Abstract
Cross-sectional associations have been found between anxiety disorders (ADs) and hypothalamic-pituitary-adrenal (HPA) axis functioning, as measured by levels of salivary cortisol, but prospective data are lacking, as are studies examining specific ADs. We have previously shown that one aspect of the diurnal rhythm of cortisol, the cortisol awakening response (CAR), prospectively predicts both new onsets and recurrences of major depressive disorder (MDD). Here we sought to examine whether it also predicts ADs. Participants (N=232) were drawn from the larger Northwestern-UCLA Youth Emotion Project, a two-site, longitudinal study of older adolescents, which aims to identify common and specific risk factors for mood and anxiety disorders. After baseline interviews for mental health diagnoses, a subset of adolescents completed a three-day cortisol sampling protocol measuring the CAR and other diurnal rhythm indices. Participants with past or current anxiety disorders at the time of cortisol measurement were excluded and Cox regression (survival analysis) was used to predict first onsets of ADs over the subsequent six years. AD onsets (N=25), the largest subset of which were social anxiety disorder (SAD) onsets (N=11), were observed over six annual follow up diagnostic interviews. Even when statistically adjusting for past and prospective MDD onsets and other covariates, a higher CAR significantly predicted increased first onsets of ADs (HR = 2.20, p < .05). A higher CAR was also a strong and significant predictor of the subset of SAD onsets (HR = 5.37, p < .005). Implications for the etiology of ADs, with a focus on SAD, are discussed.
Keywords: cortisol, cortisol awakening response, diurnal rhythms, hypothalamic pituitary adrenal axis, anxiety disorders, social anxiety disorder, social phobia, prospective, longitudinal, adolescence, non-proportional hazard models
Introduction
Alterations in hypothalamic pituitary adrenal (HPA) axis functioning are frequently observed in individuals with major depressive disorder (MDD) (Ehlert et al., 2001; Thase et al., 2002; Doane et al., 2013; Herbert, 2013). Recent meta-analytic evidence suggests that depressed individuals show higher basal cortisol, on average, than individuals without depression (Lopez-Duran et al., 2009; Knorr et al., 2010). Not all studies and not all depressed individuals show elevated cortisol, and differences are not sufficiently large for basal cortisol to be used to discriminate depressed individuals from controls (Adam et al., 2008; Knorr et al., 2010). Evidence is sufficient, however, for altered HPA axis activity to be proposed as a risk marker that potentially plays an etiological role in the development of major depressive disorder (Ehlert et al., 2001; Thase et al., 2002; Herbert, 2013). Although prospective studies are rare, our own prior research has shown that one element of the cortisol diurnal rhythm, the cortisol awakening response (i.e., increase in cortisol levels from waking to 30 or 40 minutes after waking), is a significant prospective predictor of MDD, with individuals with a higher CAR showing increased risk for MDD over the subsequent 2.5 years (Adam et al., 2010; Vrshek-Schallhorn et al., 2013). Other studies have found high morning levels (measured at 8 AM) to be a significant prospective predictor of MDD (Goodyer et al., 2000; Harris et al., 2000; Halligan et al., 2007).
Despite the fact that MDD and anxiety disorders (ADs) are highly comorbid and share many features and risk factors (e.g., Clark and Watson, 1991; Mineka et al., 1998; Watson, 2005), far less research has examined HPA-axis activity in ADs. Cross-sectional research has suggested associations between current ADs and HPA-axis activity, as indexed by salivary cortisol (see below). Prospective longitudinal studies, however, predicting first onsets of ADs from premorbid basal cortisol levels, are to our knowledge entirely absent from the literature. In the current study, we examined whether measures of basal cortisol, including the CAR, collected in late adolescence, predicted first onsets of anxiety disorders over the subsequent six years.
Cortisol, the primary hormonal product of the HPA axis, is the most frequently measured HPA-axis marker in research on emotional disorders, including ADs, in part because the non-invasive nature of salivary cortisol collection allows repeated measurement of cortisol in the context of daily life (Kirschbaum and Helhammer, 2000; Adam et al., 2008). Cortisol levels increase in response to stress, and also follow a strong circadian rhythm. The typical basal/diurnal cortisol rhythm involves high levels upon waking, a substantial (50-60%) increase in the 30-40 minutes after waking (the cortisol awakening response or CAR), and a subsequent decline over the remainder of the day, reaching a low point or nadir around midnight (Weitzman et al., 1971; Kirschbaum and Hellhammer, 1989; Pruessner et al., 1997).
Anxiety disorders (such as panic disorder with or without agoraphobia, specific phobia, social anxiety disorder, and generalized anxiety disorder) and related disorders (obsessive-compulsive disorder, post-traumatic stress disorder, and acute stress disorder)1 are very prevalent. They also tend to be chronic in nature (e.g., Ferdinand and Verhuist, 1995; Pollack et al., 1996), and contribute high disease burden and cost to society (Greenberg et al., 1999; Kessler and Greenberg, 2002). In addition, individuals with anxiety disorders often go on to develop later MDD (Fava, 2000; Stein et al., 2001). Among the anxiety disorders, social anxiety disorder (SAD; also known as social phobia) is one of the most common mental disorders in the U.S. (Kessler et al., 2005), and is often associated with a high degree of impairment, including increased rates of unemployment, absenteeism from work, financial dependence on the state, use of prescription medications, and drug dependency (Lecrubier et al., 2000; Patel et al., 2002).
SAD is of particular theoretical interest in relation to HPA-axis activity (Schiefelbein and Susman, 2006), and the CAR in particular. The hallmark of social anxiety disorder is fear of negative social evaluation (American Psychiatric Association, 2000), and compared to other types of stressors, social evaluative threat has been found to be the most powerful activator of the HPA axis in studies of experimentally-induced stress (Dickerson and Kemeny, 2004). Social anxiety symptoms in adolescents are associated with perceived loneliness, perceived peer rejection and peer conflict (Storch and Masia-Warner, 2004; La Greca and Harrison, 2005). Perceived peer rejection and peer conflict have in turn been associated with cortisol activation (Flinn and England, 1995; Stroud et al., 2009) and perceived loneliness has been associated with a higher cortisol awakening response, in particular (Steptoe et al., 2004; Adam et al., 2006; Doane and Adam, 2010).
Establishment of effective peer and romantic relationships are some of the core developmental challenges of adolescence and the transition to adulthood (Roisman et al., 2004). Recent theory and research proposes the cortisol awakening response as an allostatic response, helping to mobilize the body's physiological resources to cope with anticipated daily challenges, including social engagement (Adam et al., 2006). Following these proposals, one would expect to see an elevated CAR in response to either anticipated or perceived acute social challenges. Elevations in the CAR have, however, been proposed to be costly in the long-term. The high and rapidly increasing levels of cortisol found in the morning hours and associated with the CAR in particular, are likely to occupy low-affinity glucocorticoid receptors in limbic regions; alterations to these receptor systems have in turn been implicated in the development of mood and anxiety disorders and their associated symptomatology (Adam et al., 2010; Herbert, 2013).
In summary, the anxiety experienced in SAD is strongly social in nature, the HPA axis is highly socially-sensitive, the CAR in particular appears to be responsive to daily social challenges, and an elevated CAR is likely to impact brain regions relevant to depression and anxiety. For these reasons, we hypothesize that alterations in the CAR are the most likely basal cortisol predictor of SAD.
