Abstract
Minimally invasive glaucoma surgeries (MIGS) can improve the conventional, pressure dependent outflow by bypassing or ablating the trabecular meshwork or create alternative drainage routes into the suprachoroidal or subconjunctival space. They have a highly favorable risk profile compared to penetrating surgeries and lower intraocular pressure with variable efficacy that may depend on the extent of outflow segments accessed. Since they are highly standardized procedures that use clear corneal incisions, they can elegantly be combined with cataract and refractive procedures to improve vision in the same session. There is a growing need for surgeons to become proficient in MIGS to address the increasing prevalence of glaucoma and cataracts in a well-informed, aging population. Techniques of visualization and instrumentation in an anatomically highly confined space with semi-transparent tissues are fundamentally different from other anterior segment surgeries and present even experienced surgeons with a substantial learning curve. Here, we provide practical tips and review techniques and outcomes of TM bypass and ablation MIGS.
Keywords: iStent, Hydrus, Trabectome, minimally invasive glaucoma surgery
INTRODUCTION
There are compelling needs for ophthalmic surgeons to become proficient in minimally invasive glaucoma surgeries (MIGS) to address the increasing lifespan and glaucoma prevalence.[1, 2] Fewer patients and physicians are willing to accept the significant risks of traditional glaucoma surgeries that occur even in the most experienced hands: leakage, endophthalmitis, hypotony, hardware erosion, damage to ocular tissues and other complications that are vision threatening occur with an additive probability of 77% of trabeculectomies and 58% of tubes.[3] Since cataracts and refractive errors commonly coexist and cataract removal can further lower intraocular pressure (IOP),[4] normalize anterior segment anatomy,[5] and reduce release of pseudoexfoliative material or pigment,[6] it is useful to address them in the same session. Expedited recovery, absence of postoperative procedures and ability to avoid conjunctival scarring, hypotony,[7] and choroidal effusion are additional benefits.[8, 9]
MIGS require skills fundamentally different from traditional glaucoma surgery and may present even experienced anterior segment surgeons with a significant learning curve. Excellent gonioscopy skills and access to a surgical microscope with outstanding optics, large tilt capabilities, and illumination with a high Kelvin color temperature (e. g. a xenon high intensity discharge light source) are crucial to properly visualize the lacy and often non-pigmented trabecular meshwork (TM). MIGS can be divided into procedures that improve pressure-dependent outflow by (1) bypassing or eliminating the TM, and ones that (2) create alternative drainage routes by shunting aqueous humor into the suprachoroidal or subconjunctival space. Here, we review techniques and results of TM bypass and ablation surgeries and share key steps from our own experience in making them more successful. The sections are ordered by the extent of access to outflow segments, reflecting recent insights from canalography into how discontinuous and septated Schlemm’s canal (SC) is in vivo.[10, 11]
TRABECULAR MESHWORK BYPASS DEVICES
iStent Inject
Concept and Technique
The second generation iStent Inject (Glaukos Corporation, Laguna Hills, CA, USA), is 0.4 mm long, 0.3 mm wide and consists of titanium with heparin coating. Two bullet-shaped stents are preloaded in an injector. This allows for perpendicular implantation into TM and penetration of the tip into Schlemm’s canal (SC) of both stents without withdrawing the inserter, avoiding hypotony and reflux of blood from the collector channels and SC.[12] Trabecular meshwork stents increase outflow by allowing aqueous to directly bypass the trabecular meshwork. Since SC is discontinuous and septated, flow is not circumferential;[10, 11] outflow segments may be accessed over approximately 60° with a single stent although individual anatomy varies.[13] Placement of a second stent may increase the number of drainage segments accessed and potentially lower IOP further.
The preoperative regimen is identical to standard phacoemulsification.[14] Following cataract extraction, intracameral acetylcholine is used for miosis. The chamber is deepened with viscoelastic. Over-inflation must be avoided as it can collapse SC and lead to implantation of the device into the outer wall of SC instead of the TM, while insufficient viscoelastic prevents proper angle visualization. After rotating the microscope the first stent is inserted with a gentle push into nasal TM under direct gonioscopic view and released from the inserter. The second stent is then placed at least two clock hours away from the first one (to increase the chance of accessing a different drainage segment). Viscoelastic and refluxing blood are aspirated and the stent placement is confirmed gonioscopically. The eye is pressurized with saline. A fluoroquinolone is given four times per day for one week, along with prednisolone acetate 1% 4-6 times a day and tapered weekly as for all procedures discussed here.
Results
Outflow facility doubled after inserting one stent and doubled again with two stents in an anterior chamber (AC) perfusion model.[12] Twenty patients in a consecutive series had a mean IOP of 20 mmHg on 1. 3 medications which was reduced to 17 mmHg (14% decrease) on 0. 3 drops 1 year after combined phacoemulsification and 2 stent placements.[15] Side effects included transient IOP spikes > 30 mmHg at postoperative day 1 in 15%. With four patients, although two stents were thought to be inserted, only one could be found. No patient lost vision.
iStent G1
Concept and Technique
The first generation iStent G1 (Glaukos Corporation, Laguna Hills, CA, USA) is three times larger than the iStent Inject. After angle visualization described above, this device is gently leaned against the TM but slightly left towards the opposite side of the chamber to provide a more pointed engagement for easier entry (Figure 2). Using a gentle sweeping motion, the stent is pushed through the TM while the base is kept parallel to the iris.[16] The stent is released and gently tapped with a leftward motion to properly insert it and drive it through the TM or free it from the outer wall.
