Abstract
This is the first study to explore genetic and environmental contributions to individual differences in emotion regulation in toddlers, and the first to examine the genetic and environmental etiology underlying the association between emotion regulation and working memory. In a sample of 304 same-sex twin pairs (140 MZ, 164 DZ) at age 3, emotion regulation was assessed using the Behavior Rating Scale of the Bayley Scales of Infant Development (BRS; Bayley, 1993), and working memory was measured by the visually cued recall (VCR) task (Zelazo et al., 2002) and several memory tasks from the Mental Scale of BSID. Based on model-fitting analyses, both emotion regulation and working memory were significantly influenced by genetic and nonshared environmental factors. Shared environmental effects were significant for working memory, but not for emotion regulation. Only genetic factors significantly contributed to the covariation between emotion regulation and working memory.
Keywords: emotion regulation, working memory, genetics, twins, toddlerhood
Introduction
What is Emotion Regulation?
Emotion regulation is a multifaceted construct that encompasses regulation of multiple components engaging in emotion, including internal affective experience, external expressive behaviors, emotion-related physiological reactions, attentional process, cognition, and motivation (Eisenberg & Fabes, 2006; Gross & Thompson, 2007). Emotion regulation allows individuals to link widely separated systems to one another and to achieve the intersystem integration producing a series of organized responses that adapt to the social surroundings and the situational demands (Thompson, Meyer, & Jochem, 2009). Accordingly, based on the environmental demands, individuals regulate a group of emotion-related processes to accomplish individual goals. In other words, emotion regulation involves not only the modulation of affects, but also emotion-related physiological, attentional, cognitive, motivational, and behavioral processes. The direction of the changes in these domains depends on the situational demands and individual goals. Individuals could increase, decrease or maintain current emotion-related states to satisfy the social demands (Eisenberg & Fabes, 2006).
Emotion regulation is crucial for adaptive functioning across the life span. Appropriate emotion regulation fosters desirable developmental outcomes such as high social competence (Spinrad et al., 2006), healthy peer relations (Calkins, Gill, Johnson, &Smith, 1999; Keane & Calkins, 2004), good academic performance (Eisenberg, Sadovsky, & Spinrad, 2005), appropriate school readiness (Raver, 2002), and proper school adjustment (Miller, Gouley, Seifer, Dickstein, & Shields, 2004). Children with good emotion regulation skills can cope with separation-related feelings (e.g., anxiety, fear, and frustration due to the separation from caregivers), and enjoy pleasurable feelings of social interaction (e.g., appropriate levels of excitement, and low levels of social anxiety) (Carthy, Horesh, Apter, Edge, & Gross, 2010). Thus, competent emotion regulation is essential for children to establish and maintain healthy socialization, and to achieve academic success, or even broader, to pursue happiness.
Individual Differences in Emotion Regulation
There are varying degrees of capacity for emotion regulation among children (Calkins 1994). Different children of the same age can have distinct emotional reactions in the same situation. For example, consider children’s reactions to a novel situation such as the first day at daycare center; some children are excited, but others are uncomfortable and cry. Young children’s failure to properly manage their emotions may lead to problems in social competence, or even bring about developmental psychopathology, as both internalizing and externalizing problems have been associated with atypical emotion regulation in children (Eisenberg et al., 2001; Kim & Cicchetti, 2010). Accordingly, it is important to understand why children differ significantly in the ways in which they experience and express their emotions as knowledge of the factors that influence variability in emotion regulation can inform about possible interventions for at risk children.
There is an abundance of research explaining various sources of individual differences in emotion regulation. First, from an ecological perspective, children develop the ability to modulate emotional processes within their families and the broad social contexts in which they interact with other people. Parents, as an important part of children’s ecological system, play a crucial role in the variability of emotion regulation. Research has shown that maternal behaviors such as separation and depression may dampen the development of emotion regulation (Blandon, Calkins, Keane, & O’Brien, 2008; Field, 1994). Second, variations in emotion regulation have been linked with physiological processes (e.g., vagal tone, and vagal regulation) which are influenced by parasympathetic nervous system (Blandon et al., 2008; Cole, Zahn-Waxler, Fox, Usher, & Welsh, 1996; Porges, 2003; Santucci et al., 2008), however, there is no consensus on which index (resting vagal tone, or the change of vagal tone) can better predict emotion regulation (Eisenberg, Valiente, & Sulik, 2009). Third, individual differences in emotion regulation have been attributed to temperamental domains such as effortful control, inhibiting a dominant response and/or to activate a subdominant response (i.e., inhibitory control, a subtype of effortful control) and planning and detecting errors using executive attentional skills; and emotionality, emotional reactivity, or, the threshold, latency, frequency and intensity of emotional experience (Santucci et al., 2008). Both effortful control and emotionality are closely tied to emotion regulation. Effortful control facilitates emotion-related attentional and behavioral modulation involved in emotion regulation (Eisenberg & Fabes, 2006; Fox & Calkins, 2003), while emotionality is negatively correlated with emotion regulation in children of different ages (Eisenberg et al., 1993; Eisenberg et al., 1997).
Although these sources of variability in emotion regulation have been well-studied, the possibility of genetic effects on individual differences in emotion regulation has largely gone unexplored. There is, however, a substantial body of research that has found genetic influences on related temperamental traits such as effortful control (including inhibitory control and attentional control) (Goldsmith, Pollak, &Davidson, 2008; Gagne & Saudino, 2010; Kochanska, Philibert, Barry, 2009) and emotionality (Ganiban, Saudino, Ulbricht, Neiderhiser, & Reiss, 2008; Gjone & Stevenson, 1997; Holmes & Hariri, 2003; Krueger, South, Johnson, & Iacono, 2008; Schmitz et al., 1996), and on physiological regulation (Boomsma, van Baal, & Orlebeke, 1990; Propper et al., 2008). These findings of related constructs hint that emotion regulation may also be genetically influenced, but as indicated earlier, emotion regulation is not identical to temperamental effortful control and emotionality. To date, only one genetically-informative study has examined genetic influences on one aspect of emotion regulation (i.e., gaze aversion) during early infancy (Soussignan et al., 2009). Gaze aversion, an emotion regulation strategy used by infants, was found to be influenced by modest genetic and substantial nonshared environmental factors in 5-month-old infant twins (Soussignan et al., 2009). Although it is a good start at exploring the etiology of individual differences in emotion regulation, this study is limited by the narrow definition of emotion regulation. As indicated earlier, emotion regulation is a complex and multidimensional construct that encompasses a variety of behaviors (Gross & Thompson, 2007). Gaze aversion in infants taps only emotion-related attention processes, and is a regulatory strategy that is much less frequently used by older children (Mangelsdorf, Shapiro, & Marzolf, 1995). To manage emotions, toddlers are more likely to use behavioral avoidance, self-soothing, and self-distraction from emotion-elicited stimuli to neutral ones, which are broader and more complex than gaze aversion (Mangelsdorf, et al., 1995). Moreover, toddlers display attempts to modulate the situations by initiating regulatory behaviors related to social contexts (Kopp, 1982). For example, they can attempt to direct the interactions with strangers via vocal commands (Mangelsdorf, et al., 1995), or suppress negative facial expression in disappointing situations (Cole, 1986). Hence, the narrow measure of emotion regulation in the previous twin study highlights a need of a more comprehensive assessment of emotion regulation as well as examination at other ages. The first goal of the present study was to address this gap in the literature by investigating the genetic and environmental etiology of individual differences in emotion regulation in toddler twins. Our hypothesis was that both genetic and environmental factors would have an impact on individual differences in emotion regulation in toddlers.
Emotion Regulation and Working Memory
Cognitive functioning facilitates emotion regulation (Sroufe, 1996; Thompson, Meyer, & Jochem, 2009). Particularly, working memory has been examined as a correlate of emotion regulation in numerous studies. Nevertheless, less is known about the relation between emotion regulation and working memory in childhood, nor the genetic/environmental mechanism underlying the relation. To address this issue, the second aim of the current study has focused on the association between emotion regulation and working memory in toddlerhood.
