Abstract
PURPOSE
To test whether greater vigorous physical activity (kilometers per week run) and greater cardiorespiratory fitness (10-km race performance in meters per second) reduce the incidence of clinically diagnosed cataract.
METHODS
Prospective cohort study of self-reported clinical diagnosis of cataract in nondiabetic, nonvegetarian, and nonsmoking male (n = 29,025) and female runners (n = 11,967).
RESULTS
Incident cataracts were reported by 733 (2.53%) men and 179 (1.50%) women during (mean ± SE) 7.74 ± 0.01 and 7.42 ± 0.02 years of follow-up, respectively. The risk for incident cataract increased with BMI, such that the risk in men > 27.5 kg/m2 was 88% larger than in men <20 kg/m2. Men’s cataract risk declined significantly in relation to running distance (P = 0.01), even when adjusted for BMI. Men who ran ≥64 km/wk had 35% lower risk for cataract than those reporting <16 km/wk (28% lower risk when adjusted for BMI). In addition, men with greater cardiorespiratory fitness were at significantly less risk for development of cataract than were the least fit men. This result was not accounted for by adjustment for running distance or BMI. Compared with the least fit men, those who ran faster than 4.75 m/s had 50% lower risk for incident cataract (43% lower when adjusted for km/wk and BMI).
CONCLUSIONS
These data suggest that the men’s cataract risk decreased in association with lower BMI, greater physical activity, and greater cardiorespiratory fitness, the latter being statistically independent of both BMI and physical activity. The study limitations include the absence of confirmation of the clinical diagnosis and the lack of specificity of the type of cataract.
Age-related cataract is an opacity of the crystalline lens or its envelope and is the leading cause of blindness [1]. The occurrence may arise as part of the natural aging of the nucleus or represent the cumulative effects of exposure to risk factors over time. More than one-half of Americans over the age of 65 have cataracts of some form [2].
Although the economic and quality of life benefits of postponing the development of cataract is manifest, there are few established cataract risk factors amenable to intervention. In addition to age and trauma, possible risk factors for cataract include sunlight exposure, diabetes, smoking, intake of alcoholic beverages, and obesity. [1] Currently, lack of exercise and low cardiorespiratory fitness are not recognized cataract risk factors. [1] I am aware of only one published case–control study linking cataract to insufficient physical activity [3].
This study was undertaken to test prospectively whether greater physical activity and cardiorespiratory fitness (10-km performance) affect the risk for self-reported clinically diagnosed cataracts and whether leanness associated with activity and fitness mediate these effects. Most epidemiologic cohort studies are geographically or occupationally defined and tend to include few vigorously active men and women. The National Runners’ Health Study was specifically designed to target vigorously active men and women who regularly run for exercise [4–9]. Prospective analyses of this cohort have demonstrated that running greater distances attenuates age-related weight gain [4] and reduces the incidence of diabetes [5] in proportion to the exercise dose.
METHODS
The design and methods of the National Runners’ Health Study are described elsewhere [4–9]. Briefly, recruitment of this cohort took place between 1991 and 1993 by the national distribution of a two-page questionnaire to runners identified through subscription lists of running magazine subscribers and among participants of foot race events. The questionnaire solicited information on demographics, running history, weight history, smoking habit, history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, and diabetes. Eighty percent of the original cohort was followed up prospectively and recontacted 7 years later to determine their health status. The study protocol was approved by the University of California Berkeley Committee for the Protection of Human Subjects, and all participants signed committee-approved informed consents, in accordance with the Declaration of Helsinki.
Participants reported whether they had received a diagnosis of cataract since their baseline questionnaire and provided the year of diagnosis. Running distances were reported in usual miles run per week at baseline. There were strong correlations between repeated questionnaires for self-reported running distance (r = 0.89) [4], and self-reported running distance has been shown to be significantly associated with a number of biomarkers traditionally associated with physical activity including HDL-cholesterol, triglycerides, and LDL-cholesterol concentrations, systolic and diastolic blood pressures, fasting plasma glucose concentrations, BMI, and body circumferences [7,8]. Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) show that running represents (mean ± SD) a majority of all vigorously intense activity in men and women, and of total leisure-time physical activity, respectively.