Figure 2.
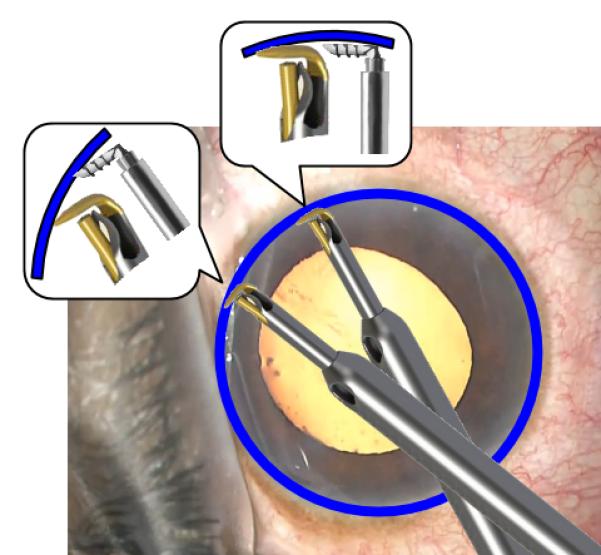
Engaging the TM with the iStent G1 and trabectome. A more pointed angle (left inset) allows for easier entry into Schlemm’s canal (blue) compared to a parallel orientation (right inset).
Results
One stent doubled the outflow facility in perfused donor eyes and insertion of two stents quadrupled it.[17] An in-vivo analysis used fluorophotometry to confirm that the placement of two stents with phacoemulsification increased trabecular outflow facility by 275% over baseline, while phacoemulsification alone increased it by only 46%.[18] A prospective study showed that IOP decreased by 25% at 6 months, while the number of medications decreased from 1. 5 to 1 (n=58).[14] Only 3 cases (5%) were advanced to trabeculectomy. At 12 months, the IOP reduction averaged 18%, and 62% patients had an IOP <= 18 mm Hg, while 26% had <= 15 mm Hg.[14] In a randomized controlled trial (RCT) that compared 36 patients who underwent stand-alone phacoemulsification to a combined group of phacoemulsification/stent placement, there was a a 17% IOP decrease at 15 months in the combined group versus a 9% decrease in the stand-alone phacoemulsification group despite being on 1 more medication.[19] Another RCT (n=240) required an entry IOP of >= 22 mm Hg following medication washout.[20] Per protocol, postoperative IOP was maintained below 21 with topical medications in both groups and mean reduction of IOP was only 1.5 mm Hg in the treatment group versus 1 in the control group. After 24 months, the IOP decrease was 8% in the combined group versus 1% in the phacoemulsification group.[21]
A 5-year study of 13 combined phacoemulsification and stent procedures found a 16% decrease from 19 mm Hg baseline while 42% of patients required no further medication.[22] A study that compared 2 with 3 stents (combined with phacoemulsification) found a 20% pressure reduction in both groups after 1 year.[23] However, only 46% of the patients with 2 stents were off of medication at 1 year versus 72% with 3 stents. A study of stand-alone stent implantation (n=10) found a 27% IOP decrease after one year and mean reduction of medications from 2. 9 to 1. 8.[24]
Complications unique to the iStent include obstruction by fibrin or peripheral anterior synechiae (4% of 111 patients[20]) and stent malposition (14%). Stent obstruction has been managed with observation or argon laser. Four of 111 patients required surgical stent repositioning or replacement. Although increased IOP following stent placement has been reported, it occurred more frequently in the control group with cataract surgery alone.[21] No patient lost more than one line of Snellen visual acuity. There are no reports of serious complications such as choroidal effusion, persistent hypotony, bleb formation, or endophthalmitis.
Hydrus: Ab Interno SC Scaffold
Concept and Technique
The Hydrus microstent scaffold (Ivantis Inc., Irvine, CA), is composed of nitinol, a nickel-titanium alloy with a 1 mm terminal lumen in the AC. This device dilates SC to a diameter of 241 microns, reducing flow resistance and bypassing TM and extends the range of circumferential flow by disrupting smaller septations.[25] There is a 15 mm long version and an 8 mm, flatter version.[26] The 15 mm stent dilates about 5 clock hours of SC and doubled the outflow facility; both devices increase outflow facility to a similar extent.[26, 27]
The Hydrus is preloaded in an injector with a bent tip for gonioscopic insertion into the nasal SC. The hydrus is advanced to engage the TM and advanced into the SC. Blood can be seen refluxing from the tip in the AC after implantation.
Results
Preliminary data on 28 patients showed that after 6 months, combined phacoemulsification and stent insertion resulted in approximately a 15% decrease in IOP from a baseline of 18 mmHg, while medications decreased from 2.4 to 0.1.[28] Another preliminary study found an approximately 35% decrease in IOP from 25 mmHg (measured after medication washout) at 12 months.[29] The two most common complications were transient hyphema in 15% and Peripheral Anterior Synechiae (PAS) formation in 10% of patients.