The link between emotion regulation and working memory may partly reflect the emotion-cognition interplay. Working memory is defined as the ability to temporarily maintain or hold information in mind and simultaneously manipulate or process it (Baddeley, 1986; Baddeley, 2000). There is a reciprocal relation between emotion regulation and working memory in which emotion regulation is as much influenced by working memory as working memory is influenced by emotion regulation (Habel et al., 2007; Schmeichel, Volokhov, & Demaree, 2008). A growing body of evidence suggests that emotion regulation and working memory are closely linked. Neural correlates involved in emotion regulation such as the prefrontal cortex (Ochsner et al, 2004; Urry et al., 2006) and the anterior cingulate cortex (Bush, Luu, & Posner, 2000; Sinha, Lacadie, Skudlarski, & Wexler, 2004) play a role in working memory (Bluhm et al., 2011; Gray, Chabris, & Braver, 2003; Kondo et al., 2004; Osaka et al., 2004). At the behavioral level, the two constructs involve limited attentional resources (Koole, 2009). Attentional interference caused by reactions to negative emotional stimuli is decreased by concurrently performing working memory task (Van Dillen & Koole, 2009). Conversely, performance on working memory tasks is reduced (i.e., slower and less correct responses) by the regulation of induced negative emotions (Habel et al., 2007). Adults with higher working memory capacity have better emotion regulation skills (Schmeichel et al., 2008). This relation has not yet been tested in children; however, infants who have higher levels of soothability, a temperament dimension linked with emotion regulation, at 8 months tend to develop better working memory at 4.5 years (Wolfe & Bell, 2007). Given the findings linking emotion regulation with working memory in adults, as well as soothability with working memory in children, we predicted that emotion regulation and working memory would be positively associated in our toddler sample.
A finding of a phenotypic association between emotion regulation and working memory raises the question of the mechanisms linking the two constructs. Multivariate behavioral genetic analyses can address this question by exploring the extent to which the association is due to common genetic and/or environmental factors. A large body of evidence indicates that individual differences in working memory are genetically influenced in school-aged children, adolescents, and adults (Ando, Ono, & Wright, 2001; Kuntsi et al., 2006; Lee et al., 2012; Luciano et al., 2001; Polderman, et al., 2006; Polderman, et al., 2007). As is the case with emotion regulation, children’s working memory can also be influenced by their experiences in the family. Parenting, as a crucial component of the family environment, may explain individual differences in working memory. Maternal behaviors facilitating children’s theory-of-mind understanding and autonomy are moderately and positively associated with working memory in infancy (Bernier, Carlson, & Whipple, 2010). In addition, maternal autonomy support at 12–15 months of age independently predicts individual differences in working memory after controlling for maternal education and child cognitive functioning at 18 months (Bernier et al., 2010). Furthermore, children with better quality of parent-child interactions and higher levels of attachment security in infancy develop greater executive functioning including working memory, set shifting, and inhibitory control at age 3 (Bernier, Carlson, Deschênes, & Matte-Gagné, 2012).
These findings of genetic and environmental effects on working memory raise the possibility that the genetic and/or environmental factors influencing working memory may overlap with those for emotion regulation, and thereby contribute to the association between the two constructs. Understanding the mechanisms linking the two domains can inform about common underlying processes and may suggest directions for future research as well as targets for possible interventions. Although not well-studied, there are hints that genes, but not environments, may contribute to links between emotion regulation and working memory in children. Molecular genetics research finds that the COMT gene has been found to be associated with both emotion regulation and working memory, but these were not within the same study (Bishop, Cohen, Fossella, Casey, & Farah, 2006; Bruder et al., 2005; Drabant et al., 2006; Dumontheil et al., 2011). With respect to the environment, higher levels of maternal emotion regulation were linked with better emotion regulation, but not working memory in school-aged children with experiences witnessing domestic violence (Samuelson, Krueger, & Wilson, 2012). It seems that genetic link might be more likely to operate the association between domains. Based on this preliminary evidence, we hypothesized that there would be a genetic covariation between emotion regulation and working memory (i.e., the link between emotion regulation and working memory in specifying that the link is, to some extent, genetic in origin).
Method
Sample
The Boston University Twin Project (BUTP) sample was recruited from birth records supplied by the Massachusetts Registry of Vital Records. As is standard for twin research, twins were selected preferentially for higher birth weight and gestational age. No twins with birth weights less than 1,750 g or with gestational ages less than 34 weeks were included in the study. This ensured that our findings were not due to prematurity or low birth weight. Data were available for 304 same-sex twin pairs (140 MZ and 164 DZ; mean age=2.99 years, SD= .08). Ethnicity was generally representative of the Massachusetts population (85.4% Caucasian, 3.2% Black, 2% Asian, 7.3% Mixed, 2.2% Other). Socioeconomic status according to the Hollingshead Four Factor Index (1975) ranged from low to upper middle class (range=20.5–66; M =50.9, SD=14.1). Zygosity was determined via DNA analyses using DNA obtained from cheek swab samples. In the cases where DNA was not available (n=3), zygosity was determined using parents’ responses on physical similarity questionnaires which have been shown to be more than 95% accurate when compared to DNA markers (Price, Freeman, Craig, Ebersole, & Plomin, 2000).
Procedure
All procedures were approved by the Boston University Institutional Review Board. Within approximately 2 weeks of their third birthday, twins completed two laboratory visits scheduled approximately 48 hours apart. On both visits, each member of the twin pair was assessed separately in either a test or a play situation. On the first visit, one of the twins participated in a standardized test situation in which the Mental Scale of the Bayley Scales of Infant Development-Second Edition (BSID-II; Bayley, 1993) and a memory task were administered. The Mental Scale of the BSID-II includes a group of cognitive tasks that were designed to evaluate children’s abilities of generalization, classification, problem solving, language, memory, and early number concepts (Fugate, 1998). Meanwhile, the other twin participated in a laboratory play situation in which episodes from the Laboratory Temperament Assessment Battery - Preschool Version (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995) and three imitation tasks (Fenstermacher & Saudino, 2007) were administered. On the second visit, the situations were reversed for each twin. Each visit was completed in less than 1.5 hours. Twins within a family were assessed by different testers.
Measures
Emotion Regulation
The Behavior Rating Scale (BRS) of the BSID-II (Bayley, 1993) was used to assess emotion regulation. The emotional regulation factor of the BRS comprises nine items (i.e., negative affect, hypersensitivity to test materials and stimuli, adaptation to change in test materials, attention to task, persistence in attempting to complete tasks, frustration with inability to complete tasks, cooperation, frenetic movement, and hyperactivity) each rated on a five-point scale. This broad measure of emotion regulation covers various domains involved in emotion regulation (i.e., emotional expressivity, attentional skills, goal-related adaptive and motivational behaviors, and social interaction based on the situational demands) and thus fits the definition of emotion regulation in our study. After both visits in our project, testers completed the BRS based on children’s observed behavior during assessments.
Although not a measure of elicited emotion regulation, the BRS emotion regulation factor has been successfully used in several prior studies of observed emotion regulation within a standard situation (e.g., Bocknek, Brophy-Herb, & Banerjee, 2009; Clark, Woodward, Horwood,& Moor, 2008; Halligan et al., in press; Lowe, Woodward, & Papile, 2005; Messinger et al., 2010; Porter, Wouden-Miller, Silva, & Porter, 2003; Raikes, Robinson, Bradley, Raikes, & Ayoub, 2007; Sylva et al., 2011). In contrast to typical emotion regulation episodes that are designed to artificially elicit frustration, in the present study both the play and test situations provided a more naturalistic measure of emotion regulation as reflected by their abilities to: modulate frustration with difficult tasks and with the removal of attractive test materials (i.e., expressed negative affect and frustration as the outcomes of their emotional modulation); to sustain attention to tasks so as to distract their attention away from their negative feelings (i.e., controlling their attentional processes); to maintain motivation in a series of tasks (i.e., persistence, and lower hypersensitivity) and socially adapt to the tester (i.e., more cooperation), both of which serve as the means of maintaining an appropriate emotional state during the sessions with the goal of completing various tasks; and to manage their high level of excitement when presented with attractive test materials (i.e., lower level of hyperactivity and frenetic movement).
