Abstract
Introduction
18F-Fluoroestradiol (FES) PET imaging provides a non-invasive method to measure estrogen receptor (ER) expression in tumors. Assessment of factors that could affect the quantitative level of FES uptake is important as part of the validation of FES PET for evaluating regional ER expression in breast cancer.
Methods
This study examines FES uptake in tumors from 312 FES PET scans (239 patients) with documented ER+ primary breast cancer. FES uptake was compared to clinical and laboratory data; treatment prior to or at time of scan; and properties of FES and its metabolism and transport. Linear mixed models were used to explore univariate, threshold-based, and multivariate associations.
Results
Sex-hormone binding globulin (SHBG) was inversely associated with FES SUV. Average FES uptake did not differ by levels of plasma estradiol, age, or rate of FES metabolism. FES tumor uptake was greater for patients with a higher body mass index (BMI), but this effect did not persist when SUV was corrected for lean body mass (LBM). In multivariate analysis, only plasma SHBG binding was an independent predictor of LBM-adjusted FES SUV.
Conclusions
Calculation of FES SUV, possibly adjusted for lean body mass, should be sufficient to assess FES uptake for the purpose of inferring ER expression. Pre-menopausal estradiol levels do not appear to interfere with FES uptake. The availability and binding properties of SHBG influence FES uptake and should be measured. Specific activity did not have a clear influence on FES uptake, except perhaps at higher injected mass/kg. These results suggest that FES imaging protocols may be simplified without sacrificing the validity of the results.
Keywords: FES PET, breast cancer, SHBG, specific activity
Introduction
Positron Emission Tomography (PET) using 16 α-[F-18]-fluoro-17-β-estradiol (FES) imaging is a method for imaging functional ER expression in-vivo, and may be used as a quantitative measure of estrogen receptor (ER) expression in breast cancer [1, 2]. FES PET may offer complementary advantages to in vitro assay of biopsy material, including the measurement of ER binding, identification of heterogeneous expression over the entire burden of disease, and measurement of the pharmacodynamic effect of ER-directed therapy [3]. The factors influencing FES uptake, however, are incompletely understood. Determining the factors that affect FES uptake, other than the desired dependency on ER expression levels, contributes to further understanding of this novel diagnostic tool and its use to measure regional ER expression.
Serum estrogen levels vary with menopausal status and drug therapy. Aromatase inhibitors reduce serum estrogen levels, and tamoxifen is an estrogen receptor blocking agent with variable impact on serum estrogen levels [4–6]. The influence of circulating estrogens in the physiologic range in humans on FES tumor uptake is unknown. Some prior reports have hypothesized that competition with circulating estrogens in pre-menopausal patients might lead to decreased FES uptake [7, 8].
FES is metabolized rapidly in vivo in both animals and humans [9, 10]. In humans, only about 20% of circulating radioactivity in the plasma is in the form of non-metabolized FES at 20 minutes after injection. The rate of FES metabolism varies somewhat between patients and may affect the availability of FES in the blood and thus the level of uptake in tumors [10].
In the blood, estrogens are transported by and bound tightly to the sex-steroid binding protein (also known as sex hormone binding globulin (SHBG)), which affects their transport and delivery [11]. Kiesewetter showed that FES binding to SHBG is similar to that for estradiol [12]. Tewson, et al. showed that approximately 45% of FES in circulating plasma is bound to SHBG, but varied between patients and was dependent on the level of SHBG in the plasma [13]. In mature rat hepatocytes, Jonson et al, [14] postulated that SHBG may potentiate the ER-mediated uptake of FES in ER+ tumors by selectively protecting the ligand from metabolism and ensuring its delivery to receptor-containing cells, and suggested that SHBG binding was necessary for an effective PET ER imaging agent. On the other hand, the “free hormone hypothesis” suggests that, like estradiol, FES bound to SHBG might be less available to tissue and thus less likely to reach the estrogen receptors [11], resulting in lower FES uptake.
Prior studies have suggested that high FES specific activity measured at injection (SAinj), and thus low injected mass, is necessary to visualize and quantify ER concentration without competition from “cold” estrogens [15]. While a limiting value of 37000 GBq/mMol (1000 Ci/mMol) has been suggested as a lower limit for acceptable SAinj, this value has not previously been tested in patients. The limits for acceptable SAinj have important implications for FES quality assurance and for the ability to image multiple patients from a single FES production run without reducing the value of the image data.
The purpose of this study was three-fold: 1) to determine if patient factors, such as age, weight, menopausal status, BMI, serum estradiol levels, and serum SHBG, affect the level of FES uptake; 2) to examine the effect specific activity has on FES uptake; 3) to determine the effect of FES metabolism and protein binding on uptake. We would expect that low serum estradiol levels, low SHBG levels and binding, and high specific activity would all predict higher FES uptake. Understanding the factors that affect FES uptake could lead to more streamlined imaging protocols.
Methods
Patients
From 1/96 to 11/06, 391 FES scans were performed at the University of Washington Medical Center. The patients were enrolled through one of several protocols at our center to assess FES uptake and metabolism, the heterogeneity of FES uptake in patients with advanced breast cancer, the correlation of FES uptake to in vitro assay of ER, and change in FES uptake during hormonal therapy. All of the patients had met the eligibility requirements of one of the approved protocols from Radioactive Drug Research Committee (RDRC) and University of Washington Human Subjects and signed informed consent.