TRABECULAR MESHWORK ABLATION DEVICES
Laser Assisted Endoscopic Trabeculostomy and Goniopuncture
Concept and Technique
Laser assisted endoscopic trabeculostomy removes TM over a circular area and was first demonstrated with a ruby laser,[30] followed by argon,[31] neodymium-doped:YAG,[32-34] erbium:YAG[35, 36] and excimer laser.[37] Dietlein et al recognized that sufficiently large ablation could only be achieved with end-firing endo-probes with 200 to 320 micron diameter and 4 to 6 mJ energy.[38] Using an erbium:YAG laser (Sklerotom 2.9, Endognost system; Schwind) at a wavelength of 2.94 microns, perforations are 300 microns.[39] A study on cadaver eyes confirmed that there was little thermal damage and the outflow more than doubled[40] after 18 pulses of 16 mJ to the nasal TM. Endoscopic excimer laser trabeculostomy (ELT, AIDA, TUI-Laser, Munich, Germany)[41, 42] involves a 308 nm wavelength ablation over a 500 micron area.[43] The instrument tip is inserted across the anterior chamber and guided gonioscopically to the TM. Successful application causes TM blanching and bubbles. As with other angle surgeries, successful penetration into SC produces blood reflux when IOP is below episcleral venous pressure.
Results
A prospective study (n=59) using the erbium:YAG laser demonstrated a 30% decrease in IOP from a baseline of 23 mm Hg on 1 less medication at 1 year.[44] 71% of patients achieved a 20% decrease in IOP. Fibrin reaction necessitating surgery occurred in 5%. When retrospectively compared to a group of 17 patients who underwent combined phacoemulsification with trabeculectomy ab externo,[45] the laser group demonstrated a 34% IOP decrease versus 29% in the trabeculectomy group at 3 years, although the trabeculectomy group was on significantly fewer medications.
Similarly, after 8 excimer applications resulted in a 32% IOP decrease from a baseline of 25 mm Hg at 2 years with 1.5 fewer medications (n=21). A larger study (n=75) showed a 30% decrease from a baseline of 24 mm Hg after 1 year, although the number of medications did not decrease.[41] Only 46% of patients maintained a 20% IOP decrease to less than 21 mm Hg. When ELT was compared to 180° SLT in an RCT,[42] treatments lowered the IOP by 30% and 21%, respectively, with a similar survival time. There was an IOP spike on postoperative day 1 in 20% of the ELT group versus 13% in the SLT group. No vision-threatening complications were reported. A recent one-year-study found that patients with a baseline IOP <= 21 (mean 16.5 mmHg) showed a mean IOP decrease of 12% with 1.1 less medications compared to a decrease of 37% on 0.6 less medications in patients with a baseline IOP above 21 (mean 25.8 mmHg).[46] Long-term failure is typically caused by migration of immediately adjacent[47] or remote cell types.[48] A concern is that light scatter with a wavelength of 308 nm can be mutagenic.[49]
Trabeculectomy Ab Interno with the Trabectome
Concept and Technique
Ab interno trabeculectomy with the trabectome (AIT, Neomedix Corp., Tustin, CA) is a plasma surgery ablation technique that uses a bipolar 550 kHz electrode tip to remove the primary resistance to outflow, the TM.[50, 51] Because the mechanism is not cautery but ionization and disintegration, heat dissipation is highly confined: histology showed no thermal damage to adjacent tissues or outer wall although margins of the immediate ablation area did occasionally show coagulation effects.[52] TM can be ablated over 180°, providing access to many more drainage segments compared with bypass stents, increasing chance of success and lower IOPs, thereby broadening indication criteria to advanced glaucomas, acute and chronic angle closure, or inactive neovascular glaucoma. Between 5-6 collector orifices may be exposed per millimeter of Schlemm’s canal (Figure 2), which may have lower flow speeds and a lower risk to aspirate iris compared to the single intake of stents.
Through a paracentesis, the eye is well pressurized with intracameral lidocaine, and a 1. 6 mm iris-parallel, uni-planar main incision is created 2 mm anterior to the limbus to prevent iris prolapse. The inner third is flared to improve mobility, and eliminate striae from torque. Hypotony is induced to allow reflux of blood into SC highlighting the intended ablation site. The irrigation bottle is raised high on continuous irrigation. No viscoelastic is used to avoid optical interfaces and trapping of ablation bubbles. After insertion of the trabectome, the TM is engaged (Figure 2) with the tip at a 45° upward angle at the level of the scleral spur (Figure 4) and SC is entered with an upward, then left movement. The footplate of the trabectome tip appears obscured by the lacy TM when properly inserted. The TM should feed into the space between the footplate and electrode tips and not push up against the leading, golden electrode. Any outward push against the outer wall must be strictly avoided not to damage collector intakes. Excessive energy above 1 mW can cause coagulation necrosis during ablation, which is seen as blackening along the ablation lips and may incite wound healing with progressive failure. The first 60° are ablated continuously and should never be forceful. In a successful ablation, the outer wall of SC will appear white. During the remaining 30°, the gonioscopy lens is rotated into the direction of ablation, the wrist increasingly supinated and range of view increased by floating the heel of the goniolens (Figure 5). The tip is then disengaged, rotated 180° pointing downward by rolling it between the thumb, index and middle finger and progressively pronating the wrist. The TM is engaged at the original starting point and ablation proceeds into the opposite direction. Removal of the trabectome from the eye midway results in hyphema and so should be avoided. At conclusion, a small amount of viscoelastic can be retained to protect against hyphema from hypotony.[53-55] In addition to the postoperative drops described above, 1% pilocarpine is used four times a day for one month and three times per day for another month to keep the iris flat and away from the angle.[56] Since most flow occurs where the TM was removed[57] a significant pressure lowering effect from pilocarpine-mediated tension on the scleral spur and TM is not seen.[58]
Figure 4.