BMI was calculated as self-reported weight in kilograms divided by the square of self-reported height in meters. Self-reported waist circumferences were elicited by the question, “Please provide, to the best of your ability, your body circumference in inches.” without further instruction. Elsewhere, strong correlations have been reported between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [4], and for self-reported running distances versus self-reported BMIs and waist circumferences in cross-sectional analyses [7,8]. Self-reported waist circumferences are somewhat less precise, as indicated by their correlations with reported circumferences on a second questionnaire (r = 0.84) and with their clinical measurements (r = 0.68) [4]. Determination of intakes of meat, fish, and fruit were based on the questions: “During an average week, how many servings of beef, lamb, or pork do you eat,” “…servings of fish do you eat,” and “…pieces of fruit do you eat?” Alcohol intake was estimated from corresponding questions for 4-oz. (112 mL) glasses of wine, 12-oz. (336 mL) bottles of beer, and mixed drinks and liqueurs. Alcohol was computed as 10.8 g per 4-oz glass of wine, 13.2 g per 12 oz. bottle of beer, and 15.1 g per mixed drink. Correlations between these responses and values obtained from 4-day diet records in 110 men were r = 0.65 for alcohol intake, r = 0.46 for red meat, r = 0.38 for fruit, and r = 0.19 for fish. For this report, baseline cardiorespiratory fitness was defined as speed in meters per second of the participant’s best 10-km race time during the previous 5 years (reported as finish time in minutes). Published data support the use of running performance to approximate maximum oxygen consumption (VO2max) [10,11].
Statistical Analyses
The Cox proportional hazards model (JMP software version 5.0; SAS Institute, Cary, NC) was used to estimate the dose–response relationships of incident cataract to baseline body weight and circumferences, weekly distances run, and cardiorespiratory fitness. Reported weekly intakes of alcohol, meat, fish, and fruit, along with age, hypertension, and BMI were used as covariates. A quadratic term was included for age to accommodate the accelerated risk for cataract with aging (quadratic term significant at P < 0.0001).
RESULTS
There were 29,025 men and 11,967 women who were nonsmoking, nonvegetarian, and nondiabetic at baseline who reported average weekly running distance, height, weight and age at baseline. These included 733 (2.53%) men and 179 (1.50%) women who reported incident physician-diagnosed cataract during (mean ± SE) 7.74 ± 0.01 and 7.42 ± 0.02 years of follow-up, respectively.
Those reporting cataracts were significantly older (men: 57.67 ± 0.36 vs. 44.52 ± 0.06 years, P < 0.0001; women: 55.63 ± 0.86 vs. 38.65 ± 0.09 years, P < 0.0001), and the men were heavier (men: 24.15 ± 0.10 vs. 23.86 ± 0.02 kg/m2, P = 0.003; women: 21.43 ± 0.18 vs. 21.26 ± 0.02 kg/m2, P = 0.35) and reported shorter weekly running distances (men: 32.49 ± 0.72 vs. 37.95 ± 0.14 km/wk, P < 0.0001; women: 34.36 ± 1.62 vs. 36.19 ± 0.20 km/wk, P = 0.26). The incident cases also reported slower 10-km performance speeds (men: 3.58 ± 0.02 vs. 3.95 ± 0.00 m/s, P = 0.002; women: 3.14 ± 0.04 vs. 3.49 ± 0.01 m/s, P < 0.0001). Adjustment for age did not eliminate the significance of the differences between those with and without cataract for men’s distance run per week (mean difference ± SE for cataract minus noncataract: −2.73 ± 0.86 km/wk, P = 0.002), 10-km performance (−0.083 ± 0.021 m/s, P < 0.0001), BMI (0.39 ± 0.10 kg/m2, P < 0.0001), or waist circumference (1.00 ± 0.24 cm, P < 0.0001).
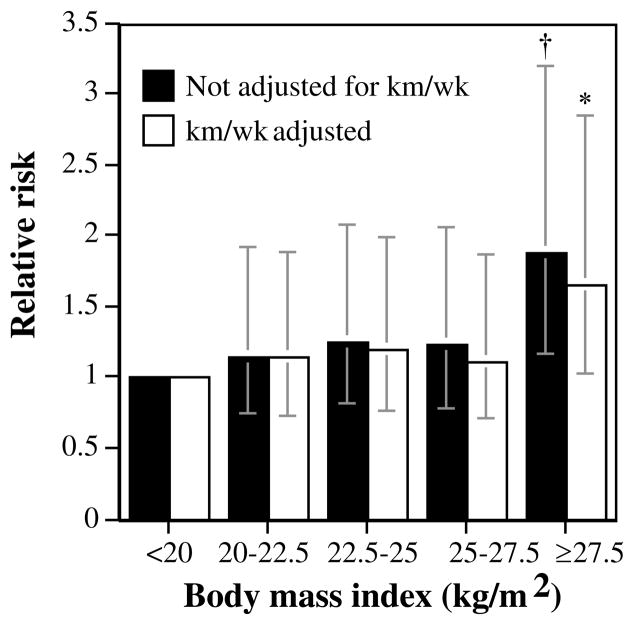
Table 1 shows a significant increase in cataract risk with increasing BMI and waist circumference in the men. Correction for differences in running distance had little effect on the relative risks. The men’s waist circumference also predicted incident cataract during follow-up, which was not accounted for by adjusting for BMI. Figure 1 shows that the relative risk for cataract in the men ≥ 27.5 kg/m2 was 88% larger than that in the men < 20 kg/m2. Although the risk for cataract appeared to increase more sharply at BMI ≥25 kg/m2, a quadratic term (i.e., BMI2) in the survival analysis model was not significant (P = 0.57), suggesting, in fact, that the increase in risk has a linear relationship with increasing BMI.
