Abstract
Sclerosing and spindle cell rhabdomyosarcoma (RMS) are rare types of RMS recently reclassified as a stand-alone pathologic entity, separate from embryonal RMS. Although sclerosing and spindle cell RMS share clinical and morphologic features, a pathogenetic link based on shared molecular alterations has not been established. Spindle cell RMS in children have been associated with a less aggressive clinical course compared to adults. Recently, recurrent MYOD1 mutations were described in 44% of adult spindle cell RMS, but no pediatric tumors or sclerosing RMS were studied for comparison. Thus, we investigate 16 RMS (5 sclerosing and 11 spindle cell) in children and adults for the presence of MYOD1 mutations by targeted PCR. Remarkably, all 5 sclerosing RMS and 4 of 11 spindle cell RMS showed the MYOD1 p.L122R hot-spot mutation. Of the 5 pediatric tumors, 2/2 sclerosing RMS and 2/3 spindle cell RMS showed MYOD1 mutations. Three of 9 MYOD1-mutant RMS showed coexistent PIK3CA mutations, while no MDM2 amplifications were identified. All 4 pediatric MYOD1-mutated RMS patients died of the disease at 12–35 months following diagnosis. In conclusion, spindle cell and sclerosing RMS show recurrent MYOD1 mutations, in keeping with a single pathologic entity, regardless of age at presentation. This group however is distinct from the infantile RMS associated with NCOA2 fusions. Although our study suggests that pediatric MYOD1-mutant RMS follow an aggressive behavior with high mortality, further studies are required to confirm this finding.
Keywords: Sclerosing rhabdomyosarcoma, Spindle cell rhabdomyosarcoma, MYOD1
INTRODUCTION
Spindle cell variant of rhabdomyosarcoma (RMS) is an uncommon subtype of RMS that was initially described in the paratesticular and head and neck sites in children, and associated with a more favorable pattern of behavior and prognosis. (Cavazzana et al., 1992, Leuschner et al., 1993) In adults, the preferred location of spindle cell RMS is the head and neck region, and unlike the pediatric tumors, seem to follow a more aggressive clinical course.(Mentzel and Kuhnen 2006, Nascimento and Fletcher 2005) A subset of spindle cell RMS display areas of prominent hyaline sclerosis and pseudo-vascular growth pattern, suggesting morphologic overlap with the even less common sclerosing type RMS. As both spindle cell and sclerosing RMS have similar clinical presentations, Mentzel and colleagues suggested that they may represent a histologic spectrum of a single pathologic entity. (Mentzel 2010, Mentzel and Katenkamp 2000, Mentzel and Kuhnen 2006) More recently, these tumors have been classified as a single entity in the latest WHO classification of soft tissue tumors. (Fletcher et al., 2013) However, very few genetic studies have addressed this issue. Our group recently described recurrent NCOA2 gene rearrangements in a small subset of spindle cell RMS occurring in the infantile/congenital setting. (Mosquera et al., 2013) Since NCOA2 gene rearrangements were only seen in infants, we speculated that spindle cell/sclerosing RMS represent a heterogenous genetic group of tumors among different age groups. More recently, Szuhai et al. reported that MYOD1 gene mutation is a frequent event in adult spindle cell RMS. (Szuhai et al., 2014) As their study did not include pediatric patients or RMS with sclerosing morphology, we sought to investigate a group of sclerosing and spindle RMS in both adult and pediatric patients for this hot spot mutation.
MATERIALS AND METHODS
Patient Selection
Archival material from adult and pediatric patients with diagnosis of spindle cell or sclerosing RMS was retrieved from the pathology files at Memorial Sloan Kettering Cancer Center. Sixteen cases were identified. The diagnosis was confirmed applying strict morphologic criteria to separate from the more common embryonal RMS and immunohistochemical profile including reactivity to desmin and myogenin. Cases that showed more than focal nuclear pleomorphism or rhabdomyoblastic differentiation were excluded from the study group. Formalin fixed paraffin embedded tissue was available on all the cases selected for the study. Eleven cases were previously included in the Mosquera et al. (Mosquera et al., 2013). The study was approved by the Institutional Review Board at MSKCC (IRB# 02-060).
PCR and Sanger sequencing
Genomic DNA was isolated either from fresh-frozen or archival paraffin tissue, as described previously (Antonescu et al., 2003) in all 16 samples. Targeted PCR was performed for the known MYOD1 exon 1 hot spot mutation and PIK3CA exon 9 and exon 20 mutations, using the following primer sequences: MYOD1 Ex1 fwd: 5′-CCTACTGTGGGCCTGCAAG-3′ and Ex1 rev: 5′-GGATCTCCACCTTGGGCAAC-3′; PIK3CA-Ex9 fwd: 5′-CCAGAGGGGAAAAATATGACAAAG-3′, PIK3CA-Ex9 rev 5′-CCATTTTAGCACTTACCTGTGACTCC-3′, PIK3CA-Ex20 fwd: 5′-CTCAATGATGCTTGGCTCTGG-3′ and PIK3CA-Ex20 rev: 5′-GTGGAATCCAGAGTGAGCTTTC-3′, using Clontech advantage 2 PCR KIT, at 64.5°C annealing temperature for 35 cycles. Direct sequencing of PCR products was performed and compared to the NCBI human MYOD1 and PIK3CA gene sequences.