Higher scores on the emotional regulation factor of the BRS reflect better levels of emotional regulation. The correlation between emotion regulation scores for the two laboratory situations was high (r =.65, p<.01), and therefore, average scores of BRS for two laboratory visits were calculated for data analysis. The emotion regulation factor showed good internal consistency in our sample (Cronbach’s α =.95). Interrater reliability based on approximately 25% of the present sample was high (r= .71, p<.01).
Working Memory
Working memory was assessed using the Visually Cued Recall task (VCR; Zelazo, Jacques, Burack, & Frye, 2002) and memory items from the Mental Scale of BSID-II. The VCR task is a combination of a pictorial memory span task and a delayed response task. Research suggests that simple span tasks such as the VCR and the Bayley items tap working memory in young children (Cowan, 2008; Engle, Tuholski, Laughlin, & Conway, 1999; Hutton & Towse, 2001; Hornung, Brunner, Reuter, & Martin, 2011). Moreover, the delay-response tasks assessing memory in infants are linked with the executive processing and the neural basis of working memory (Reznick, 2007). At the beginning of the VCR task, a puppet was introduced to the child, and in each trial of the VCR task, an 8.5 by 11 inch card with pictures of 12–18 objects was shown to the child. The child was told that the puppet would point to a certain number of objects that she/he liked on each card, and the child was asked to remember them. In each trial, after the puppet pointed to pictures, experimenters flipped the card as a short delay, and when they flipped the card back, the child was cued to identify in any order the objects that the puppet liked. From trial to trial, the child had to update their mental representations of the puppet’s preferred objects and maintain the visual images and spatial locations of the objects across the short delay. During the process of identifying the objects, the child not only needed to recognize and focus their attention on the targeting objects, but also had to differentiate the targeting objects from and direct their attention away from other attractive distractors on the card. Load was manipulated by increasing the number of objects that participants needed to remember on each card. The VCR task was discontinued when the child has failed two consecutive trials (i.e., the ceiling rule), or when the child has completed all 12 trials successfully. The highest number of objects that the child remembered was the total score of the VCR task.
The memory items from the Mental Scale of BSID-II include repeats 3 number sequences, recalls geometric forms, discriminates pictures, and remembers sequence. 1) Repeats three number sequences is a digit span task. There were two practice trials and four test trials. In each practice trial, a different digit was asked to repeat. In each test trial, series of two or three digits were tested on the child. 2) For recalls geometric forms, three different pieces (triangle, circle, square) were separately displayed to the child, while a shield was used to hide the rest pieces. The shield was then removed; and the child was asked to identify the piece that had been shown to him/her. To successfully accomplish this task, the child needed to utilize his/her abilities to memorize, differentiate shapes, and meanwhile sustain attention on the targeting shape and disorient from the distractors. 3) During discriminates pictures, a picture of one object (e.g., apple) was shown to the child. Then a picture of four objects including the one previously shown (e.g., orange, banana, pear, and apple) were displayed. The child was cued to point to the object previously shown. Four trials were administered. As with the recall of geometric shapes task, this task entailed the child’s memorization, differentiation, and attentional control. 4) For remembers sequence, behavioral sequences were verbally instructed to the child. Two practice trials and one test trial were administered. First, the child was asked to perform one action (i.e., pat the table). Second, the child was asked to perform two-step actions (i.e., pat the table and then touch the nose). Finally, as the test trial, the child was asked to perform three-step actions (i.e., pat the table, and then touch the nose, and then clap the hands). In this task, the child needed to memorize and code the verbal instructions first and then transferred the information into movements.
A factor analysis of VCR score and Bayley memory items found that these measures loaded on a single factor, which was labeled “working memory”. Internal consistency for this factor was high (cronbach’s α=.81) and a composite score was formed based on the average of two measures’ standardized scores.
Statistical Analyses
Data Transformations
Emotion regulation scores were negatively skewed (skewness= −1.38) and were normalized using the BLOM transformation in the SAS RANK procedure (skewness after transformation= −.08). Because twin correlations can be inflated by variance due to gender, all scores were residualized for gender effects (McGue & Bouchard, 1984). These residualized scores were used in all behavioral genetic analyses.
Correlational Analyses
Twin intraclass correlations were calculated as indices of indicating co-twin similarity. When MZ twins are more similar than DZ twins (i.e., MZr > DZr), genetic influences are indicated. To evaluate genetic and environmental contributions to the phenotypic correlation between emotion regulation and working memory, cross-twin cross-variable correlations were calculated. The cross-twin cross-variable correlation involved correlating the score of Twin A for emotion regulation with score of Twin B for working memory, and vice versa. Cross-twin correlations are the essence of a multivariate analysis of covariance. Genetic contributions to the covariance between emotion regulation and working memory are implied when the MZ cross-twin correlation is greater than the DZ cross-twin correlation.
Model-Fitting Analyses
A bivariate correlated factors model (Figure 1) was used to examine the extent to which genetic (A), shared environmental (C) and nonshared environmental (E) factors accounted for the variances of emotion regulation and working memory, and the covariance between the two constructs. In Figure 1, the latent factors A1, C1, and E1 refer to the genetic (additive), shared and nonshared environmental influences on emotion regulation, and A2, C2, and E2 refer to the genetic and environmental influences on working memory. The path coefficients, h, c, and e, are standardized partial regressions indicating the relative influence of the latent factors on the phenotypes. The square of these path coefficients estimates the genetic and environmental variances for each phenotype. Of particular interest in this model are the estimated parameters rg, rc and re (i.e., the genetic, shared environmental, and nonshared environmental correlations, respectively, between emotion regulation and working memory). The genetic correlation indicates the extent to which genetic effects on one phenotype correlate with genetic effects on another, independent of the heritability of each phenotype. The genetic factors that influence two phenotypes can covary perfectly even though the genetic effects on each phenotype contribute only slightly to the phenotypic variance. Thus, rg can be 1.0 even though the genetic contribution to the phenotypic correlation is only modest if the heritability of each measure is modest and the same genetic effects operate on each measure. Conversely, two phenotypes may be substantially heritable, but the genetic correlation would be zero if the genetic effects on the two phenotypes do not overlap. Similar logic applies to rc and re.
Figure 1. Bivariate Correlated Factors Model.
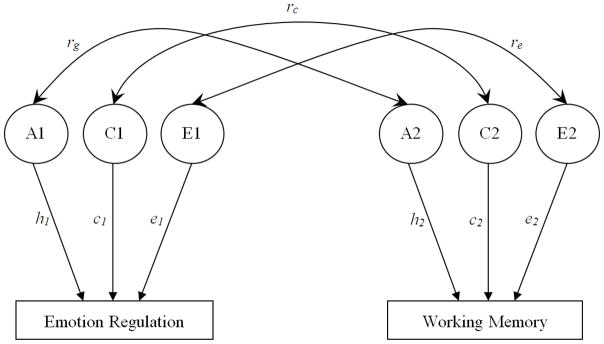
The full model includes additive genetic (A), shared environmental (C), and nonshared environmental (E) factors. The path coefficients, h, c and e, are standardized partial regression coefficients indicating the relative influence of the latent factors on the phenotypes. rg, rc and re represent the genetic, shared-environmental, and nonshared environmental correlations, respectively.
Using the model depicted in Figure 1 as the full model, various reduced models were tested. In the reduced models, rg, rc, and re, respectively, were fixed to zero but the remaining parameters were freely estimated. Models were fit to raw data using a maximum likelihood pedigree approach implemented in Mx structural equation modeling software (Neale, Boker, Xie, & Maes, 2003). This approach allows the inclusion of participants with incomplete data. The overall fit of a model can be assessed by calculating twice the difference between the negative log-likelihood (−2LL) of the model and that of a saturated model (i.e., a model in which the variance/covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated). The difference in −2LL is asymptotically distributed as χ2 with degrees of freedom equal to the difference in the number of parameters in the full model and that in the saturated model. In addition, a standard fit index, Akakie’s information criterion (AIC; AIC= Δχ2−2*Δdf) was used to assess models’ fits (Neale and Cardon, 1992). Negative AIC values indicate good fit of the model to the observed data, and the model that minimizes AIC is a better-fitting model (Akaike, 1987). Because the reduced models were nested in the full model, the relative fit of the reduced model was determined by the χ2 difference (Δχ2) between full model and the reduced model, and corresponding change in degrees of freedom (Δdf). A significant Δχ2 indicates that the parameter not included in the reduced model could not be dropped without a significant decrement in fit and is therefore, significant.
