Abstract
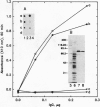
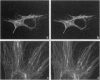
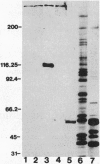
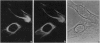
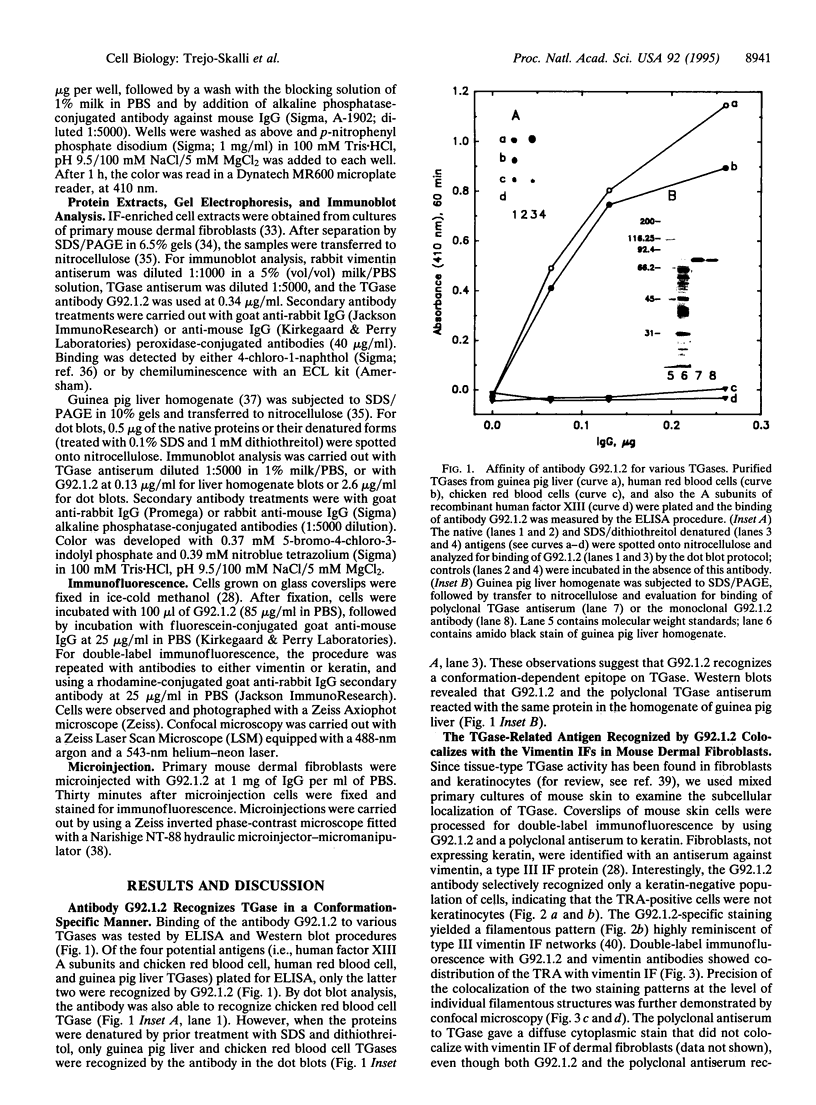
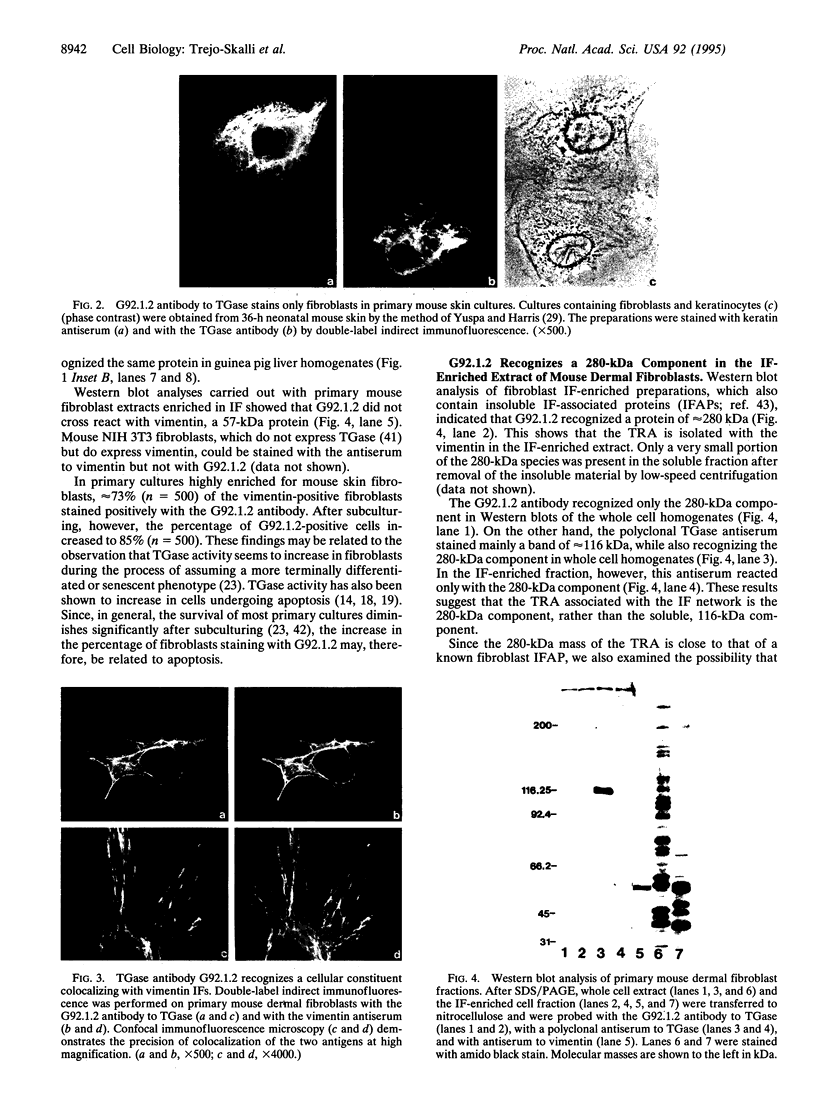
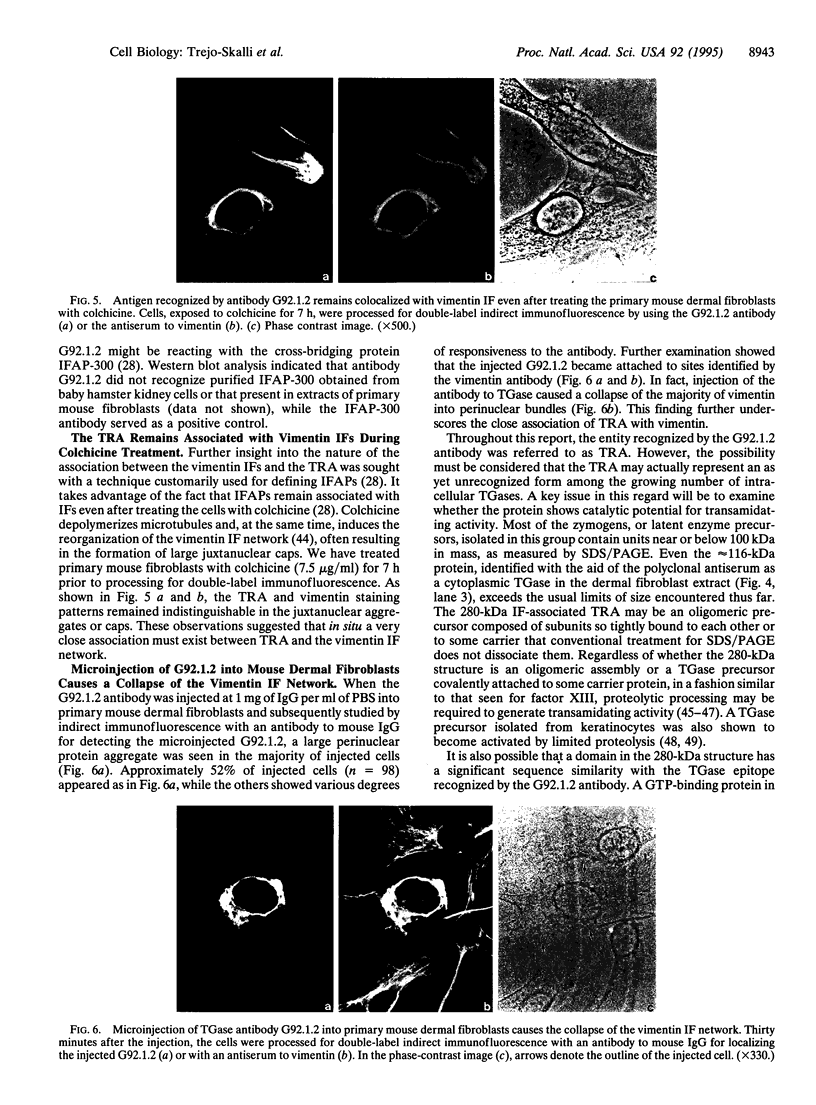
A mouse monoclonal antibody, G92.1.2, raised against guinea pig liver transglutaminase (TGase) recognizes an antigen present in primary mouse dermal fibroblasts. A filamentous pattern, bearing remarkable similarity to the vimentin intermediate filament (IF) network, is seen when these cells are fixed and processed for indirect immunofluorescence with the antibody. Double-label immunofluorescence reveals that the antigen reacting with the antibody colocalizes precisely with vimentin IF and that this colocalization is retained after the treatment of fibroblasts with colchicine, which induces a redistribution of the majority of IFs into perinuclear aggregates. These morphological observations are further supported by the finding that the protein reacting with G92.1.2 is retained in IF-enriched cytoskeletal preparations made by using nonionic detergent-containing high ionic strength solutions. Western blots of the IF fraction show that G92.1.2 recognizes a major band of approximately 280 kDa and does not cross react with vimentin. Furthermore, when the antibody is microinjected into live dermal fibroblasts, it causes a collapse of the vimentin IF network in the majority of injected cells. The results suggest that a form of TGase, or a TGase-related antigen, is closely associated with the vimentin IF network of primary cultures of mouse dermal fibroblasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birckbichler P. J., Anderson L. E., Dell'Orco R. T. Transglutaminase, donor age, and in vitro cellular senescence. Adv Exp Med Biol. 1988;231:109–117. doi: 10.1007/978-1-4684-9042-8_9. [DOI] [PubMed] [Google Scholar]
- Bishop P. D., Teller D. C., Smith R. A., Lasser G. W., Gilbert T., Seale R. L. Expression, purification, and characterization of human factor XIII in Saccharomyces cerevisiae. Biochemistry. 1990 Feb 20;29(7):1861–1869. doi: 10.1021/bi00459a028. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Lichti U. Two types of transglutaminase in the PC12 pheochromocytoma cell line. Stimulation by sodium butyrate. J Biol Chem. 1987 Aug 25;262(24):11699–11705. [PubMed] [Google Scholar]