TABLE 1.
Relative risks (95% Confidence Interval [CI]) for incident self-reported clinically diagnosed cataracts during follow-up in relation to baseline BMI and waist circumference
| No Additional Adjustment | Adjusted for km/wk | Adjusted for BMI | |
|---|---|---|---|
| Men | |||
| BMI, kg/m2 | 1.048* (1.019–1.077) | 1.035† (1.005–1.066) | |
| Waist circumference, (cm) | 1.021‡ (1.009–1.033) | 1.016† (1.004–1.029) | 1.016† (1.000–1.032) |
| Women | |||
| BMI, kg/m2 | 0.975 (0.916–1.033) | 0.969 (0.908–1.029) | |
| Waist circumference, (cm) | 1.014 (0.993–1.034) | 1.014 (0.993–1.034) | 1.036* (1.010–1.062) |
Adjusted for age and intakes of meat, fish, fruit, and alcohol and additional variables, as indicated.
Significance:
P ≤ 0.01;
P ≤ 0.05;
P ≤ 0.001.
FIGURE 1.
Relative risk from survival analyses of self-reported physician-diagnosed cataract by the men’s BMI adjusted for age and intakes of meat, fish, fruit, and alcohol and weekly running distance, as indicated. Significant differences relative to the leanest men are coded *P < 0.05; †P < 0.01. Brackets represent 95% CI. The analyses included 1,040 men who weighed <20 kg/m2, 8,045 who weighed 20 to 22.5 kg/m2, 11,571 who weighed 22.5 to 25 kg/m2, 6,049 who weighed 25 to 27.5 kg/m2, and 2,320 who weighed ≥27.5 kg/m2
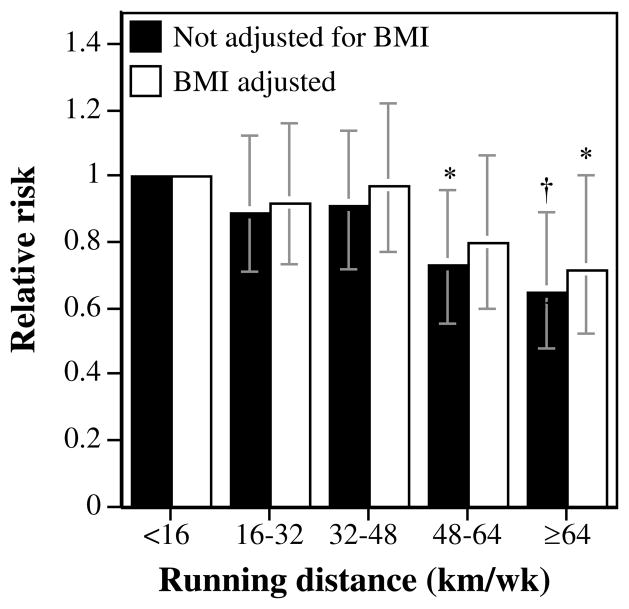
Table 2 shows that the men’s risk for incident cataract declined significantly in relation to weekly running distance (P = 0.01) even when adjusted for BMI. The men who ran ≥64 km/wk had a 35% lower risk for cataract than those reporting <16 km/wk (28% lower risk when adjusted for BMI; Fig. 2).
TABLE 2.