Several cross-sectional studies have found elevated CARs or other aspects of morning cortisol in individuals with anxiety disorders. More specifically, in one study individuals with current anxiety disorders (n=774) showed significantly greater CAR values than their remitted (n=311) and never-disordered (n=324) counterparts (Vreeburg et al., 2010). Also, among 1768 adolescents in another study, those with “persistent” anxiety problems showed higher morning cortisol values, determined by averaging the waking and 30-minute post waking cortisol values, than those without anxiety problems or current problems only. Thus, cross-sectional data suggest that the CAR, and morning cortisol more generally, may be elevated in individuals with ADs or elevated anxiety. As noted earlier, however, to our knowledge there have been no prospective studies of this association.
Taking a prospective longitudinal approach to understanding the role of the HPA axis in emotional disorders is important because it can reveal whether HPA-axis alterations precede and predict the onset of disorder, increasing the likelihood the HPA axis plays an etiological role (Adam et al., 2008; Adam et al., 2010). It is most helpful to measure HPA-axis functioning prior to first onsets of disorder; otherwise, the observed HPA axis differences may reflect the effects of experiencing a current emotional disorder, or the “scar” effects of the past experience of disorder (e.g., Dierckx et al., 2012), rather than representing a premorbid risk factor.
In the current prospective study, we utilized a large longitudinal sample to examine whether morning cortisol levels, and the CAR in particular, assessed in late adolescence among participants who had not previously experienced an AD, were associated with first onsets of ADs over the following 6 years. We also examined whether pre-morbid variations in HPA axis functioning predicted two subgroups of AD onsets: 1) first onsets of social anxiety disorder, a disorder which occurred with high prevalence in our sample, and which we and others consider to be of particular interest in relation to HPA axis activity (Schiefelbein and Susman, 2006) and 2) first onsets of anxiety disorders other than social anxiety disorder (none of which occurred with high enough prevalence in our sample to be examined as separate disorders). We expected that elevated morning cortisol, and an elevated CAR in particular, would predict first onsets of ADs. For reasons outlined earlier, we expected to find particularly strong associations between the cortisol awakening response and SAD.
Method
Participants
Participants (N=232) were drawn from the Northwestern-UCLA Youth Emotion Project, a two-site longitudinal study of older adolescents. The key aims of this project were to identify risk factors that were shared versus unique to mood or anxiety disorders (Zinbarg et al., 2010). Risk factors included a set of personality, cognitive, life event and biological factors proposed to increase risk for these disorders. Over 1,900 juniors from two diverse high schools (one in a suburb of Chicago, one in a suburb of Los Angeles) were screened for neuroticism (N) using the Revised Eysenck Personality Questionnaire (EPQ-R-N; Eysenck et al., 1985). Given that neuroticism is a known risk factor for mood and anxiety disorders (Kendler et al., 2004), individuals scoring in the top third on this measure were oversampled in order to increase the number of individuals in the sample likely to go on to develop these disorders. Of the final group of 627 who consented to the longitudinal study and completed the baseline interviews and questionnaires described below, 59% scored in the top third.
From the larger YEP sample, 491 individuals were randomly selected and invited to participate in a diary study and cortisol sampling protocol; 344 completed this protocol. Participants were excluded for one or more of the following criteria: use of corticosteroid-based medications (N=14); presence of psychotic symptoms (N=3) or bipolar disorder (N=6) at any time point; provision of insufficient cortisol data to compute cortisol indices (N=10); presence of MDD at the time of cortisol sampling (N=15) and presence of any past or present clinically significant anxiety disorders at baseline (N=58). These eliminations resulted in 232 participants (171 female, 61 male). The predominance of females over males in this sample is accounted for by the fact that individuals with high levels of neuroticism were oversampled, and females score higher, on average, on this personality trait (Costa et al., 2001). In addition, females were disproportionately likely to agree to participate in the study if invited.
On average, participants were 16.91 years of age at baseline assessment (SD=.39, females= 16.92, males = 16.88). Participants were 17.06 years of age on average at the time of the cortisol assessment (SD = .40). At the 6-year follow-up, participants were 23 years of age on average (SD = .62 years). See Table 1 and Figure 1. The sample was racially diverse: 48% Caucasian, 10% African-American, 20% Hispanic, 5% Asian/Pacific Islander, 17% Multiracial/Other. It was also socioeconomically diverse, with parent occupations ranging from unskilled labor and service work to high-level professional and business positions (See Table 1).
Table 1. Descriptive Statistics for Total Sample and for Groups with No Anxiety Disorder Onset, and Other Anxiety Disorder Onset Over Follow-up Period.
| Total Sample | No Anxiety Disorder (AD) | Social Anxiety Disorder (SAD) | AD other than SAD | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | % | % | % | % | ||||
| Male gender | 26 | 27 | 36 | 7 | ||||
| African American race | 10 | 10 | 18 | 0 | ||||
| Hispanic ethnicity | 20 | 20 | 0 | 29 | ||||
| MDD history at baseline | 16 | 15 | 36 | 7 | ||||
| Baseline risk category | ||||||||
| Low | 19.8 | 21.7 | 0 | 7.1 | ||||
| Medium | 25.9 | 26.1 | 27.3 | 21.4 | ||||
| High | 54.3 | 52.2 | 72.7 | 71.4 | ||||
| Oral contraceptives (females) | 9.4 | 9.3 | 0 | 15.4 | ||||
| Smoke Cigarettes | 4.3 | 3.9 | 0 | 14.3 | ||||
| Psychotropic medication | 6 | 4.3 | 18.2 | 21.4 | ||||
| Total Sample | No AD | SAD | AD other than SAD | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age in years | ||||||||
| Baseline | 16.91 | 0.39 | 16.89 | 0.38 | 17.28 | 0.31 | 16.95 | 0.38 |
| Cortisol testing | 17.06 | 0.40 | 17.02 | 0.39 | 17.51 | 0.30 | 17.15 | 0.35 |
| Final follow-up | 23.00 | 0.60 | 22.96 | 0.58 | 23.52 | 0.79 | 23.01 | 0.59 |
| Socioeconomic status (SES) | 46.74 | 12.96 | 46.51 | 13.27 | 49.23 | 9.30 | 48.19 | 11.06 |
| Mean hours of sleep | 7.19 | 0.91 | 7.19 | 0.09 | 7.02 | 0.92 | 7.30 | 1.06 |
| Mean wake time (military) | 6:46 | 0:51 | 6:44 | 20:24 | 6:39 | 0:48 | 7:14 | 0:46 |
| Neuroticism | -0.16 | 0.76 | -0.21 | 0.76 | 0.39 | 0.74 | 0.14 | 0.56 |
| Waking cortisol (μg/dl) | 0.44 | 0.24 | 0.44 | 0.25 | 0.38 | 0.15 | 0.46 | 0.19 |
| Total Cortisol (AUCg) | 3.44 | 1.78 | 3.39 | 1.70 | 3.43 | 0.72 | 4.18 | 3.10 |
| Cortisol awakening response(CAR) (μg/dl) | 0.16 | 0.32 | 0.15 | 0.32 | 0.27 | 0.30 | 0.23 | 0.42 |
| Diurnal cortisol slope (Slope) | -0.02 | 0.01 | -0.02 | 0.01 | -0.02 | 0.01 | -0.02 | 0.02 |
Notes: MDD history = participant has diagnosed history of MDD at baseline. Baseline risk category = whether participant was considered at low, medium or high risk for depression and anxiety based on baseline neuroticism screener. Oral contraceptives = participants uses oral contraceptives at time of cortisol assessment, Smoke cigarettes = participant smokes cigarettes at time of cortisol assessment, Psychotropic medication = participant uses a psychotropic medication at baseline or at time of cortisol assessment. SES = socioeconomic status coded from education and occupational status ratings using the Hollingshead 1975 system (scale ranges from 0 to 66, with scores 40 or above indicating trained technical, business or professional status, and scores under 20 indicating unskilled labor or service work). (N) = composite measure of neuroticism based on average of four N questionnaires (z-scored). Waking Cortisol = cortisol values measured immediately upon waking. Cortisol Awakening Response (CAR) = increase in cortisol between waking and 40-minute post-awakening. AUCg = area under the curve defined by all cortisol values taken across the waking day. Diurnal cortisol slope=rate of change (decline) in cortisol levels, per hour, from waking to bedtime.