Fluorescence in situ hybridization (FISH)
FISH on interphase nuclei from paraffin embedded 4-micron sections was performed applying custom probes using bacterial artificial chromosomes (BAC), covering and flanking CDK4 and MDM2 genes. (Table 1) BAC clones were chosen according to USCS genome browser (http://genome.uscs.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (CHORI; Oakland, CA; http://bacpac.chori.org). DNA from individual BACs was isolated according to the manufacturer’s instructions, labeled with different fluorochromes in a nick translation reaction, denatured, and hybridized to pretreated slides. Slides were then incubated, washed, and mounted with DAPI in an antifade solution. The genomic location of each BAC set was verified by hybridizing them to normal metaphase chromosomes. Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). A positive score was interpreted when at least 20% of the nuclei showed amplification. Nuclei with incomplete set of signals were omitted from the score. In addition all cases were tested for NCOA2 and NCOA1 gene rearrangements as previously described (Mosquera et al., 2013).
Table 1.
List of BAC Clones Used for Fluorescence-in-situ-hybridization.
| BAC clones | Cytoband | Genes | GP-starting | GP-ending |
|---|---|---|---|---|
| RP11-936I7 | 12q13.3 - 12q14.1 | T-DDIT3/M-CDK4 | 58010762 | 58186776 |
| RP11-1143G9 | 12q15 | T-CPSF6/MDM2 | 69625618 | 69775056 |
RESULTS
Clinicopathologic Features
The clinicopathologic features are summarized in Table 2. There were 5 children (all females), ranging in age from 3–15 years and 11 adults (8 males, 3 females) with an age range of 21–76 years. Histologically, the 16 cases were classified as 5 sclerosing RMS and 11 spindle cell RMS. The 11 spindle cell RMS included 3 pediatric (aged 2, 10 and 13 years) and 8 adult cases (aged 21–62 years). The anatomic location of the spindle cell RMS included: buttock (2), thigh (2), paraspinal region, pelvis, forearm, spermatic cord, abdominal wall, and shoulder. In one case, the patient presented with lung metastasis from a paraspinal primary tumor. Morphologically, the spindle cell RMS were composed of monomorphic, undifferentiated spindle cells arranged in long intersecting fascicles. (Figures 2 and 3) Three cases each showed either a growth pattern reminiscent of leiomyosarcoma or of fibrosarcoma, with streaming cells organized in long fascicles with a distinctive ‘herring-bone’ appearance. Two cases showed geographic areas of necrosis with perivascular preservation of tumor cells, resembling a malignant peripheral nerve sheath tumor-like morphology. Four of the cases showed rare scattered pleomorphic cells between the spindle cells. Tumors typically showed minimal if any sign of rhabdomyoblastic differentiation. Immunohistochemical stains performed at the time of diagnosis showed all tumors were positive for desmin and focally positive for myogenin, confirming the diagnosis of RMS.
Table 2.
Clinicopathologic features, MYOD1 and PIK3CA mutation status and follow-up
| Case # | Age/Sex | Location | Diagnosis | MYOD1 exon1 | Therapy | Follow-up
|
|||
|---|---|---|---|---|---|---|---|---|---|
| LR | DR | duration (mo) | status | ||||||
| 1*# | 34y / F | maxilla | Sclerosing RMS | p.L122R (heterozygous) | Chemo | DR | 41 | NED | |
| 2* | 39y / M | lower leg | Sclerosing RMS | p.L122R (heterozygous) | Chemo | 12 | NED | ||
| 3* | 76y / M | thigh | Sclerosing RMS | p.L122R (homozygous) | Chemo | DR | 17 | AWD | |
| 4*#¥ | 13y / F | chest wall | Sclerosing RMS | p.L122R (homozygous) | Chemo & RT | LR | DR | 21 | DOD |
| 5*#¥ | 14y / F | infra-temporal fossa | Sclerosing RMS | p.L122R (homozygous) | Chemo | LR | DR | 26 | DOD |
| 6* | 10y / F | paraspinal | Spindle cell RMS | p.L122R (homozygous) | Chemo & RT | LR | DR | 35 | DOD |
| 7* | 2y / F | buttock | Spindle cell RMS | p.L122R (heterozygous) | Chemo & RT | DR | 12 | DOD | |
| 8* | 21y / M | pelvis | Spindle cell RMS | p.L122R (homozygous) | Chemo | LR | DR | 30 | AWD |
| 9 | 35y / M | forearm | Spindle cell RMS | p.L122R (heterozygous) | Chemo | 4 | AWD | ||
| 10* | 13y / F | thigh | Spindle cell RMS | WT | Chemo | 32 | NED | ||
| 11* | 30y / M | spermatic cord | Spindle cell RMS | WT | |||||
| 12* | 60y / M | abdominal wall | Spindle cell RMS | WT | |||||
| 13 | 50y / M | buttock | Spindle cell RMS | WT | Chemo & RT | 8 | NED | ||
| 14 | 31y / M | lung | Spindle cell RMS | WT | Chemo & RT | DR | 36 | AWD | |
| 15 | 28y / F | shoulder | Spindle cell RMS | WT | RT | LR | 36 | NED | |
| 16 | 62y / F | thigh | Spindle cell RMS | WT | Chemo & RT | 8 | NED | ||
WT, wild type; RT, radiation therapy; LR, local recurrence; DR, distant recurrence; NED, no evidence of disease; AWD, alive with disease; DOD, died of disease.