Relative Risks (95% CI) for Incident Self-Reported Clinically Diagnosed Cataracts during Follow-up in Relation to Baseline Cardiorespiratory Fitness and Physical Activity
| Men | Women | |||
|---|---|---|---|---|
| Cardio-Respiratory Fitness (m/s) | Physical Activity (km/wk) | Cardio-Respiratory Fitness (m/s) | Physical Activity (km/wk) | |
| All | ||||
| All Physical activity only, no BMI adjustment | 0.993* (0.990–0.997) | 0.999 (0.992–1.006) | ||
| Physical activity only, BMI adjusted | 0.995† (0.991–0.999) | 0.998 (0.991–1.006) | ||
| Fitness subset | ||||
| Cardiorespiratory fitness only, no BMI adjustment | 0.704‡ (0.592–0.838) | 0.773 (0.526–1.128) | ||
| Physical activity only, no BMI adjustment | 0.993* (0.989–0.997) | 0.994 (0.985–1.003) | ||
| Cardiorespiratory fitness & physical activity together, no BMI adjustment | 0.754† (0.624–0.910) | 0.996 (0.991–1.000) | 0.827 (0.550–1.222) | 0.995 (0.986–1.005) |
| Cardiorespiratory fitness only, BMI adjusted | 0.724* (0.602–0.872) | 0.711 (0.470–1.063) | ||
| Physical activity only, BMI adjusted | 0.994§ (0.990–0.998) | 0.993 (0.983–1.002) | ||
| Cardiorespiratory fitness and physical activity together, BMI adjusted | 0.765* (0.629–0.930) | 0.996 (0.991–1.001) | 0.766 (0.498–1.157) | 0.994 (0.985–1.004) |
All results adjusted for age (age and age2), and weekly intakes of meat, fish, fruit, and alcohol. The fitness subset refers to the 85% of the men and 76% of the women who had completed a 10-km foot race during the previous 5 years.
Significance levels for relative risk:
P ≤ 0.001;
P ≤ 0.01;
P ≤ 0.0001;
P ≤ 0.05.
FIGURE 2.
Relative risk shown by survival analyses of self-reported physician-diagnosed cataract by the men’s running distance adjusted for age and intakes of meat, fish, fruit, and alcohol and BMI as indicated. Significant differences relative to the least-active men are coded (*P < 0.05; †P < 0.01). Brackets represent 95% CI. The analyses included 3331 men who ran <16 km/wk, 7965 who ran 16 to 32 km/wk, 8547 who ran 32 to 48 km/wk, 4946 who ran 48 to 64 km/wk, and 4236 who ran ≥64 km/wk.
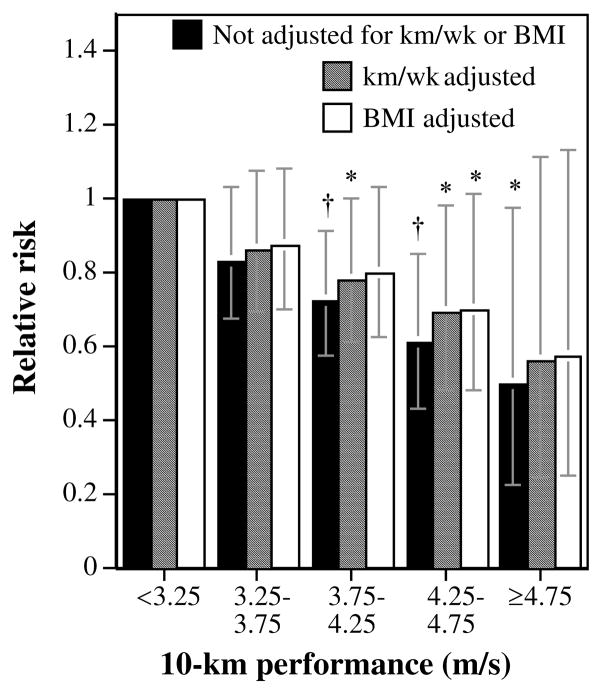
Ten-kilometer performance times were provided by 24,427 (84.2%) men and 9,047 (75.6%) women. Table 2 shows that the fitter men were at significantly less risk for development of cataract than were the least fit men and that adjustment for physical activity had only a minor effect on the magnitude of the risk reduction. The reduction in risk associated with performance remained significant when adjusted for BMI. Figure 3 suggests that the decline in the men’s risk with speed was linear, and largely independent of weekly distance run. Compared with the least fit men, those who ran faster than 4.75 m/s had 50% lower risk for incident cataract (43% lower when adjusted for km/wk and BMI). The fittest men were also at significantly less risk than were the men who ran between 3.25 and 3.75 m/s (P = 0.02). Table 2 shows that adjustment for 10-km performance weakened the significance of the relationship between physical activity and cataract (P = 0.07).
FIGURE 3.
Relative risk shown by survival analyses of self-reported physician-diagnosed cataract by 10-km performance times adjusted for age and intakes of meat, fish, fruit, and alcohol and additional variables, as indicated. Significant difference relative to the slowest men and women are coded (*P <0.05; †P <0.01). Brackets represent 95% CI. The analyses included 2244 men who ran <3.25 m/s, 6522 who ran 3.25 to 3.75 m/s, 8593 who ran 3.75 to 4.25 m/s, 5090 who ran 4.25 to 4.75 m/s, and 1978 who ran ≥4.75 m/s.