For this report, from patients imaged with FES at our center, we selected patients who had a documented ER+ primary breast cancer, with at least one disease site greater than 1.5 cm to avoid severe partial volume effects, and were not taking ER blocking agents such as tamoxifen or fulvestrant for at least 6 weeks prior to scanning. Multiple scans for the same patient were included. Of the 391 FES scans performed, 312 met these criteria. The exclusions were due to no visible viable tumor (n=35), ER− primary disease (n=11), patient on tamoxifen or fulvestrant at the time of the study (n=29), or technical problems (n=4). The remaining 312 studies of 239 patients were analyzed for this report. Of the 51 patients with multiple scans 15% (36/239) had 2 scans, and 6% (15/239) had 3 or more scans,.
FES synthesis
The synthesis procedure 16α-[F-18]-fluoro-17β-estradiol (FES) follows that reported by Lim et al and modified by Romer et al [13, 16, 17]. Reagents and solvents for synthesis and purification were obtained from ABX Biochemicals, Sigma Aldrich Chemical Co., Mallinckrodt Baker, or from USP suppliers and were used without further purification unless otherwise noted. 18F was produced using either a Siemens Eclipse or a Scanditronix MC-50 cyclotron. Quality control tests were performed immediately following each synthesis to evaluate radiochemical and chemical purity and to calculate specific activity of each dose. Prior to May of 2000 (n=45), the specific activity of each dose was measured using HPLC analysis and UV detection and are not used in this analysis. After May 2000, (n=267), the analysis was performed with HPLC-MS (HPLC- Mass Spectrometry) (Waters 2690 and MicroMass ZMD (ES−)). Specific activity was recorded as GBq/mMol (Ci/mMol) at the time of injection. A typical injection of FES consisted of approximately 185 MBq (5.0 mCi) (range 103.6–296 MBq (2.8–8.0 mCi)) of radiopharmaceutical in 20 mL of isotonic phosphate buffered saline containing less than 15% of ethanol by volume. The mass injected per unit patient weight (μmole/kg) was also recorded.
PET imaging
All imaging was performed on a GE Advance tomograph (Waukesah, WI). The protocol for PET imaging has been previously described [10, 18]. Briefly, following an attenuation scan of 20–25 minutes, FES was injected over 2 minutes. Sixty minutes of dynamic imaging over the main area of interest was followed by a five field-of-view (FOV) sweep to further assess the extent of disease.
Blood Analysis
Whenever possible, a second venous access site was used for blood sampling during the scan for measures of FES metabolism and SHBG binding.
The methods for processing the plasma used for HPLC metabolite analysis to determine blood radioactivity present as FES versus labeled metabolites (%FES) have been previously published [10]. Briefly, for HPLC analysis, a 50 μl sample of filtered plasma collected at 5, 20, or 60 minutes post injection was injected onto a 4.6 × 250 mm (10μm) C-18 column (Econosil Alltech, Deerfield IL). The column eluant fractions were collected and counted in the same gamma counter (Packard Cobra, Meridian, CT) used for the blood samples. The blood samples collected at 20 minutes after injection were used in this analysis.
The methods for SHBG analysis at the time of the study have also been previous reported [13, 19]. A plasma sample collected prior to injection was spiked with a small quantity ((~0.074 MBq (~ 2 μCi)) of FES. Four hundred μL of the plasma was placed into each of 2 tubes. Non-radioactive dihydrotestosterone (DHT) (Steraloids Inc, Wilton NH) 0.75 ng/100 μL, was added to one of the tubes. The tubes were incubated then cooled and an equal volume of saturated ammonium sulfate solution was added. The tubes were centrifuged and the supernatant was decanted into a separate tube. An equal volume of water was added to the tube containing the pellet. Samples were then counted in the same well detector gamma counter as all other samples. Specific binding of the tracer was calculated as:
Pellet and supernatant refer to the background corrected counts in those fractions and the DHT subscript refers to the sample to which an excess of DHT was added to displace the FES.
Additional clinical and laboratory measures
For most patients, serum was collected prior to FES injection, was frozen and then sent for assay of plasma SHBG levels (nM) with dual antibody immunoassay (ARUP laboratories™ operating procedure CORE-99375 (Salt Lake City, Utah).
Blood samples, taken just prior to injection, were also collected and sent to Laboratory Services at the University of Washington for standard clinical assays of estradiol, testosterone and albumin. Estradiol detectable limits by this assay were 20 pg/mL and testosterone detectable limits were 0.5 ng/mL or 0.2 ng/mL as reported by Laboratory Services. Estradiol levels below 30 pg/mL were considered to be postmenopausal per guidance of the UWMC laboratory. Albumin was reported in g/dL (normal range 3.5–5.2 g/dL).
Image analysis
As in earlier studies [2, 10, 18], the primary FES uptake measure used in this analysis was the mean SUV of peak ROIs on summed images over the last 30 minutes of imaging (30–60 minutes). ROIs were placed using the aid of correlative anatomic imaging (mammogram, ultrasound, or CT), the PET transmission scan, and/or FDG-PET images, as needed. Square ROIs of 16 pixels each (~1.5 cm) were placed over the tumor site on three adjacent imaging planes. SUV was calculated by the commonly used formula: SUV = tissue activity (μCi/mL)/(injected dose/patient weight) (mCi/Kg). For patients with more than 1 tumor visible in the dynamic field-of-view, the mean SUVs for up to 3 tumors was averaged. To avoid the confounding effects of severe partial volume effects, only lesions with diameters of 1.5 cm or greater were included.
An alternative measure of FES uptake was analyzed to further examine the association between FES uptake and patient weight. SUV corrected for lean body mass, SUVLBM, substituted lean body mass (LBM) for patient weight in calculating SUV, with LBM calculated using the James method [20].