Cases used in the prior report by Mosquera et al.
Cases with coexistent PIK3CA helical mutations;
Cases included in Shukla et al.
Figure 2.
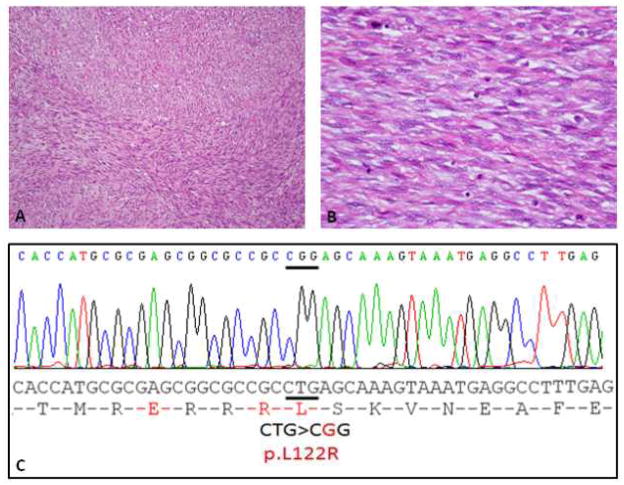
Spindle cell RMS arising in the paraspinal region of a 10 year-old girl (Case 4). (A, B) Tumor is composed of a monomorphic spindle cell proliferation, arranged in tight intersecting fascicles (H&E 100×), which at high power display deceptively bland nuclei, with open chromatin and scant fibrillary cytoplasm (H&E 400×). (C) ABI direct sequencing showing a homozygous MYOD1 p.L122R mutation.
Figure 3.
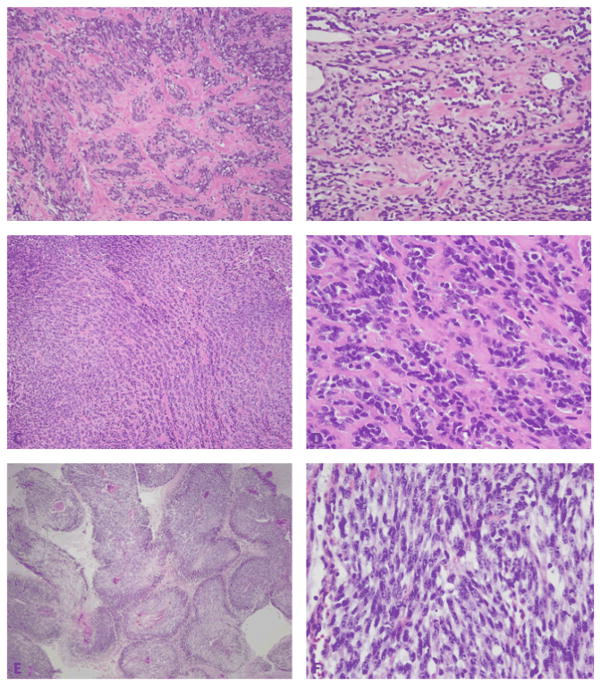
Pathologic spectrum of MYOD1-mutant RMS. (A, B) Sclerosing RMS from the thigh in a 76 year-old male (Case 3, H&E 100×) showing cellular clustering in a densely sclerotic background; (B) which at higher power display a distinctive pseudo-vascular growth pattern (Case 3, H&E, 200×). (C) Sclerosing RMS in a 15 year-old girl (case 5, H&E, 100×) showing small nests of cells in a sclerotic background; (D) which at higher magnification reveal small blue round cells with minimal cytoplasm embedded in a collagenous stroma (case 5, H&E, 400×). (E) Spindle cell RMS from the buttock in a 2 year-old girl (Case 7, H&E,100×) showing perivascular distribution of viable spindle cells with intervening geographic necrosis, reminiscent of an MPNST-like morphology; (F) higher power showing elongated spindle cells with hyperchromatic nuclei arranged in a fascicular pattern (Case 7, H&E 400×).
The 5 sclerosing RMS occurred in 2 children (aged 13 and 15 years) and 3 adults (aged 34, 39 and 76 years). The sclerosing RMS cases occurred in the maxilla, infratemporal fossa, lower leg, thigh and chest wall. Histologically, the sclerosing RMS showed spindle cells with rare, if any, rhabdomyoblasts embedded in a collagenous, densely hyalinized background. A pseudo-vascular pattern was noted focally in most cases. (Figures 1 and 3) In 3 cases the post-chemotherapy resection showed prominent areas of differentiating rhabdomyoblasts consistent with therapy effect.
