crosslinking blocks on degradation. Throughout an incubation period in Ca2+-free phosphate-buffered saline (PBS), unary gels exhibited faster decreases in the value of E with reductions in the MW of the polymer, as expected[11] (Fig. 1a). The reductions in MWG of the polymer did not alter the rate of decrease in E for the high-MW unary hydrogels. Binary MVG gels also maintained 50 % of the initial E value for 3 weeks, similar to the unary high-MW hydrogels. In addition, no significant change in Q was observed with binary MVG gels and other high-MW unary hydrogels. In contrast, binary MVM hydrogel I containing the crosslinking zone size mismatch demonstrated a faster reduction in E and increase in Q than the low-MW unary gels (i.e., reduction of E by 90 % within 2 weeks, followed by complete dissolution within 4 weeks). The decrease in E of the binary MVM gels was also faster than the unary MVM gels, which were initially softer. The rates of change in the E and Q values of binary MVM gels could also be mediated by varying the weight fraction of low-MW MVG in the gels (not shown). Changes in the MW of the polymers used to form binary MVM gels (i.e., binary MVM gels II) or in the incubation condition (i.e., incubating gels in Ca2+-containing cell-culture medium) did not alter the accelerated degradation of gels formed with a mismatch in the size of the crosslinking zone (Fig. 1b).
Figure 1.
In-vitro and in-vivo degradation of hydrogels prepared with polymer chains having different molecular weight (MW) or MWG. In-vitro experiments showed that binary MVM hydrogels possessing a mismatch in the size of crosslinking junctions exhibited faster reductions in elastic modulus (E) (a),(b), and reductions in the number of crosslinks (N) (c), as compared with other hydrogels formed from polymers of a uniform MW or a mix of high-and low-MW polymers all having the same size of segments to form crosslinking junctions. Hydrogels possessing a crosslinking zone size mismatch (i.e., gels consisting of MVG and MVM) also showed a faster reduction in E, as compared with unary MVG gels (b). Data in (a) and (b) were obtained from gels incubated in PBS and α-MEM, respectively. In-vivo experiments conducted via implantation of gel disks into SCID mice demonstrated that binary MVG gels maintained their structure over the entire time period (~100 days) (d), while binary MVM gels fragmented, or disappeared (e). Measurements of E from retrieved gels revealed an increase in E for unary MVG and binary MVG gels, and decrease in E of binary MVM gels over time (f). -●- Unary MVG hydrogels, -○- unary MVM hydrogels, -■- unary low-MW MVG hydrogels, -◆- binary MVG hydrogels, -◇- binary MVM hydrogels, and -▲-hydrogels consisting of high-MW MVG and high-MW MVM. E0: elastic modulus measured after 1 day.
A calculation of the number of crosslinking junctions (N) of the gels in these conditions demonstrated that N of binary MVG gels decreased in a linear manner after the first week (i.e., N ∝ (Time)) (Fig. 1c), like other high-MW unary gels (not shown). In contrast, N of binary MVM gels decreased following a power law (i.e., N ∝ (Time)−α) (Fig. 1c). Raising the fraction of low-MW MVG in the binary MVM gels I from 0.25 to 0.50 increased the value of α from 0.2 to 1.3. The control over degradation rate by the size mismatch of crosslinking segments was also observed at other alginate concentrations (i.e., ranging from 2 to 5 wt.-%. In addition, the reductions in MW of polymers while maintaining the difference in MWG, led to another increase in the degradation rate of the gels, likely due to the enhanced dissolution of polymers following dissociation. These results thus suggested that the degradation kinetics of the gels can be modulated over a broad range with the proper selection of G block size, polymer molecular weights, and total polymer concentration.
In-vitro degradation studies enable one to predict in-vivo degradation behavior. Unary MVG, binary MVG, and binary MVM gel I disks were implanted into the backs of SCID mice, and explanted at various times. Both unary and binary MVG gels largely maintained their original shapes without any visual evidence of breakdown (Fig. 1d), as expected from the in-vitro studies. In contrast, binary MVM hydrogel disks were largely fragmented after two weeks, followed by an extensive fragmentation, or disappearance after 100 days (Fig. 1e). Histological examination of thin sections of the gels also confirmed that binary MVG gels maintained their structure over 100 days, with little infiltration of fibrous tissues into the gels. In contrast, binary MVM gels were fragmented after the infiltration of fibrous tissues (not shown). The unary and binary MVG gels demonstrated small initial decreases in E, followed by an increase in E after the first 2 weeks (Fig. 1f). This later increase is likely to be attributed to the growth of calcium phosphate phases in the gels (data not shown). In contrast, binary MVM gels exhibited a constantly decreasing E value over time, as observed in vitro.