Statistical analysis
Our overall goal was to identify factors that affect FES uptake besides the level of ER expression. Ideally this would also consider an independent measure of ER expression, such as assay of biopsy material, in the analysis. However, biopsy of all lesions in the large population of patients studied, most with multiple sites of metastatic disease, was neither practical nor ethical. We instead tested for an association of these factors with FES uptake level in a cohort of patients documented as having ER-expressing tumors. Although ER expression levels varied across patients, the large number of patients studied permits fairly robust tests to screen for other factors that might affect FES uptake. Few, if any of the factors tested, for example specific activity and FES metabolism, have a known or suspected independent association with ER expression. Some factors, for example, menopausal status, could have a minor association with ER expression levels; however, selecting only patients with ER-expressing tumors mitigated the possible effect of such associations.
Associations between factors and FES SUV uptake were first explored through scatterplots and smoothed local linear polynomial (LOWESS) fits [21]. Following suitable transformations to satisfy linear model assumptions, univariate and multivariate linear mixed models were fitted with random intercepts to account for patient-level clustering. Potential linear, non-linear, and threshold-based patterns were also explored based on mechanisms proposed in the FES literature as described in the Introduction.
To address the relationship between FES specific activity measured at injection (SAinj) and FES uptake at low values of SAinj, we examined a subset of scans with low SAinj (≤1200 Ci/mMol, based upon prior reports [7, 8]) separately. A post hoc power calculation [22] was used to estimate the strength of association that could be observed with 93 scans conducted with SAinj ≤44,400 GBq/mMol (≤1200 Ci/mMol). Observed values for the subset with low SAinj and the full sample were used to choose appropriate transformations, and to estimate standard deviations for SAinj and log(SUV). Observed standard deviations were used to interpret correlation coefficients as slopes, using the easily derived relationship between correlation and univariate linear regression [23]. Fisher’s Z transformation was used to normalize the sample correlation coefficient, so that a sample size calculation for the normal distribution could approximate the minimum detectable effect size for the association between SAinj and FES SUV. For a linear model with SAinj (standard deviation = 10,360 GBq/mMol (280 Ci/mMol)) predicting log(FES SUV) (standard deviation = 0.66), a correlation of 0.28 would correspond to a 7% difference in SUV for scans similar for a 3,700 GBq/mMol (100 Ci/mMol) difference in specific activity, and a 39% difference in SUV for a 18,500 GBq/mMol (500 Ci/mMol) difference in SAinj (within the 0–44,400 GBq/mMol (0–1200 Ci/mMol) range for SAinj). Similarly, a correlation of 0.32 corresponds to SUV differences of 8% and 46% for a 3700 GBq/mMol (100 Ci/mMol) and a 18,500 GBq/mMol (500 Ci/mMol) point difference in SAinj. For two-sided tests at the α = .05 level, a sample of 93 scans would have 80% power to detect a correlation of 0.28, and 90% power to detect a correlation of 0.32.
Three hundred twelve studies were included in the initial analyses. A sensitivity analysis excluded scans with a least one known lesion that was qualitatively ER− by FES imaging (n=80) to account for the possibility of loss of ER expression with recurrence and metastasis [24], since ER+ status was pathologically confirmed only for the primary breast tumor at the time of initial diagnosis for most patients. The sensitivity analysis also excluded patients who were on chemotherapy at the time of the scan (n=50, including 21 who also had at least one qualitatively ER− lesion by FES imaging), due to the known effects of chemotherapy on tumor viability.
Results
Table 1 summarizes the characteristics of the 312 FES studies analyzed for 239 breast cancer patients with previously documented ER+ primary tumors. Nearly all scans (298/312) were conducted in patients with advanced stage breast cancer (Stage III or IV). Two hundred ninety-eight of the scans were conducted on female patients (67% postmenopausal), and 14 on males. The average age at scan time was 54 years (range 23–88). The weight range was 46–156 kg (mean 74 kg), and the average body mass index (BMI) was 27 (range 18–55). Patients’ serum estradiol levels were 30 pg/ml or greater for 27% (82/309) of scans, and testosterone levels were 0.5 ng/ml or greater for 25% (75/296). Excluding scans where therapy history was uncertain, 51% (155/301) were performed in patients with remote prior exposure to tamoxifen, 57% (170/297) with remote prior radiation therapy, and 74% (220/298) with remote prior chemotherapy.
Table 1.
Descriptive Characteristics for N=312 FES PET scans in N= 239 patients
| N total (N=312 if blank) | N with characteristic (% of N) | Mean (SD) | Range | |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age (years) | 54 (12) | 23 – 88 | ||
| Female sex | 298 (96%) | |||
| Pre-menopausal (female only) | 298 | 98 (33%) | ||
| Advanced stage breast cancer† | 298 (96%) | |||
| HER2 positive primary tumor | 293 | 62 (21%) | ||
| Any FES negative sites | 311 | 80 (26%) | ||
| Weight (kg) | 74 (17) | 46 – 156 | ||
| BMI (kg/m2) | 27 (6) | 18 – 55 | ||
| Prior Treatment | ||||
| Prior chemotherapy | 298 | 220 (74%) | ||
| Prior radiation | 297 | 170 (57%) | ||
| Prior tamoxifen | 301 | 155 (51%) | ||
| Currently undergoing chemotherapy | 306 | 50 (16%) | ||
| Blood Assays | ||||
| Serum Estradiol (pg/mL) | 309 | 21 (43) | 0 – 567 | |
| Testosterone (ng/mL) | ||||
| men | 12 | 3.8 (2.8) | 0 – 9.0 | |
| women* | 284 | 0.3 (0.7) | 0 – 9.7 | |
| Albumin (g/dL) | 280 | 3.6 (0.4) | 2.3 – 5.1 | |
| Serum SHBG (nM) | 284 | 55 (37) | 3 – 287 | |
| FES metabolism (%at 20 min)** | 278 | 29 (13) | 4 – 83 | |
| FES Characteristics for Imaging | ||||
| FES specific activity at injection (GBq/mMol) | 267 | 156,917 (228,068) | 7992–1,671,216 | |
| FES injected Activity dose (MBq) | 185.0 (25.9) | 103.6 – 296 | ||
| FES injected per patient weight (nmol/kg) | 267 | 0.05 (0.05) | 0.001 – 0.31 | |
| SHBG Binding (%FES bound to SHBG) | 293 | 32 (15) | 0 – 66 |
Advanced Stage Breast cancer refers to Stage III or IV.