DISCUSSION
The results show that higher doses of vigorous physical activity and greater cardiorespiratory fitness reduced the risk for incident cataract in a large prospective study of runners (Table 2). Some, but not all of these associations, were attributable to the leanness of the faster, higher mileage runners. Lack of vigorous physical activity and low cardiorespiratory fitness have heretofore been unrecognized as cataract risk factors. Their associations are not unexpected given the conditions that predispose men and women prematurely to age-related cataract.
Physical Activity and Cardiorespiratory Fitness
Oxidation has a pivotal role in the formation of age-related nuclear cataracts [12]. and systemic inflammation is also thought to be involved in its etiology [13]. High-density lipoproteins (HDL) have both antioxidative and anti-inflammatory activities [14,15] in addition to their better-known cardioprotective role in reverse cholesterol transport. Exercise-induced increases in HDL-cholesterol concentrations are well documented from training studies [16,17]. Among runners, there is a pronounced dose–response relationship between weekly running distance and HDL levels, with plasma concentrations increasing incrementally with each 16-km/wk increase through at least 64 km/wk [7,8]. Men with innately higher HDL-cholesterol levels are also predisposed to run longer distances [17]. Plasma HDL-cholesterol and triglyceride concentrations are inversely related, and in men, triglyceride concentrations decline incrementally with each 16 km/wk increase in running distance through 64 km/wk [7].
Several observations suggest an association between cataract and low HDL or high triglycerides. In the Beaver Dam Eye Study, HDL-cholesterol concentrations were inversely related to cortical cataract in women, and higher ratios of total to HDL-cholesterol were concordantly related to posterior sub-capsular cataract (PSC) in men [18]. Another study reported that patients in the upper 5% of total cholesterol concentration or having a low-density lipoprotein to HDL-cholesterol ratio >5 had seven-fold greater odds of lenticular opacification if their HDL-cholesterol fell below 1.5 [19]. Results in other studies have failed to show an association between cataracts and plasma HDL-cholesterol concentrations [20,21]. Although hypertriglyceridemia was found in the Framingham study to predict incident PSC prospectively during follow-up in men only [22] and patients with PSC are reported to have significantly higher plasma triglyceride concentrations than patients with nuclear or cortical cataract [23], PSC is by far the less frequent type of opacity, representing only approximately 5% of cases. Yet another study showed that the odds of having cataract were 86% higher in Lithuanian women with elevated triglycerides [24].
An inverse association between cardiorespiratory fitness and cataract is suggested by the relationships to C-reactive protein, a marker for systemic inflammation. C-reactive protein levels predict incident cataract prospectively [13] and are inversely correlated with cardiorespiratory fitness (i.e., VO2max) [23]. Association studies show cardiorespiratory fitness correlates significantly with other inflammation markers as well, including cytokine (IL-6), fibrinogen, and white blood cell counts [25]. In part, the decrease in CRP with cardiorespiratory fitness may be mediated by the leanness of fitter runners. Clinical trials show that CRP levels decrease with weight loss [26] and there is a strong inverse association between 10-km performance and body weight [9].
Diabetes is a risk factor for PSC and cortical cataract [1]. Prediabetic endocrine dysfunction may expose lens cells to higher glucose concentrations, which may be cataractogenic by modifying lens protein through oxidative stress of glycation. Even in the absence of diabetes, glucose intolerance [27] and insulin resistance [28,29] are related to increased risk for cataract. Cross-sectionally, plasma glucose concentrations have been shown to decline in association with running distance in these runners [7] and at least one study has reported a positive association between fasting glucose concentrations and risk for cortical opacity [30]
Gout [31–33] and blood pressure [1] have also been associated with cataract, and among runners, the risks for these diseases decline in association with both greater running distance and greater cardiorespiratory fitness [9,34]. Serum uric acid concentrations are also associated with both cataract risk [35] and physical inactivity [7]. Cross-sectionally, systolic and diastolic blood pressures decline with weekly running distance and 10-km performance [9].
To my knowledge, only one other study has reported a relationship between cataract and physical activity. Paunksnis et al. [3] compared lens opacity and reported physical activity in 110 patients admitted to a university hospital for cataract surgery and 50 age-matched control subjects. Total physical activity was calculated from walking and minutes spent at moderate and vigorous activities. The less active subjects had seven-fold greater odds for cataract of the right eye (P<0.0001) and 4.4-fold greater odds for cataract of the left eye (P <0.001) when compared to active patients. They also reported that cortex lens opacity of the left eye was inversely correlated with physical activity.