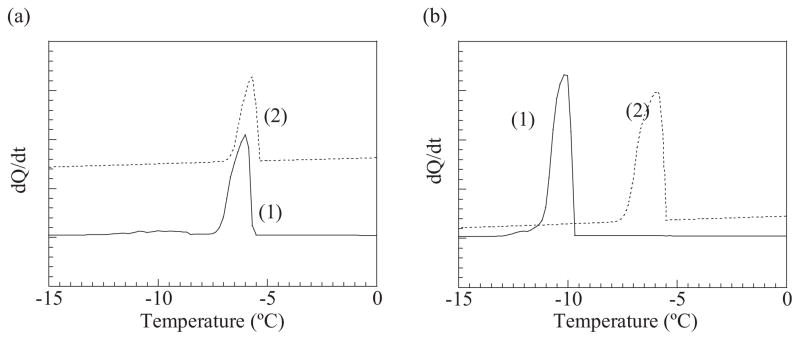
The importance of size match/mismatch in the crosslinked junctions in the degradation rate was reconfirmed by following the changes in the pore size of the gels that were evaluated from the freezing point of water (Eq.2, Experimental). The freezing point of water within the fresh binary MVG hydro-gels was higher than that for fresh binary MVM gels (Fig. 2). This result illustrates that binary MVG gels possessed larger pores than the binary MVM gels. This is likely due to the stiffer and poorly packed polymer chains that are present in binary MVG gels.[12,13] Incubating the binary MVG gels for two weeks led to limited changes in the freezing point of water (Fig. 2a), while binary MVM gels exhibited a significant increase in the freezing point, which represented an increase in the average pore diameter from 6 to 9 nm (Fig. 2b). This result clearly confirms that binary MVM gels underwent a more rapid loss of crosslinks over time, causing larger pores within the gels, than the MVG gels.
Figure 2.
Changes in the freezing point of water entrapped in the pores of gels were measured with differential scanning calorimetry (DSC). The binary MVG gels showed little changes in the freezing point of water during the incubation of the gels over two weeks (a). In contrast, the increases in the freezing point of water in binary MVM gels indicated an increase in the pore diameter of the gels, likely due to significant degradation of the gels (b). Curves (1) and (2) correspond to gels incubated for one day and two weeks, respectively.
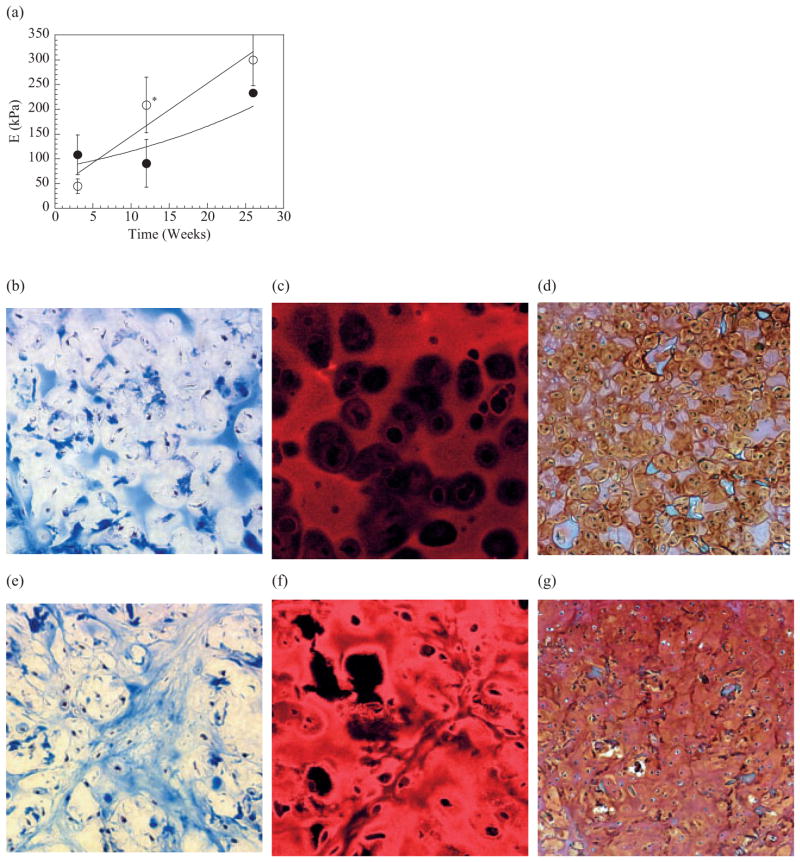
To establish that this approach (to regulate gel degradation) is useful in tissue regeneration applications, chondrocytes were transplanted using binary MVG and MVM gels. Tissues explanted after 12 weeks demonstrated characteristics of cartilage tissue (eg., white opalescence, qualitatively stiff). However, the quality of the engineered tissues was greatly dependent upon the degradation rate of the gels. Tissues that were grown using the more rapidly degrading binary MVM gels were largely more rigid than tissues from binary MVG gels. This qualitative evaluation was confirmed by mechanical testing which demonstrated higher elastic moduli (E) of tissue from binary MVM gels compared to binary MVG gels after 12 weeks in vivo (Fig. 3a). A histological evaluation of the engineered tissues also indicated that the degradation rate affected the cellularity and deposition of collagen, specifically type II collagen, and glycosaminoglycan (GAG) within the engineered tissues. Deposition of collagen in cartilagenous tissues that were engineered by using binary MVG gels was limited mainly to the interstitial space between large gel fragments (Figs. 3b,c), and islands of tissue containing glyco-saminoglycans (GAGs) (Fig. 3d). In contrast, tissues engineered by using binary MVM gels demonstrated abundant chondrocytes within lacunae, as is observed with normal cartilage, and extensive and more uniform collagen and GAG deposition surrounding small areas of residual gel (Figs. 3e-g). The higher intensity of the type II collagen and GAG staining in tissues constructed using binary MVM gels, as compared to those using binary MVG gels, also suggests the formation of more mature cartilage tissue in the rapidly degrading gels.
Figure 3.