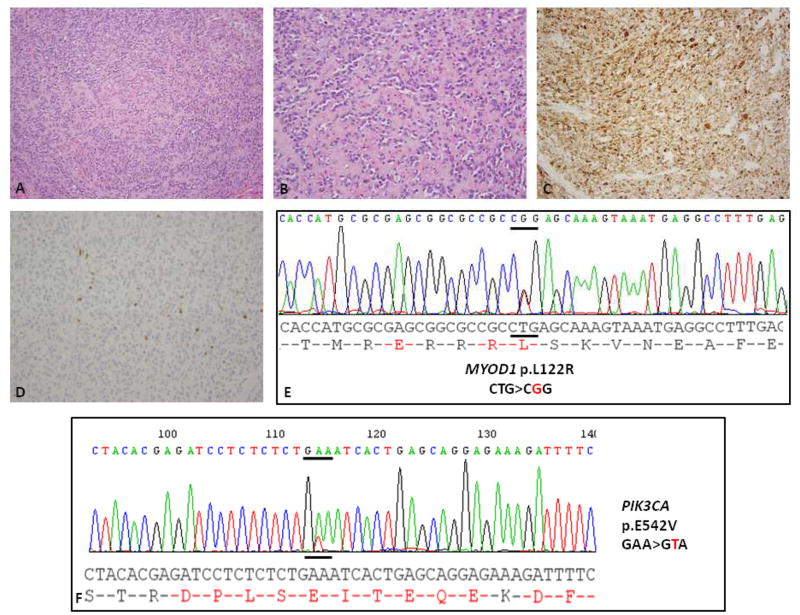
Figure 1.
Sclerosing RMS of the maxillary region in a 34 year-old woman (Case 1). (A, B) Histology showing a cellular neoplasm within a sclerotic background (H&E, 100×); at higher power undifferentiated round to ovoid cells are distributed in a pseudo-vascular pattern (H&E, 200×). (C, D) Immunohistochemical stains show diffuse positivity for desmin (C) and patchy positivity for myogenin (D). ABI direct sequencing showing a (E) heterozygous MYOD1 p.L122R mutation and (F) PIK3CA p.E542V mutation.
The morphologic and immunohistochemical features of all cases were in keeping with prior descriptions of spindle cell and sclerosing RMS in literature. (Cavazzana et al., 1992, Chiles et al., 2004, Folpe et al., 2002, Leuschner et al., 1993, Mentzel and Katenkamp 2000)
Recurrent p.L122R MYOD1 mutations present in both sclerosing and spindle cell RMS
Cases were analyzed by PCR for mutations in MYOD1 gene exon 1. All of the 5 sclerosing RMS cases showed a p.L122R (c. T365G) mutation, with 3 cases showing a homozygous pattern, while remaining 2 had heterozygous mutations. Of the 11 spindle cell RMS cases, 4 cases showed the same p.L122R mutation with 2 cases showing homozygous mutations (Figure 2) and 2 cases showing a heterozygous mutation (Figure 1). The remaining 7 spindle cell RMS (6 adults and one child) did not show MYOD1 mutations. No mutations were identified in the DNA extracted from the normal tissue obtained from one case of spindle cell RMS (Case 8).
All except one pediatric tumor showed MYOD1 mutations: 2/2 sclerosing RMS and 2/3 spindle cell RMS. All of the 3 adult sclerosing RMS were also positive for this genotype. Only two of the 8 adult spindle cell RMS showed MYOD1 mutation.
Concurrent PIK3CA mutations are present in MYOD1-mutant sclerosing RMS
As rare patients with ERMS have been shown to harbor PIK3CA mutations, either in the helical domain (E542K, E545K) or kinase domain (H1047R), along with MYOD1 mutations (Kohsaka et al., 2014) (Shukla et al., 2012), we further investigated all of the 16 cases in the study for mutations at these PIK3CA hot-spots. Three of the 9 MYOD1-mutant RMS cases showed PIK3CA mutations in exon 9 (E542K, E542V and E545K), while no kinase domain (exon 20) mutations were identified. Remarkably, all these 3 cases with MYOD1 and PIK3CA mutations had a sclerosing phenotype, 2 occurring in children (Cases 4 and 5) and one in an adult (Case 1). None of the 7 RMS lacking MYOD1-mutations showed PIK3CA mutations.
No MDM2 gene amplifications were identified in spindle cell/sclerosing RMS
As MDM2 gene amplification has been previously documented in spindle cell RMS (Bouron-Dal Soglio et al., 2009; Kikuchi et al., 2013), FISH analysis for the presence of MDM2 gene abnormalities was performed on 8 cases (3 sclerosing and 5 spindle cell RMS). However, no MDM2 gene copy number abnormalities were identified in any of the cases analyzed, regardless of the MYOD1 mutation status. Three cases were diploid and 5 others showed aneuploidy changes, with 3–4× copies of both MDM2 and the chromosome 12 centromeric control probes. No NCOA2 or NCOA1 gene rearrangements were identified by FISH in any of the cases tested.