Atraumatic entry into Schlemm’s canal can be most easily achieved by angling the tip 45° upward, directly anterior to the scleral spur, to avoid collapse of the canal during engagement. The tip is then moved up, in and along Schlemm’s canal (dotted line).
Figure 5.
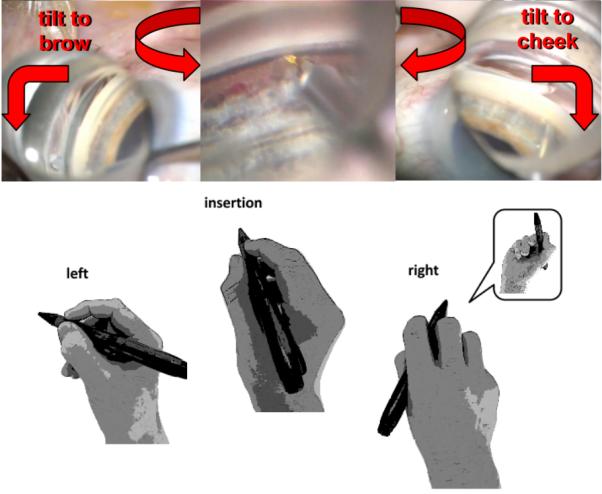
Gonioscopic view and hand position in angle surgery (trabectome shown). After engagement and SC entry at the nasal TM slightly off to the left, ablation is continued for 90° while the lens is rotated in the same direction and the eye (right eye shown) is tilted towards the brow. The same is repeated for the opposite direction. Gonioscopic prism power is increased by lifting the lens off the cornea to float in saline at the final clock hours.
Results
Over nearly one decade, more than 50,000 AIT cases with adult and infantile glaucoma have been documented. More than 5000 were recorded in detail and followed long-term via a repository that includes the submitted first cases of new surgeons and is available to researchers.[59] In the largest study of 1878 cases, some patients who had been followed for 6 years are still maintaining a 38% IOP reduction.[60] In a retrospective study (n=1127) with 5 years follow-up, the definition of failure was similar to that of the Tube versus Trabeculectomy study,[61] with failure being defined as any one of: final IOP above 21 mm Hg, less than a 20% decrease from baseline IOP, or further surgery.[62] Using this definition, the failure rate after 4 years was approximately 53%. Risk factors for failure are lower baseline IOP[63] and younger age.[64]
We analyzed our own outcomes of 192 consecutive combined AIT and cataract surgeries[65] in a retrospective study approved by the institutional review board. The 180° ablation technique above was applied to all patients consisting of 65% primary open-angle, 10% low pressure, 7% pseudoexfoliation and 4% angle closure glaucoma. The IOP decreased from 20.1 ± 8.2 mmHg to 14.5 ±4.5 mmHg (28% decrease, p<0. 05). Of the patients who had 2 years of follow-up, the IOP decrease was maintained at a 23% decrease (p<0.05), although the number of topical medications had then increased by an average of 0.5. After 1 year, 81% of patients had an IOP <= 18, 52% of patients had an IOP <=15, and 27% had an IOP <= 12 (p<0. 05). Best corrected mean visual acuity improved from 20/50 at baseline to 20/32 (p<0. 05). One patient needed further surgery (aqueous shunt) and 3 were managed with additional laser procedures (2 selective laser trabeculoplasties and 1 cyclophotocoagulation).
A common postoperative observation is reflux of blood from the collector channels indicating free communication with the drainage system[64] but only one case needing a surgical intervention for hyphema has been reported.[66] Peripheral anterior synechiae may occur in 24%[67] and can be lysed with photocoagulation or with the Nd:YAG laser.[56] Transient postoperative IOP elevations of more than 10 mmHg occur in 4[68] – 10%[69] of patients consistent with other MIGS. Other complications reported in less than 1% include cyclodialysis cleft,[64] transient hypotony, lens injury, aqueous misdirection,[60] and choroidal hemorrhage.[60]
SURGICAL COMPARISONS
There has been no published study directly comparing these MIGS to each other and the studies discussed have different patient populations and indications for surgery. From the available data, laser trabeculostomy was reported to lower the IOP by 30%,[41, 42] compared to the single published paper on the second generation iStent with a 14% IOP decrease with 2 stents.[15] The overall mean reported IOP reduction for the first generation iStent was near 20%[14, 22, 70] and 25% for AIT with phacoemulsification[69, 71] and 35% without phacoemulsification.[59, 62, 69] the mechanism of MIGS procedures discussed here cannot be expected to produce IOP reductions that can be expressed as a simple percentage of preoperative IOP as may be the case with topical medications. Rather, postoperative IOP will be determined by outflow resistance that is downstream of the trabecular meshwork and independent of preoperative IOP. As a result, a patient with high preoperative IOP will typically have a postoperative pressure similar to someone with relatively low preoperative IOP. Increasing the extent of TM bypass (Hydrus) or removal (Trabectome) to access more outflow segments,[13] may improve surgical success rates and possibly also reduce IOP more effectively while the reverse is seen for the incidence of transient, postoperative hyphema. All of these procedures are highly standardized and faster than traditional glaucoma surgery.