8% (23/284) of women had testosterone levels >0.7ng/mL
Percent of total blood activity present as FES at 20 minutes after injection determined by HPLC
The average FES dose injected was 185 MBq (5.0 mCi ) with a range of 103.6–296 MBq (2.8–8.0 mCi ). Radiochemical purity of the FES was measured and approved for each production run. Specific activity was available for 267 scans with a mean of 156,917 GBq/mMol (4,241 Ci/mMol) (range 7,992–1,671,216 GBq/mMol (216–45,168 Ci/mMol)) at time of injection.
The average SUV was 2.1 (range 0.13–9.6). For 35% of scans, the SUV was of a single tumor in the dynamic field-of-view. For other scans, reported SUV was the average of two (22%) or three (43%) tumors. Natural log transformations of SUV values, BMI, specific activity, and nmol of FES injected/kg were performed to satisfy linear model assumptions.
The left side of Table 2 summarizes univariate associations between average FES SUV and patient characteristics (age, sex, menopausal status, disease stage, tumor HER2-neu status, any FES negative sites, BMI), prior treatment (prior chemotherapy, radiation, or tamoxifen use and concurrent chemotherapy), blood assays (serum estradiol, testosterone, albumin, serum SHBG, rate of FES metabolism (% of plasma activity present as FES at 20 minutes post injection)), and FES characteristics for imaging (dose, SAinj, nmol of FES injected/kg, fractional binding of SHBG to FES). To interpret regression parameters with log(FES SUV) as the outcome, results are presented as the ratio of FES SUV for values of the predictor variable that are one unit (or another measure chosen to be approximately the difference between the third or first quartile and the median) apart.
Table 2.
Univariate linear mixed models predicting log(average FES SUV), with random intercept to account for patients with multiple scans. In the sensitivity analysis, patients with concurrent chemotherapy and/or sites of apparent ER loss without FES uptake above background are excluded. Associations with p < 0.05 noted in italic type
| All eligible scans N=312 scans except where noted | Sensitivity analysis N=203 except where noted | |||||
|---|---|---|---|---|---|---|
| Factor | N Scans | Ratio of mean FES SUV versus factor* | p-value for factor effect | N Scans | Ratio of mean FES SUV versus factor* | p-value for factor effect |
| Patient Characteristics | ||||||
| Age | 1.05 (10 years older) | 0.18 | 1.02 | 0.69 | ||
| Male sex | 1.16 | 0.38 | 1.01 | 0.94 | ||
| Pre-menopausal (female only) | 298 | 1.08 | 0.42 | 194 | 1.00 | 0.98 |
| Advanced (vs early) | 1.11 | 0.62 | 1.34 | 0.18 | ||
| HER2-positive tumor | 293 | 0.89 | 0.22 | 194 | 0.86 | 0.13 |
| Any negative sites | 311 | 0.44 | <0.001 | -- | -- | |
| BMI (kg/m2)** | 1.11 (15% higher) | <0.001 | 1.14 | <0.001 | ||
| Prior Therapy | ||||||
| Prior chemotherapy | 298 | 1.14 | 0.16 | 196 | 1.23 | 0.05 |
| Prior radiation | 297 | 0.93 | 0.37 | 195 | 1.07 | 0.52 |
| Prior tamoxifen | 301 | 1.08 | 0.37 | 197 | 1.12 | 0.20 |
| Concurrent chemotherapy | 306 | 0.77 | 0.02 | -- | -- | |
| Blood Assays | ||||||
| Serum estradiol ≥ 30 pg/mL | 309 | 1.04 | 0.58 | 201 | 0.94 | 0.47 |
| Testosterone ≥ 0.5 ng/mL | 296 | 1.17 | 0.05 | 190 | 0.93 | 0.44 |
| Albumin (g/dL) | 280 | 0.95 (0.3 g/dL higher) | 0.17 | 178 | 0.95 | 0.13 |
| Serum SHBG (nM)*** | 284 | 0.91 (~20 nM higher) | <0.001 | 183 | 0.92 | 0.009 |
| FES metabolism (% at 20 min)**** | 278 | 0.96 (10% pts higher) | 0.19 | 177 | 0.97 | 0.39 |
| FES characteristics for Imaging | ||||||
| Dose (mCi) | 0.98 (0.5 mCi higher) | 0.46 | 0.99 | 0.80 | ||
| Specific activity (Ci/mMol)** | 267 | 1.02 (2x higher) | 0.49 | 168 | 1.03 | 0.34 |
| FES injected per patient weight (nmol/kg)** | 267 | 0.97 (2x higher) | 0.20 | 168 | 0.95 | 0.16 |
| SHBG (% binding) | 293 | 0.91 (10% pts higher) | <0.001 | 186 | 0.91 | 0.002 |
Mean FES SUV for all patients with factor versus those without. For continuous measures that ratio is listed as a function of the listed change in the factor, given in parentheses.
log transformation
square root transformation
Percent of total blood activity present as FES at 20 minutes after injection determined by HPLC
Concurrent chemotherapy and patients with one or more qualitatively negative lesions were each associated with lower FES uptake (p=0.02 and p=<0.001 respectively). A sensitivity analysis (described above) repeated the univariate analysis excluding patients with negative FES PET scans and/or concurrent chemotherapy, and is described on the right side of Table 2.
