Abstract
Graves’ hyperthyroidism is an autoimmune disease occurring spontaneously in humans caused by autoantibodies that stimulate the thyrotropin-receptor. In mice, inducing Graves’-like hyperthyroidism requires in vivo expression of the thyrotropin-receptor using plasmid or adenovirus vectors. However, mice with different genetic backgrounds vary markedly in their susceptibility to induced hyperthyroidism. Further, in some strains major disparities exist between the induction of hyperthyroidism and detection of thyroid-stimulating antibodies. To break tolerance, all Graves’ mouse models involve immunization with human thyrotropin-receptor DNA and the standard thyroid-stimulating antibodies bioassay utilizes cells expressing the human thyrotropin-receptor. We hypothesized, and now report, that disparities between hyperthyroidism and thyroid-stimulating antibody bioactivity are explained, at least in part, by differential antibody recognition of the human versus the mouse thyrotropin-receptor. The genetic basis for these species differences was explored using genotyped, recombinant-inbred mouse strains. We report that loci in the immunoglobulin heavy chain variable region as well as in the MHC region contribute in a strain-specific manner to the development of antibodies specific for the human or the mouse thyrotropin-receptor. The novel finding of a role for immunoglobulin heavy chain variable region gene involvement in thyroid-stimulating antibody epitopic specificity provides potential insight into genetic susceptibility in human Graves’ disease.
Keywords: Graves’ disease, immunoglobulin heavy chain variable region, thyroid stimulating antibody, thyroid stimulating hormone receptor
INTRODUCTION
The thyroid is the organ most commonly affected in human autoimmunity.Hashimoto’s thyroiditis is associated with autoantibodies to thyroid peroxidase and thyroglobulin and hypothyroidism affects 4.6 % of the US population 1. Hyperthyroidism in Graves’ disease, prevalent in 0.5 - 1% of women 1,2, is caused by autoantibodies to the thyrotropin receptor (TSHR) that mimic thyroid stimulation by thyrotropin (TSH) (reviewed in 3). Autoimmune hyperthyroidism only develops spontaneously in humans. Nevertheless, in other autoimmune disease, induced animal models have provided important clues to elucidating the pathogenesis and genetic basis of their human counterparts, such as experimental autoimmune encephalomyelitis 4 (a model for multiple sclerosis) and collagen-induced arthritis 5.
Induction of hyperthyroidism in mice is not as straightforward as the two foregoing induced autoimmune diseases. Even though highly-purified, disease-specific antigen (unequivocally the TSHR) is available, conventional immunization with TSHR protein and adjuvant cannot induce hyperthyroidism . Although this approach generates high titers of TSHR antibodies, these are incapable of activating the TSHR on the surface of intact cells (reviewed in 6). To produce Graves’ hyperthyroidism in mice, it is necessary to express in vivo the TSHR or it’s A-subunit using plasmid or adenovirus vectors (reviewed in 6). However, mice with different genetic backgrounds vary markedly in their susceptibility to induction of hyperthyroidism.
Virtually all mouse models of induced hyperthyroidism involve in vivo expression of the TSHR without additional adjuvant (for example 7,8). Human TSHR cDNA is generally employed, because of its general availability and because its use bypasses the need to overcome self tolerance with potent adjuvants. [In one study, hyperthyroidism was induced by injecting B cells expressing mouse-TSHR with the adjuvant cholera toxin B 9]. Importantly, following immunization with the human TSHR, the induced thyroid stimulating antibodies (TSAb) must stimulate the mouse TSHR to produce hyperthyroidism in vivo.
It has been recognized for fifty years that TSAb in human Graves’ patients stimulate the mouse (m) TSHR in vivo 10 and early bioassays for TSAb utilized a rat thyroid cell line (FRTL5 11). Following the molecular cloning of the TSHR, Chinese hamster ovary (CHO) cells expressing the recombinant human (h) TSHR have progressively supplanted rat thyroid cells in TSAb assays 12. In BALB/c mice made hyperthyroid by immunization with human TSHR-adenovirus, serum thyroxine levels correlated with TSAb activity when measured with FRTL5 rat thyroid cells 7. Unexpectedly, with the human hTSHR bioassay, TSAb activities in hyperthyroid mice correlated poorly with serum thyroxine levels. For example, some euthyroid BALB/c mice had high TSAb activity while other hyperthyroid animals were TSAb negative 13. Similarly, some C3H/He mice were hyperthyroid despite very low levels of TSAb assayed using hTSHR expressing CHO cells 14. BALB/c mice are far more susceptible than C57BL/6 mice to hTSHR-adenovirus induced hyperthyroidism 15. Nevertheless, when assayed with hTSHR-CHO cells, TSAb activities were comparable in these two mouse strains 15.
To explore the foregoing inconsistencies in the relationship between the degree of murine hyperthyroidism and TSAb activity, we generated mouse-TSHR expressing CHO cells16. Unlike with hTSHR-CHO cells, when assayed with mTSHR-CHO cells TSAb levels were higher in BALB/c than in C57BL/6 mice, more consistent with the greater susceptibility of the former to induced hyperthyroidism 16. With this background, in the present study we hypothesized that TSAb generated in genetically diverse strains of mice and assayed with hTSHR- and mTSHR-CHO cells as separate traits would provide insight into the genetic basis for variability in TSHR antibody functional activity in different mouse strains. We report that loci in the MHC region as well as in the immunoglobulin heavy chain variable (IgVH) region contribute in a strain-specific manner to the development of antibodies specific for the human- or the mouse TSHR.
METHODS AND MATERIALS
Mouse strains and immunization
Adenoviruses, mouse strains and immunization protocols were previously described 14,17. Briefly, we used adenovirus encoding the human A-subunit (TSHR amino acids 1-289; A-subunit-Ad)8 and null-adenovirus (Con-Ad) 18. Propagation, purification and determination of particle virus number was reported previously 7. Female mice (5-8 weeks) of the following strains were obtained (Jackson Laboratory, Bar Harbor, Maine):- (a) C3H/HeJ and C57BL/6J (parental BXH strains); (b) RI CXB1/ByJ through CXB7/ByJ; CXB8/HiAJ through CXB13/HiAJ; (c) RI BXH2-, 4-, 6 through 11-, 14-, 19- TyJ, BXH20/ KccJ , BXH22/KccJ and B6cC3-1/KccJ. Parental strains are referred to as C3H, B6 (Jackson or Bailey strains, J or By), and RI strains as CXB1, CXB 2 or BXH2, BXH4 etc.