CONCLUSIONS
With an improved risk profile, MIGS offer the ability to intervene earlier, to lower IOP efficiently and improve vision function in the same session, thereby reducing noncompliance and costs.[72] Procedures are highly standardized and faster than traditional glaucoma surgery. It is likely that the amount of IOP reduction is related to the extent of access to outflow segments while the reverse has been reported for the amount of transient, postoperative hyphema. To prevent stents from blocking (Figure 6) or missing prominent collector channels and drainage segments, it would be desirable to assess the intended location by canalography.[11] Procedures discussed here require a sufficiently working conventional outflow system downstream of the TM. Still preclinical MIGS may be able to use alternative outflow pathways that could be used to bypass it if MIGS described here fail.[73, 74] Key steps in all MIGS are properly identifying SC, avoiding undue outward pressure and confirming proper placement of devices or ablation instruments. Since techniques and visualization are considerably different from traditional glaucoma and cataract surgery, surgeons beginning MIGS should be prepared for a significant learning curve.
Figure 6.
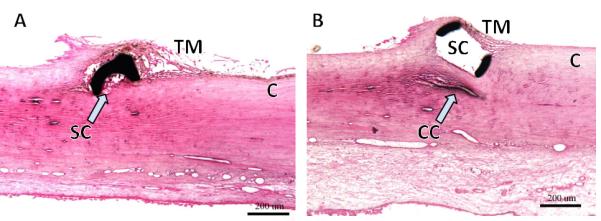
Challenges encountered by microstents. (A) Section through microstent in Schlemm's canal (SC) shows that the lumen can become partially occluded. B) Scaffold device that distends SC effectively but may compress adjacent collector channels (CC; courtesy of Ivantis Inc., Irvine, CA).
Figure 1.
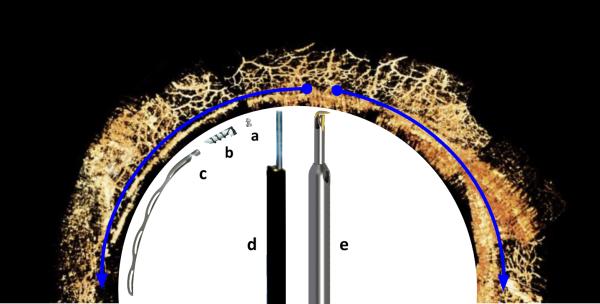
Procedures enhancing conventional outflow by degree of angle access. Left half: trabecular meshwork bypass stents (counter clockwise): a) iStent Inject, b) iStent G1 and c) Hydrus. Right half: trabecular meshwork ablation devices (clockwise): d) laser assisted endoscopic goniopuncture with single, circular entry and e) trabectome with plasma ablation tip that can remove TM up to 180° (blue half-circle arrows). The naturally segmented and discontinuous Schlemm’s canal limits the extent of access to drainage segments that can be achieved with bypass stents (Background canalography image courtesy of The Glaucoma Imaging Group, UPMC Eye Center, University of Pittsburgh School of Medicine).
Figure 3.
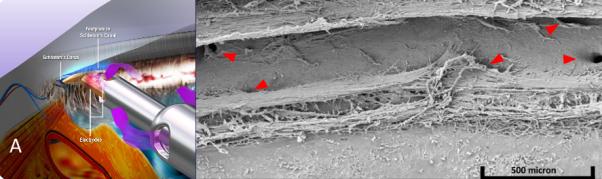
Trabectome. A) Schematic of trabectome ablating TM, opening a view to the white inner wall of Schlemm’s canal (courtesy of Neomedix Corp. , Tustin, CA) and B) scanning electron microscopy of Schlemm’s canal following successful TM ablation with a collector channel seen clearly at far right (courtesy of Douglas H. Johnson for Neomedix).
Acknowledgments
FUNDING STATEMENT
This work was supported in part by the National Institute of Health grant number R01EY013178 and K08EY022737 and an unrestricted grant from Research to Prevent Blindness.
Footnotes
COMPETING INTERESTS
Dr. Kevin Kaplowitz has no competing interests.
Dr. Joel Schuman has no competing interests.