Results
Descriptive Statistics
Means and standard deviations (SD) of emotion regulation and working memory by gender and zygosity are presented in Table 1. The mean emotion regulation score for the full sample was 40.03 indicating that, on average the twins, were well regulated, however, more important to our research questions, there was considerable variation in emotion regulation scores (SD=4.71, range 17.5 to 45). There was also substantial variation in working memory (M= −.02, SD=.90, range −2.93 to 2.70). We evaluated mean differences for gender and twin type using generalized estimating equations (GEE) implemented in the SAS GENMOD procedure to account for dependence in the data due to the fact that our sample comprised pairs of twins. GEE are an extension of the standard generalized linear models that allow modeling of correlated data (Liang & Zeger, 1986; Zeger & Liang, 1986). For both variables, females scored significantly higher than males (emotion regulation: z=2.74, p< .05; working memory: z=2.83, p< .05), indicating females have better emotion regulation and higher working memory capacity. MZ and DZ twins did not significantly differ in both variables (emotion regulation: z=1.85, p=.06; working memory: z=0.84, p=.40) thus fulfilling a basic assumption of the twin method.
Table 1.
Means (SD) for Emotion Regulation and Working Memory at age 3 by Sex and Zygosity
| Age | Males
|
Females
|
Effect size
|
|||
|---|---|---|---|---|---|---|
| MZ twins | DZ twins | MZ twins | DZ twins | Sex | Zygosity | |
| Emotion Regulation | 39.16 (5.08) | 39.72 (4.92) | 40.06 (4.44) | 41.20 (4.10) | −.25 | −.17 |
| n | 140 | 177 | 140 | 150 | ||
| Working Memory | −.22 (0.99) | −.08 (0.84) | .10 (.83) | .12 (.92) | −.28 | −.07 |
| n | 138 | 176 | 140 | 149 | ||
Note. Effect size was estimated by Cohen’s d. MZ=monozygotic; DZ=dizygotic. Mean values of working memory are based on z-transformed scores.
Correlations
Emotion regulation and working memory were moderately correlated (r=.49, p< .001). Twin intraclass correlations and cross-twin cross-variable correlations are presented in Table 2. For both variables, the intraclass correlations for MZ twins exceeded those for DZ twins, suggesting genetic influences on both domains. For working memory, DZ correlations exceeded one-half the MZ correlation, suggesting that shared environmental effects also influenced the variation in this capacity. The cross-twin cross-variable correlation for MZ twins was higher than that for DZ twins, suggesting that genetic influences may contribute to the phenotypic correlation between emotion regulation and working memory, which can be tested by more powerful multivariate genetic model-fitting analyses.
Table 2.
Twin Intraclass Correlations and Cross-Twin Cross-Trait Correlations
| Variables | Twin Zygosity
|
|
|---|---|---|
| MZ twins | DZ twins | |
| Emotion Regulation | .53** | .27** |
| Working Memory | .62** | .46** |
| Cross Correlations | .43** | .30** |
Note. ER=emotion regulation; WM=working memory; MZ=monozygotic; DZ=dizygotic.
p<.001.
Model-Fitting Analyses
Table 3 presents the fit statistics for the model-fitting analyses. Comparative model fitting showed that dropping the genetic correlation (rg) resulted in a significant Δχ2 (p=.011, as indicated by the ‘Relative fit of model’ p-value on the right hand side of the table), indicating that there was a significant genetic correlation between emotion regulation and working memory. The model constraining rg to zero also yielded a poorer fit to the data according to the AIC (Reduced model AIC= −8.161; full model AIC= −12.603). On the other hand, dropping both the shared and the nonshared environmental correlations between emotion regulation and working memory (i.e., rc and re) from the full model did not lead to a significant difference in fit from the full model (p =.083), suggesting that shared and nonshared environment did not contribute significantly to the observed covariance between variables. Although the AIC for the full model was slightly lower than that for the reduced model with no environmental covariation (−12.603 vs −11.632), the AIC differences between models that are less than 2 are viewed as trivial (Millar, 2011) and the best model by virtue of parsimony is the reduced model which drops the nonsignificant parameters. Nonetheless, to avoid artificial inflation of parameter estimates (i.e., due to the fact that in reduced models non-significant shared environmental variance is absorbed into genetic variance), parameter estimates and their 95% confidence intervals are presented from the full bivariate ACE model in Table 4. Individual differences in emotion regulation were significantly influenced by genetic factors, which accounted for 43% of the variance (p< .05). Shared environmental effects on emotion regulation were modest (9%) and not significant (p> .05). The remaining variance (48%) in emotion regulation was due to significant nonshared environmental influences (p< .05). Working memory showed significant and moderate heritability (29%, p< .05) shared environmental influences (32%, p< .05) and nonshared environmental influences (39%, p< .05). There was a significant and substantial genetic covariance between the two constructs (rg= .76, p< .05).1 This suggests that there is considerable overlap in genes influencing both emotion regulation and working memory. The non-significant shared environment correlation was not substantively meaningful because of the large confidence intervals around the estimate [−1.0, 1.0], and the fact that there were no significant shared environmental factors for emotion regulation. The nonshared environmental contribution to the association between emotion regulation and working memory was modest and not significant (p> .05). Thus, as indicated by our tests of alternative models, only genetic factors significantly contributed to the covariation between emotion regulation and working memory.
Table 3.
Fit Statistics for Models of Associations Between Emotion Regulation and Working Memory
| Overall fit of modela
|
Relative fit of modelb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| −2LL | df | χ2 | Δdf | p | AIC | Δχ2 | Δdf | p | |
| Saturated Model | 2948.577 | 1182 | |||||||
| Full Model | 2957.974 | 1193 | 9.397 | 11 | .585 | −12.603 | |||
| Drop rg | 2964.416 | 1194 | 15.839 | 12 | .199 | −8.161 | 6.442 | 1 | .011 |
| Drop rc | 2961.079 | 1194 | 12.502 | 12 | .406 | −11.498 | 3.106 | 1 | .078 |
| Drop re | 2961.210 | 1194 | 12.633 | 12 | .396 | −11.367 | 3.236 | 1 | .072 |
| Drop rc & re | 2962.945 | 1195 | 14.368 | 13 | .348 | −11.632 | 4.972 | 2 | .083 |
Note. −2LL = log-likelihood statistic; df = degree of freedom; χ2= chi-square fit statistic = −2LL difference between a model and the saturated model; AIC = Akaike’s information criterion; Δχ2 = chi-square difference between reduced model and full model.
Overall fit of the model is determined by the difference in −2LL of each model and that of the saturated model.
Relative fit of the model determined by the χ2 difference (Δχ2) between full bivariate ACE model and the reduced model.
Table 4.
Estimates of Genetic and Environmental Variances and Covariances (95% Confidence Interval)
| Variance Estimates
|
Covariance Estimates
|
|||||
|---|---|---|---|---|---|---|
| h2 | c2 | e2 | rg | rc | re | |
| Emotion Regulation | .43* [.16,.58] | .09 [.00,.30] | .48* [.39,.60] | .76* [.31,1.00] | 1.00 [−1.00,1.00] | .14 [−.01,.29] |
| Working Memory | .29* [.03,.58] | .32* [.06,.54] | .39* [.31,.49] | |||
Note. h2= genetic variance; c2= shared environmental variance; e2= nonshared environmental variance. rg, rc and re denote the genetic, shared environmental, and nonshared environmental correlations between WM and ER, respectively.
p<.05.