Mice were immunized with A-subunit-Ad (108 particles/injection) on 3 occasions at 3 weekly intervals. Blood was drawn one week after the second injection and mice were euthanized four weeks after the third immunization. Six mice were studied for each CXB or BXH strain (except for CXB5; only 2 animals were available). The number of parental animals immunized with A-subunit-Ad were 10 C3H/J and 10 B6/J mice. Additional parental strain mice were immunized with Con-Ad (108 particles/injection): 5 C3H/J and 5 B6/J. All studies were approved by the Institutional Animal Care and Use Committee and performed with the highest standards of care in a pathogen-free facility. All sera had previously been characterized for thyroxine and TSHR antibodies measured by inhibition of TSH binding (TBI) or ELISA 15,17.
TSAb activity measured using mouse-TSHR and human-TSHR expressing cells
TSAb was assayed by stimulation of cAMP generation in CHO cells expressing the human TSHR 14 and the mouse TSHR 16. TSHR-CHO cells in 96 well plates were incubated (80-90 min, 37°C) with test sera diluted 1:20 in Ham’s F12 containing 10 mM Hepes, pH 7.4 and 1 mM isobutylmethylxanthine. After aspirating the medium, intracellular cAMP was extracted with ethanol, evaporated to dryness and resuspended in Dulbecco’s phosphate buffered saline. Samples (12 μl) were assayed using the LANCE cAMP kit (Perkin Elmer, Boston MA). Sera from CXB mice and their parental strains were analyzed for TSAb using mTSHR and hTSHR bioassays; sera from BXH mice and their parental strains were tested for TSAb using mTSHR-CHO cells (data for hTSHR-CHO cells were previously reported 14.
Human and mouse TSAb levels were expressed as a percentage of cAMP values attained with sera from control adenovirus immunized mice and these values were used for linkage analysis (see below). An additional trait used in linkage analysis is the TSAb ratio for mTSHR:hTSHR (BXH strains), and hTSHR:mTSHR (CXB strains). Because maximum cAMP induction differed in the two bioassays, for visual comparisons TSAb values for individual mice were normalized by assigning 100% to the mean value for the strain with the highest response. as follows:- BXH strains, mouse-TSHR 485% (BXH9) and human-TSHR 1229% (BXHcC3-1); CXB strains:- mouse-TSHR 555 % (CXB6) and human-TSHR 945% (CXB6).
Statistical analyses
Significant differences between responses in different groups were determined by Mann Whitney rank sum test or, when normally distributed, by Student’s t test. Multiple comparisons were performed using analysis of variance (ANOVA). Tests were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA). Data in bar graphs are shown as mean + SEM.
Genetic linkage analysis
Putative quantitative trait loci (QTL) involved in TSAb induced by A-subunit-Ad immunization of CXB and BXH strains were mapped using the genotype files for these recombinant inbred strains generated by Williams et al. 19 available at http://www.nervenet.org and embedded in GeneNetwork (www.genenetwork.org). The probability of linkage between our traits and previously mapped genotypes was estimated at ~1 centiMorgan intervals (~2 megabase; Mb) along the entire genome, except for the Y chromosome. To establish criteria for suggestive and significant linkage, a permutation test was performed (1000 permutations at 1-centimorgan intervals) 20. This test compares the peak likelihood ratio statistics (LRS; LRS = LOD x 4.6, where LOD is the logarithm of the odds) obtained for a given data set with the peak LRS score obtained for 1000 random permutations of the same data set.
The primary phenotype data have been entered into the mouse CXB and BXH Phenotype databases in GeneNetwork (www.genenetwork.org) under the trait accession identifiers: CXB 10683 through 101690; and BXH 10147, 10148; 10155 through 10160). These data can be found by searching the CXB or BXH; databases for the name “McLachlan”. In the Results we refer to the identification (GN) specific trait numbers so that readers can verify and extend the data analysis. Because GN interval maps connect directly to the UCSC (University of California Santa Cruz) Genome Browser, it is possible to explore the gene complement of chromosomal intervals together with the QTL profile.
CXB and BXH data were combined (when appropriate) to provide a data set from 26 RI strains sharing one parental strain (B6). As described for BXH and BXD RI mice 21 and CXB and BXH mice 14, we calculated the probability associated with a X2 value equal to:- 2(lnPBXD + lnPCXD) with 4 degrees of freedom, where lnPBXD and lnPCXD are the natural logarithms of the probabilities derived independently for the two RI strains in the same chromosomal interval. For the combined analysis, the Gene Network program only provides a LOD score; for comparison with the LRS scores provided by the standard analysis using this program an LRS value of 4.61 is equivalent to a LOD score of 1.0.
RESULTS
TSAb in sera from parental and recombinant inbred strains bioassayed using cells expressing the mouse and human TSHR
C3H and B6 mice were immunized three times with adenovirus encoding the human TSHR A-subunit. Sera were drawn one week after the second injection and one month after the third injection and were tested for TSAb in bioassays using eukaryotic cells expressing the mouse or the human TSHR. As we observed previously 14, TSAb levels determined using the human TSHR were far lower in C3H than in B6 parental strains after the second A-subunit immunization (Fig. 1A). This observation is paradoxical because C3H mice are more susceptible to hyperthyroidism than B6 mice 14. A possible explanation for this phenomenon was that, despite immunization with the human TSHR A-subunit, the TSAb generated in the C3H mice preferentially recognized the mouse TSHR. This hypothesis was confirmed when the same sera were tested with cells expressing the mouse TSHR. In the latter assay, TSAb activities were comparable in C3H and B6 parental strains (Fig. 1B). This difference for each strain can be expressed as the ratio of TSAb activities determined with the mouse and human TSHR assays (Fig. 1C). Similar data were obtained with the sera obtained at the time of euthanasia, four weeks after the third immunization (Supplementary Information Fig. S1).
Figure 1.