Figure 1.

Timeline of study procedures. SCID = Structured Clinical Interview for DSM-IV, non-patient edition (SCID; First et al., 2001). Average ages at time of assessments are provided in parentheses.
Procedures
Demographic Data
Gender, date of birth, self-reported racial/ethnic group were collected at screening. Variables for African-American and Hispanic race/ethnicity were created for potential use as covariates, given previous findings from this study showing significant differences in basal/diurnal cortisol for African American and Hispanic youth as compared to European American youth (DeSantis et al., 2007). Socioeconomic status (SES), coded from parental education level and occupation using the Hollingshead system (Hollingshead, 1975), was assessed at baseline and examined as a potential covariate due to prior studies showing associations between SES and cortisol diurnal rhythms (Cohen et al., 2006).
Diagnostic Assessment
The presence or absence of clinically significant mood and anxiety disorders was ascertained using the Structured Clinical Interview for DSM-IV, non-patient edition (SCID; First et al., 2001). Although DSM-V was recently released (American Psychiatric Association, 2013), this longitudinal study was conducted while DSM-IV was in effect (American Psychiatric Association, 2000). As a result, DSM-IV definitions of ADs and DSM-IV diagnostic criteria were employed in the current study. The baseline SCID assessed lifetime diagnoses of mood and anxiety disorders. The six subsequent annual follow-up SCIDs assessed diagnoses occurring during the interim since the previous SCID. DSM-IV anxiety disorders assessed and included in the present analyses were: panic disorder with or without agoraphobia, specific phobia, social anxiety disorder, obsessive-compulsive disorder, generalized anxiety disorder, post-traumatic stress disorder and acute stress disorder. DSM-IV past or present diagnoses of major depressive disorder (MDD) at baseline, and new onsets of MDD at each follow-up were also assessed.
Interviewers were extensively trained (see Zinbarg et al., 2010) graduate students or BA level research assistants who were blind to the results of previous assessments and diagnoses. All diagnoses were finalized by consensus in supervision meetings with a postdoctoral-level psychologist. Inter-rater reliability was assessed for approximately 10% of SCIDs in the larger study. Kappa values adjusted due to departure from equiprobable distributions (i.e., low base rates of diagnoses) across the SCID assessments ranged from .82 to .94 for MDD and from .72 to .85 for anxiety disorders. These represent a lower bound estimate for the reliability, given that all SCIDs were diagnosed by consensus of the group.
Personality Assessment
As previously described (Zinbarg et al., 2010), baseline levels of neuroticism (N) were measured using four separate scales: the Eysenck Personality Questionnaire-Revised Neuroticism Scale (EPQ-R-N; Eysenck et al., 1985) completed at the screening, the Behavioral Inhibition Scale (BIS)2 from the Behavioral Inhibition Scale/Behavioral Activation Scale (BIS/BAS; Carver and White, 1994), the Big Five Mini-Markers Neuroticism Scale (Saucier, 1994), and the IPIP NEO Revised Personality Inventory (IPIP NEO-PI-R; Goldberg, 1999). These scales were standardized (mean = 0, SD =1) and averaged to create a composite N score. Supporting the decision to use this composite, confirmatory factor analysis revealed that a single-factor model provided excellent fit (comparative fit index = 1.00, root mean square error of approximation = .013, standardized root mean square residual = .008) to the observed covariances among the four scales (Zinbarg et al., 2010).
Cortisol Assessment
The cortisol collection protocol used here has been described in detail previously (Adam et al., 2010; Doane and Adam, 2010). Cortisol was collected by passive drool on three consecutive weekdays, six times daily: upon waking, 40 minutes after waking, at bedtime, and at three unanticipated times signaled by a programmed watch beep throughout the remainder of the day. Thus, cortisol samples were taken: immediately upon waking (S1); 40 minutes after waking (S2); in the mid-morning approximately 3h post-waking (S3); in the mid-afternoon approximately 8h post-waking (S4); in the mid-evening approximately 12h post-waking (S5); and immediately before bedtime (S6). For the scheduled samples, participants were instructed not to eat, drink, or brush their teeth during the 30 minutes prior to the expected sample collection times. The unanticipated beeps were scheduled so as to avoid typical major mealtimes. Participants were asked to store samples in their refrigerators after collection, and returned samples to our team by way of a drop off location at their local school, or by regular postal mail. Cortisol samples are stable in saliva at room temperature for several days, and are not affected by the handling associated with a regular postal journey (Clements and Parker, 1998). Samples were stored at -20°C after being returned to the lab. They were later shipped to Trier, Germany, and were assayed in duplicate using time-resolved fluorescent-detection immunoassay (DELFIA; Dressendörfer et al., 1992). Intra-assay variation ranged from 4.0% to 6.7%, while inter-assay variation ranged from 7.1% to 9.0%.
Diurnal Cortisol Indices
Raw cortisol data were logarithmically transformed due to positive skew prior to use in analyses, but raw salivary cortisol values in μg/dL are presented in figures for ease of interpretation. Three indices of diurnal cortisol were calculated: the cortisol awakening response or CAR (cortisol value 40 minutes after waking minus value at waking, an index of cortisol increase in the 40 minutes post-awakening), the diurnal cortisol slope (a regression line fit to all measures between waking and bedtime except the measure taken at 40 minutes after waking, an index of rate of decline per hour in cortisol from waking to bedtime), and total cortisol across the day (the area under the curve of all six cortisol measures with respect to ground, AUCg). For the CAR and AUC, indices were calculated separately for each day and averaged; for the diurnal cortisol slope, regression-based slopes were fit using all 3 days of cortisol data. Waking cortisol levels (S1, taken immediately upon waking) were also examined due to past research suggesting their importance in predicting mood disorders (Goodyer et al., 2000; Halligan et al., 2007), and in response to recommendations that waking cortisol levels also be examined when examining the CAR (Clow et al., 2010b).
Health Behavior Covariates
Health behavior variables were measured by questionnaire or in a short diary entry completed at the time of each saliva sample. We focused on variables that have previously been found to influence HPA axis functioning that could be plausibly related to anxiety disorders. Thus, they may confound the association between HPA axis measures and anxiety outcomes. The covariates included: time of waking (Kudielka and Kirschbaum, 2003), hours of sleep (Zeiders et al., 2011), use of oral contraceptives (Kirschbaum, 1999), (Meulenberg, 1987), nicotine use (smoking cigarettes) (Badrick et al., 2007), and use of psychotropic medications (Hibel et al., 2007). Time of waking and hours of sleep were continuous, and were standardized for use in analyses. Medication and smoking variables were dichotomous and were coded as 1 if participants reported any use. Health behavior covariates were only retained in final models if they showed significant associations with any of the anxiety disorder outcomes.
Analytic Plan
We conducted Cox regression models (survival analyses; Cox, 1972) using a person-year dataset, in which each participant possesses one line of data per year of study participation. Anxiety disorder onsets (no = 0, yes = 1) were dated according to the year at which they were first diagnosed. Non-proportional hazards models were used in order to test whether the strength of the relation between cortisol and future anxiety disorders varied with the passing of time over the 6-year follow up period. Unlike proportional hazards models, such models do not assume that the hazard associated with a particular predictor is constant across time, permitting the examination of interactions with time (Singer and Willett, 2003). We adopted this non-proportional hazards approach because we have previously shown that the CAR's strength of prediction for major depressive episode onsets varied significantly over time, such that prediction was initially significant, but no longer significantly predicted depressive episode onsets occurring beyond 2.5 years of follow-up (Vrshek-Schallhorn et al., 2013). Interactions of the cortisol variables with time were calculated by multiplying the year of assessment, centered at the median (3rd follow-up), by each cortisol variable. Models yield hazard ratios (HRs) and their 95% confidence intervals (CIs) for each predictor. Continuous predictors such as the cortisol indices (CAR, AUC, cortisol slope) were standardized to facilitate interpretation of HRs.