Cartilagenous tissues formed via implantation of hydrogel disks containing primary bovine chondrocytes into SCID mice. The more rapidly degrading binary MVM gels led to a greater increase in the elastic modulus (E) of the engineered tissue over time, even though the MVG gels were initially more stiff a) Use of binary MVG gels for chondrocyte transplantation appeared to limit the development of new cartilage tissues over the entire time period (24 weeks) as a diffuse staining for collagen b) immunofluorescent staining for type II collagen c) and a staining for GAGs d) were observed in these tissues. In contrast, the binary MVM gels led to a higher cellularity and larger deposition of collagen e) specifically type II collagen f) and GAGs g) in the engineered tissues. * indicates differences between two conditions were statistically different (p<0.05). Tissues shown in (b) and (e) were stained with Mason’s Trichrome blue, and type II collagen in samples in (c) and (f) was visualized with a rhodamine-conjugated secondary antibody. Samples in (d) and (g) were stained with Safarin-O. All tissue sections were obtained from samples implanted for 24 weeks, and analyzed. Size bars (bar = 50 or 100 μm) are shown in figures.
The results of this study demonstrate a new material design that allows one to control the degradation of hydrogels, without utilizing conventional chemical or enzymatic degradation pathways. We showed the faster loss of crosslinks within gels that possessed geometrically mismatched crosslinked junctions, as indicated by reductions in mechanical rigidity; increases in the swelling ratio, and changes in the pore size of the gels. We interpret these results to indicate that the size match/mismatch between ionically crosslinking blocks in polymer chains altered the resistance of the gels to an ion-exchange process, due to the changes in hydrophobicity of the crosslinked junctions. The crosslink junctions composed of the G blocks with an equivalent MWG are proposed to be highly stable against ion exchange, because the majority or perhaps all of the anionic carboxylic acids in the sugar residues comprising the G block are involved in the crosslinking, thus making the crosslinking junctions hydrophobic. In contrast, a crosslinking junction formed from two G blocks having different MWG samples would leave carboxylic acids not participating in the crosslinking, and would increase the hydrophilicity of the junction. In this situation, counterions could more readily access the hydrophilic crosslinking junctions, thus accelerating the dissociation between G blocks, irrespective of the total MW of polymers. The kinetics of the ion-exchange process and consequent gel degradation could also be controlled by the fraction of crosslinked junctions in the gel possessing a size mismatch. Modification of the gel degradation rate with this approach was effective in regulating the quality of engineered cartilage tissues. This was probably due to the fact that the accelerated degradation provided space that was essential for new tissue formation. It has been previously reported that the degradation rate of gels encapsulating chondrocytes can regulate their ability to form cartilage-like tissues in vitro.[14] However, the data provided in this manuscript is the first demonstration of this relationship in vivo. It is also possible that host cells migrated into the rapidly degrading gels more effectively, and participated in the tissue formation coupled with the transplanted chondrocytes. We envisage that adjusting the differences between MWG samples in a more refined manner, potentially via the biosynthesis of these polymers[15] may broaden the ranges of the degradation rates, achievable with this system. The method described here to control gel degradation may also be reproduced with synthetic block copolymers consisting of ion-ically or hydrophobically crosslinkable blocks and non-crosslinkable blocks.