Dr. Nils Loewen is a trabectome wet lab trainer for Neomedix Inc.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–7. doi: 10.1136/bjo.2005.081224. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116(5):653–8. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 3.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–14. doi: 10.1016/j.ajo.2011.10.024. e1 doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shingleton BJ, Laul A, Nagao K, et al. Effect of phacoemulsification on intraocular pressure in eyes with pseudoexfoliation: single-surgeon series. J Cataract Refract Surg. 2008;34(11):1834–41. doi: 10.1016/j.jcrs.2008.07.025. doi: 10.1016/j.jcrs.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol. 2011;129(10):1283–90. doi: 10.1001/archophthalmol.2011.272. doi: 10.1001/archophthalmol.2011.272. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi PC, Dietlein TS, Krieglstein GK. Bimanual trabecular aspiration in pseudoexfoliation glaucoma: an alternative in nonfiltering glaucoma surgery. Ophthalmology. 1998;105(5):886–94. doi: 10.1016/S0161-6420(98)95032-1. doi: 10.1016/S0161-6420(98)95032-1. [DOI] [PubMed] [Google Scholar]
- 7.Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003;110(6):1185–91. doi: 10.1016/S0161-6420(03)00227-6. doi: 10.1016/S0161-6420(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 8.Jalkh AE, Avila MP, Trempe CL, et al. Diffuse choroidal thickening detected by ultrasonography in various ocular disorders. Retina. 1983;3(4):277–83. doi: 10.1097/00006982-198300340-00009. [DOI] [PubMed] [Google Scholar]
- 9.Speaker MG, Guerriero PN, Met JA, et al. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98(2):202–9. doi: 10.1016/s0161-6420(91)32316-9. discussion 10. [DOI] [PubMed] [Google Scholar]
- 10.Kagemann L, Wollstein G, Ishikawa H, et al. Identification and assessment of Schlemm's canal by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(8):4054–9. doi: 10.1167/iovs.09-4559. doi: 10.1167/iovs.09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis AW, Kagemann L, Wollstein G, et al. Morphometric analysis of aqueous humor outflow structures with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(9):5198–207. doi: 10.1167/iovs.11-9229. doi: 10.1167/iovs.11-9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahler CK, Hann CR, Fjield T, et al. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthalmol. 2012;153(6):1206–13. doi: 10.1016/j.ajo.2011.12.017. doi: 10.1016/j.ajo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Rosenquist R, Epstein D, Melamed S, et al. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989;8(12):1233–40. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel D, Garcia-Feijoo J, Garcia-Sanchez J, et al. Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther. 2008;25(5):453–64. doi: 10.1007/s12325-008-0062-6. doi: 10.1007/s12325-008-0062-6. [DOI] [PubMed] [Google Scholar]
- 15.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. Br J Ophthalmol. 2013 doi: 10.1136/bjophthalmol-2012-302394. doi: 10.1136/bjophthalmol-2012-302394. [DOI] [PubMed] [Google Scholar]
- 16.Nichamin LD. Glaukos iStent Trabecular Micro-Bypass. Middle East Afr J Ophthalmol. 2009;16(3):138–40. doi: 10.4103/0974-9233.56227. doi: 10.4103/0974-9233.56227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahler CK, Smedley GT, Zhou J, et al. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthalmol. 2004;138(6):988–94. doi: 10.1016/j.ajo.2004.07.035. doi: 10.1016/j.ajo.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Barrientos Y, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51(7):3327–32. doi: 10.1167/iovs.09-3972. doi: 10.1167/iovs.09-3972. [DOI] [PubMed] [Google Scholar]
- 19.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407–12. doi: 10.1016/j.jcrs.2009.10.031. doi: 10.1016/j.jcrs.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–67. doi: 10.1016/j.ophtha.2010.07.007. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Craven ER, Katz LJ, Wells JM, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–45. doi: 10.1016/j.jcrs.2012.03.025. doi: 10.1016/j.jcrs.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96(5):645–9. doi: 10.1136/bjophthalmol-2011-300218. doi: 10.1136/bjophthalmol-2011-300218. [DOI] [PubMed] [Google Scholar]