Pediatric MYOD1-Mutated RMS Followed a Highly Aggressive Clinical Course
Follow-up information (Table 1) was available in 14 of the 16 cases with a follow-up duration of 4–41 months. One patient developed local recurrence (LR), 4 patients distant recurrence (DR) and 4 developed both LR and DR. The sites of distant recurrences included lung, mediastinum, breast, abdominal soft tissue, spleen and chest wall. Six of the patients had no evidence of disease (NED) at last follow-up ranging from 8–41 months, 3 patients were alive with disease (AWD) at 17, 30 and 36 months, and 4 patients died of the disease (DOD) at 12–35 months following diagnosis. Case 9 was a recent case with short follow-up, patient presently undergoing chemoradiation after an established diagnosis on a core biopsy.
All 4 pediatric MYOD1-mutated RMS (2 spindle cell, 2 sclerosing) patients DOD at 12–35 months following diagnosis. All these 4 patients received intensive, multi-agent chemotherapy and 3 of them received radiation therapy to the primary site of tumor for local control. Cases 4 and 5, treated primarily at our institution, developed isolated local or loco-regional recurrence as their first site of relapse before developing distant metastasis. All except one patient developed local recurrences and all 4 developed distant recurrences to various sites including the lung, mediastinum, breast, abdominal soft tissue, spleen and chest wall.
All 5 MYOD1-mutated adult RMS (3 sclerosing, 1 spindle cell) patients received chemotherapy; one developed LR and 3 developed DR to the lungs. Case 8, treated primarily at our institution with intensive, multi-agent chemotherapy, developed loco-regional recurrence as the first site of relapse and subsequently lung metastases 30 months after presentation. Two of the patients were NED at 12 and 41 months follow-up and 2 were AWD at 17 and 30 months follow-up.
Of the 7 cases of spindle cell RMS lacking MYOD1 mutations, follow-up was available on 5 cases, ranging from 8–36 months. One patient developed LR and one developed distant recurrence in the lungs and bone. Four patients were NED at 8–36 months follow-up and one patient was AWD at 36 months follow-up.
DISCUSSION
The term spindle cell RMS was first introduced by the German-Italian Cooperative Sarcoma Study (Cavazzana et al., 1992) to distinguish it from the more common ERMS group, based on its distinctive clinicopathologic features and favorable outcome. Spindle cell RMS was defined as a bland spindle cell proliferation, with eosinophilic, fibrillary cytoplasm, resembling true smooth muscle differentiation. The cells were typically arranged in intersecting long fascicles, reminiscent of the “herring bone” pattern of adult-type fibrosarcoma. In all tumors, evidence of rhabdomyoblasts with cross-striations were noted, as well as occasional areas of classic ERMS, composed of a mixture of spindle, stellate, and round cells embedded in a myxoid stroma. The findings were then confirmed in a larger study of 800 RMS tumors from the Intergroup RMS Study (IRS)(Leuschner et al., 1993). Patients with spindle cell RMS of non-paratesticular sites had more extensive disease compared with patients with tumors in paratesticular location. (Leuschner et al., 1993) Spindle cell RMS in adults was initially reported by Rubin et al. (Rubin et al., 1998) and subsequently by other authors. (Mentzel and Kuhnen 2006, Nascimento and Fletcher 2005) and described as cellular proliferation of spindle cells with morphology closely resembling fibrosarcoma, leiomyosarcoma or MPNST. In contrast with the favorable behavior reported in the pediatric age group, spindle cell RMS in adults was shown to follow an aggressive course and had a predilection for head and neck location.