Depression was covaried in two ways. Whether participants had experienced one or more clinically significant depressive episodes prior to cortisol measurement was covaried as “depression history” (no = 0, yes = 1). Further, prospective major depressive onsets were indicated with a dummy variable in a time-varying fashion during the follow-up, specific to the year in which a depressive episode occurred and for all subsequent years after that onset.
A maximum of one new anxiety disorder onset per participant was included in the model. No participants were diagnosed with first onsets of two separate anxiety disorders at the same follow-up interview. Given that individuals with past anxiety disorders were purposefully excluded from our analyses, all onsets represent first-ever onsets of an anxiety disorder. After a new onset was experienced, the participant's remaining person-years were excluded from the model, as is necessary for Cox regression. Participants without new anxiety disorder onsets remained in the model until the year corresponding to their last follow-up SCID assessment.
We examined anxiety disorder onsets in two ways. First, we examined whether our baseline cortisol variables predicted the onset of any anxiety disorder (one model for all anxiety disorders combined). Second, we examined whether our cortisol variables predicted social anxiety disorder onsets when such onsets were modeled separately from the rest of the anxiety disorders (resulting in two additional models – one for social anxiety disorder onsets, and one for anxiety disorder onsets other than social anxiety disorder).
Model building/Selection of covariates
The following steps were employed in building our Cox regression models predicting: 1) all ADs, 2) social anxiety disorder, and 3) ADs other than social anxiety disorder. For each model, we automatically included a set of key analytic variables, including the cortisol variables, baseline neuroticism, MDD history, and our MDD onset covariate. When any cortisol variable was found to be significant, we also added the interaction of that variable with time to examine whether the prediction remained consistent over time. We then added each potential covariate separately to the model, including: gender, race/ethnicity, socioeconomic status, time of waking, hours of sleep, smoking, oral contraceptive use, and use of psychotropic medications. The key analytic variables were left in the model regardless of significance level. Covariates were retained in all models if they were significantly related to any one of the three anxiety disorder outcomes, for ease of comparison across models. Due to high correlations among several cortisol indices, cortisol predictors were examined in several models: the waking value and CAR in one model, and then the slope and AUCg in separate models.
Results
Excluding participants with a lifetime clinically significant AD at baseline, there were 25 prospective first onsets of ADs over the 6 years of follow-up, including 11 social anxiety disorder cases and 14 non-social anxiety disorder cases (8 specific phobia, 2 panic disorder, 2 generalized anxiety disorder, 1 obsessive-compulsive disorder, 1 post-traumatic stress disorder). Although more AD onsets were seen among females (20 onsets) than males (5 onsets), this was not a statistically significant effect [χ2 (1) = .573, p = .45]. See Table 1 for descriptive statistics on the total sample, participants who did not develop an AD over the follow-up, those who developed social anxiety disorder, and participants who developed an AD other than social anxiety disorder. A total of 1,053 person-time points were available for examination of all anxiety disorder onsets, 1,078 for examination of social anxiety disorder onsets, and 1,079 for non-social anxiety disorder anxiety disorder onsets.
Identification of Covariates
As noted above, neuroticism, MDD history, and MDD onsets over the follow-up period were included in all models on theoretical grounds. Of the potential covariates examined for inclusion in our final models, gender, time of waking, and baseline use of psychotropic medications were all significantly related to at least one AD outcome, and thus retained in all final models. Due to collinearity between psychotropic medication use and the baseline MDD history variable, MDD history and psychotropic medication use were combined into a single variable indicating whether the individual had a history of MDD or had reported psychotropic medication use either at baseline or at the time of cortisol assessment. Nicotine use, oral contraceptive use, race/ethnicity, socioeconomic status and hours of sleep were not significantly related to any AD outcomes and were therefore not retained in the final models. The same set of covariates was used for each AD outcome for ease of comparison of models across outcomes.
Prospective Prediction of All Anxiety Disorders from Cortisol Indices
Turning to the primary analyses of interest, the association between cortisol indices and AD outcomes, we first examined the associations between the baseline CAR and AD onsets, and then examined the diurnal cortisol slope and AUCg as predictors of AD onsets. As recommended in recent reviews on the CAR (Clow et al., 2010b), we included both waking cortisol levels and the size of the CAR as predictors when examining the CAR in order to differentiate between elevated morning cortisol levels more generally (high waking levels) and the size of the cortisol awakening response (high increase in cortisol after waking).
Predicting to all ADs, the HR for baseline neuroticism approached conventional levels of significance as a predictor of increased risk of AD onset (β = 0.43, SE(β) = 0.24, HR = 1.54, 95% CI = [.97-2.44], p = .066). There was also a non-significant trend for a history of MDD/psychotropic medication use prior to the cortisol assessment to predict increased risk for the development of an AD (β = 0.73, SE(β) = 0.44, HR = 2.07, 95% CI = [.87-4.93], p = .099). Among the cortisol variables, the CAR was a significant prospective predictor of increased risk of AD onsets (β = 0.79, SE(β) = 0.38, HR = 2.20, 95% CI = [1.05-4.62], p = .036). Waking cortisol levels, by contrast, were not significantly associated with AD onsets; further, the CAR×Time interaction was not significant (see Table 2). In separate models (not shown), total cortisol across the day (AUCg) and the slope of the diurnal cortisol rhythm were examined as predictors of new AD onsets. Neither variable was a significant predictor of AD onsets (slope: β = -.35, SE(β) = 0.21, HR = .704, 95% CI = [.47-1.06], p = .094; AUCg: β = .15, SE(β) = 0.25, HR = 1.16, 95% CI = [.72-1.88], p = .535).
Table 2. Results of Cox Regressions Model of Morning Cortisol Predicting Anxiety Disorder Onsets Over Subsequent Six Years: All Anxiety Disorders; Social Anxiety Disorders; All Other Anxiety Disorders.