Body Weight
Consistent with other studies, Table 1 shows that incident cataract is associated with greater BMI. The Framingham Study reported that cortical opacity increased with BMI and that posterior subcapsular opacity was associated with weight gain, whereas nuclear opacity was unrelated to BMI [36]. The Physicians Health Study reported that posterior subcapsular and nuclear opacity increased with BMI [37,38]. The Nurses’ Health Study reported that women with a BMI ≥30 or a waist circumference ≥ 89 cm had a greater prevalence of posterior subcapsular opacity than women with a BMI <25 or waist circumference ≤80 cm, whereas nuclear and cortical opacities were unrelated to BMI [39]. The Blue Mountain Eye Study reported that the odds ratio for cortical cataract and PSC were 1.6 and 2.1 in heavier (> 30 kg/m2) and leaner subjects, respectively [40]. The analyses suggest that greater waist circumference increases the risk for development of cataract in both men and women independent of overall BMI (Table 1). The choice to report waist circumference rather than waist-to-hip ratio is based on the former’s stronger association with visceral fat [41]. The Physicians’ Health Study reported that incident nuclear and PSC cataract and cataract extraction were associated with a greater waist-to-hip ratio, which persisted when adjusted for BMI [38]. A higher prevalence of posterior subcapsular opacity in women with a greater waist circumference was also reported by the Nurses’ Health Study [39].
In addition to demonstrating prospectively that baseline running distance predicted lower risk for cataract, the findings also showed that the lower risk was attributed in part to the leanness of the higher mileage runners (Table 2). A reduction in cataract, mediated by body weight, would not diminish the public health significance of promoting high-levels of vigorous activity, because the leanness of the runners is largely the consequence of running. Self-selection accounts for only 26% of the differences in BMI between running levels in men [6]. High-mileage runners are leaner because they gain less weight as they age than less active men [4], in addition to losing weight acutely when starting to run or increasing distance [17]. Specifically, the data show that men who run ≥48 km/wk had one half the average annual weight gain of those who run <24 km/wk [4]. The attenuation of age-related average weight gain is disproportionately due to the prevention of more extreme gains in weight [4], which the nonlinear relationship in Figure 1 suggests would have the greatest impact in preventing cataract.
Potential Confounders
Although the study did not specifically ask about indoor treadmill use, it is expected that almost all runners, particularly longer distance runners, accumulated their mileage outdoors and were exposed to sunlight in proportion to their running distance. Ecological and case–control studies suggest that sunlight exposure, specifically ultraviolet B radiation, is a risk factor for cataract [1]. The inverse relationship observed between self-reported cataract and distance run suggests that the apparent protective effects of running longer distances compensate for any additional risk produced by sun exposure. Similarly, running appears to compensate for any additional risk from greater corticosteroids use in treating running-related injuries [1]. Aspirin has been reported to reduce cataract risk in some but not all studies [1]. Although higher mileage runners take more aspirin than lower mileage runners, aspirin use in these runners was not found to be associated with cataract risk, and adjusting for its use did not change the results (analyses not displayed). Aspirin use was greater in slower than faster runners, and therefore any protective effects of aspirin would be contradictory to the lower cataract risk observed in with faster runners.
Limitations
The two primary limitations of these analyses are that they are based on self-report and they do not identify the type of cataract. The proportion of subjects who received dilated eye examination to properly assess cataract is not known. The presence of a cataract may not be reported to patients if thought to be clinically insignificant with respect to the best-corrected visual acuity obtainable during a routine eye examination. Misclassification of the presence or absence of cataract is also a possibility given that different types of opacity may have very different impact on visual function and that early cataract in the absence of subjective visual impairment may be diagnosed only by an ophthalmic slit lamp examination and not by a general physician. In addition to being less reliable than clinically verified diagnoses, the self-report provides no information on the location of the cataract, which affects its etiology. Although these analyses are specific to runners and the higher doses of more vigorous exercise that characterize this population, the biological mechanisms relating cataract risk to fitness and physical activity are unlikely to differ from those in other populations, and probably apply proportionately to smaller doses of less vigorous activity. The higher doses of more intense physical activity in this study provide greater statistical power for observing relationships that may extend in a dose-dependent manner throughout the range of activity levels practiced in the general population.
Based on data reported by others, it is not expected that the observed differences in self-reported clinically diagnosed cataract were due to less frequent clinical visits among leaner, higher mileage runners. Schaumberg et al. [38] report that leaner women tend to have more frequent eye examinations than heavier women. The Health Professionals Study reported that the more vigorously active male participants had more routine medical check-ups than did less active men [42], and there was no difference in routine medical check-up by activity level in the Nurses Health Study [43]. The prospective design specifically excluded preexisting cataract from being counted as incident events; however, blurry vision associated with subclinical lens opacity may affect either running distance or intensity, although there is no direct evidence of such a correlation.