- 23.Belovay GW, Naqi A, Chan BJ, et al. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–7. doi: 10.1016/j.jcrs.2012.07.017. doi: 10.1016/j.jcrs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Buchacra O, Duch S, Milla E, et al. One-year analysis of the iStent trabecular microbypass in secondary glaucoma. Clin Ophthalmol. 2011;5:321–6. doi: 10.2147/OPTH.S15025. doi: 10.2147/OPTH.S15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hann CR, Bentley MD, Vercnocke A, et al. Imaging the aqueous humor outflow pathway in human eyes by three-dimensional micro-computed tomography (3D micro-CT) Exp Eye Res. 2011;92(2):104–11. doi: 10.1016/j.exer.2010.12.010. doi: 10.1016/j.exer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulati V, Fan S, Hays CL, et al. A novel 8-mm Schlemm's canal scaffold reduces outflow resistance in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2013;54(3):1698–704. doi: 10.1167/iovs.12-11373. doi: 10.1167/iovs.12-11373. [DOI] [PubMed] [Google Scholar]
- 27.Camras LJ, Yuan F, Fan S, et al. A novel Schlemm's Canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2012;53(10):6115–21. doi: 10.1167/iovs.12-9570. doi: 10.1167/iovs.12-9570. [DOI] [PubMed] [Google Scholar]
- 28.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi: 10.1097/ICU.0b013e32834ff1e7. doi: 10.1097/ICU.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 29.One-Year Results of an Intracanalicular Microstent Combined With Cataract Surgery for IOP Reduction; Proceedings of the American Academy of Ophthalmology; Chicago. American Academy of Ophthalmology; Nov 10-13, 2012. 2012. [Google Scholar]
- 30.Krasnov MM. [Laser puncture of the anterior chamber angle in glaucoma (a preliminary report)] Vestn Oftalmol. 1972;3:27–31. [PubMed] [Google Scholar]
- 31.Ticho U, Cadet JC, Mahler J, et al. Argon laser trabeculotomies in primates: evaluation by histological and perfusion studies. Invest Ophthalmol Vis Sci. 1978;17(7):667–74. [PubMed] [Google Scholar]
- 32.Epstein DL, Melamed S, Puliafto CA, et al. Neodymium: YAG laser trabeculopuncture in open-angle glaucoma. Ophthalmology. 1985;92(7):931–7. doi: 10.1016/s0161-6420(85)33932-5. [DOI] [PubMed] [Google Scholar]
- 33.Melamed S, Pei J, Puliafito CA, et al. Q-switched neodymium-YAG laser trabeculopuncture in monkeys. Arch Ophthalmol. 1985;103(1):129–33. doi: 10.1001/archopht.1985.01050010135037. [DOI] [PubMed] [Google Scholar]
- 34.Dietlein TS, Jacobi PC, Krieglstein GK. Ab interno infrared laser trabecular ablation: preliminary short-term results in patients with open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1997;235(6):349–53. doi: 10.1007/BF00937282. [DOI] [PubMed] [Google Scholar]
- 35.Hill RA, Baerveldt G, Ozler SA, et al. Laser trabecular ablation (LTA) Lasers Surg Med. 1991;11(4):341–6. doi: 10.1002/lsm.1900110405. [DOI] [PubMed] [Google Scholar]
- 36.Hill RA, Stern D, Lesiecki ML, et al. Effects of pulse width on erbium:YAG laser photothermal trabecular ablation (LTA) Lasers Surg Med. 1993;13(4):440–6. doi: 10.1002/lsm.1900130408. [DOI] [PubMed] [Google Scholar]
- 37.Vogel M, Lauritzen K, Quentin CD. [Targetted ablation of the trabecular meshwork with excimer laser in primary open-angle glaucoma] Ophthalmologe. 1996;93(5):565–8. doi: 10.1007/s003470050040. [DOI] [PubMed] [Google Scholar]
- 38.Dietlein TS, Jacobi PC, Krieglstein GK. Erbium: YAG laser ablation on human trabecular meshwork by contract delivery endoprobes. Ophthalmic Surg Lasers. 1996;27(11):939–45. [PubMed] [Google Scholar]
- 39.Feltgen N, Mueller H, Ott B, et al. Combined endoscopic erbium:YAG laser goniopuncture and cataract surgery. J Cataract Refract Surg. 2003;29(11):2155–62. doi: 10.1016/s0886-3350(03)00241-4. [DOI] [PubMed] [Google Scholar]
- 40.McHam ML, Eisenberg DL, Schuman JS, et al. Erbium: YAG laser trabecular ablation with a sapphire optical fiber. Exp Eye Res. 1997;65(2):151–5. doi: 10.1006/exer.1996.0274. doi: 10.1006/exer.1996.0274. [DOI] [PubMed] [Google Scholar]
- 41.Wilmsmeyer S, Philippin H, Funk J. Excimer laser trabeculotomy: a new, minimally invasive procedure for patients with glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244(6):670–6. doi: 10.1007/s00417-005-0136-y. doi: 10.1007/s00417-005-0136-y. [DOI] [PubMed] [Google Scholar]
- 42.Babighian S, Caretti L, Tavolato M, et al. Excimer laser trabeculotomy vs 180 degrees selective laser trabeculoplasty in primary open-angle glaucoma. A 2-year randomized, controlled trial. Eye (Lond) 2010;24(4):632–8. doi: 10.1038/eye.2009.172. doi: 10.1038/eye.2009.172. [DOI] [PubMed] [Google Scholar]
- 43.Babighian S, Rapizzi E, Galan A. Efficacy and safety of ab interno excimer laser trabeculotomy in primary open-angle glaucoma: two years of follow-up. Ophthalmologica. 2006;220(5):285–90. doi: 10.1159/000094616. doi: 10.1159/000094616. [DOI] [PubMed] [Google Scholar]
- 44.Feltgen N, Mueller H, Ott B, et al. Endoscopically controlled erbium:YAG goniopuncture versus trabeculectomy: effect on intraocular pressure in combination with cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2003;241(2):94–100. doi: 10.1007/s00417-002-0557-9. doi: 10.1007/s00417-002-0557-9. [DOI] [PubMed] [Google Scholar]