Several factors hypothesized to affect FES uptake did not appear to have a significant impact on uptake, within the observed ranges of these variables and FES uptake. Figure 1A shows FES SUV by values of circulating estradiol. Although it appears that FES SUV is slightly higher for moderate estradiol values (20–30 pg/mL), this is not supported by hypothesized relationships or by quantitative findings. Differences in average FES SUV were not observed for estradiol levels above versus below 30 pg/mL, a cutoff point used to indicate post-menopausal levels (comparing left and right sides of dashed line in Figure 1A, p=0.58 and p=0.47 for univariate and sensitivity analysis respectively). Furthermore, no relationship was found between estradiol levels and FES SUV for the 82 studies with plasma estradiol ≥ 30 pg/mL (110 fmol/mL) (within right side of dashed line in Figure 1A, p = 0.26). Similarly, FES uptake was not related to testosterone and albumin levels: the “borderline significant” univariate result (17% higher expected FES SUV for testosterone ≥ 0.5 ng/mL, p=0.05) was not reinforced by the sensitivity analysis (7% lower SUV, p=0.44). FES uptake showed no relationship to the rate of FES metabolism (fraction of total activity present as FES at 20 minutes) (p = 0.19). Age, sex, disease stage, and tumor HER2-neu status -- prognostic and predictive factors for disease outcomes in patients with ER+ primary breast cancer -- did not have a significant relationship with FES uptake in this analysis (Table 2).
Figure 1.
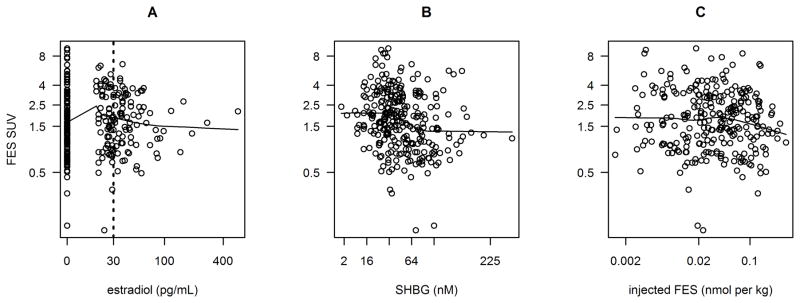
Scatterplots of log(FES SUV) plotted against estradiol (A), SHBG binding (B), and injected FES (C). Fitted curves are locally weighted scatterplot smoothing (LOWESS) with smoother span 2/3.
The plasma levels of SHBG and fractional FES binding to SHBG were both associated with FES uptake, with higher SHBG (nM) and SHBG percent binding associated with lower FES SUV (p<0.001). For example, a patient with an observed SHBG of 31 nM (near the 25th percentile) and FES SUV of 1.89 would be expected to have an FES SUV of 1.57 (17% lower) with SHBG of 72 nM (near the 75th percentile). In the sensitivity analysis, the correlation between plasma levels of SHBG (nM) and FES uptake was of lower magnitude, but still significant (p=0.009). Figure 1B shows the negative trend of SHBG (nM) with FES uptake.
Analyzing the effect of specific activity by considering the FES injected molar dose/kg provides a measure of the competition of “cold” FES with radioactive FES for binding to ER. Injected FES mass per kg patient weight did not appear to have a statistically significant negative relationship with FES uptake, (3% lower SUV expected if injected FES mass/kg was doubled, p=0.20, Table 2). In the sensitivity analysis, the effect was greater, a 5% lower SUV if injected FES mass/kg was doubled (p=0.16). In the LOWESS fit (Figure 1C) there appeared to be no relationship for injected FES mass lower than approximately 0.2 nmol/kg and a slight downward slope for higher injected mass per kg. These findings suggest that the exogenous dose of FES does not saturate ER. Although a small effect of injected mass on FES uptake at low SAinj values cannot be completely excluded, the magnitude of this effect is, at worst case, small.
Specific activity at injection (SAinj) did not have a significant association with FES uptake in either the full univariate or sensitivity analysis (p = 0.49 and 0.34 respectively, Table 2). The scatterplot and LOWESS fit (Figure 2A) suggest a slight increase in FES SUV over the range of values between 5,550 and 44,400 Gbq/mMol (between 150 and 1200 Ci/mMol). With 93 scans of SAinj ≤44,400 GBq/mMol ((≤1200 Ci/mMol) (73 of which had SAinj ≤37,000 GBq/mMol (≤ 1000 Ci/mMol)) the log transformation of SA was not required to fit a linear mixed model predicting log(FES SUV) (Figure 2B). The model predicted that a 100 Ci/mMol increment in SAinj (within the range of 4,440–44,400 GBq/mMol (120–1200 Ci/mMol)) would be associated with a 2% higher FES SUV, but the effect was not statistically significant (p = 0.49). Recall that for a 3,700 GBq/mMol (100 Ci/mMol) difference in SAinj, we estimated 80% power to detect a difference of 7% in expected FES SUV, and 90% power to detect a difference of 8%.