To address the possibility that the covariance between emotion regulation and working memory may have arisen because our composite emotion regulation measure included the behavioral ratings from the test situation in which working memory was assessed, we also looked at the association between the two constructs using only the BRS emotion regulation ratings for the play situation. The results were similar to those for our multi-situation composite measure. Emotion regulation in the play situation was significantly correlated with working memory (.36, p< .05). Model-fitting analyses, indicated significant genetic influences on emotion regulation during the play situation (h2 =.24, 95% CI=.01–.40). Although the estimate of heritability for the emotion regulation in play situation was lower than that for the composite measure, the overlapping confidence intervals suggest that the heritabilities do not significantly differ. More important to our research question regarding the link between emotion regulation and working memory, the covariance between the two constructs was due to genetic effects (rg = .86, 95% CI=.06–1.00). Thus, the association between emotion regulation and working memory is not simply due to the fact that working memory was assessed within the same context as part of our emotion regulation measure.
Discussion
Individual differences in emotion regulation have been linked with parental behaviors, physiological processes, and temperamental variability; however, genetic influences as a potential source of variability in emotion regulation in early childhood have gone largely unexplored. The present findings support our hypotheses that variations in emotion regulation in toddlerhood are attributed to significant genetic and environmental effects, and emotion regulation is associated with working memory, which is due to significant genetic overlap on the two constructs.
Emotion regulation at age 3 was significantly influenced by genetic and nonshared environmental factors, which is consistent with the previous findings of modest genetic and large nonshared environmental influences on gaze aversion in 5-month-old infants (Soussignan et al., 2009). Our study using a more comprehensive assessment of emotion regulation broadens our knowledge of the etiology underlying individual differences in emotion regulation of toddlers. Rather than being a single construct, emotion regulation involves processes tied to a variety of psychological domains including experiential, cognitive, behavioral, motivational, and social subsystems (Eisenberg & Fabes, 2006; Gross & Thompson, 2007). Emotion regulation, as indicated by a combination of emotional expressivity, attentional skills, goal-related adaptive and motivational behaviors, and interaction based on the situational demands in our study, is genetically influenced.
Although our finding of genetic influences is consistent with the earlier study of gaze aversion in infancy, our estimate of heritability was higher and nonshared environmental effects were lower. This difference in the magnitude of effects may be due to age differences. Temperament research has shown that when developmental differences in genetic influences on temperament are found, it tends to be in the direction of increased genetic variance in the populations studied (i.e., higher estimates of heritability across age) (e.g., Braungart, Plomin, DeFries, & Fulker, 1992; Ganiban, Saudino, Ulbricht, Neiderhiser, & Reiss, 2008; Stevenson & Fielding, 1985). Emotion regulation could show a similar developmental pattern of increasing genetic effects across age. Alternatively, it is possible that the differences in the magnitude of genetic and environmental effects reflect differences in the behaviors examined across the two studies. Longitudinal studies assessing the same behaviors across age are needed to address this question.
Genetic influences on the individual differences in emotion regulation, a hybrid of different psychological systems, may illustrate the widespread and intensive effects of genetic factors. It is possible that the sources of variability in emotion regulation such as parental behaviors, physiological processes, and temperamental traits are influenced by genetic effects, which are overlapping with those on emotion regulation. In other words, the connections between physiological processes, parenting, temperaments and emotion regulation might be mediated by genetic factors.
The finding of significant genetic influences on emotion regulation does not mean that the environment is unimportant. Noticeably, nonshared environmental influence contributes to almost half of the variance of emotion regulation in toddlers. Differential parenting can be considered as a within-family effect (Boyle et al., 2004), and may serve as a possible source of nonshared environments affecting emotion regulation. Positive parental behaviors can promote children’s emotion regulation (Eisenberg et al., 2005), and parents may treat their children differently resulting in the variation in emotion regulation. In addition, maternal harshness has a more detrimental influence on emotion regulation than paternal harshness (Chang, Schwartz, Dodge, & McBride-Chang, 2003), and each child within a family may have differential interactions with mother and father, and consequently might develop different capacities for emotion regulation. The issue of differential parental treatment as a source of nonshared environmental influences, however, is complicated in that parents may treat their children differently in response to their children’s genetically influenced characteristics. Parenting behaviors linked with emotion regulation might be explained by genotype-environment correlations. Genetic effects on parenting behaviors have been shown to be, in part, child-driven effects in that they represent the genetic contributions of children to their parents’ behavior (McGuire, 2003). For example, genetic influences on negative parenting can, to some extent, be explained by genetic influences on children’s antisocial behaviors, depression and personality, suggesting an evocative gene-environment correlation between child traits and parenting behaviors (Larsson, Viding, Rijsdijk, & Plomin, 2006; Pike, McGuire, Hetherington, Reiss, & Plomin, 1996; South, Krueger, Johnson, & Iacono, 2008).
Regarding sources of variation in working memory, studies of school-aged children, adolescents, and adults have indicated that moderate genetic and nonshared environmental factors, but not shared environmental factors, have a significant impact on individual differences in working memory (Ando et al., 2001; Kuntsi et al., 2006; Lee et al., 2012; Luciano et al., 2001; Polderman, et al., 2006; Polderman, et al., 2007). Although well-studied in older samples, no prior studies have examined genetic influences on working memory in toddlerhood, a period when working memory is undergoing substantial development (Reznick, 2009). Our study replicates prior findings of significant genetic and nonshared environmental effects on working memory; however, in contrast to research with older samples, in our toddler sample, we found that shared environmental influences also contribute to individual differences in working memory. This difference in the relative importance of shared environmental effects across age mirrors the findings from behavioral genetics work on general cognitive ability that finds that shared environmental factors are significant and substantial in early, but not late, childhood (Bishop et al., 2003). This similarity in developmental patterns of etiology likely reflects the fact that working memory is a component of cognitive abilities. Indeed, working memory tasks often appear on standardized test of intelligence (e.g., Wechsler Intelligence Scales, and Stanford–Binet Intelligence Scales). The finding of shared environmental variance on working memory and general cognitive ability in early, but not late, childhood is likely due to the fact that young twins spend most of their time with their family, and shared family experiences contribute to the twin resemblances in cognitive abilities; however, as they get older and spend less time within the same environment, individual experiences of each twin outside the family outweigh shared environmental influences (Plomin, DeFries, McClearn, & McGuffin, 2008).
Of particular interest, the current finding of a genetic overlap between emotion regulation and working memory lends support for the connections between emotional and cognitive functioning. Emotion regulation and working memory are linked, not only in terms of phenotypic manifestations, but also in terms of underlying genetic etiology. The fact that this finding remained when emotion regulation was assessed within only the play situation or using a more restrictive measure suggests that it is not simply an epiphenomenon of a shared context in which both domains were assessed or a function of our broad definition of emotion regulation. To some extent, it is not surprising that common genes influence both working memory and emotion regulation given that the same neural substrates have been associated with both constructs (Bluhm et al., 2011; Bush et al., 2000; Gray et al., 2003; Kondo et al., 2004; Ochsner et al, 2004; Osaka et al., 2004; Sinha et al., 2004; Urry et al., 2006). Because common brain regions are involved for emotion regulation and working memory, genes influencing the structures or functions of those brain regions may have an impact on both emotion regulation and working memory. For instance, the prefrontal cortex plays a crucial role in both emotion regulation (Ochsner et al, 2004; Urry et al., 2006) and working memory (Bluhm et al., 2011; Gray et al., 2003). In early childhood, developmental changes in synaptic density and myelination occur in the prefrontal cortex (Durston & Casey, 2006; Huttenlocher & Dabholkar, 1997). These structural and related functional changes in prefrontal cortex support the developmental improvement in both inhibitory control (i.e., a temperamental trait closely related to emotion regulation) and working memory in childhood (Durston & Casey, 2006; Tsujimoto, 2008). It is also possible that the two constructs are genetically correlated because both phenotypes might reflect common basic skills. For example, attentional control might be involved in both. Accordingly, genetic factors influencing attentional control may subsequently have an impact on both emotion regulation and working memory. Molecular genetics research, to some extent, indirectly illustrates the genetic link between the two constructs. The COMT gene has been found to modulate prefrontal brain activity (Liu et al., 2010) and influence the performance of the attentional control task (Blasi et al., 2005), and has been associated with emotion regulation and working memory in separate studies (Bishop, Cohen, Fossella, Casey, & Farah, 2006; Bruder et al., 2005; Drabant et al., 2006; Dumontheil et al., 2011). The common genes related to both emotion regulation and working memory need to be further confirmed in the same group of people. Nevertheless, the substantial genetic overlap between the genetic effects involved in emotion regulation and working memory have important implications for identifying candidate genes. Future molecular genetics studies can target candidate genes for emotion regulation based on results of working memory, and vice versa.