Experimental
Characterization of Alginate
Molecular weights of alginates (supplied from FMC Technologies) were determined with a size-exclusion chromatographic system equipped with triple detectors (Viscotek) including a laser refractometer (LR 40), a differential viscometer (T60), and a right angle laser light scattering detector (RALLS). Fractions of G and M residues in alginate molecules were determined with a circular dichroism (CD) spectrometer (AVIV 202). The molecular weights of G blocks isolated by acid-base and thermal treatments of alginate molecules [7] were measured with the size-exclusion chromatographic system.
Preparation of Hydrogels and Characterization of Gel Properties
Hydrogels were formed by mixing alginate solutions with 0.2 g mL−1 CaSO4 (Sigma) aqueous slurries at a molar ratio of 1.0:0.3. The mixtures were cast between two glass plates separated by spacers with 2 mm thickness. Gel disks having a diameter of 6 or 12 mm were punched after 2 h, and incubated in minimum essential medium alpha medium (α-MEM Gibco) for one week, followed by incubation in phosphate-buffered saline (Gibco) or α-MEM at 37 °C until the testing of the physical properties. The medium was exchanged once a week. To avoid biological contamination during the incubation, all components of the gels and medium were pre-sterilized.
Compressive elastic moduli (E) of hydrogels were measured with a mechanical testing system (MTS Bionix 100) at 25 °C The slopes of stress versus strain curves obtained from a constant deformation at 1 mm min−1 were used in order to calculate the elastic moduli. Assuming alginate hydrogels follow an affined network model, the shear modulus (S) was also obtained from the slope of stress versus −(λ − λ−2) plot, where λ is the ratio of the deformed length to the undeformed length of the hydrogel. Degree of swelling (Q), was calculated from Q= ν2−1=[(1/ρp)(Qm/ρw+1/ρp)−1]−1where ρp is the polymer density (0.88 g cm−3), ρw is the density of water, and Qm is the mass ratio of absorbed water to the dried gel. Effective number of crosslinks (N) was determined from S and Q, based on the rubber elasticity theory [16]
| (1) |
where R is the gas constant (8.314 J moL−1 K−1), and T is the temperature at which the modulus was measured. Tests were performed on quadruplicate gels. To monitor the degradation of gels, the measurements of E and Q were conducted on a weekly basis for 30 to 35 days.
In-Vivo Gel Degradation Experiment
Hydrogel disks (6 mm diameter) were subcutaneoulsy implanted into the backs of male 4–5 week old anesthetized CB-17 SCID mice (Taconic Farms Inc., German-town, NY) (n = 4/time point condition). Hydrogel disks were harvested after 2, 4, and 14 weeks, mechanically tested, and processed for histological analysis by paraffin embedding, sectioning, and staining with hematoxylin and eosin.
Measurement of Pore Size of the Gel
The pore size of the gels was measured following Iza’s procedure, which utilizes ambient differential scanning calorimetry (DSC, Perkin Elmmer) [17]. In this measurement, the enthalpic change of a gel placed in the chamber was monitored, while decreasing the temperature from 15 to −30 °C at a rate of 5°Cmin−1. Since the behavior of water (i.e., freezing point of water molecule) entrapped within the pores of the gels depends on the pore size, the heat emitted under cooling and at the freezing point of water were related to the pore structure of the gels. From the heat measured (Q) versus temperature curves, the volume distribution of the pores (dV/dR) and pore size (R) were calculated, following these equations,
| (2) |
where Ttri is the triple point temperature of water, k is a factor depending on the cooling rate and mass of sample, and Wa is the energy of solidification of water expressed by −5.56 × 10−2(T − Ttri)2 −7.43(T − Ttri) −332.