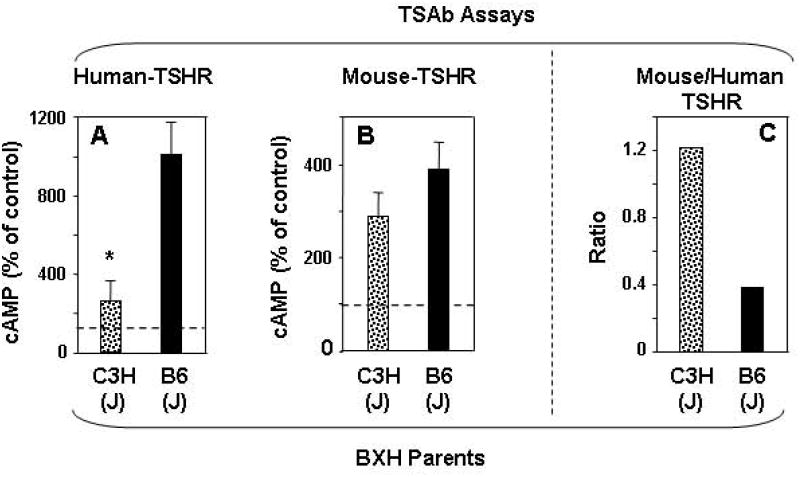
TSAb specific for the mouse-TSHR and the human-TSHR in parental strains of BXH mice, C3H and B6 mice. Sera were tested one week after two immunizations with A subunit-Adenovirus. A) Human -TSHR TSAb (previously reported in 14; B) Mouse-TSHR TSAb; C) Ratio of mouse-TSHR:human-TSHR TSAb. TSAb values (mean + SEM) are expressed as % cAMP generated sera from control adenovirus immunized mice; indicated by dashed lines (panels A, B); number of mice C3H, n=10; B6, n=10; * p =0.006 (Rank sum test) . The TSAb ratios were calculated from the means for mouse- and human-TSHR TSAb.
Based on the foregoing differences between B6 and C3H mice in TSAb determined in mouse and human TSHR assays, we performed similar assays on 13 recombinant inbred BXH strains derived from the two parental strains. BXH strains had been immunized and tested as described above for the parental B6 and C3H mice. Because maximum stimulation levels differed in the two bioassays (Fig.1) and also because TSAb activities induced in the different strains varied widely, to facilitate comparison the data were normalized to the recombinant strain giving the highest mean TSAb value, expressed as 100% (Methods). Six of the 13 BXH strains (BXH-2,-4,-6, -7, -9 and 19) developed significantly higher mouse-TSAb than human-TSAb activity after two immunizations (Fig 2A).
Figure 2.
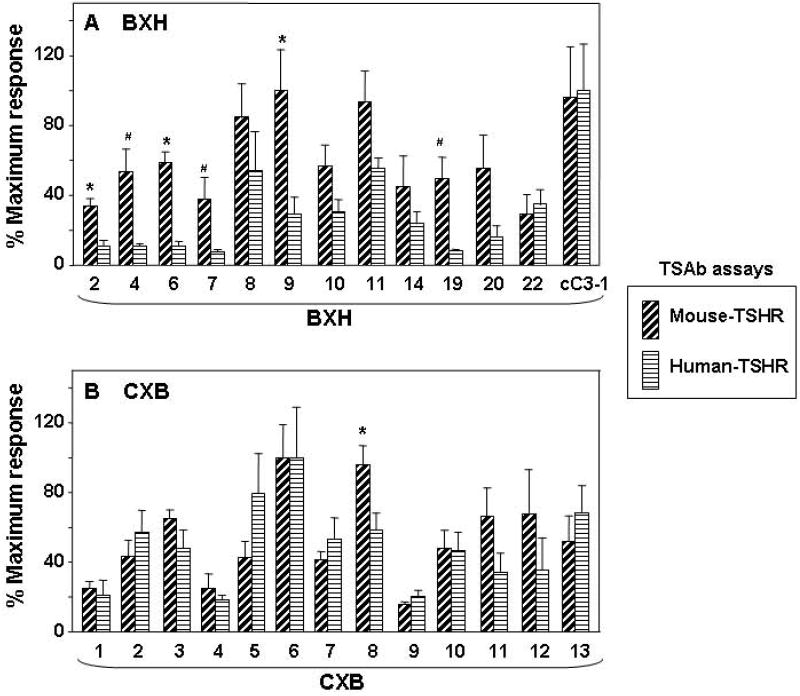
Comparison of mouse-TSHR TSAb versus human-TSHR TSAb in recombinant inbred strains BXH (panel A) and CXB (panel B) after two immunizations with A-subunit (human)-Adenovirus. The data are shown as paired bar graphs (mean + SEM) of mouse-TSHR TSAb and human-TSHR TSAb for each strain. Because the maximum stimulation was higher with the human-TSHR than with the mouse-TSHR, TSAb values were expressed as a percentage of the mean maximum levels attained as follows:- BXH strains, mouse-TSHR 485% (BXH9) and human-TSHR 1229% (BXHcC3-1); CXB strains:- mouse-TSHR 555 % (CXB6) and human-TSHR 945% (CXB6). Significant differences between mouse- and human-TSHR TSAb:- A) * p<0.019 (t test), # p<0.010 (rank sum test); B), * p=0.030 (t test).
In previous studies, TSHR A-subunit adenovirus immunization of BALB/c(J) and B6(J) mice generated similar TSAb activities when measured in the standard human TSHR assay 15. Recombinant inbred CXB strains were derived from parental BALB/c and B6 mice of the Bailey strains (By, rather than J). We had previously investigated the 13 CXB recombinant inbred strains 14, but had not assayed TSAb activities in these mice following immunization with TSHR A-subunit adenovirus. On doing so in the present study, we found that, in sera tested one week after the second immunization, most CXB strains had comparable TSAb activities measured in the mouse TSHR and human TSHR assays and only one strain (CXB8) had significantly higher TSAb activity in the assay using the human vs the mouse TSHR assay (Fig. 2B).
Linkage analysis in BXH and CXB strains for TSAb determined in mouse and human TSHR assays
For these analyses, we used the raw TSAb data (not the values normalized to 100% for better visualization, as shown in Fig. 2). Putative quantitative trait loci (see Methods) were analyzed for the following traits after the second immunization with human TSHR A-subunit adenovirus:- a) TSAb assayed with human TSHR-expressing cells; b) TSAb assayed with mouse TSHR-expressing cells and; c) the ratio between these two TSAb assay values.
In 13 BXH recombinant inbred strains, the whole genome scan revealed highly significant linkage between the ratio of TSAb values determined using the mouse vs. human TSHR assays and a locus on chromosome (Chr 12)(Fig. 3A). The Linkage Ratio Statistic (LRS) of 20.433 is equivalent to a LOD (logarithm of the odds) score of 4.432 (Table 1). Similarly, whole genome scanning for the 13 CXB strains revealed the strongest linkage between a Chr 12 locus and TSAb assayed with mouse TSHR-expressing cells (Fig. 3B). However, data from the same CXB sera assayed for activity using the human TSHR provided evidence for linkage between human TSHR TSAb and loci on Chr 17 and Chr X linked (Fig. 3C). The linkage data obtained in the present study taken together with our previous findings 14,17 (summarized in Table 1) permit the following general conclusions:-
Figure 3.