Notably, genetic and/or environmental effects may be dynamic rather than static over time (i.e., genetic and environmental effects can change across age). For instance, there was an age × genotype interaction on visuospatial working memory capacity (Dumontheil et al., 2011). Specifically, the influence of the Val158Met polymorphism of the COMT gene on working memory changes with age. Individuals with the Met/Met genotype had lower levels of working memory before age 10, but had higher levels after age 10, compared to people who are Val carriers (Dumontheil et al., 2011). Moreover, the Val158Met polymorphism of the COMT gene was associated with positive affect (a temperamental trait related to emotion regulation) at 6–7 months, but not at 18–20 months (Sheese, Voelker, Posner, & Rothbart, 2009), which might hint the possible developmental change of genetic effects on emotion regulation. Given the evidence of varying genetic effects across age, it is possible that the genetic covariation between emotion regulation and working memory could change across age. Future studies are needed to explore the longitudinal pathways that underpin the development of emotion regulation and working memory (e.g., a genetically informative cross-lagged analysis).
The results also have clinical implications for clinicians and have the potential to indirectly inform treatments for psychopathology associated with deficits in working memory or emotion regulation. Patients with attention deficit hyperactivity disorder, mood disorders, anxiety disorders, personality disorders, and schizophrenia have impairments in emotion regulation (Gross, 1998; Martel, 2009; van der Meer, van’t Wout, & Aleman, 2009), as well as working memory dysfunction (Canuto et al., 2010; Caudle et al., 2007; Goldstein et al., 2010; McClure et al., 2007; Rhodes, Park, Seth, & Coghill, 2012; Schecklmann et al., 2011). The genetic link between emotion regulation and working memory provides a potential mechanism by which deficits in both emotion regulation and working memory may arise in the disorders. Given the overlapping mechanisms underlying individual differences in both emotion regulation and working memory, individuals with impaired emotion regulation may benefit from treatment strategies of improving working memory, and vice versa. Thus, there is a possibility of improving both emotion regulation and working memory by interventions targeted at only one of them.
Some potential limitations of this study should be acknowledged. First, our measurement of emotion regulation relied on observer ratings that may be situation-specific and constrained to children’s behavior during two lab visits. Children’s behavior outside the laboratory situations may differ. Nonetheless, the laboratory measures provided us with standard situations in which to assess emotion regulation, and assured that individual differences in emotion regulation were not simply due to contextual factors. Future work may benefit from including other informants, situations, and methods of data collection so as to broaden the generalizability of these findings. Second, although our sample included over 600 children, it is a relatively small twin sample and does not afford sufficient power to examine sex effects on the etiology of individual differences in emotion regulation. Consistent with prior research (Sylva et al., 2011), we found that males displayed lower levels of emotion regulation as compared to females. However, gender differences on the mean level of emotion regulation do not inform about sex differences at the level of etiology (i.e., genetic and environmental influences on the variance of emotion regulation). This can be addressed by twin analyses with large samples that include opposite sex twins. Third, because our sample was young, our measure of working memory does not differentiate between the subcomponents of executive attention, verbal storage, and visual-spatial storage. Therefore, we were unable to analyze the links between the different subcomponents of working memory and emotion regulation. Better measures of working memory in toddlers are needed before this can be explored. Given that working memory begins to emerge in infancy or toddlerhood, and this emerging working memory might be qualitatively different from adult working memory (Reznick, 2007), difficulties with obtaining good measures of working memory in very young children can be foreseen. Finally, our finding of a genetic correlation between emotion regulation and working memory does not clarify the causal mechanisms of the relation between emotion regulation and working memory. It remains unclear that impairments of emotion regulation can lead to working memory dysfunction, or vice versa. Future studies are needed to explore the longitudinal pathways that underpin the development of emotion regulation and working memory.
In conclusion, the present study provides the first empirical evidence that individual differences in emotion regulation in toddlerhood are influenced by genetic and nonshared environmental factors, and importantly, that common genetic effects contribute to the association between emotion regulation and working memory. These findings enable us to understand the relation between emotional and cognitive functioning in a broad sense, and provide insight into the interpretations of the mechanisms that underlie the emotion-cognition interaction. Our results have potential implications for molecular genetic studies. Given the genetic overlap between emotion regulation and working memory, results from molecular genetics studies on one construct can help identify candidate genes associated with the other. The current results also have clinical implications that people with problems in emotion regulation may benefit from treatment strategies of improving working memory, and vice versa.
Acknowledgments
The Boston University Twin Project (BUTP) is supported by grant MH062375 to Dr. Saudino from the National Institute of Mental Health. The authors gratefully acknowledge the families in the BUTP, the BUTP staff and volunteers.
Footnotes
A reviewer suggested that we restrict the measure of emotion regulation to include only 4 of the original 9 items on the BRS for emotion regulation (i.e., negative affect, hypersensitivity to test materials and stimuli, adaptation to change in test materials, frustration with inability to complete tasks). This restricted measure of emotion regulation yielded similar results. That is, emotion regulation was moderately correlated with working memory (r =.38, p <.05); the heritability of the restricted measure of emotion regulation was significant (h2 = .40, 95% CI=.07–.51); and the covariance between the two was due to genetic effects (rg = .86, 95% CI=.20–1.00).