Sclerosing RMS in adults was first recognized in small case series by Mentzel and Katenkamp (2000) and Folpe et al. (2002). It was defined as a ‘sclerosing, pseudovascular RMS in adults’, showing an extensive hyalinized matrix, which may mimic osteosarcoma or angiosarcoma. Although the focal alveolar architecture and the primitive round cytologic appearance suggested that it may be closely related to ARMS, the occasional presence of strap cells, the low level of myogenin expression and the absence of FOXO1-related fusions, pointed more toward an ERMS variant. The anatomic location from these combined series showed an equal distribution for head and neck and limbs, with 3 cases each. (Folpe et al., 2002, Mentzel and Katenkamp 2000) Subsequently, sclerosing RMS was investigated in a large cohort of children and adolescents from the Intergroup RMS Study (IRS)/Children Oncology Group.(Chiles et al., 2004) The authors studied 13 sclerosing RMS patients, showing a 1:1 gender ratio and being equally distributed between head and neck and extremities. The clinical behavior of sclerosing RMS in pediatric age group was quite favorable, reminiscent of the outcome reported in the pediatric spindle cell RMS. (Cavazzana et al., 1992) Subsequently, Mentzel et al. suggested that due to overlapping morphologic features, spindle cell RMS and sclerosing RMS may represent a morphological spectrum of a distinct variant of RMS, separate from ERMS and ARMS, although unifying genetic abnormalities are still to be discovered.(Mentzel 2010)
Rare single case reports have outlined the genetic alterations in sclerosing and spindle cell RMS. One case of a pediatric head and neck spindle cell RMS showed an abnormal hypotriploid karyotype with numerous structural rearrangements.(Gil-Benso et al., 1999) Another case of spindle cell RMS in the cheek of an 18-year-old girl, showed a 2q36-37 breakpoint in an unbalanced der(2)t(2;7)(q36-37;q3?), outside the 2q35 PAX3 locus.(Debiec-Rychter et al., 2003)
From the IRS study, 3 of 13 sclerosing RMS had molecular/genetic analysis, showing the presence of a PAX3-FOXO1 fusion in one case, numerous marker chromosomes in the second, and extra-copies of chromosomes 11 and 19 in a third.(Chiles et al., 2004) Two other pediatric sclerosing RMS have been karyotyped showing numerous structural and numerical abnormalities, including double minute chromosomes, in one case (8-year-old girl, suprapubic/intra-abdominal), while a balanced t(5;20)(q31;p13) was identified in the other case (17-year-old girl, lower leg).(Zambrano et al., 2006) A closely located 20p12 break was described as part of a t (2;20)(q35;p12), possibly involving the PAX3 locus, in a 2-year-old girl with a buttock ERMS with an undifferentiated morphology (Ho et al., 2004). In a more recent study of 21 RMS (17 spindle cell, 4 sclerosing), Mosquera et al. (Mosquera et al., 2013) reported recurrent NCOA2 gene rearrangements in 3 congenital/infantile cases of spindle cell RMS with fusions involving SRF and TEAD1 skeletal muscle-related transcription factors. None of the adult spindle cell RMS or four sclerosing RMS included in the study showed NCOA2 rearrangements.
Recently, Szuhai et al.(Szuhai et al., 2014) reported that MYOD1 transactivating mutations are frequent events in adult spindle cell RMS. They identified homozygous mutations at p.L122 of the MYOD1 gene leading to p.L122R change, in 7 of 17 (44%) spindle cell RMS cases. None of the cases examined in their study were sclerosing subtype and no pediatric RMS were included.
MYOD1 gene encodes a nuclear protein that belongs to the basic helix-loop-helix (bHLH) family of transcription factors and the myogenic factors subfamily. The bHLH domains consist of 12–15 aminoacid segments consisting of basic residues and a region consisting of two amphipathic alpha-helixes of 15 aminoacids each. It regulates muscle cell differentiation by inducing cell cycle arrest, a prerequisite for myogenic initiation. The protein is also involved in muscle regeneration. It activates its own transcription, which may stabilize commitment to myogenesis. Mutation of Leu 122 to Arg in MYOD1 has been shown to confer reduced transcriptional activation at MYOD1 sites, together with enhanced binding to MYC sites. (Van Antwerp et al., 1992) Anand et al., in a subsequent study of 19 RMS samples (13 ERMS, 6 ARMS) and 14 RMS cell lines, showed no evidence of mutations in the MYOD1, MYF5, MYF6 and MYOG genes. (Anand et al., 1994). Szuhai et al. also analyzed MYF5, MYF6 and MYOG for mutations at homologous residues as MYOD1 and found no abnormalities. (Szuhai et al., 2014)
In order to answer the question of a shared pathogenesis among these two different histologic phenotypes and age groups, our study investigated a cohort of pediatric and adult spindle cell and sclerosing RMS. We identified homozygous MYOD1 p.L122R mutations in 5 cases and heterozygous p. L122R mutations in 4 tumors. All of the 5 sclerosing RMS, presenting either in pediatric or adult age-group, showed mutations of the MYOD1 gene. Four of the 11 (36%) spindle cell RMS showed MYOD1 mutations, 2 each in children and adults. In contrast to the study by Szuhai et al. (Szuhai et al., 2014) where only homozygous MYOD1 mutations were identified, 4/9 MYOD1 mutant tumors in our study showed heterozygous mutations. Our study confirms that spindle cell and sclerosing RMS share genetic abnormalities thereby providing a strong genetic basis in supporting the classification of these two groups as a single entity. Furthermore, MYOD1 mutations were consistently identified in all RMS displaying sclerosing morphology, regardless of pediatric or adult clinical presentation, in keeping with a homogeneous genetic entity. Although combining our results with Szuhai et al, MYOD1 hot spot mutations occur in both pediatric and adult patients; more than half of adult spindle cell RMS cases remain unclassified genetically, being negative for both NCOA2-related fusions and MYOD1 mutations. These findings suggest that spindle cell RMS is a more heterogeneous group beyond age-related clinical differences.