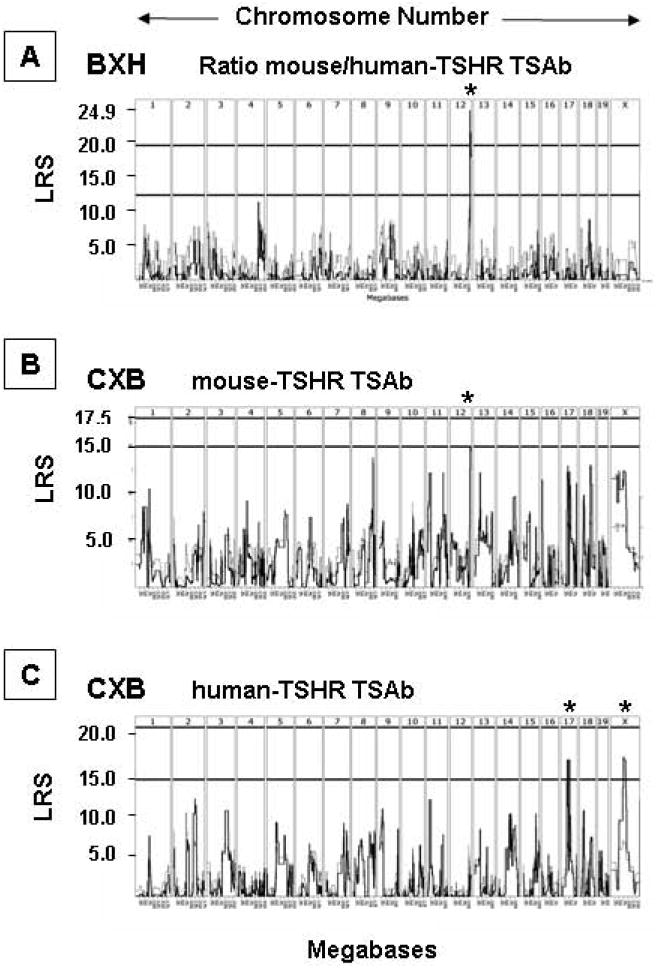
Whole genome interval mapping in BXH and CXB strains for TSAb traits after two A-subunit-Ad immunizations: A) human-TSHR TSAb in CXB mice; B) mouse-TSHR TSAb in CXB mice; C) Ratio mouse:human (m:h) TSHR TSAb in BXH mice. Chromosomes 1 to 19 and X are indicated at the top of each panel and likelihood ratio statistics (LRS) values on the vertical axis. The horizontal lines (panels A and C) indicate the LRS values above which a trait is associated with a particular chromosome [indicated by asterisk(s)]. In panel A, the upper horizontal line indicates the level for a significant association. GeneNetwork identifiers: A) GN 10683; B) GN 10685; C) GN 10157.
Table 1.
Chromosomal linkage for TSAb in CXB and BXH mice immunized twice with TSHR A-subunit adenovirus. TSAb activity was measured with mouse-TSHR cells and human-TSHR cells; these data were used to calculate the TSAb ratios (human:mouse TSHR, h:m or mouse: human TSHR, m:h). Loci and chromosomal locations (megabases, Mb) are presented for LRS scores suggestive of linkage. Possible candidate genes are included with the corresponding (or likely) human (Hu) chromosome in parentheses.
| Strain | LRS | Chr | Locus | Mb | Candidate | Hu |
|---|---|---|---|---|---|---|
|
| ||||||
| human-TSHR TSAb
| ||||||
| BXH a | 13.628 | 12 | rs13459138 | 113.270 | Igh V genes | 14 |
| rs3705923 | 117.869 | Igh V genes | ||||
| rs3692361 | 118.161 | |||||
| rs3679276 | 120.329 | |||||
|
| ||||||
| CXB | 16.787 | 17 | gnf17.035.152 | 34.663 | MHC region | 6 |
| rs6395893 | 38.786 | genes | ||||
| D17Mit66 | 47.186 | |||||
| rs3690039 | 48.900 | |||||
| 16.787 | X | rs13483834 | 72.576 | ll1rapl1 | X | |
| rs13483877 | 83.201 | |||||
|
| ||||||
| mouse-TSHR TSAb
| ||||||
| CXB | 14.518 | 12 | rs13481658 | 113.595 | Igh V region | 14 |
| rs3711281 | 118.633 | genes | ||||
|
| ||||||
| TSAb Ratio | ||||||
|
| ||||||
| BXH m:h b | 20. 433 | 12 | CEL-12_10454502266.496 | 110.245 | 14 | |
| CEL-12_10470961366.496 | 110.409 | |||||
| D12Mit133 | 110.746 | (Dio3) | 14 | |||
| rs13481651 | 111.769 | |||||
| 18.323 | 12 | rs13459138 | 113.271 | Igh V genes | 14 | |
| rs3705923 | 117.869 | Igh V genes | ||||
| rs3692361 | 118.161 | |||||
| rs3679276 | 120.329 | |||||
|
| ||||||
| CXB h:m | 11.552 | 12 | D12Mit132 | 107.374 | (Dio3) | 14 |
| rs13481636 | 108.001 | |||||
previously reported 14;
similar observations for h:m TSHR TSAb (but lower LRS values). Candidate genes: MHC region including:C2 (34.470663), Hspa1 (34.577246);Tnf (34.807461 and Ltb (34.802573); ll1rapl1, interleukin 1 receptor accessory protein-like 1; Dio3, deiodinase type 3 (110.727 Mb); Igh V region genes, immunoglobulin heavy chain variable region genes. LRS = LOD X 4.61. GeneNetwork (GN) Identifiers:- human-TSHR TSAb, BXH GN10147; CXB GN10683; mouse-TSHR TSAb, CXB GN10685; TSAb Ratio: BXH m:h GN10157; CXB h:m GN10686. No linkage was observed for BXH mouse-TSHR TSAb GN10155.
First, loci on the terminal portion of Chr 12 are linked to the following traits involving TSAb after two immunizations with human TSHR A-subunit adenovirus:-
TSAb measured in the human-TSHR assay for BXH mice (Fig. 4A) and in the mouse-TSHR assay for CXB mice (Fig. 4A);
the mouse:human-TSHR TSAb ratio in BXH (Fig. 4B) and the human:mouse-TSHR ratio in CXB mice (Fig. 4B).