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behavior Genetics. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford, UK: Clarendon; 1986. [Google Scholar]
- Baddeley AD. The episodic buffer a new component of working memory. Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Development. 2010;81(1):326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Deschênes M, Matte-Gagné C. Social factors in the development of early executive functioning: a closer look at the caregiving environment. Developmental Science. 2012;15(1):12–24. doi: 10.1111/j.1467-7687.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- Bishop EG, Cherny SS, Corley R, Plomin R, DeFries JC, Hewitt JK. Developmental genetic analysis of general cognitive ability from 1 to 12 years in a sample of adoptees, biological siblings, and twins. Intelligence. 2003;31:31–49. [Google Scholar]
- Bishop SJ, Cohen JD, Fossella J, Casey BJ, Farah MJ. COMT genotype influences prefrontal response to emotional distraction. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(1):62–70. doi: 10.3758/cabn.6.1.62. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O’Brien M. Individual differences in trajectories of emotion regulation processes: the effects of maternal depressive symptomatology and children’s physiological regulation. Developmental Psychology. 2008;44(4):1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA. Default network connectivity during a working memory task. Human Brain Mapping. 2011;32(7):1029–1035. doi: 10.1002/hbm.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocknek EL, Brophy-Herb HE, Banerjee M. Effects of parental supportiveness on toddlers’ emotion regulation over the first three years of life in a low-income African American sample. Infant Mental Health Journal. 2009;30(5):452–476. doi: 10.1002/imhj.20224. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, van Baal GC, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. Acta geneticae medicae et gemellologiae. 1990;39(2):181–191. doi: 10.1017/s0001566000005419. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Jenkins JM, Georgiades K, Cairney J, Duku E, Racine Y. Differential-maternal parenting behavior: estimating within- and between-family effects on children. Child Development. 2004;75(5):1457–1476. doi: 10.1111/j.1467-8624.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- Braungart JM, Plomin R, DeFries JC, Fulker D. Genetic influences on tester-rated infant temperament as assessed by Bayley’s Infant Behavior Record: Nonadoptive and adoptive siblings and twins. Developmental Psychology. 1992;28:40–47. [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biological Psychiatry. 2005;58(11):901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Origins and outcomes of individual differences in emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:53–72. [PubMed] [Google Scholar]
- Calkins SD, Gill KA, Johnson MC, Smith C. Emotional reactivity and emotion regulation strategies as predictors of social behavior with peers during toddlerhood. Social Development. 1999;8:310–341. [Google Scholar]
- Canuto A, Giannakopoulos P, Moy G, Rubio MM, Ebbing K, Meiler-Mititelu C, Weber K. Neurocognitive deficits and personality traits among euthymic patients with mood disorders in late life. Journal of the Neurological Sciences. 2010;299(1–2):24–29. doi: 10.1016/j.jns.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, Edge MD, Gross JJ. Emotional reactivity and cognitive regulation in anxious children. Behaviour Research and Therapy. 2010;48(5):384–393. doi: 10.1016/j.brat.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, Stanley MA. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. American Journal of Geriatric Psychiatry. 2007;15(8):680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- Chang L, Schwartz D, Dodge KA, McBride-Chang C. Harsh parenting in relation to child emotion regulation and aggression. Journal of Family Psychology. 2003;17(4):598–606. doi: 10.1037/0893-3200.17.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CA, Woodward LJ, Horwood LJ, Moor S. Development of emotional and behavioral regulation in children born extremely preterm and very preterm: biological and social influences. Child Development. 2008;79(5):1444–1462. doi: 10.1111/j.1467-8624.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Cole PM. Children’s spontaneous control of facial expression. Child Development. 1986;67:1686–1706. [Google Scholar]
- Cole PM, Zahn-Waxler C, Fox NA, Usher BA, Welsh JD. Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology. 1996;105(4):518–529. [PubMed] [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Progress in Brain Research. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Archives of General Psychiatry. 2006;63(12):1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Influence of the COMT genotype on working memory and brain activity changes during development. Biological Psychiatry. 2011;70(3):222–229. doi: 10.1016/j.biopsych.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA. Emotion regulation and children’s socioemotional competence. In: Balter L, Tamis-LeMonda CS, editors. Child psychology: A handbook of contemporary issues. New York, NY: Psychology Press; 2006. pp. 357–381. [Google Scholar]
- Eisenberg N, Fabes RA, Bernzweig J, Karbon M, Poulin R, Hanish L. The relations of emotionality and regulation to preschoolers’ social skills and sociometric status. Child Development. 1993;64:1418–1438. [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Shepard SA, Murphy BC, Guthrie IK, Jones S, Maszk P. Contemporaneous and longitudinal prediction of children’s social functioning from regulation and emotionality. Child Development. 1997;68:642–664. [PubMed] [Google Scholar]
- Eisenberg N, Hofer C, Vaughan J. Effortful Control and Its Socioemotional Consequences. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 287–306. [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL. Associations of emotion-related regulation with language skills, emotion knowledge, and academic outcomes. New Directions for Child and Adolescent Development. 2005;109:109–118. doi: 10.1002/cd.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Sulik MJ. How the study of regulation can inform the study of coping. In: Skinner EA, Zimmer-Gembeck MJ, editors. Coping and the development of regulation. New Directions in Child and Adolescent Development. Vol. 124. San Francisco, CA: Jossey-Bass; 2009. pp. 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children’s effortful control, and externalizing problems: a three-wave longitudinal study. Child Development. 2005;76(5):1055–1071. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology. General. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fenstermacher SK, Saudino KJ. Toddler see, toddler do? Genetic and environmental influences on laboratory-assessed elicited imitation. Behavior Genetics. 2007;37(5):639–647. doi: 10.1007/s10519-007-9160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. The effects of mother’s physical and emotional unavailability on emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:208–227. [PubMed] [Google Scholar]
- Fox N, Calkins S. The development of self-control of emotion: intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. [Google Scholar]
- Fugate MH. Review of the Bayley Scales of Infant Development. In: Impara JC, Plake BS, Murphy LL, editors. The thirteenth mental measurements yearbook. 2. Lincoln, NE: The university of Nebraska-Lincoln; 1998. pp. 93–95. [Google Scholar]
- Gagne JR, Saudino KJ. Wait for it! A twin study of inhibitory control in early childhood. Behavior Genetics. 2010;40(3):327–337. doi: 10.1007/s10519-009-9316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganiban JM, Saudino KJ, Ulbricht J, Neiderhiser JM, Reiss D. Continuity and change in temperament during adolescence. Journal of Personality and Social Psychology. 2008;95:222–236. doi: 10.1037/0022-3514.95.1.222. [DOI] [PubMed] [Google Scholar]
- Gjone H, Stevenson J. A longitudinal twin study of temperament and behavior problems: Common genetic or environmental influences? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(10):1448–1456. doi: 10.1097/00004583-199710000-00028. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Pollak SD, Davidson RJ. Developmental Neuroscience Perspectives on Emotion Regulation. Child Development Perspectives. 2008;2:132–140. doi: 10.1111/j.1750-8606.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. The laboratory temperament assessment battery-preschool version: Description of procedures. 1995 Unpublished manuscript. [Google Scholar]
- Goldstein KE, Hazlett EA, Savage KR, Berlin HA, Hamilton HK, Zelmanova Y, New AS. Dorso- and ventro-lateral prefrontal volume and spatial working memory in schizotypal personality disorder. Behavioural Brain Research. 2011;218(2):335–340. doi: 10.1016/j.bbr.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6(3):316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2(3):271–299. [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. pp. 3–27. [Google Scholar]
- Habel U, Koch K, Pauly K, Kellermann T, Reske M, Backes V, Schneider F. The influence of olfactory-induced negative emotion on verbal working memory: individual differences in neurobehavioral findings. Brain Research. 2007;1152:158–170. doi: 10.1016/j.brainres.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Halligan S, Cooper P, Fearon P, Wheeler S, Crosby M, Murray L. The longitudianl development of emotion regulation capacities in children at risk for externalizing disorders. Development and Psychopathology. doi: 10.1017/S0954579412001137. in press. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes, Brain and Behavior. 2003;2(6):332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Hornung C, Brunner M, Reuter R, Martin R. Children’s working memory: Its structure and relationship to fluid intelligence. Intelligence. 2011;39:210–221. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hutton UMZ, Towse JN. Short-term memory and working memory as indices of children’s cognitive skills. Memory. 2001;9(4–6):383–394. doi: 10.1080/09658210042000058. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry. 2010;51:706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Philibert RA, Barry RA. Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. Journal of Child Psychology and Psychiatry. 2009;50(11):1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H. Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage. 2004;21(1):2–14. doi: 10.1016/j.neuroimage.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Koole SL. The psychology of emotion regulation: An integrative review. Cognition and Emotion. 2009;23:4–41. [Google Scholar]