| Variable | β | SE(β) | Exp(β) | 95.0% CI for | p-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model 1: All Anxiety Disorders (N=25) | ||||||
| Gender | 0.12 | 0.56 | 1.13 | 0.38 | 3.34 | 0.832 |
| Neuroticism | 0.43 | 0.24 | 1.54 | 0.97 | 2.44 | 0.066 |
| MDD History | 0.73 | 0.44 | 2.07 | 0.87 | 4.93 | 0.099 |
| MDD Onsets | 0.50 | 0.51 | 1.65 | 0.60 | 4.49 | 0.331 |
| Time of Waking | 0.28 | 0.25 | 1.32 | 0.81 | 2.14 | 0.263 |
| Waking Cortisol | 0.24 | 0.25 | 1.27 | 0.77 | 2.08 | 0.346 |
| Cortisol Awakening Response | 0.79 | 0.38 | 2.20 | 1.05 | 4.62 | 0.036 |
| CAR × Time | -0.19 | 0.15 | 0.83 | 0.62 | 1.11 | 0.208 |
| Model 2: Social Phobias (N=11) | ||||||
| Gender | 1.92 | 0.87 | 6.79 | 1.23 | 37.40 | 0.028 |
| Neuroticism | 0.64 | 0.35 | 1.89 | 0.95 | 3.77 | 0.070 |
| MDD History | 1.39 | 0.67 | 4.01 | 1.08 | 14.88 | 0.038 |
| MDD Onsets | 1.47 | 0.78 | 4.34 | 0.94 | 20.17 | 0.061 |
| Time of Waking | -0.19 | 0.32 | 0.83 | 0.45 | 1.54 | 0.551 |
| Waking Cortisol | 0.49 | 0.39 | 1.62 | 0.76 | 3.47 | 0.211 |
| Cortisol Awakening Response | 1.68 | 0.55 | 5.37 | 1.85 | 15.66 | 0.002 |
| CAR × Time | -0.47 | 0.19 | 0.63 | 0.43 | 0.91 | 0.015 |
| Model 3: All Other Anxiety Disorders (N=14)a | ||||||
| Gender | -1.63 | 1.07 | 0.20 | 0.02 | 1.58 | 0.126 |
| Neuroticism | 0.22 | 0.31 | 1.25 | 0.68 | 2.30 | 0.477 |
| MDD History | 0.16 | 0.68 | 1.17 | 0.31 | 4.46 | 0.815 |
| MDD Onsets | -0.38 | 0.80 | 0.69 | 0.14 | 3.31 | 0.639 |
| Time of Waking | 0.97 | 0.40 | 2.64 | 1.21 | 5.75 | 0.015 |
| Waking Cortisol | 0.00 | 0.36 | 1.00 | 0.49 | 2.04 | 0.997 |
| Cortisol Awakening Response | 0.13 | 0.47 | 1.14 | 0.46 | 2.87 | 0.776 |
| CAR × Time | 0.09 | 0.20 | 1.10 | 0.74 | 1.63 | 0.640 |
Notes: MDD History = participant has diagnosed history of MDD at baseline or reported use of psychotropic medication at baseline or time of cortisol assessment. MDD Onsets = participant has onset of MDD over the follow-up period. Waking Cortisol = cortisol values at waking. Cortisol Awakening Response (CAR) = increase in cortisol between waking and 40-minute post-awakening. CAR × Time = interaction between CAR and year of assessment (change in the impact of CAR variable over time). Continuous variables (e.g. neuroticism, time of waking) were entered in the model as z-scores to aid interpretation; dichotomous variables (i.e., gender, MDD history) were entered as dummy variables.
Specific phobia (n = 8), Generalized Anxiety Disorder (n = 2), Obsessive-compulsive disorder (n = 1), Panic Disorder (n = 2) and PTSD (n=1).
Prospective Prediction of Social Anxiety Disorder from Cortisol Indices
The CAR was a strong and significant predictor of SAD onsets (N=11) over the subsequent 6 years (β = 1.68, SE(β) = 0.55, HR = 5.37, 95% CI = [1.85-15.66], p = .002). In addition, the CAR × Time interaction effect was significant (β = -.47, SE(β) = 0.19, HR = .63, 95% CI = [.43-.91], p = .015), suggesting that the strength of prediction from the CAR to SAD declined over time. Specifically, the CAR predicted SAD onsets significantly to the fourth annual follow-up, but not significantly thereafter (see Figure 2). Waking cortisol levels did not significantly predict SAD onsets (see Table 3). Male gender also significantly predicted increased risk for SAD (HR = 6.79, p = .028); this finding should be regarded with caution, however, given the low number of males in the sample and low overall number of male SAD onsets (N=5). Prospective MDD onsets over the follow-up period (HR=4.34, p = .061) and baseline neuroticism (HR = 1.89, p = .070) both approached significance as predictors of SAD onsets. Neither diurnal cortisol slope, nor total cortisol across the day (AUCg) were significant predictors of SAD onsets when they were substituted for waking cortisol and the CAR in the model (slope: β = -.51, SE(β) = 0.33, HR = .60, 95% CI = [.31-1.48], p = .12; AUCg: β = .31, SE(β) = .40, HR = 1.37, 95% CI = [.63-2.98], p = .43).
Figure 2.
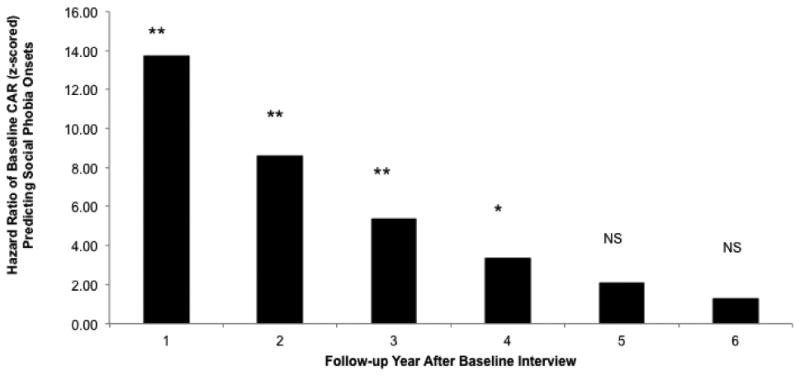
Model-estimated hazard ratios illustrate the observed CAR × Time interaction predicting social anxiety disorder onsets. Bars represent HRs for z-scored baseline natural log transformed CAR in year intervals after baseline. * = p < .05, ** = p < .01, NS = not significant, p > .05.
Although the HR coefficient for the CAR predicting to SAD onsets was notably larger in size than the CAR coefficient when predicting ADs more generally, tests comparing their inferential confidence intervals showed that they overlapped slightly, suggesting that the coefficients are not significantly different from one another (Tryon, 2001). Thus, the CAR is a significant predictor both of the full set of ADs, and of the subset of SAD cases, but the effect size for SAD is not statistically significantly stronger.
Prospective Prediction of Other Anxiety Disorders from Cortisol Indices
None of the baseline cortisol variables were significant predictors of the combined non-social anxiety disorder onsets, including the CAR (β = .13, SE(β) = 0.47, HR = 1.14, 95% CI = [.46-2.87], p = .776), waking cortisol (β = .00, SE(β) = 0.36, HR = 1.00, 95% CI = [.49-2.04], p = .997), the cortisol diurnal slope (β = -.16, SE(β) = 0.27, HR = .85, 95% CI = [.50-1.45], p = .556), or total (AUCg) cortisol (β = .14, SE(β) = 0.30, HR = 1.16, 95% CI = [.64-2.09], p = .628). The only significant predictor of the ADs other than SAD was time of waking, with individuals waking later in the day having an increased risk for ADs other than SAD (see Table 2).
Discussion
This study provides the first evidence of a prospective relation between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. The strength of prediction to first onsets of the total group of ADs did not decline significantly over time. The CAR was also a strong and significant predictor of first onsets of one specific type of anxiety disorder, social anxiety disorder (SAD). Prediction to SAD declined significantly over time, but the CAR remained a significant predictor of first SAD onsets for four years following the baseline cortisol assessment. Analyses predicting to anxiety disorders other than social anxiety disorder did not show significant effects of the CAR. The “other anxiety” disorder category combined a wide range of disorders, however, some of which might be predicted by cortisol variables if examined separately with sufficient numbers of onsets in future research.
It is worth noting that many anxiety disorder onsets (N=58) had already occurred at the time of the baseline assessment of this study. This is consistent with research showing that many anxiety disorders are first diagnosed during early and mid-adolescence (Merikangas et al., 2010), and in some cases, even pre-adolescence (Weissman et al., 1999). Our study began in late adolescence. Thus, future research on first onsets of anxiety disorders would benefit from assessing participants starting at an earlier age, in order to capture more cases of first onset ADs, and to examine the role that puberty may play in HPA-axis changes and risk for ADs.