SUMMARY
The economic and quality-of-life benefits of postponing the development of cataract is manifest. Heretofore, there have been few established risk factors amenable to intervention for achieving postponement. Although the limitation of self-reported clinically diagnosed cataract in the absence of a defined protocol for their diagnoses is acknowledged, these data nevertheless suggest the potential for vigorous physical activity to delay the onset of cataract. Vigorous exercise and high cardiorespiratory fitness attenuate age-related weight gain [4], raise HDL-cholesterol and lower plasma triglyceride concentrations [7,8], and reduce the risk for diabetes [5], hypertension [7,9], and gout [34])—all risk factors for cataract. Given these affects, it would have been all the more surprising if vigorous activity and fitness had not decreased cataract risk. The failure of other population studies to report this association may relate to the need for activity to be vigorous and fitness to be physiologically significant.
Acknowledgments
Supported in part by Grants HL-45652 and HL-072110 from the National Heart Lung and Blood Institute and Grant DK-066738 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure: P.T. Williams, None
References
- 1.Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 2.Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol. 1981;91:381–395. doi: 10.1016/0002-9394(81)90293-2. [DOI] [PubMed] [Google Scholar]
- 3.Paunksnis A, Kusleika S, Kusleikaite M. The relationship of the intensity of lens opacity with physical activity. Medicina (Kaunas) 2006;42:738–743. [PubMed] [Google Scholar]
- 4.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc. 2007;39:801–809. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PT. Changes in vigorous physical activity and incident diabetes in male runners. Diabetes Care. 2007;30:2838–2842. doi: 10.2337/dc07-1189. [DOI] [PubMed] [Google Scholar]
- 6.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obesity (Silver Spring) 2008;16:102–106. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 7.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–198. [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PT. High-density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Engl J Med. 1996;334:1298–1303. doi: 10.1056/NEJM199605163342004. [DOI] [PubMed] [Google Scholar]
- 9.Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch Intern Med. 1998;158:237–245. doi: 10.1001/archinte.158.3.237. [DOI] [PubMed] [Google Scholar]
- 10.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann N Y Acad Sci. 1977;301:484–494. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–204. [PubMed] [Google Scholar]
- 12.Taylor A. Cataract: relationship between nutrition and oxidation. J Am Coll Nutr. 1993;12:138–146. doi: 10.1080/07315724.1993.10718294. [DOI] [PubMed] [Google Scholar]
- 13.Schaumberg DA, Ridker PM, Glynn RJ, Christen WG, Dana MR, Hennekens CH. High levels of plasma C-reactive protein and future risk for age-related cataract. Ann Epidemiol. 1999;9:166–171. doi: 10.1016/s1047-2797(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 14.Klimov AN, Gurevich VS, Nikiforova AA, et al. Antioxidative activity of high density lipoproteins in vivo. Atherosclerosis. 1993;100:13–18. doi: 10.1016/0021-9150(93)90063-z. [DOI] [PubMed] [Google Scholar]
- 15.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Wood PD, Stefanick ML, Dreon DM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 17.Williams PT, Wood PD, Haskell WL, Vranizan K. The effects of running mileage and duration on plasma lipoprotein levels. JAMA. 1982;247:2674–2679. [PubMed] [Google Scholar]
- 18.Klein BE, Klein R, Lee KE. Cardiovascular disease, selected cardiovascular disease risk factors, and age-related cataracts: the Beaver Dam Eye Study. Am J Ophthalmol. 1997;123:338–346. doi: 10.1016/s0002-9394(14)70129-1. [DOI] [PubMed] [Google Scholar]
- 19.Meyer D, Parkin D, Maritz FJ, Liebenberg PH. Abnormal serum lipoprotein levels as a risk factor for the development of human lenticular opacities. Cardiovasc J S Afr. 2003;14:60–64. [PubMed] [Google Scholar]
- 20.Miglior S, Bergamini F, Migliavacca L, Marighi P, Orzalesi N. Metabolic and social risk factors in a cataractous population: a case-control study. Dev Ophthalmol. 1989;17:158–164. doi: 10.1159/000417021. [DOI] [PubMed] [Google Scholar]
- 21.Marks RG, Hale WE, Perkins LL, May FE, Stewart RB. Cataracts in Dunedin Program participants: an evaluation of risk factors. J Cataract Refract Surg. 1988;14:58–63. doi: 10.1016/s0886-3350(88)80065-8. [DOI] [PubMed] [Google Scholar]