Figure 2.
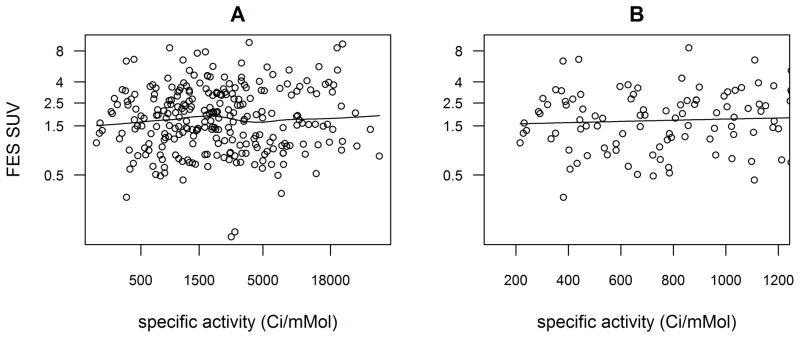
Scatterplots of log(FES SUV) plotted against specific activity at time of injection (SAinj), for all scans (A), and for subset of scans with SAinj ≤44,400 GBq/mMol (≤1200 Ci/mMol) (B). Fitted curves are locally weighted scatterplot smoothing (LOWESS) with smoother span 2/3.
A significant correlation was found between BMI and FES uptake (p<0.001) for both the univariate and sensitivity analyses. In the univariate analysis FES SUV would be expected to be 11% higher for a BMI that was 15% higher. In the sensitivity analysis, the relationship was somewhat stronger with the SUV expected to be 14% higher for a 15% higher BMI. Figure 3A shows a scatterplot of FES SUV (on a log scale) and BMI (also logged). Throughout the range of observed BMI, FES SUV is predicted to be higher for higher BMI. This could be due to the known limitations of weight-based SUV [25] or to actual differences in disease characteristics (with respect to estrogen receptor activity) between heavier and lighter women with (mostly) advanced breast cancer. If the effect were due to the weight-based SUV estimation, it should not be evident when uptake is measured by lean-body-mass corrected SUV (SUVLBM), which attempts to account for the known discrepancy between distribution volume and weight for high BMI patients. Figure 3B shows that no relationship was apparent between FES SUV and BMI when SUV was adjusted for lean body mass using the method of James (p = 0.76) [20]. Figure 3C, a scatterplot of corrected and uncorrected FES SUV, illustrates the varying impact of lean body mass correction for individual patients’ SUV measures.
Figure 3.
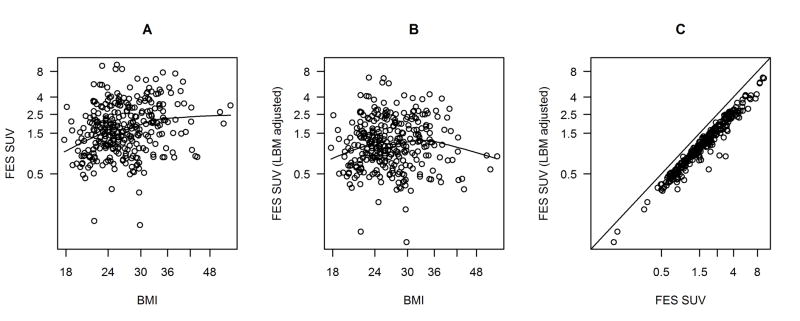
Exploring the relationship between BMI and FES SUV: log(FES SUV) plotted against log(BMI) (A); log of lean body mass-adjusted FES SUV plotted against log(BMI) (B); comparison of FES SUV and lean body mass-adjusted FES SUV (C), with line showing equality. Fitted curves are locally weighted scatterplot smoothing (LOWESS) with smoother span 2/3.
Finally, we explored multivariate models. BMI was still an independent predictive factor in models predicting log(FES SUV) by SHBG binding and BMI. We explored further multivariate models using FES SUVLBM as the outcome (Table 3). Controlling for SHBG, predicting values of SUVLBM, no additional predictors were statistically significant. For the full sample (n=284, excluding scans with no SHBG information), SHBG predicted SUVLBM with magnitude comparable to that in the analysis with unadjusted SUV (p = 0.01). For SHBG near the 25th percentile (31 nM), the predicted average SUVLBM was 1.22, compared to 1.06 at the 75th percentile (72 nM). In the sensitivity analysis dataset, SHBG predicted SUVLBM as 5% lower for a difference in sqrt(SHBG) of 1.5 (p = 0.08).
Table 3.
Linear mixed model with the square root of SHBG predicting log(lean body mass-adjusted FES SUV), with random intercept to account for patients with multiple scans. In the sensitivity analysis, patients with concurrent chemotherapy and/or sites of apparent ER loss without FES uptake above background are excluded
| Patients with SHBG analysis N=284 | p-value | Sensitivity analysis of patients who had SHBG analysis N=183 scans | p-value | |
|---|---|---|---|---|
| SHBG (nM)** | predicted SUVLBM 7% lower when difference in sqrt(SHBG) is 1.5 | 0.01 | predicted SUVLBM 5% lower when difference in sqrt(SHBG) is 1.5 | 0.08 |
Discussion
We analyzed biological factors that might influence FES uptake to better understand factors that may contribute to variability of FES uptake measures. We gathered data on these factors for over 300 scans through various FES PET imaging protocols. To screen for factors that might affect uptake, we tested for associations between FES SUV in tumors and several clinical, physical, or chemical factors. Of all the factors tested through univariate analysis, we found that concurrent chemotherapy, BMI, and SHBG had significant association with FES uptake. We also found that plasma estradiol levels and FES catabolism rates did not have a significant association with FES uptake.