Figure 4.
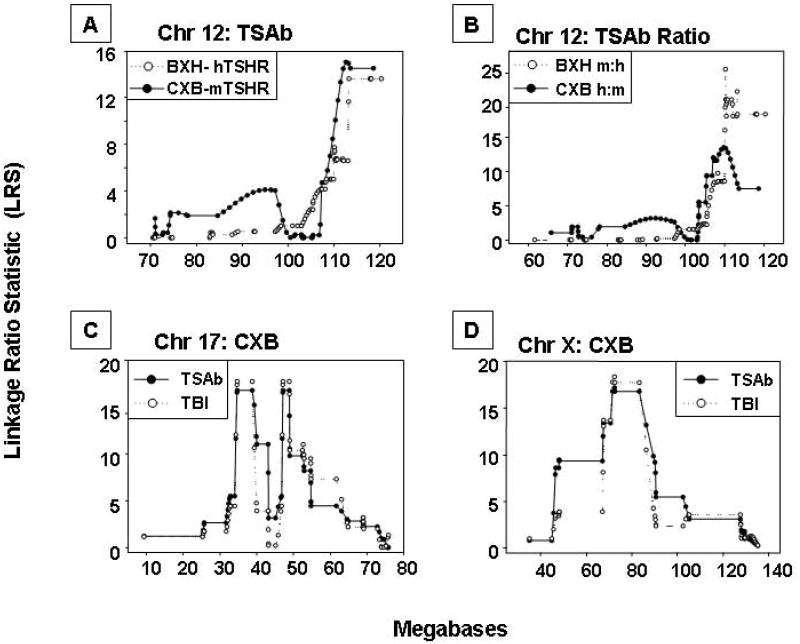
Individual chromosomes in CXB and BXH mice associated with TSAb activity or TSH binding inhibition (TBI) after two A-subunit-Ad immunizations in the present and previous studies 14,17: A) Chr 12: Mouse-TSHR TSAb for CXB mice and human-TSHR TSAb for BXH mice; B) Chr 12: Ratios for mouse:human TSHR TSAb for BXH mice and human:mouse TSAb for CXB mice; C) Chr 17: TBI (inhibition of TSH binding) for CXB mice and human-TSHR TSAb; D) Chr X: TBI (inhibition of TSH binding) for CXB mice and human-TSHR TSAb. Chromosomal distances (megabases) are on the horizontal axes and Likelihood Ratio Statistics are on the vertical axes. GeneNetwork identifiers: A) CXB GN 10685 and BXH GN 10147; B) BXH GN10157 and CXB GN 10686; C) GN 10512 and GN 10683; D)GN 10512 and GN 10683.
Second, loci on other chromosomes were shared by TSAb and non-TSAb traits:-
In CXB mice, there is linkage between human-TSHR TSAb values and loci on Chr 17 (Fig. 4C) and Chr X (4D);
Similar loci on these Chr previously identified in CXB mice for TSHR antibodies measured by TSH binding inhibition (TBI) 17 are indicated in Fig. 4C and D.
Combined linkage analysis in BXH and CXB mice
Combining the data for BXH and CXB strains strengthened the linkage between TSAb values (using either the mouse- or human-TSHR or the ratio between these assays) with loci on Chr 12 (Table 2). This combined analysis provides statistical data as LOD (not LRS) scores. LOD scores of 3.49 to 3.56 were obtained for the Chr 12 region from 109.73 to 118.161 Mb, which is dominated by immunoglobulin H chain genes (Fig. 5). The highest LOD scores (>5.8) were obtained for TSAb ratios linked to Chr 12 loci between 110.306 and 111.967 Mb, a region which includes the gene for type 3 deiodinase (Dio3) 22.
Table 2.
Combined linkage analysis for TSAb in BXH and CXB recombinant inbred mice (26 strains) immunized twice with TSHR A-subunit-adenovirus.
| Trait | Chr | Chr Interval (Mb) | X 2 | p | LOD |
|---|---|---|---|---|---|
|
| |||||
| TSAb | |||||
|
| |||||
| CXB mouse-TSHR TSAb and BXH human-TSHR TSAb |
12 | 109.730 -110.187 | 20.95 | 0.000324 | 3.490 |
| 110.245 -110.306 | 20.05 | 0.000488 | 3.312 | ||
| 110.882- 111.769 | 19.44 | 0.000643 | 3.192 | ||
| 113.181- 113.187 | 19.92 | 0.000519 | 3.285 | ||
| 113.595 -118.161 | 21.13 | 0.000298 | 3.526 | ||
|
| |||||
| Ratio | |||||
|
| |||||
| CXB human:mouse TSHR and BXH mouse:human TSHR |
12 | 108.577 - 109.137 | 21.06 | 0.00030858 | 3.511 |
| 110.306 -110.409 | 33.80 | 8.19781E-07 | 6.086 | ||
| 110.882 - 111.967 | 32.53 | 1.49236E-06 | 5.826 | ||
Linkage analysis in BXH and CXB strains for TSAb following the third immunization
TSAb assays performed on sera one month after the third immunization replicated the foregoing data with sera obtained after the second immunization. However, with greater stimulation to the immune system, in addition to the loci on Chr 12, 17 and X, a number of other loci in BXH and CXB mice were linked to TSAb traits, for example on Chr 9 and 10 (Supplementary Information Table S1). The potential significance of these loci is unknown because the genes therein are unidentified.
DISCUSSION
Before considering our present findings, it should be emphasized that two assays are used to assess TSHR antibody levels in Graves’ patients and induced animal models of this disease. Clinically, the most standardized and cost-effective assay involves antibody mediated TSH binding inhibition (TBI). This assay utilizes purified human or porcine TSHR. A bioassay for functional thyroid stimulating antibodies (TSAb) is more complex and involves measuring the cAMP response to stimulation in non-thyroidal cells expressing the recombinant human TSHR. Early studies with the animal model of Graves’ disease revealed that background genes in different mouse strains had a major effect on the development of hyperthyroidism. Thus, although TSHR antibodies (measured as TBI) were induced in several mouse strains by immunization with TSHR-expressing adenovirus, BALB/c mice were the most susceptible to developing hyperthyroidism 7,23. C57BL/6 mice, which develop high TSHR antibody levels , rarely become thyrotoxic 7.