There were no significant associations between waking cortisol and later anxiety disorders, nor were the slope of the diurnal cortisol rhythm or total cortisol across the day significant prospective predictors of anxiety disorders. Rather, effects were specific to the CAR. This finding is similar to our previously reported results, in which the CAR, but not other aspects of diurnal cortisol activity, predicted onsets of major depressive disorder in this sample (Adam et al., 2010; Vrshek-Schallhorn et al., 2013). Importantly, however, the current analyses used both MDD history and prospective onsets of MDD as covariates, suggesting that the prediction of anxiety disorders from the CAR is not attributable to comorbid MDD diagnoses.
The mechanisms underlying the association of the CAR with future onsets of anxiety disorders will require further investigation. A number of possibilities exist. Our analyses removed anyone with a past or present AD at baseline, such that we predicted only first onsets of any of the anxiety disorders our participants developed. Thus, in the context of our data, an elevated CAR can be interpreted as a risk marker for future ADs, rather than a scar marker for the experience of past ADs. However, our analyses do not rule out the possibility that early subclinical symptoms of anxiety may be contributing to an increased CAR. Future evaluations of symptom-level anxiety could examine whether an elevated CAR is a marker of prodromal AD symptoms. In the current analyses, we covaried continuous levels of neuroticism, which are moderately to highly correlated with anxiety symptomatology in this sample (Sutton et al., 2011). Still, future analyses should examine whether the cortisol awakening response predicts future continuous anxiety symptoms as well as disorders, while covarying concurrent symptom level measures of general and social anxiety. Such an approach would be consistent with the research domain criteria (RDoC) approach currently being developed and promoted at the National Institute of Mental Health (Craske, 2012). The RDoC approach attempts to replace diagnostic categories with a continuous dimensional description of symptoms, as well as of underlying biological markers of vulnerability and symptomatology. The current paper suggests that the HPA axis, and the CAR in particular, should be considered as a unit of analysis of vulnerability for social fear.
Associations between the CAR and future ADs could possibly be explained by genetic factors, given the substantial genetic component to the cortisol awakening response (Wüst et al., 2000). As a result, an elevated CAR might be a reflection of genetic risk for anxiety. Future analyses could examine specific polymorphisms found to be related to the CAR and whether they are, in turn, associated with anxiety symptoms and ADs. Beyond genetics, the CAR may also be simply an indicator of an underlying trait neurobiological vulnerability that contributes both to an elevated CAR and to risk for ADs. While alterations in limbic system neurocircuitry or neurochemistry are obvious candidates, recent theory and research on the neurobiology of the CAR response suggests that central circadian circuitry, and signaling from the suprachiasmic nucleus in particular, may play an important role in regulating the size of the CAR response (Clow et al., 2010a).
Possible explanations may also lie at the level of social experience. The CAR has been shown to be strongly affected by psychosocial experience (Chida and Steptoe, 2009). Thus, it is worth considering whether either recent (proximal to the time of CAR measurement) or earlier experiential influences on the CAR could play a role. There are substantial current state influences on the CAR. The CAR is increasingly being interpreted as a preparatory response, helping to organize the individual for anticipated daily demands by helping to overcome sleep inertia, sharpening morning cognitive functioning, and mobilizing energy resources to prepare the individual for action (Adam et al., 2006; Clow et al., 2010b). Thinking about its role in social anxiety disorder in particular, an increased CAR in those who go on to develop SAD may be a reflection of relatively high levels of anticipated daily social demand. This hypothesis should be examined in future work that investigates the determinants of an elevated CAR in those at risk for SAD. Along these lines of thinking, we might expect to see the CAR particularly elevated on days that higher levels of social interaction are anticipated, and to see a higher CAR in response to anticipated social interaction among those at risk for social anxiety disorder, compared to those without.
Only a few studies of early or chronic adverse experience and the CAR have been conducted. Some studies show an elevated cortisol awakening response but others show a reduced CAR among those with adverse early experiences such as parental loss, or childhood trauma (Chida and Steptoe, 2009). More consistent associations have been found between elevated general life stress and an increased CAR (Chida and Steptoe, 2009). Of relevance to social anxiety disorder in particular, several studies report associations between perceived social problems and an elevated CAR in both adolescents and adults (Wüst et al., 2000; Ellenbogen et al., 2006). Loneliness and perceived social exclusion have also been related to social anxiety disorder symptoms (Storch et al., 2003) and with an elevated cortisol awakening response (Wüst et al., 2000; Steptoe et al., 2004; Adam et al., 2006; Doane and Adam, 2010). Perceived social exclusion, loneliness and a high CAR may therefore reflect an interrelated set of risk factors for the development of social anxiety disorder. Moreover, several studies have shown that experiences of loneliness precede and predict subsequent CAR elevations (Adam et al., 2006; Doane and Adam, 2010). As a result, although further research is required to fully reveal their causal ordering, we hypothesize that perceived social exclusion and loneliness may contribute to an elevated CAR, which may in turn place the individual at risk for mood and anxiety disorders.
Whether the CAR plays a causal etiological role in the development of social anxiety disorder, or is simply an indicator of the presence of increased risk remains to be determined. In identifying a possible biological pathway for the effects of elevated CAR, it is worth considering the impact of high morning cortisol levels on corticosteroid receptor populations in brain regions involved in the activation and regulation of the stress response, and of known relevance to mood and anxiety disorders (e.g. prefrontal cortex, hippocampus, amygdala) (Wüst et al., 2000; Herbert, 2013). The effects of cortisol in the brain are mediated by way of high affinity mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs) which have a 10× lower affinity (de Kloet et al., 2007). Due to these differential affinities, high GR occupation typically only occurs at times of stress, or at the peak of the cortisol diurnal rhythm. The high and rapidly increasing cortisol levels associated with a high CAR would likely result in substantial occupation of glucocorticoid receptors (GR). A higher than normal CAR could, over time, result in changes in the GR population and an imbalance the MR/GR ratio that has been proposed to play a role in the etiology of emotional disorders (de Kloet et al., 2007; Adam et al., 2010).
It is also of interest to consider why major depressive disorder (Adam et al., 2010; Vrshek-Schallhorn et al., 2013), and anxiety disorders (as well as SAD in particular) are all significantly predicted by an increased CAR. MDD and several of the anxiety disorders, including SAD, share several common features, including increased interpersonal sensitivity and low levels of positive affect (Dozois and Frewen, 2006). As noted earlier, perceived social problems and social exclusion appear to be related to an elevated CAR. Meta-analytic findings are more mixed regarding the role of positive affect in the CAR (Chida and Steptoe, 2009), but the role of positive affect in the CAR is worthy of further examination.
Our finding of increased risk for social anxiety disorder among males was unexpected given past epidemiological work showing higher rates of social anxiety disorder among females (Merikangas et al., 2010; Kessler et al., 2012). Given the small number of male SAD onsets (N=5), we give little weight to this finding unless it is replicated in future prospective research of this nature. Our finding that later time of waking predicts onsets of anxiety disorders other than social anxiety disorder suggests that further attention may be warranted to the role of sleep, circadian rhythms, and circadian timing (morningness/eveningness) in the development of anxiety disorders.