- Kopp C. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Krueger RF, South S, Johnson W, Iacono W. The Heritability of Personality is not Always 50%: Gene-Environment Interactions and Correlations between Personality and Parenting. Journal of Personality. 2008;76(6):1485–1522. doi: 10.1111/j.1467-6494.2008.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Börger N, van der Meere J, Rijsdijk F, Asherson P. Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychological Medicine. 2006;36(11):1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Viding E, Rijsdijk F, Plomin R. A genetic factor explains most of the variation in the psychopathic personality. Journal of Abnormal Psychology. 2006;115:221–230. doi: 10.1037/0021-843X.115.2.221. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lee T, Mosing MA, Henry JD, Trollor JN, Ames D, Martin NG, Sachdev PS. Genetic influences on four measures of executive functions and their covariation with general cognitive ability: The older Australian twins study. Behavior Genetics. 2012;42(4):528–538. doi: 10.1007/s10519-012-9526-1. [DOI] [PubMed] [Google Scholar]
- Liu B, Song M, Li J, Liu Y, Li K, Yu C, Jiang T. Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. Journal of Neuroscience. 2010;30(1):64–69. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Woodward B, Papile LA. Emotional regulation and its impact on development in extremely low birth weight infants. Journal of Developmental and Behavioral Pediatrics. 2005;26(3):209–213. doi: 10.1097/00004703-200506000-00008. [DOI] [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Smith GA, Geffen GM, Geffen LB, Martin NG. Genetic covariance among measures of information processing speed, working memory, and IQ. Behavior Genetics. 2001;31:581–592. doi: 10.1023/a:1013397428612. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf S, Shapiro J, Marzolf D. Developmental and temperamental differences in emotional regulation in infancy. Child Development. 1995;66:1817–1828. [PubMed] [Google Scholar]
- Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. Journal of Child Psychology and Psychiatry. 2009;50(9):1042–1051. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- Millar RB. Maximum Likelihood Estimation and Inference: With Examples in R, SAS and ADMB. New York: John Wiley & Sons; 2011. [Google Scholar]
- Messinger D, Lambert B, Bauer CR, Bann CM, Hamlin-Smith K, Das A. The relationship between behavior ratings and concurrent and subsequent mental and motor performance in toddlers born at extremely low birth weight. Journal of Early Intervention. 2010;32(3):214–233. doi: 10.1177/1053815110380917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MM, Romero MJ, Bowie CR, Reichenberg A, Harvey PD, Siever LJ. Visual-spatial learning and memory in schizotypal personality disorder: continued evidence for the importance of working memory in the schizophrenia spectrum. Archives of Clinical Neuropsychology. 2007;22(1):109–116. doi: 10.1016/j.acn.2006.11.004. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ., Jr Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- McGuire S. The heritability of parenting. Parenting: Science & Practice. 2003;3:73–94. [Google Scholar]
- Miller AL, Gouley KK, Seifer R, Dickstein S, Shields A. Emotions and behaviors in the Head Start classroom: associations among observed dysregulation, social competence, and preschool adjustment. Early Education & Development. 2004;15:147–165. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 6. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Osaka N, Osaka M, Kondo H, Morishita M, Fukuyama H, Shibasaki H. The neural basis of executive function in working memory: an fMRI study based on individual differences. Neuroimage. 2004;21(2):623–631. doi: 10.1016/j.neuroimage.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depressive symptoms and antisocial behaviour: A multivariate genetic analysis. Developmental Psychology. 1996;32:590–603. [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 5. New York: Worth; 2008. [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, Carbone MA, Cox M. Gene-environment contributions to the development of infant vagal reactivity: the interaction of dopamine and maternal sensitivity. Child Development. 2008;79(5):1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Polderman TJC, Gosso MF, Posthuma D, van Beijsterveldt TC, Heutink P, Verhulst FC, Boomsma DI. A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurologica Belgica. 2006;106:191–207. [PubMed] [Google Scholar]
- Polderman TJC, Posthuma D, Stins JF, De Sonneville LMJ, Verhulst FC, Boomsma DI. Genetic analyses of the stability of executive functioning during childhood. Biological Psychology. 2007;76:11–20. doi: 10.1016/j.biopsycho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic contributions to social behavior. Physiology and Behavior. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porter CL, Wouden-Miller M, Silva SS, Porter AE. Marital harmony and conflict: Links to infants’ emotional regulation and cardiac vagal tone. Infancy. 2003;4:297–307. [Google Scholar]
- Price TS, Freeman B, Craig I, Ebersole L, Plomin R. Infant zygosity can be assigned by parent questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Raikes HA, Robinson JL, Bradley RH, Raikes HH, Ayoub CC. Developmental trends in self-regulation among low-income toddlers. Social Development. 2007;16:128–149. [Google Scholar]
- Raver CC. Emotions matter: Making the case for the role of young children’s emotional development for early school readiness. Social Policy Report. 2002;16:3–8. [Google Scholar]
- Reznick JS. Working memory in infants and toddlers. In: Oakes L, Bauer P, editors. Short- and long-term memory in infancy and early childhood: Taking the first steps toward remembering. Oxford, England: Oxford University Press; 2007. pp. 3–26. [Google Scholar]
- Reznick JS. Working Memory in Infants and Toddlers. In: Courage M, Cowan N, editors. The development of memory in infancy and childhood. New York, NY: Psychology Press; 2009. pp. 343–365. [Google Scholar]
- Rhodes SM, Park J, Seth S, Coghill DR. A comprehensive investigation of memory impairment in attention deficit hyperactivity disorder and oppositional defiant disorder. Journal of Child Psychology and Psychiatry. 2012;53(2):128–137. doi: 10.1111/j.1469-7610.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- Rothbart M. Becoming who we are: temperament and personality and development. NY: The Guilford Press; 2011. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology, Vol. 3. Social, emotional, and personality development. 6. New York: Wiley; 2006. pp. 99–166. [Google Scholar]
- Rothbart M, Sheese B. Temperament and emotion regulation. In: Gross J, editor. Handbook of emotion regulation. New York, NY US: Guilford Press; 2007. pp. 331–350. [Google Scholar]
- Samuelson KW, Krueger CE, Wilson C. Relationships Between Maternal Emotion Regulation, Parenting, and Children’s Executive Functioning in Families Exposed to Intimate Partner Violence. Journal of Interpersonal Violence. 2012;27(17):3532–3550. doi: 10.1177/0886260512445385. [DOI] [PubMed] [Google Scholar]
- Santucci AK, Silk JS, Shaw DS, Gentzler A, Fox NA, Kovacs M. Vagal tone and temperament as predictors of emotion regulation strategies in young children. Developmental Psychobiology. 2008;50(3):205–216. doi: 10.1002/dev.20283. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Dresler T, Beck S, Jay JT, Febres R, Haeusler J, Fallgatter AJ. Reduced prefrontal oxygenation during object and spatial visual working memory in unpolar and bipolar depression. Psychiatry Research. 2011;194(3):378–384. doi: 10.1016/j.pscychresns.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology. 2008;95(6):1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Schmitz S, Saudino KJ, McGuire S, Reiss D, Hetherington EM, Plomin R. Genetic and environmental influences on temperament in middle childhood: Analyses of teacher and tester ratings. Child Development. 1996;67:409–422. [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Posner MI, Rothbart MK. Genetic variation influences on the early development of reactive emotions and their regulation by attention. Cognitive Neuropsychiatry. 2009;14(4–5):332–355. doi: 10.1080/13546800902844064. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Annals of the New York Academy of Sciences. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- Soussignan R, Boivin M, Girard A, Pérusse D, Liu X, Tremblay RE. Genetic and environmental etiology of emotional and social behaviors in 5-month-old infant twins: influence of the social context. Infant Behavior & Development. 2009;32(1):1–9. doi: 10.1016/j.infbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- South SC, Krueger RF, Johnson W, Iacono WG. Adolescent personality moderates genetic and environmental influences on relationships with parents. Journal of Personality and Social Psychology. 2008;94:899–912. doi: 10.1037/0022-3514.94.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA. Emotional development: The organization of emotional life in the early years. New York: Cambridge University Press; 1996. [Google Scholar]
- Spinrad TL, Eisenberg N, Cumberland A, Fabes RA, Valiente C, Shepard SA, Guthrie IK. Relation of emotion-related regulation to children’s social competence: a longitudinal study. Emotion. 2006;6(3):498–510. doi: 10.1037/1528-3542.6.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J, Fielding J. Ratings of temperament in families of young twins. British Journal of Developmental Psychology. 1985;3:143–152. [Google Scholar]
- Sylva K, Stein A, Leach P, Barnes J, Malmberg LE FCCC-team. Effects of early child-care on cognition, language, and task-related behaviours at 18 months: an English study. British Journal of Developmental Psychology. 2011;29:18–45. doi: 10.1348/026151010X533229. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59(2–3):25–52. [PubMed] [Google Scholar]
- Thompson RA, Meyer SC, Jochem R. Emotion regulation. Reprinted. In: Benson JB, Haith MM, editors. Social and emotional development in infancy and early childhood. San Diego: Academic Press; 2009. pp. 119–128. [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. How automatic is “automatic vigilance? Effects of working memory load on interference of negative emotional information. Cognition and Emotion. 2009;23:1106–1117. [Google Scholar]
- Wolfe CD, Bell MA. The integration of cognition and emotion during infancy and early childhood: regulatory processes associated with the development of working memory. Brain and Cognition. 2007;65(1):3–13. doi: 10.1016/j.bandc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Zelazo PD, Jacques S, Burack J, Frye D. The relation between theory of mind and rule use: Evidence from persons with autism-spectrum disorders. Infant and Child Development (Special Issue: Executive function and its development) 2002;11:171–195. [Google Scholar]