Because of this strain difference and the dominance of the BALB/c phenotype in F1 offspring of the BALB/c x B6 cross 15, we studied recombinant inbred CXB mice (derived from these parental strains) for genetic factors that control the variability in TSHR antibodies and hyperthyroidism. Our findings suggested that loci on different chromosomes were responsible for TSHR antibody induction versus thyroid function 17. Extending these studies to BXH mice, derived from C3H/He and B6 parental strains, we confirmed the importance of different gene sets controlling immune responses versus thyroid function. In this second study, in addition to measuring TBI, we assayed functional TSAb. We were puzzled to observe that, despite very low TSAb levels, C3H/He mice and some BXH strains became hyperthyroid 14. Our present data resolve this conundrum. Remarkably, despite genetic immunization with a vector expressing the human TSHR A-subunit, C3H/He mice and some BXH strains develop TSAb that preferentially recognize the mouse TSHR, not the human TSHR used in the conventional bioassay. Of course, hyperthyroidism can only develop by activation of the mouse TSHR in vivo. Better antibody recognition of a related antigen than the immunogen, termed “heteroclicity”, is a well established phenomenon. For example, preferential antibody recognition of bovine or porcine insulin after immunization with human insulin 24 and greater affinity early in the immune response for mouse- than for pigeon- cytochrome c after immunization with the latter antigen 25.
In humans, TSAb cross-reactivity with TSHR from different species was first recognized in the late 1950’s, before the immunoglobulin nature of TSHR antibodies was recognized. A component of Graves’ serum was found to stimulate the guinea pig thyroid with a prolonged time course compared with TSH, hence the name “Long Acting Thyroid Stimulator” (abbreviated LATS) 10. When the assay was adapted for use in mice, sera from some patients did not cross-react with the mouse thyroid and were LATS negative. The first commercialized TBI assay also involved antigen cross-reactivity, namely porcine TSHR 26. Correlation between TBI measured with the porcine TSHR versus the recombinant human TSHR is generally good (for example 27. In retrospect, the few exceptions, involving better recognition of porcine- than human-TSHR, likely represent antibody heteroclicity.
Parenthetically, we wish to comment on our present findings of relatively minor differences between TSAb measured in the human- and mouse-TSHR assays in the CXB recombinant inbred strains (BALB/c crossed with B6 mice). These data would appear to conflict with our previous report that, following human TSHR immunization, mouse-specific TSAb predominated over human-TSAb activity in BALB/c mice, and vice versa for B6 mice 16. The explanation for this apparent discrepancy is that the latter studies utilized BALB/c and B6 both of the Jackson substrain 16, whereas CXB mice are derived from the Bailey strains of BALB/c and B6. The clear-cut difference between BALB/c susceptibility versus B6 resistance to hyperthyroidism in Jackson-substrains 7,15 is muted in the Bailey-substrains 17. Moreover, in a direct comparison, fewer Bailey-BALB/c than Jackson-BALB/c (BALB/cByJ versus BALB/c/J) mice developed hyperthyroidism in response to TSHR A-subunit adenovirus immunization 28. The Bailey substrains of BALB/c and B6 mice have been bred separately from their Jackson counterparts for over 50 years 28
The most important outcome of our study, with potential implications for the genetics of human Graves’ disease, is the discovery of novel candidate genes besides the previously observed linkage to the MHC gene region 14,17. In BXH strains, preferential TSAb recognition of the mouse- versus human-TSHR in BXH mice was significantly linked to loci on Chr 12 between 110.245 and 111.769 Mb (LRS of 20.433; LOD of 4.43). This region includes the gene for type 3 thyroid deiodinase (Dio3), an enzyme involved in thyroid hormone inactivation 22. Increasing the power of genetic analysis by combining the TSAb species specificity data for the BXH and CXB mice increased the LOD score for this chr 12 region to >5.8.
Potentially of greater pathophysiological importance were loci slightly further upstream on Chr 12. Thus, LOD scores of 3.49 to 3.56 were obtained for the Chr 12 region from 113.270-120.329 Mb. Combining linkage data for the CXB and BXH strains narrowed this region to 113.595 -118.161 Mb. Remarkably, in this broad region, 32/66 (48%) of the genes markers encode the variable regions (IgV H) of immunoglobulin heavy chains (Supplementary Table S2), which are logical candidate genes for influencing antibody specificity. Moreover, the high frequency of VH genes in this locus, taken together with earlier findings that an IgV H gene controlled antibody heteroclicity (reactivity to steryl-oxazolone versus the immunogen furyloxazolone 29 ), strongly suggests that VH gene differences between mouse strains underlie the susceptibility (or lack thereof) to develop antibodies capable of activating the TSHR.
In early studies, it was reported that two genes, linked to immunoglobulin heavy chains (Gm) and HLA, controlled susceptibility to Graves disease 30. Because the chromosomal region encompassing genes for immunoglobulin heavy chain variable- and constant- regions is large (~ 3 million bases in mice 31), these observations for humans are potentially similar to ours in mice. However, similar associations were made for Hashimoto’s thyroiditis 32, a condition in which TSAb are absent. Moreover, later studies found no evidence for genes associated with the immunoglobulin H chain loci in Graves’ or Hashimoto’s diseases 33,34.
Why have immunoglobulin VH germline gene loci not previously been identified in genetic linkage and genome wide association studies in human Graves’ disease? In our opinion, ascertainment of traits for inclusion in these studies has been too broad. Greater stratification than a simple diagnosis of Graves’ disease is necessary. In Graves’ patients, TSHR antibodies are present at very low concentrations and are rarely (if ever) detected in family members without clinical disease. In addition, TSHR antibody levels are modulated by, and may disappear after, therapy with anti-thyroid drugs, surgery or radioiodine (for example 35,36). For these reasons, to our knowledge, no studies in humans have tested the genetic susceptibility for TSHR antibodies separately from hyperthyroidism, as has been done for autoantibodies to thyroid peroxidase and thyroglobulin 37. One advantage of the Graves’ animal model, using genetically identical mouse strains subject to identical environmental factors, is the ability to separate susceptibility to TSHR antibody development from variations in thyroid function 14,17. Our present data suggest that insight into the genetics of human Graves’ disease would be facilitated by restricting genetic analysis to untreated patients and which are subdivided according to TSAb levels or even divided into TSAb antibody subsets including perhaps differential TSAb activity for the human- versus the mouse-TSHR.