- Cai D., Ben T., De Luca L. M. Retinoids induce tissue transglutaminase in NIH-3T3 cells. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1119–1124. doi: 10.1016/0006-291x(91)91681-2. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-dependent affinity purification of transglutaminase from rat liver cytosol. Cell Calcium. 1986 Feb;7(1):29–39. doi: 10.1016/0143-4160(86)90028-x. [DOI] [PubMed] [Google Scholar]
- Dadabay C. Y., Pike L. J. Rapid increases in the transglutaminase activity of A431 cells following treatment with epidermal growth factor. Biochemistry. 1987 Oct 20;26(21):6587–6591. doi: 10.1021/bi00395a004. [DOI] [PubMed] [Google Scholar]
- Dell'Orco R. T., Anderson L. E., Conway E., Birckbichler P. J. Variable transglutaminase activity in human diploid fibroblasts during in vitro senescence. Cell Biol Int Rep. 1985 Oct;9(10):945–956. doi: 10.1016/s0309-1651(85)90115-8. [DOI] [PubMed] [Google Scholar]
- Fesus L. Biochemical events in naturally occurring forms of cell death. FEBS Lett. 1993 Aug 9;328(1-2):1–5. doi: 10.1016/0014-5793(93)80952-q. [DOI] [PubMed] [Google Scholar]
- Fesus L., Davies P. J., Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991 Dec;56(2):170–177. [PubMed] [Google Scholar]
- Fesus L., Thomazy V. Searching for the function of tissue transglutaminase: its possible involvement in the biochemical pathway of programmed cell death. Adv Exp Med Biol. 1988;231:119–134. doi: 10.1007/978-1-4684-9042-8_10. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Kojiro M., Chiu J. F. Induction of apoptosis by transforming growth factor-beta 1 in the rat hepatoma cell line McA-RH7777: a possible association with tissue transglutaminase expression. Hepatology. 1993 Oct;18(4):945–953. doi: 10.1002/hep.1840180428. [DOI] [PubMed] [Google Scholar]
- Gentile V., Thomazy V., Piacentini M., Fesus L., Davies P. J. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cellular morphology and adhesion. J Cell Biol. 1992 Oct;119(2):463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Goldman A. E., Green K. J., Jones J. C., Jones S. M., Yang H. Y. Intermediate filament networks: organization and possible functions of a diverse group of cytoskeletal elements. J Cell Sci Suppl. 1986;5:69–97. doi: 10.1242/jcs.1986.supplement_5.5. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L. Versatility of biotin-labeled lectins and avidin-biotin-peroxidase complex for localization of carbohydrate in tissue sections. J Histochem Cytochem. 1982 Feb;30(2):157–161. doi: 10.1177/30.2.7037937. [DOI] [PubMed] [Google Scholar]