Limitations and Future Directions
This study has several limitations, each of which suggest directions for future research. First, the limited number of anxiety disorder onsets was problematic. We consider it an advantage that we only predicted first onsets of ADs, thus reducing the possibility that an elevated CAR is a “scar” of the past experience of an anxiety disorder. Nevertheless, future analyses could examine whether the CAR plays a role in the maintenance or exacerbation of ADs over time. Although our current analyses covaried past diagnoses of MDD and MDD onsets over follow-up, with larger numbers of AD onsets, it would also be interesting to examine whether the CAR behaves differently in prediction of pure AD cases, as compared to AD cases that are comorbid with past or present diagnoses of MDD. Additional comorbidities, such as externalizing symptoms, should also be considered. Gender differences could also be examined further in future studies with an increased number of AD cases; current analyses covary the impact of gender, but it would be informative to examine whether the CAR is equally predictive of AD onsets for males vs. females. Our relatively low number of AD onsets also prevented us from drawing firm conclusions about the prospective relation of the CAR to each of the specific anxiety disorders. Although we did not find significant associations between the CAR and the combined group of anxiety disorders other than social phobia, it is possible that specific disorders within that combined group (such as GAD) would also be predicted by the CAR if they were examined individually in future studies. As noted earlier, utilization of continuous symptom-level data is another potential solution to low numbers of formal clinical cases, and is another a potentially important approach to examining the role of the CAR in the development of various types of anxiety symptoms.
Second, to alleviate the participation burden on adolescents during a relatively intensive data collection over the course of three days, we chose to measure the CAR with two saliva samples (waking and 40 minutes after waking) rather than the original and more complete CAR protocol of sampling cortisol at waking and every 15-20 minutes thereafter for the next hour (a total of 4-5 samples). Our less intensive CAR measure may have missed the true peak of the CAR for some individuals, particularly given that cortisol levels, on average, remain elevated even 60 minutes after waking (Wust et al., 2000). However, similar strategies have been used extensively in past research (Adam and Kumari, 2009), and if anything, less precise measurement of the CAR should have hampered our ability to find an effect. A third limitation is that objective measures of compliance with the timing of collection of saliva samples were not employed. Noncompliance with requested sample timing can significantly impact estimation of diurnal cortisol rhythm, including the CAR (Dockray et al., 2008; DeSantis et al., 2010).
Implications for Intervention
If the CAR plays an etiological role in the emergence of anxiety disorders, interventions aimed at reducing the size of the CAR might reduce their onsets. As previously noted, anxiety disorders have high prevalence, impairment and cost and burden to society. Given the importance of variation in the CAR in responding to daily demands (Adam et al., 2006), psychosocial interventions aimed at reducing the average CAR while still maintaining its plasticity and responsivity to daily changes might be a desirable approach.
Adam et al. (Adam et al., 2008) proposed that intervention research, in particular random assignment interventions including measures of HPA axis function, could help to address the etiological role of the HPA axis in the development of disorder. Lupien and colleagues (Lupien et al., 2013) demonstrated that an early adolescent, school-based stress-reduction program was effective at reducing cortisol levels for a subset of youth; those youth with decreases in cortisol, in turn, showed decreased risk for subsequent depressive symptoms. However, anxiety disorders were not assessed, and cortisol measures were taken at school such that measures of the cortisol awakening response were not available. Future stress-reduction interventions, examining anxiety outcomes, and utilizing home-based cortisol assessments across the full day (including measurement of the CAR), would test the feasibility and efficacy of modifying the CAR through social/behavioral interventions, and provide additional insights into the role of the cortisol awakening response in the development of mood and anxiety disorders.
Conclusions
The cortisol awakening response predicts onsets of a combined group of anxiety disorders, as well as smaller group of social anxiety disorder onsets in older adolescents and young adults. This was true even when covarying lifetime depression (another disorder we have shown the CAR to predict) at the time of cortisol measurement as well future onsets of depression over the follow-up period. Additional research with a larger number of onsets of anxiety disorders would allow examination of the prospective relation of cortisol awakening response to specific anxiety disorders other than social anxiety. Future research should delineate the biological mechanisms and psychological predictors of the CAR in relation to risk for depression and social anxiety disorder, and incorporate measures of the CAR into intervention studies designed to prevent and reduce anxiety and depressive symptoms and disorders.
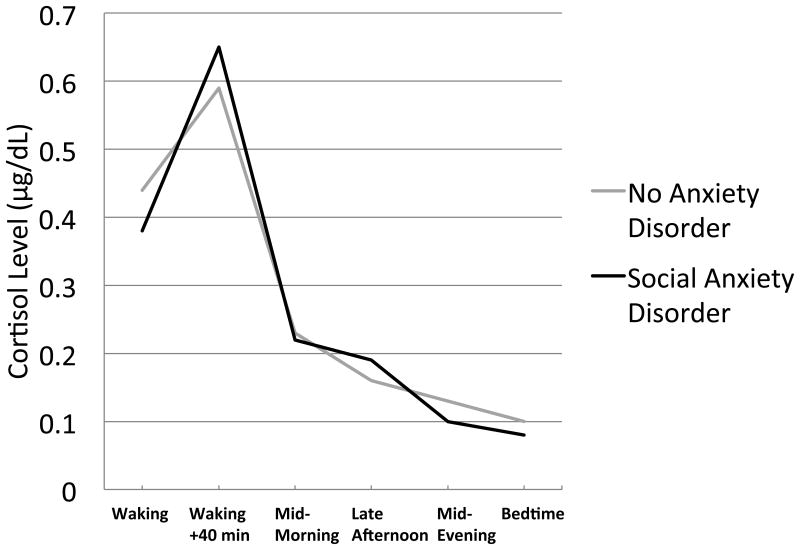
Figure 3.
Baseline 3-day averages of raw salivary cortisol level (μg/dl) across the day for never anxiety-disordered participants and participants who experience a first onset of social anxiety disorder within the six-year follow-up.
Acknowledgments
We thank the participants of the Youth Emotion Project for their ongoing participation; their generosity, commitment and openness has made this research possible. This research was supported by a William T. Grant Foundation Scholars Career Award to EKA, a Faculty Fellowship from the Institute for Policy Research at Northwestern University to EKA, a two-site grant from the National Institute of Mental Health to SM and REZ (R01-MH065652) and MGC (R01-MH065651), and a postdoctoral NRSA from the National Institute of Mental Health to SVS (F32-MH091955). SVS is now at University of North Carolina – Greensboro. A portion of these results was presented by EKA at the annual meetings of the American Psychosomatic Society (2012) and the International Society of Psychoneuroendocrinology (2013).
Role of the Funding Source: The funding sources for this study did not influence the analyses or results presented in this manuscript or the conclusions drawn from this research.
Footnotes
These three disorders were previously classified as anxiety disorders in DSM-IV (American Psychiatric Association, 2000); DSM-V (American Psychiatric Association, 2013) classifies them as a distinct but related category of disorder.
The BIS measures anxiety, which is often included as a facet of neuroticism in neuroticism scales, such as the IPIPNEO-PI-RN.
Conflict of Interest: None of the authors have any conflicts of interest to declare with respect to this research.