Besides our novel findings pointing to VH germline genes in Chr 12 as candidates for genetic susceptibility to TSAb generation, our present and previous linkage data 14,17 provide evidence for other loci underlying this trait:-
TSHR antibodies (determined in TSAb and TBI assays) in CXB mice were linked to the same Chr 17 loci including MHC class I and II, complement components, tumor necrosis factor and lymphotoxin. These data support observations in humans in which MHC class II has long been associated with autoimmune thyroid disease (reviewed in 38) and very recently MHC class I 39.
The highest LRS or LOD scores for TSAb species specificity were associated with the Chr 12 region including the gene encoding Deiodinase 3 (Dio3) 22, a seleno-enzyme that catalyzes the conversion of thyroid hormones to inactive metabolites. However, because it is difficult to envisage how Dio3 could contribute to the pathogenesis of Graves’ disease, TSAb activity is more likely linked to another closely associated, presently unidentified gene.
TSAb generation in CXB mice linked to Chr X loci between 72.5 and 83.3 Mb, supporting our previous finding with TSHR antibodies detected in the TBI assay 17. There are no obvious candidate genes in this region, with the possible exception of interleukin 1 receptor accessory protein-like 1 (Il1rapl1). In addition to the foregoing distal Chr X loci, TSHR antibody linkage to more proximal Chr X loci (32-44 Mb) was noted for TBI in BXH mice 14. Again, there are no logical candidate genes in this region. As noted previously, although not confirmed in all data sets 40,41, associations with the X chromosome have been reported for human Graves’ disease 42-44.
In conclusion, measuring mouse-TSAb versus human-TSAb in mice with different and known genetic backgrounds provides novel insight into the genetic basis for susceptibility to induction of antibodies capable of activating the TSHR. Most important, we provide the first evidence suggesting involvement of immunoglobulin VH genes in this process. These data suggest that re-examination of these candidate genes in human Graves’ disease is warranted, using improved subject ascertainment and stratification, for example by including only patients with untreated disease of recent onset, of the same gender, and focusing on TSHR antibodies rather than a simple past or present history of disease.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants DK54684 (S.M.M.), DK19289 (B.R), U01AA13499, U24AA13513, P20-DA-21131, U01CA105417and U24RR021760 (R.W.W.). R.W.W. and GeneNetwork are also supported by Integrative Neuroscience Initiative on Alcoholism-National Institute on Alcohol Abuse and Alcoholism (INIA-NIAAA); Biomedical Informatics Research Network-National Center for Research Resources (BIRN-NCRR); Mouse Models of Human Cancers Consortium-National Cancer Institute (MMHCC-NCI) and a Human Brain Project. We are also grateful for contributions by Dr. Boris Catz, Los Angeles.
Non-standard abbreviations
- IgV H
immunoglobulin heavy chain variable region
- LRS
Linkage ratio statistic
- RI mice
recombinant inbred mice
- TSAb
thyroid stimulating antibody
- TBI
TSH binding inhibition
- TSHR
thyroid stimulating hormone receptor
Footnotes
Conflict of Interest: The authors declare that no conflict of interest exists.
References
- 1.Hollowell JG, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpump MPJ, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport B, McLachlan SM. The thyrotropin receptor in Graves’ disease. Thyroid. 2007;17:911–922. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 4.Ercolini AM, Miller SD. Mechanisms of immunopathology in murine models of central nervous system demyelinating disease. J Immunol. 2006;176:3293–3298. doi: 10.4049/jimmunol.176.6.3293. [DOI] [PubMed] [Google Scholar]
- 5.Joe B. Quest for arthritis-causative genetic factors in the rat. Physiol Genomics. 2006;27:1–11. doi: 10.1152/physiolgenomics.00034.2005. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan SM, Nagayama Y, Rapoport B. Insight into Graves’ hyperthyroidism from animal models. Endocr Rev. 2005;26:800–832. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- 7.Nagayama Y, et al. A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002;168:2789–2794. doi: 10.4049/jimmunol.168.6.2789. [DOI] [PubMed] [Google Scholar]
- 8.Chen C-R, et al. The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–1904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaithamana S, Fan J, Osuga Y, Liang SG, Prabhakar BS. Induction of experimental autoimmune Graves’ disease in BALB/c mice. J Immunol. 1999;163:5157–5164. [PubMed] [Google Scholar]
- 10.McKenzie JM. Delayed thyroid response to serum from thyrotoxic patients. Endocrinol. 1958;62:865–868. doi: 10.1210/endo-62-6-865. [DOI] [PubMed] [Google Scholar]
- 11.Vitti P, et al. Graves’ IgG stimulation of continuously cultured rat thyroid cells: a sensitive and potentially useful clinical assay. J Endocrinol Invest. 1982;5:179–182. doi: 10.1007/BF03349476. [DOI] [PubMed] [Google Scholar]
- 12.Ludgate M, et al. Use of the recombinant human thyrotropin receptor (TSH-R) expressed in mammalian cell lines to assay TSH-R autoantibodies. Molec Cell Endocrinol. 1990;73:R13–R18. doi: 10.1016/0303-7207(90)90050-i. [DOI] [PubMed] [Google Scholar]
- 13.Muehlberg T, Gilbert JA, Rao PV, McGregor AM, Banga JP. Dynamics of thyroid-stimulating and -blocking antibodies to the thyrotropin receptor in a murine model of Graves’ disease. Endocrinol. 2004;145:1539–1545. doi: 10.1210/en.2003-1456. [DOI] [PubMed] [Google Scholar]
- 14.McLachlan SM, et al. Shared and unique susceptibility genes in a mouse model of Graves’ disease determined in BXH and CXB recombinant inbred mice. Endocrinol. 2008;149:2001–2009. doi: 10.1210/en.2007-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CR, et al. Susceptibility rather than resistance to hyperthyroidism is dominant in a thyrotropin receptor adenovirus-induced animal model of Graves’ disease as revealed by BALB/c-C57BL/6 hybrid mice. Endocrinol. 2004;145:4927–4933. doi: 10.1210/en.2004-0716. [DOI] [PubMed] [Google Scholar]