Shukla et al. (Shukla et al., 2012) reported the presence of PIK3CA mutations in a small subset (5%, 3/60) of ERMS patients, two of these cases being reclassified as sclerosing RMS and included in this series (see Table 2). More recently, the same group identified MYOD1 L122R mutation in 10% (10/104) of ERMS patients (Kohsaka et al., 2014), 3 of them showing concurrent mutations in PIK3CA hot spots (E542K, E545K, H1047R). In contrast, none of the 56 ERMS lacking MYOD1 mutations harbored PIK3CA mutations. Based on this possible association, we investigated our cohort for similar PIK3CA gene mutations. Interestingly, 3 of the 9 MYOD1-mutant spindle cell/sclerosing RMS showed helical domain PIK3CA mutations (E542K, E542V and E545K), all showing a sclerosing phenotype (2 pediatric and one adult). None of tumors lacking MYOD1 mutations showed PIK3CA mutations. This data suggests that a subset of sclerosing RMS show coexisting MYOD1 and PIK3CA mutations, which may be responsible for the highly aggressive clinical behavior.
Isolated case reports of sclerosing RMS have been reported to have amplifications of chromosome 12q13-15.(Bouron-Dal Soglio et al., 2009, Kikuchi et al., 2013) Based on SNP genotyping of one sclerosing rhabdomyosarcoma, Bouron-Dal Soglio et al. reported a highly aneuploid profile and specific amplification of the MDM2/HMGA2 amplicon. In our study, FISH for MDM2, performed on 3 cases of sclerosing RMS and 5 cases of spindle cell RMS, showed no copy numbers alterations in this region, including 3 cases lacking MYOD1-mutations.
Clinical follow-up information shows that local and distant recurrences are frequent events in MYOD1-mutated RMS with 4 cases showing local recurrence, 7 distant recurrences, including 4 cases that showed both local and distant recurrences. The 4 pediatric tumors with MYOD1 mutations particularly showed an aggressive behavior with all of them succumbing to the disease at 12–35 months follow-up after undergoing chemotherapy and radiation therapy. Two cases treated primarily at our institution with intensive multidrug chemotherapy and radiation, developed isolated local or loco-regional recurrence as their first site of relapse and subsequently developing distant metastasis. This finding is in contrast to earlier reports in literature (Leuschner et al., 1993, Chiles et al., 2004) of sclerosing and spindle cell RMS in children having a good prognosis compared to tumors with similar morphology in adults. Although it could be speculated that coexistent MYOD1 and PIK3CA mutations in sclerosing RMS might portend a worse prognosis, two children from this study with MYOD1-mutant spindle cell RMS had a similarly aggressive clinical outcome, without coexistent PIK3CA mutations.
In conclusion, sclerosing and spindle cell RMS show recurrent MYOD1 mutations and should be considered as a single pathologic entity. A subset of sclerosing RMS also show coexisting PIK3CA mutations. Our study also confirms that the pediatric and adult spindle cell/sclerosing RMS show similar genetic alterations, which are distinct from the infantile/congenital RMS with associated NCOA2 related fusions. Although our study suggests that MYOD1-mutated RMS in pediatric population show a highly lethal prognosis regardless of multimodality chemoradiation therapy, additional studies including larger number of cases are required to confirm this finding.
Acknowledgments
Grant support: PO1 CA047179-15A2 (CRA), P50 CA 140146-01 (CRA), Cycle for Survival (LW, CRA).
The authors would like to thank Alyne Manzo for preparation of composite figures and Milagros Soto for editorial assistance.
Footnotes
Disclosure / Conflict of interest: none
References
- Anand G, Shapiro DN, Dickman PS, Prochownik EV. Rhabdomyosarcomas do not contain mutations in the DNA binding domains of myogenic transcription factors. J Clin Invest. 1994;93(1):5–9. doi: 10.1172/JCI116982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, Robson M, Maki R, Brennan MF, Ladanyi M, DeMatteo RP, Besmer P. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9(9):3329–3337. [PubMed] [Google Scholar]