For factors related to FES SAinj, our results indicate that any effect of lower specific activities on FES uptake is quite modest. However, because of a small, though not statistically significant, observed negative relationship between injected FES mass/kg and FES SUV for injected mass greater than 0.2 nmole/kg it seems prudent to limit injected mass to this value. For a typical FES activity dose of 222 MBq (6 mCi) and typical female patient weight of approximately 60 kg, FES SAinj of 18,500 GBq/mMol (500 Ci/mMol) or greater, at injection, should not require any dose limitation to reduce mass injected. For SAinj less than 18,500 GBq/mMol (500 Ci/mMol), and particularly for smaller patients, it may be desirable to reduce the injected activity dose of FES to limit the injected mass.
Fractional FES binding to SHBG and plasma SHBG levels, both indicators of FES binding to SHBG, had a significant association with FES SUV (p<0.001 for both measures). For both SHBG measures the association with FES uptake was inverse, suggesting that higher levels of SHBG binding limit FES uptake at the tumor site. For SHBG at the observed 25th percentile (31 nM), the predicted average SUVLBM was 1.22, compared to 1.06 at the 75th percentile (72 nM). These results show that there is a significant association between SHBG and FES uptake and therefore, SHBG should be measured in each patient. It is also interesting to note that the observed inverse association between SHBG binding and FES uptake is consistent with the free hormone hypothesis which states that the levels of hormone transfer to tissue receptors depends upon the amount of non-bound hormone [26]. On the other hand, prior studies of PET ER imaging agents with poor SHBG binding support that some level of binding is needed for a functional ER imaging agent [14]. Taken together, these observations suggest an optimal range of SHBG binding for PET ER imaging, not surprisingly, at levels close to physiologic levels for estradiol.
Body mass index appeared to have a significant correlation to FES uptake. The likely explanation for this is that weight overestimates tracer distribution values at higher BMI, resulting in an artifactually high SUV, as has been shown for 18F-Fluorodeoxyglucose (FDG) PET [25, 27, 28]. No relationship was evident when FES SUV values were adjusted for lean body mass rather than weight in kg. When comparing uptake in patients, adjusting for LBM may be helpful to reduce the error associated with tracer distribution.
Equally important in this study to the factors that were significantly associated with FES uptake are some of the factors that were not significantly associated with FES uptake. In our patient group, which included a sizeable number of pre-menopausal patients (33%), circulating estradiol levels did not appear to effect FES uptake. Looking at pre-menopausal, post-menopausal, and male patients separately, uptake still did not show a significant relationship with estradiol. This finding is consistent with our previous study showing a good correlation between FES uptake and ER expression for both pre- and post-menopausal patients [2], and further implies that ER levels are not saturated by endogenous estradiol in these patients.
Also, the association between the rate of FES catabolism (%FES) and SUV was not significant. This is consistent with the fact that we did not find an improvement in correlation with in vitro ER assay using FES measures accounting for metabolism, versus for simple uptake measures like SUV [2]. It also allows for simplification in the blood processing by eliminating the need for HPLC analysis of blood samples.
This study has some important limitations. We could not directly adjust for the known effect of tumor ER expression level on FES uptake, but rather tested for associations of factors besides ER expression with uptake in a population with ER-expressing tumors. To reduce the variability of ER expression, we performed sensitivity analysis excluding patients without apparent FES uptake at one or more sites of known and active disease, indicating that ER expression may have been lost. We also observed low uptake in some patients with concurrent chemotherapy and excluded these patients in the sensitivity analysis. Even with these exclusions, the patient population in this analysis was heterogeneous, and the resulting expected variability in FES uptake due to variable ER expression might obscure more subtle associations of some factors with FES uptake. However, the relatively large number of studies provided a means of screening for significant factors that affect FES uptake into tumors.
Conclusion
Higher SHBG levels and binding correlated with lower FES uptake, and should therefore be measured for each patient. The correlation between BMI and FES SUV uptake suggests a need for LBM correction when comparing SUV values between patients. A non-significant trend was observed suggesting a negative relationship between injected mass/kg patient weight and FES uptake at low specific activity. It may therefore be prudent to limit injected dose at lower specific activities for smaller patients. Pre-menopausal levels of estradiol do not appear to affect FES uptake, nor does FES metabolism suggesting that FES metabolism may not need to be measured for individual patients. These findings should allow simpler, more targeted protocols for FES imaging in breast cancer patients with ER+ disease.