- 16.Misharin A, et al. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinol. 2009;150:1051–1060. doi: 10.1210/en.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aliesky HA, et al. Probing the genetic basis for thyrotropin receptor antibodies and hyperthyroidism in immunized CXB recombinant inbred mice. Endocrinol. 2006;147:2789–2800. doi: 10.1210/en.2006-0160. [DOI] [PubMed] [Google Scholar]
- 18.Chen CR, Aliesky HA, Guo J, Rapoport B, McLachlan SM. Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves’ disease induced using thyrotropin receptor-expressing adenovirus. Thyroid. 2006;16:427–434. doi: 10.1089/thy.2006.16.427. [DOI] [PubMed] [Google Scholar]
- 19.Williams RW, Gu L, Qi S, Lu L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-11-research0046. Research0046.1-0046.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RW, Strom RC, Goldowitz D. Natural variation in neuron number in mice is linked to a major quantitative trait locus on Chr 11. J Neurosci. 1998;18:138–146. doi: 10.1523/JNEUROSCI.18-01-00138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez A, Martinez ME, Croteau W, St Germain DL. Complex organization and structure of sense and antisense transcripts expressed from the DIO3 gene imprinted locus. Genomics. 2004;83:413–424. doi: 10.1016/j.ygeno.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Nagayama Y, McLachlan SM, Rapoport B, Niwa M. A major role for non-MHC genes, but not for micro-organisms, in a novel model of Graves’ hyperthyroidism. Thyroid. 2003;13:233–238. doi: 10.1089/105072503321582024. [DOI] [PubMed] [Google Scholar]
- 24.Mirza IH, Wilkin TJ. Antibody specificity in the immune response to insulin. Int Arch Allergy Appl Immunol. 1989;89:261–263. doi: 10.1159/000234957. [DOI] [PubMed] [Google Scholar]
- 25.Minnerath JM, Wakem LP, Comfort LL, Sherman F, Jemmerson R. The BALB/c mouse B-cell response to pigeon cytochrome c initiates as a heteroclitic response specific for the self antigen mouse cytochrome c. Proc Natl Acad Sci U S A. 1995;92:12379–12383. doi: 10.1073/pnas.92.26.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shewring GA, Rees Smith B. An improved radioreceptor assay for TSH receptor antibodies. Clin Endocrinol. 1982;17:409–417. doi: 10.1111/j.1365-2265.1982.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 27.Kakinuma A, et al. Comparison of recombinant human thyrotropin receptors versus porcine thyrotropin receptors in the thyrotropin binding inhibition assay for thyrotropin receptor autoantibodies. Thyroid. 1999;9:849–855. doi: 10.1089/thy.1999.9.849. [DOI] [PubMed] [Google Scholar]
- 28.Seetharamaiah GS, Land KJ. Differential Susceptibility of BALB/c and BALB/cBy mice to Graves’ hyperthyroidism. Thyroid. 2006;16:651–658. doi: 10.1089/thy.2006.16.651. [DOI] [PubMed] [Google Scholar]
- 29.Makela O, Matoso-Ferreira A, Kaartinen M. Fine specificity of the immune response to oxazolones III. Antibodies but not contact sensitivity specific for 2-furyloxazolone are controlled by an Igh-V gene in the mouse. Eur J Immunol. 1983;13:1017–1022. doi: 10.1002/eji.1830131213. [DOI] [PubMed] [Google Scholar]
- 30.Uno H, Sasasuk T, Tamai H, Matsumoto H. Two major genes, linked to HLA and Gm, control susceptibility to Graves’ disease. Nature. 1981;292:768–770. doi: 10.1038/292768a0. [DOI] [PubMed] [Google Scholar]
- 31.Retter I, et al. Sequence and Characterization of the Ig Heavy Chain Constant and Partial Variable Region of the Mouse Strain 129S1. J Immunol. 2007;179:2419–2427. doi: 10.4049/jimmunol.179.4.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamai H, et al. Immunogenetics of Hashimoto’s and Graves’ diseases. J Clin Endocrinol Metab. 1985;60:62–66. doi: 10.1210/jcem-60-1-62. [DOI] [PubMed] [Google Scholar]
- 33.Weetman AP, et al. Immunogenetics of Graves’ ophthalmopathy. Clin Endocrinol. 1988;28:619–628. doi: 10.1111/j.1365-2265.1988.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 34.Barbesino G, Tomer Y, Concepcion E, Davies TF, Greenberg DA. Linkage analysis of candidate genes in autoimmune thyroid disease. I. Selected immunoregulatory genes. J Clin Endocrinol Metab. 1998;83:1580–1589. doi: 10.1210/jcem.83.5.4813. [DOI] [PubMed] [Google Scholar]
- 35.McGregor AM, et al. Carbimazole and the autoimmune response in Graves’ disease. N Engl J Med. 1980;303:302–307. doi: 10.1056/NEJM198008073030603. [DOI] [PubMed] [Google Scholar]
- 36.Chiovato L, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351. doi: 10.7326/0003-4819-139-5_part_1-200309020-00010. [DOI] [PubMed] [Google Scholar]
- 37.Tomer Y, Greenberg DA, Barbesino G, Concepcion E, Davies TF. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab. 2001;86:1687–1693. doi: 10.1210/jcem.86.4.7372. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson EM, Tomer Y. The Genetic Basis of Thyroid Autoimmunity. Thyroid. 2007;17:949–961. doi: 10.1089/thy.2007.0153. [DOI] [PubMed] [Google Scholar]
- 39.Simmonds MJ, et al. A novel and major association of HLA-C in Graves’ disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet. 2007;16:2149–2153. doi: 10.1093/hmg/ddm165. [DOI] [PubMed] [Google Scholar]
- 40.Tomer Y, et al. Common and unique susceptibility loci in Graves and Hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73:736–747. doi: 10.1086/378588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor JC, et al. A Genome-wide Screen in 1119 Relative Pairs with Autoimmune Thyroid Disease. J Clin Endocrinol Metab. 2006;91:646–653. doi: 10.1210/jc.2005-0686. [DOI] [PubMed] [Google Scholar]
- 42.Barbesino G, Tomer Y, Concepcion E, Davies TF, Greenberg D. Linkage analysis of candidate genes in autoimmune thyroid disease: II. Selected gender-related genes and the X-chromosome. J Clin Endocrinol Metab. 1998;83:3290–3295. doi: 10.1210/jcem.83.9.5091. [DOI] [PubMed] [Google Scholar]
- 43.Tomer Y, Barbesino G, Greenberg DA, Concepcion E, Davies TF. Mapping the major susceptibility loci for familial Graves’ and Hashimoto’s diseases: evidence for genetic heterogeneity and gene interactions. J Clin Endocrinol Metab. 1999;84:4656–4664. doi: 10.1210/jcem.84.12.6216. [DOI] [PubMed] [Google Scholar]
- 44.Imrie H, et al. Evidence for a Graves’ disease susceptibility locus at chromosome Xp11 in a United Kingdom population. J Clin Endocrinol Metab. 2001;86:626–630. doi: 10.1210/jcem.86.2.7191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