- Jeong J. M., Murthy S. N., Radek J. T., Lorand L. The fibronectin-binding domain of transglutaminase. J Biol Chem. 1995 Mar 10;270(10):5654–5658. doi: 10.1074/jbc.270.10.5654. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Jones J. C., Goldman R. D. Fractionation of desmosomes and comparison of the polypeptide composition of desmosomes prepared from two bovine epithelial tissues. J Cell Biochem. 1988 Mar;36(3):223–236. doi: 10.1002/jcb.240360304. [DOI] [PubMed] [Google Scholar]
- Korsgren C., Lawler J., Lambert S., Speicher D., Cohen C. M. Complete amino acid sequence and homologies of human erythrocyte membrane protein band 4.2. Proc Natl Acad Sci U S A. 1990 Jan;87(2):613–617. doi: 10.1073/pnas.87.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAND L., DOOLITTLE R. F., KONISHI K., RIGGS S. K. A NEW CLASS OF BLOOD COAGULATION INHIBITORS. Arch Biochem Biophys. 1963 Aug;102:171–179. doi: 10.1016/0003-9861(63)90168-1. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K. ACTIVATION OF THE FIBRIN STABILIZING FACTOR OF PLASMA BY THROMBIN. Arch Biochem Biophys. 1964 Apr;105:58–67. doi: 10.1016/0003-9861(64)90235-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieska N., Yang H. Y., Goldman R. D. Purification of the 300K intermediate filament-associated protein and its in vitro recombination with intermediate filaments. J Cell Biol. 1985 Sep;101(3):802–813. doi: 10.1083/jcb.101.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Barnes N., Bruner-Lorand J. A., Hawkins M., Michalska M. Inhibition of protein cross-linking in Ca2+-enriched human erythrocytes and activated platelets. Biochemistry. 1987 Jan 13;26(1):308–313. doi: 10.1021/bi00375a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Hsu L. K., Siefring G. E., Jr, Rafferty N. S. Lens transglutaminase and cataract formation. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1356–1360. doi: 10.1073/pnas.78.3.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Velasco P. T., Murthy S. N., Wilson J., Parameswaran K. N. Isolation of transglutaminase-reactive sequences from complex biological systems: a prominent lysine donor sequence in bovine lens. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11161–11163. doi: 10.1073/pnas.89.23.11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino G., Annicchiarico-Petruzzelli M., Piredda L., Candi E., Gentile V., Davies P. J., Piacentini M. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol. 1994 Oct;14(10):6584–6596. doi: 10.1128/mcb.14.10.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni Y., Iwanaga S., Konishi K. A peptide released from plasma fibrin stabilzing factor in the conversion to the active enzyme by thrombin. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1393–1403. doi: 10.1016/0006-291x(73)91141-8. [DOI] [PubMed] [Google Scholar]
- Murthy S. N., Wilson J., Zhang Y., Lorand L. Residue Gln-30 of human erythrocyte anion transporter is a prime site for reaction with intrinsic transglutaminase. J Biol Chem. 1994 Sep 9;269(36):22907–22911. [PubMed] [Google Scholar]
- Nakaoka H., Perez D. M., Baek K. J., Das T., Husain A., Misono K., Im M. J., Graham R. M. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994 Jun 10;264(5165):1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- Nara K., Nakanishi K., Hagiwara H., Wakita K., Kojima S., Hirose S. Retinol-induced morphological changes of cultured bovine endothelial cells are accompanied by a marked increase in transglutaminase. J Biol Chem. 1989 Nov 15;264(32):19308–19312. [PubMed] [Google Scholar]
- Piacentini M., Autuori F. Immunohistochemical localization of tissue transglutaminase and Bcl-2 in rat uterine tissues during embryo implantation and post-partum involution. Differentiation. 1994 Jun;57(1):51–61. doi: 10.1046/j.1432-0436.1994.5710051.x. [DOI] [PubMed] [Google Scholar]
- Radek J. T., Jeong J. M., Murthy S. N., Ingham K. C., Lorand L. Affinity of human erythrocyte transglutaminase for a 42-kDa gelatin-binding fragment of human plasma fibronectin. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3152–3156. doi: 10.1073/pnas.90.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Mehrpouyan M., O'Callahan W., Parenteau N. L., Rubin A. L. Keratinocyte transglutaminase: differentiation marker and member of an extended family. Epithelial Cell Biol. 1992 Jul;1(3):128–137. [PubMed] [Google Scholar]