- Bouron-Dal Soglio D, Rougemont AL, Absi R, Barrette S, Montpetit A, Fetni R, Fournet JC. SNP genotyping of a sclerosing rhabdomyosarcoma: reveals highly aneuploid profile and a specific MDM2/HMGA2 amplification. Hum Pathol. 2009;40(9):1347–1352. doi: 10.1016/j.humpath.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Cavazzana AO, Schmidt D, Ninfo V, Harms D, Tollot M, Carli M, Treuner J, Betto R, Salviati G. Spindle cell rhabdomyosarcoma. A prognostically favorable variant of rhabdomyosarcoma. Am J Surg Pathol. 1992;16(3):229–235. doi: 10.1097/00000478-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Chiles MC, Parham DM, Qualman SJ, Teot LA, Bridge JA, Ullrich F, Barr FG, Meyer WH Soft Tissue Sarcoma Committee of the Children’s Oncology G. Sclerosing rhabdomyosarcomas in children and adolescents: a clinicopathologic review of 13 cases from the Intergroup Rhabdomyosarcoma Study Group and Children’s Oncology Group. Pediatr Dev Pathol. 2004;7(6):583–594. doi: 10.1007/s10024-004-5058-x. [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter M, Hagemeijer A, Sciot R. Spindle-cell rhabdomyosarcoma with 2q36 approximately q37 involvement. Cancer Genet Cytogenet. 2003;140(1):62–65. doi: 10.1016/s0165-4608(02)00647-7. [DOI] [PubMed] [Google Scholar]
- Fletcher C, Bridge JA, Hogendoorn PC, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. IARC; Lyon: 2013. [Google Scholar]
- Folpe AL, McKenney JK, Bridge JA, Weiss SW. Sclerosing rhabdomyosarcoma in adults: report of four cases of a hyalinizing, matrix-rich variant of rhabdomyosarcoma that may be confused with osteosarcoma, chondrosarcoma, or angiosarcoma. Am J Surg Pathol. 2002;26(9):1175–1183. doi: 10.1097/00000478-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Gil-Benso R, Carda-Batalla C, Navarro-Fos S, Pellin-Perez A, Llombart-Bosch A. Cytogenetic study of a spindle-cell rhabdomyosarcoma of the parotid gland. Cancer Genet Cytogenet. 1999;109(2):150–153. doi: 10.1016/s0165-4608(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Wettach GR, Ryan CW, Hung A, Hooper JE, Beadling C, Warrick A, Corless CL, Olson SB, Keller C, Mansoor A. MDM2 Amplification and PI3KCA Mutation in a Case of Sclerosing Rhabdomyosarcoma. Sarcoma. 2013;2013:520858. doi: 10.1155/2013/520858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka S, Shukla N, Ameur N, Lim D, Viale A, Socci ND, Qin L-X, Sciot R, Bridge JA, Singer S, Wexler L, Barr FG, Dogan S, Fletcher JA, Ladanyi M. A Recurrent Point Mutation in MYOD1 Defines a Clinically Aggressive Subset of Embryonal Rhabdomyosarcoma. Mod Pathol. 2014 Feb;27 Supplement 2(Abstract 68):20A–21A. doi: 10.1038/ng.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner I, Newton WA, Jr, Schmidt D, Sachs N, Asmar L, Hamoudi A, Harms D, Maurer HM. Spindle cell variants of embryonal rhabdomyosarcoma in the paratesticular region. A report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol. 1993;17(3):221–230. doi: 10.1097/00000478-199303000-00002. [DOI] [PubMed] [Google Scholar]
- Mentzel T. Spindle cell rhabdomyosarcoma in adults: a new entity in the spectrum of malignant mesenchymal tumors of soft tissues. Pathologe. 2010;31(2):91–96. doi: 10.1007/s00292-009-1249-6. [DOI] [PubMed] [Google Scholar]
- Mentzel T, Katenkamp D. Sclerosing, pseudovascular rhabdomyosarcoma in adults. Clinicopathological and immunohistochemical analysis of three cases. Virchows Arch. 2000;436(4):305–311. doi: 10.1007/s004280050451. [DOI] [PubMed] [Google Scholar]
- Mentzel T, Kuhnen C. Spindle cell rhabdomyosarcoma in adults: clinicopathological and immunohistochemical analysis of seven new cases. Virchows Arch. 2006;449(5):554–560. doi: 10.1007/s00428-006-0284-4. [DOI] [PubMed] [Google Scholar]
- Mosquera JM, Sboner A, Zhang L, Kitabayashi N, Chen CL, Sung YS, Wexler LH, LaQuaglia MP, Edelman M, Sreekantaiah C, Rubin MA, Antonescu CR. Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosomes Cancer. 2013;52(6):538–550. doi: 10.1002/gcc.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento AF, Fletcher CD. Spindle cell rhabdomyosarcoma in adults. Am J Surg Pathol. 2005;29(8):1106–1113. [PubMed] [Google Scholar]
- Rubin BP, Hasserjian RP, Singer S, Janecka I, Fletcher JA, Fletcher CD. Spindle cell rhabdomyosarcoma (so-called) in adults: report of two cases with emphasis on differential diagnosis. Am J Surg Pathol. 1998;22(4):459–464. doi: 10.1097/00000478-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Shukla N, Ameur N, Yilmaz I, Nafa K, Lau CY, Marchetti A, Borsu L, Barr FG, Ladanyi M. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin Cancer Res. 2012;18(3):748–757. doi: 10.1158/1078-0432.CCR-11-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuhai K, de Jong D, Leung WY, Fletcher CD, Hogendoorn PC. Transactivating mutation of the MYOD1 gene is a frequent event in adult spindle cell rhabdomyosarcoma. J Pathol. 2014;232(3):300–307. doi: 10.1002/path.4307. [DOI] [PubMed] [Google Scholar]
- Van Antwerp ME, Chen DG, Chang C, Prochownik EV. A point mutation in the MyoD basic domain imparts c-Myc-like properties. Proc Natl Acad Sci U S A. 1992;89(19):9010–9014. doi: 10.1073/pnas.89.19.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Perez-Atayde AR, Ahrens W, Reyes-Mugica M. Pediatric sclerosing rhabdomyosarcoma. Int J Surg Pathol. 2006;14(3):193–199. doi: 10.1177/1066896906290558. [DOI] [PubMed] [Google Scholar]