- 22.Hiller R, Sperduto RD, Reed GF, D’Agostino RB, Wilson PW. Serum lipids and age-related lens opacities: a longitudinal investigation: the Framingham Studies. Ophthalmology. 2003;110:578–583. doi: 10.1016/S0161-6420(02)01762-1. [DOI] [PubMed] [Google Scholar]
- 23.Jahn CE, Janke M, Winowski H, et al. Identification of metabolic risk factors for posterior subcapsular cataract. Ophthalmic Res. 1986;18:112–116. doi: 10.1159/000265424. [DOI] [PubMed] [Google Scholar]
- 24.Paunksnis A, Bojarskiene F, Cimbalas A, Cerniauskiene LR, Luksiene DI, Tamosiunas A. Relation between cataract and metabolic syndrome and its components. Eur J Ophthalmol. 2007;17:605–614. doi: 10.1177/112067210701700420. [DOI] [PubMed] [Google Scholar]
- 25.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–1379. doi: 10.1152/japplphysiol.01028.2006. IOVS, January 2009, Vol. 50, No. 1. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Karasik A, Modan M, Halkin H, Treister G, Fuchs Z, Lusky A. Senile cataract and glucose intolerance: the Israel Study of Glucose Intolerance Obesity and Hypertension (The Israel GOH Study) Diabetes Care. 1984;7:52–56. doi: 10.2337/diacare.7.1.52. [DOI] [PubMed] [Google Scholar]
- 28.Gutman A, Andreus A, Adler JH. Hyperinsulinemia, insulin resistance and cataract formation in sand rats. Isr J Med Sci. 1975;11:714–722. [PubMed] [Google Scholar]
- 29.Clayton RM, Cuthbert J, Duffy J, et al. Some risk factors associated with cataract in S.E. Scotland: a pilot study. Trans Ophthalmol Soc UK. 1982;102:331–336. [PubMed] [Google Scholar]
- 30.Bunce GE, Kinoshita J, Horwitz J. Nutritional factors in cataract. Annu Rev Nutr. 1990;10:233–254. doi: 10.1146/annurev.nu.10.070190.001313. [DOI] [PubMed] [Google Scholar]
- 31.Leske MC, Chylack LT, Jr, Wu SY. The Lens Opacities Case-Control Study: risk factors for cataract. Arch Ophthalmol. 1991;109:244–251. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 32.The Italian-American Cataract Study Group. Risk factors for age related cortical, nuclear, and posterior subcapsular cataracts. Am J Epidemiol. 1991;133:541–553. [PubMed] [Google Scholar]
- 33.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–465. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 34.Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. 2008;87:1480–1487. doi: 10.1093/ajcn/87.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leske MC, Wu SY, Hyman L, et al. Biochemical factors in the lens opacities: case-control study. The Lens Opacities Case-Control Study Group. Arch Ophthalmol. 1995;113:1113–1119. doi: 10.1001/archopht.1995.01100090039020. [DOI] [PubMed] [Google Scholar]
- 36.Hiller R, Podgor MJ, Sperduto RD, et al. A longitudinal study of body mass index and lens opacities: the Framingham studies. Ophthalmology. 1998;105:1244–1250. doi: 10.1016/s0161-6420(98)97029-4. [DOI] [PubMed] [Google Scholar]
- 37.Glynn RJ, Christen WG, Manson JE, Bernheimer J, Hennekens CH. Body mass index: an independent predictor of cataract. Arch Ophthalmol. 1995;113:1131–1137. doi: 10.1001/archopht.1995.01100090057023. [DOI] [PubMed] [Google Scholar]
- 38.Schaumberg DA, Glynn RJ, Christen WG, Hankinson SE, Hennekens CH. Relations of body fat distribution and height with cataract in men. Am J Clin Nutr. 2000;72:1495–1502. doi: 10.1093/ajcn/72.6.1495. [DOI] [PubMed] [Google Scholar]
- 39.Jacques PF, Moeller SM, Hankinson SE, et al. Weight status, abdominal adiposity, diabetes, and early age-related lens opacities. Am J Clin Nutr. 2003;78:400–405. doi: 10.1093/ajcn/78.3.400. [DOI] [PubMed] [Google Scholar]
- 40.Younan C, Mitchell P, Cumming R, Rochtchina E, Panchapakesan J, Tumuluri K. Cardiovascular disease, vascular risk factors and the incidence of cataract and cataract surgery: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10:227–240. doi: 10.1076/opep.10.4.227.15905. [DOI] [PubMed] [Google Scholar]
- 41.Despres JP, Prud’homme D, Pouliot MC, Tremblay A, Bouchard C. Estimation of deep abdominal adipose-tissue accumulation from simple anthropometric measurements in men. Am J Clin Nutr. 1991;54:471–477. doi: 10.1093/ajcn/54.3.471. [DOI] [PubMed] [Google Scholar]
- 42.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–425. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 43.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk for cholecystectomy in women. N Engl J Med. 1999;341:777–784. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]