Chondrocyte Transplantation in Gels and Histological Evaluation of Explants
Primary bovine chondrocytes isolated from calf forelimbs [18] were transplanted in binary MVG and MVM gels at a density of 40 × 106 cells per mL. Cell-alginate solutions were mixed with CaSO4 aqueous slurries to form the gels. The gel disks were implanted in anesthetized CB-17 SCID mice (n= 4/time point condition), and were retrieved after 2, 12, and 24 weeks. The elastic moduli of the explants were measured to assess mechanical rigidity. Then, the explants were fixed in 10 % buffered formalin, dehydrated in ethanol, embedded in paraffin. Sectioned samples were stained with Mason’s Trichrome blue and Safarin-O. The sectioned samples were also immunostained with a primary antibody directed against type II collagen (Collagen II mouse monoclonal antibody, Neo Markers) using a rhodamine conjugated secondary antibody (Rhodamine goat anti-mouse IgG, Jackson Immunolab.) for detection [19]. The immunostained samples were imaged by collecting the fluorescence emitted between 580 and 620 nm, with excitation at 543 nm, using laser scanning confocal microscopy (Leica).
Contributor Information
Dr. Hyun Joon Kong, Division of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138 (USA)
Dr. Eben Alsberg, Biomedical Engineering Department, University of Michigan, 2200 Bonisteel Avenue, Ann Arbor, Ml 48109 (USA)
Dr. Darnell Kaigler, Department of Biological and Material Sciences, Dentistry, University of Michigan, Ann Arbor, Ml 48109 (USA)
Prof. Kuen Yong Lee, College of Chemical Engineering, Hanyang University, Seoul 133-791 (South Korea)
Prof. David J. Mooney, Email: mooneyd@deas.harvard.edu, Division of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138 (USA)
References
- 1.Ratner BD, Hoffman AS. In: Hydrogels for Medical and Related Applications. Andrade JD, editor. Vol. 31. American Chemical Society; Washington DC: 1976. p. 1. [Google Scholar]
- 2.Lee KY, Mooney DJ. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 3.Sawhney AS, Pathak CP, Hubbell JA. Macromolecules. 1993;26:581. [Google Scholar]
- 4.Anseth KS, Burdick JA. MRS Bull. 2002;27:130. [Google Scholar]
- 5.Luo Y, Kirker KR, Prestwich GD. J Controlled Release. 2000;69:169. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 6.Tabata Y, Ikada Y. Adv Drug Delivery Rev. 1998;31:287. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 7.Bouhadir KH, Hausman DS, Mooney DJ. Polymer. 1999;40:3575. [Google Scholar]
- 8.Lutolf MR, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Nat Biotechnol. 2003;21:1513. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 9.Smidsrod O, Skjak-Braek G. Trends Biotechnol. 1990;8:71. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 10.Kong HJ, Lee KY, Mooney DJ. Polymer. 2002;43:6239. [Google Scholar]
- 11.Alsberg E, Kong HJ, Hirano Y, Smith MK, Alberiruti A, Mooney DJ. J Dent Res. 2003;82:903. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 12.Franse MWCP, Nijenhuis K. J Mol Struct. 2000;554:1. [Google Scholar]
- 13.Stokke BT, Draget KI, Smidsrod O, Yuguchi Y, Urakawa H, Kajiwara K. Macromolecules. 2000;33:1853. [Google Scholar]
- 14.Bryant SJ, Durand KL, Anseth KS. J Biomed Mater Res. 2003;67:1430. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 15.Ott CM, Day DF. Trends Polym Sci. 1995;3:402. [PMC free article] [PubMed] [Google Scholar]
- 16.Anseth KS, Bowman CN, Brannon-Peppas L. Biomaterials. 1996;17:1647. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 17.Iza M, Woerly S, Danumah C, Kaliaguine S, Bousmina M. Polymer. 2000;41:5885. [Google Scholar]
- 18.Paige KY, Cima LG, Yaremchuck MJ, Vacanti JP, Vacanti CA. Plast Reconstr Surg. 1995;96:1390. doi: 10.1097/00006534-199511000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Miralles G, Baudoin R, Dumas D, Baptiste D, Hubert P, Stoltz JF, Dellacherie E, Mainard D, Netter P, Payan E. J Biomed Mater Res. 2001;57:268. doi: 10.1002/1097-4636(200111)57:2<268::aid-jbm1167>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]