Acknowledgments
This work was supported by NIH grants CA42045 (Kenneth Krohn, PI) and CA72064. The authors would like to thank Phil Petra for his expertise in SHBG assays. We also thank the radiochemistry staff, the nuclear medicine technologists and the physicists in the Dept. of Radiology for their assistance with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–8. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–74. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 3.Mankoff DA, Link JM, Linden HM, Sundararajan L, Krohn KA. Tumor receptor imaging. J Nucl Med. 2008;49 (Suppl 2):149S–63S. doi: 10.2967/jnumed.107.045963. [DOI] [PubMed] [Google Scholar]
- 4.Key TJ. Serum oestradiol and breast cancer risk. Endocr Relat Cancer. 1999;6:175–80. doi: 10.1677/erc.0.0060175. [DOI] [PubMed] [Google Scholar]
- 5.Zeleniuch-Jacquotte A, Toniolo P, Levitz M, et al. Endogenous estrogens and risk of breast cancer by estrogen receptor status: a prospective study in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1995;4:857–60. [PubMed] [Google Scholar]
- 6.Rossi E, Morabito A, Di Rella F, et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009;27:3192–7. doi: 10.1200/JCO.2008.18.6213. [DOI] [PubMed] [Google Scholar]
- 7.Katzenellenbogen JA. Designing steroid receptor-based radiotracers to image breast and prostate tumors. J Nucl Med. 1995;36:8S–13S. [PubMed] [Google Scholar]
- 8.Katzenellenbogen JA, Welch MJ, Dehdashti F. The development of estrogen and progestin radiopharmaceuticals for imaging breast cancer. Anticancer Res. 1997;17:1573–6. [PubMed] [Google Scholar]
- 9.Mathias CJ, Welch MJ, Katzenellenbogen JA, et al. Characterization of the uptake of 16 alpha-([18F]fluoro)-17 beta-estradiol in DMBA-induced mammary tumors. Int J Rad Appl Instrum B. 1987;14:15–25. doi: 10.1016/0883-2897(87)90156-5. [DOI] [PubMed] [Google Scholar]
- 10.Mankoff DA, Tewson TJ, Eary JF. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-16 alpha-fluoroestradiol (FES) Nucl Med Biol. 1997;24:341–8. doi: 10.1016/s0969-8051(97)00002-4. [DOI] [PubMed] [Google Scholar]
- 11.Murad F. Hormones and hormone antagonists. 8. New York: Pergamon Press; 1990. [Google Scholar]
- 12.Kiesewetter DO, Kilbourn MR, Landvatter SW, Heiman DF, Katzenellenbogen JA, Welch MJ. Preparation of four fluorine- 18-labeled estrogens and their selective uptakes in target tissues of immature rats. J Nucl Med. 1984;25:1212–21. [PubMed] [Google Scholar]
- 13.Tewson TJ, Mankoff DA, Peterson LM, Woo I, Petra P. Interactions of 16alpha-[18F]-fluoroestradiol (FES) with sex steroid binding protein (SBP) Nucl Med Biol. 1999;26:905–13. doi: 10.1016/s0969-8051(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 14.Jonson SD, Bonasera TA, Dehdashti F, Cristel ME, Katzenellenbogen JA, Welch MJ. Comparative breast tumor imaging and comparative in vitro metabolism of 16alpha-[18F]fluoroestradiol-17beta and 16beta-[18F]fluoromoxestrol in isolated hepatocytes. Nucl Med Biol. 1999;26:123–30. doi: 10.1016/s0969-8051(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 15.Katzenellenbogen JA, Mathias CJ, VanBrocklin HF, Brodack JW, Welch MJ. Titration of the in vivo uptake of 16 alpha-[18F]fluoroestradiol by target tissues in the rat: competition by tamoxifen, and implications for quantitating estrogen receptors in vivo and the use of animal models in receptor-binding radiopharmaceutical development. Nucl Med Biol. 1993;20:735–45. doi: 10.1016/0969-8051(93)90160-v. [DOI] [PubMed] [Google Scholar]
- 16.Lim JL, Zheng L, Berridge MS, Tewson TJ. The use of 3-methoxymethyl-16 beta, 17 beta-epiestriol-O-cyclic sulfone as the precursor in the synthesis of F-18 16 alpha-fluoroestradiol. Nucl Med Biol. 1996;23:911–5. doi: 10.1016/s0969-8051(96)00126-6. [DOI] [PubMed] [Google Scholar]
- 17.Romer J, Fuchtner F, Steinbach J, Johannsen B. Automated production of 16alpha-[18F]fluoroestradiol for breast cancer imaging. Nucl Med Biol. 1999;26:473–9. doi: 10.1016/s0969-8051(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 18.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–9. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 19.Fattah DI, Chard T. Simplified method for measuring sex-hormone binding globulin. Clin Chem. 1981;27:1277–9. [PubMed] [Google Scholar]
- 20.Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol. 1981;11:523–6. doi: 10.1111/j.1365-2125.1981.tb01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–36. [Google Scholar]
- 22.Levine D, Bankier AA, Halpern EF. Submissions to Radiology: Our top 10 list of statistical errors. Radiology. 2009;253:288–90. [Google Scholar]
- 23.Fisher LD, van Belle G. Biostatistics: A methodology for the health sciences. New York: Wiley; 1993. [Google Scholar]
- 24.Kuukasjarvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–9. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara Y, Zasadny KR, Neuhoff AW, Wahl RL. Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology. 1999;213:521–5. doi: 10.1148/radiology.213.2.r99nv37521. [DOI] [PubMed] [Google Scholar]
- 26.Hryb DJ, Khan MS, Romas NA, Rosner W. The control of the interaction of sex hormone-binding globulin with its receptor by steroid hormones. J Biol Chem. 1990;265:6048–54. [PubMed] [Google Scholar]
- 27.Erselcan T, Turgut B, Dogan D, Ozdemir S. Lean body mass-based standardized uptake value, derived from a predictive equation, might be misleading in PET studies. Eur J Nucl Med Mol Imaging. 2002;29:1630–8. doi: 10.1007/s00259-002-0974-3. [DOI] [PubMed] [Google Scholar]
- 28.Graham MM, Peterson LM, Hayward RM. Comparison of simplified quantitative analyses of FDG uptake. Nucl Med Biol. 2000;27:647–55. doi: 10.1016/s0969-8051(00)00143-8. [DOI] [PubMed] [Google Scholar]