- Rice R. H., Rong X. H., Chakravarty R. Proteolytic release of keratinocyte transglutaminase. Biochem J. 1990 Jan 15;265(2):351–357. doi: 10.1042/bj2650351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefring G. E., Jr, Apostol A. B., Velasco P. T., Lorand L. Enzymatic basis for the Ca2+-induced cross-linking of membrane proteins in intact human erythrocytes. Biochemistry. 1978 Jun 27;17(13):2598–2604. doi: 10.1021/bi00606a022. [DOI] [PubMed] [Google Scholar]
- Smith B. D., La Celle P. L., Siefring G. E., Jr, Lowe-Krentz L., Lorand L. Effects of the calcium-mediated enzymatic cross-linking of membrane proteins on cellular deformability. J Membr Biol. 1981;61(2):75–80. doi: 10.1007/BF02007633. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. Postsynthetic modifications of mammalian epidermal alpha-keratin. Biochemistry. 1979 Dec 11;18(25):5664–5669. doi: 10.1021/bi00592a022. [DOI] [PubMed] [Google Scholar]
- Sung L. A., Chien S., Chang L. S., Lambert K., Bliss S. A., Bouhassira E. E., Nagel R. L., Schwartz R. S., Rybicki A. C. Molecular cloning of human protein 4.2: a major component of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1990 Feb;87(3):955–959. doi: 10.1073/pnas.87.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin. Biochemistry. 1974 Feb 12;13(4):750–756. doi: 10.1021/bi00701a018. [DOI] [PubMed] [Google Scholar]
- Thacher S. M., Rice R. H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985 Mar;40(3):685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom K. L., Borisy G. G., Goldman R. D. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):549–553. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weraarchakul-Boonmark N., Jeong J. M., Murthy S. N., Engel J. D., Lorand L. Cloning and expression of chicken erythrocyte transglutaminase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9804–9808. doi: 10.1073/pnas.89.20.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Notides A. C., Pabalan S. S., Lorand L. Transamidase reactions involved in the enzymic coagulation of semen: isolation of -glutamyl- -lysine dipeptide from clotted secretion protein of guinea pig seminal vesicle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2322–2325. doi: 10.1073/pnas.69.8.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Lieska N., Goldman A. E., Goldman R. D. A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985 Feb;100(2):620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Harris C. C. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]