Contributors: Emma Adam – designed the study, conducted data analyses, drew conclusions, wrote first draft of manuscript, revised and submitted manuscript
Suzanne Vrshek-Schallhorn – designed the study, conducted data analyses, contributed to writing the methods and results sections
Ashley Kendall – contributed to the literature review and introduction, helped prepare tables and figures, commented on manuscript drafts
Susan Mineka – designed the larger study from which these data were taken, commented on the data analyses and manuscript drafts
Richard Zinbarg - designed the larger study from which these data were taken, commented on the data analyses and manuscript drafts
Michelle Craske - designed the larger study from which these data were taken, commented on the data analyses and manuscript drafts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic– pituitary–adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Development and Psychopathology. 2008;20:975–1001. doi: 10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Publishing; Washington, D.C: 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Publishing; Washington, D.C: 2013. [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The Relationship between Smoking Status and Cortisol Secretion. Journal of Clinical Endocrinology and Metabolism. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews. 2010a;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Thorn L. The cortisol awakening response in context. International Review of Neurobiology. 2010b;93:153–175. doi: 10.1016/S0074-7742(10)93007-9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic Status Is Associated With Stress Hormones. Psychosomatic Medicine. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Costa PT, Terracciano A, McCrae RR. Gender Differences in Personality Traits Across Cultures: Robust and Surprising Findings. Journal of Personality and Social Psychology. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Cox D. Regression models and life-tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34:187–220. [Google Scholar]
- Craske MG. THE R-DOC INITIATIVE: SCIENCE AND PRACTICE. Depression and Anxiety. 2012;29:253–256. doi: 10.1002/da.21930. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, DeRijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nature clinical practice Endocrinology & metabolism. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Mendelsohn K, Doane LD. Concordance between self-reported and actual wake-up times in ambulatory salivary cortisol research: Implications for the cortisol response to awakening. International Journal of Behavioral Medicine. 2010;17:74–78. doi: 10.1007/s12529-009-9053-5. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dierckx B, Dieleman G, Tulen JH, Treffers PD, Utens EM, Verhulst FC, Tiemeier H. Persistance of anxiety disorders and concomitant changes in cortisol. Journal of Anxiety Disorders. 2012;26:635–641. doi: 10.1016/j.janxdis.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and psychopathology. 2013;25:629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dozois DJA, Frewen PA. Specificity of cognitive structure in depression and social phobia: A comparison of interpersonal and achievement content. Journal of affective disorders. 2006;90:101–109. doi: 10.1016/j.jad.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Dressendörfer R, Kirschbaum C, Rohde W, Stahl F, Strasburger C. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker CD, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;31:1164–1180. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6:21–29. [Google Scholar]
- Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, Rosenbaum JF. Anxiety disorders in major depression. Comprehensive Psychiatry. 2000;41:97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- Ferdinand RF, Verhuist FC. Psychopathology from adolescence into young adulthood: An 8-year follow-up study. American Journal of Psychiatry. 1995;152:1586–1594. doi: 10.1176/ajp.152.11.1586. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I disorders-non-patient edition. New York State Psychiatric Institute, Biometrics Research Department; New York: 2001. [Google Scholar]
- Flinn M, England BG. Childhood stress and family environment. Current Anthropology. 1995;36:854–866. [Google Scholar]
- Goldberg L. A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. In: Mervielde I, Deary I, De Fruyt F, Ostendorf F, editors. Personality Psychology in Europe. Tilburg University Press; Tilburg, The Netherlands: 1999. pp. 7–28. [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, Sondeijker FE, Ormel J, Verhulst FC. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatrica Scandinavica. 2007;116:137–144. doi: 10.1111/j.1600-0447.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JRT, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Brown GW, Cleary SE, Shiers HM, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. British Journal of Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Herbert J. Cortisol and depression: three questions for psychiatry. Psychological Medicine. 2013;43:449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Cicchetti D, Rogosch F. Salivary Biomarker Levels and Diurnal Variation: Associations With Medications Prescribed to Control Children's Problem Behavior. Child Development. 2007;78:927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The Interrelationship of Neuroticism, Sex, and Stressful Life Events in the Prediction of Episodes of Major Depression. American Journal of Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello J, Green JG, Gruber MJ, McLaughlin KA, Petukhova M, Sampson NA, Zaslavsky AM, Merikangas KR. Severity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry. 2012;69:381–389. doi: 10.1001/archgenpsychiatry.2011.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Greenberg PE. Neuropsychopharmacology: The Fifth Generation of Progress. Baltimore, MD: Lippincott Williams and Wilkins; 2002. The economic burden of anxiety and stress disorders; pp. 981–992. [Google Scholar]
- Kirschbaum C, Helhammer D. Salivary cortisol. In: Fink G, editor. Encyclopedia of Stress. Academic Press; San Diego: 2000. pp. 379–383. [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psycho-biological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka B, Gaab J, Schommer NC, Helhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35:1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Harrison HM. Adolescent peer relations, friendships, and romantic relationships: Do they predict social anxiety and depression? Journal of Clinical Child and Adolescent Psychology. 2005;34:49–61. doi: 10.1207/s15374424jccp3401_5. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Wittchen HU, Faravelli C, Bobes J, Patel A, Knapp M. A European perspective on social anxiety disorder. European Psychiatry. 2000;15:5–16. doi: 10.1016/s0924-9338(00)00216-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Trépanier L, Juster RP, Marin MF, Francois N, Sindi S, Wan N, Findlay H, Durand N. The DeStress for Success Program: Effects of a stress education program on cortisol levels and depressive symptomatology in adolescents making the transition to high school. Neuroscience. 2013;249:74–81. doi: 10.1016/j.neuroscience.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg PM, Ross HA, Swinkels LM, Benraad TJ. The effect of oral contraceptives on plasma-free and salivary cortisol and cortisone. Clinica Chimica Acta. 1987;165:370–385. doi: 10.1016/0009-8981(87)90183-5. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Patel A, Knapp M, Henderson J, Baldwin D. The economic consequences of social phobia. Journal of Affective Disorders. 2002;68:221–233. doi: 10.1016/s0165-0327(00)00323-2. [DOI] [PubMed] [Google Scholar]
- Pollack M, Otto M, Sabatino S, Majcher D, Worthington J, McArdle E, Rosenbaum A. Relationship between childhood anxiety to adult panic disorder: Correlates and influence on course. American Journal of Psychiatry. 1996;153:376–381. doi: 10.1176/ajp.153.3.376. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Saucier G. Mini-Markers: A Brief Version of Goldberg s Unipolar Big-Five Markers. Journal of Personality Assessment. 1994;63:506–516. doi: 10.1207/s15327752jpa6303_8. [DOI] [PubMed] [Google Scholar]
- Schiefelbein VL, Susman EJ. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. The Journal of Early Adolescence. 2006;26:397–413. [Google Scholar]
- Singer J, Willett J. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; USA: 2003. [Google Scholar]
- Stein MB, Fuetsch M, Muller N, Hofler M, Lieb R, Wittchen HU. Social anxiety disorder and the risk of depression: A prospective community study of adolescents and young adults. Archives of General Psychiatry. 2001;58:251–256. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Storch EA, Brassard MR, Masia-Warner CL. The relationship of peer victimization to social anxiety and loneliness in adolescence. Child Study Journal. 2003;33:1–18. doi: 10.1016/j.adolescence.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Storch EA, Masia-Warner C. The relationship of peer victimization to social anxiety and loneliness in adolescent females. Journal of Adolescence. 2004;27:351–362. doi: 10.1016/j.adolescence.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JM, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Rose RD, Waters AM, Nazarian M, Mor N. The relationships of personality and cognitive styles with self-reported symptoms of depression and anxiety. Cognitive therapy and research. 2011;35:381–393. doi: 10.1007/s10608-010-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Jindal R, Howland RH. Biological aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression Guilford, New York. 2002. pp. 192–218. [Google Scholar]
- Tryon WW. Evaluating statistical difference, equivalence, and indeterminacy using inferential confidence intervals: an integrated alternative method of conducting null hypothesis statistical tests. Psychological Methods. 2001;6:371–386. [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Hoogendijk WJ, Smit JH, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic Medicine. 2010;72:340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine. 2013;43:483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Wickramaratne P, Goldstein RB, Adams P, Greenwald S, Ryan ND, Dahl RE, Steinberg D. Children with prepubertal-onset major depressive disorder and anxiety grown up. Archives of general psychiatry. 1999;56:794. doi: 10.1001/archpsyc.56.9.794. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. Journal of Clinical Endocrinology and Metabolism. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise and health. 2000;2:79. [PubMed] [Google Scholar]
- Zeiders K, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescents. Journal of Adolescent Health. 2011;48:566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg R, Mineka S, Craske M, Griffith J, Sutton J, Rose R, Nazarian M, Mor N, Waters A. The Northwestern-UCLA youth emotion project: Associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48:347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]