- 45.Philippin H, Wilmsmeyer S, Feltgen N, et al. Combined cataract and glaucoma surgery: endoscope-controlled erbium:YAG-laser goniotomy versus trabeculectomy. Graefes Arch Clin Exp Ophthalmol. 2005;243(7):684–8. doi: 10.1007/s00417-004-1004-x. doi: 10.1007/s00417-004-1004-x. [DOI] [PubMed] [Google Scholar]
- 46.Toteberg-Harms M, Hanson JV, Funk J. Cataract surgery combined with excimer laser trabeculotomy to lower intraocular pressure: effectiveness dependent on preoperative IOP. BMC Ophthalmol. 2013;13:24. doi: 10.1186/1471-2415-13-24. doi: 10.1186/1471-2415-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dietlein TS, Jacobi PC, Schroder R, et al. Experimental erbium: YAG laser photoablation of trabecular meshwork in rabbits: an in-vivo study. Exp Eye Res. 1997;64(5):701–6. doi: 10.1006/exer.1996.0254. doi: 10.1006/exer.1996.0254. [DOI] [PubMed] [Google Scholar]
- 48.van der Zypen E, Fankhauser F, England C, et al. Morphology of the trabecular meshwork within monkey (Macaca speciosa) eyes after irradiation with the free-running Nd:YAG laser. Ophthalmology. 1987;94(2):171–9. doi: 10.1016/s0161-6420(87)33480-3. [DOI] [PubMed] [Google Scholar]
- 49.Kochevar IE. Cytotoxicity and mutagenicity of excimer laser radiation. Lasers Surg Med. 1989;9(5):440–5. doi: 10.1002/lsm.1900090503. [DOI] [PubMed] [Google Scholar]
- 50.Grant WM. Facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1955;54(2):245–8. doi: 10.1001/archopht.1955.00930020251012. [DOI] [PubMed] [Google Scholar]
- 51.Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54(6):879–83. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- 52.Francis BA, See RF, Rao NA, et al. Ab interno trabeculectomy: development of a novel device (Trabectome) and surgery for open-angle glaucoma. J Glaucoma. 2006;15(1):68–73. doi: 10.1097/01.ijg.0000196653.77836.af. [DOI] [PubMed] [Google Scholar]
- 53.McDonnell PJ, Taban M, Sarayba M, et al. Dynamic morphology of clear corneal cataract incisions. Ophthalmology. 2003;110(12):2342–8. doi: 10.1016/S0161-6420(03)00733-4. doi: 10.1016/S0161-6420(03)00733-4. [DOI] [PubMed] [Google Scholar]
- 54.Chawdhary S, Anand A. Early post-phacoemulsification hypotony as a risk factor for intraocular contamination: in vivo model. J Cataract Refract Surg. 2006;32(4):609–13. doi: 10.1016/j.jcrs.2006.01.020. doi: 10.1016/j.jcrs.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Shingleton BJ, Wadhwani RA, O'Donoghue MW, et al. Evaluation of intraocular pressure in the immediate period after phacoemulsification. J Cataract Refract Surg. 2001;27(4):524–7. doi: 10.1016/s0886-3350(00)00641-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Harasymowycz P. Goniopuncture in the Treatment of Short-term Post-Trabectome Intraocular Pressure Elevation: A Retrospective Case Series Study. J Glaucoma. 2012 doi: 10.1097/IJG.0b013e3182595042. doi: 10.1097/IJG.0b013e3182595042. [DOI] [PubMed] [Google Scholar]
- 57.Fellman RL, Grover DS. Episcleral Venous Fluid Wave: Intraoperative Evidence for Patency of the Conventional Outflow System. J Glaucoma. 2012 doi: 10.1097/IJG.0b013e31827a06d8. doi: 10.1097/IJG.0b013e31827a06d8. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman PL, Barany EH. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976;15(10):793–807. [PubMed] [Google Scholar]
- 59.Minckler D, Baerveldt G, Ramirez MA, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc. 2006;104:40–50. [PMC free article] [PubMed] [Google Scholar]
- 60.Vold SD. Ab interno trabeculotomy with the trabectome system: what does the data tell us? Int Ophthalmol Clin. 2011;51(3):65–81. doi: 10.1097/IIO.0b013e31821e5da3. doi: 10.1097/IIO.0b013e31821e5da3. [DOI] [PubMed] [Google Scholar]
- 61.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803. doi: 10.1016/j.ajo.2011.10.026. e2 doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minckler D, Mosaed S, Dustin L, et al. Trabectome (trabeculectomy-internal approach): additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:149–59. discussion 59-60. [PMC free article] [PubMed] [Google Scholar]
- 63.Vold SD, Dustin L. Impact of laser trabeculoplasty on Trabectome(R) outcomes. Ophthalmic Surg Lasers Imaging. 2010;41(4):443–51. doi: 10.3928/15428877-20100525-06. Trabectome Study G. doi: 10.3928/15428877-20100525-06. [DOI] [PubMed] [Google Scholar]
- 64.Jea SY, Francis BA, Vakili G, et al. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology. 2012;119(1):36–42. doi: 10.1016/j.ophtha.2011.06.046. doi: 10.1016/j.ophtha.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 65.Kaplowitz K, Chen X, Loewen N. Two Year Results for 180 Degree Trabectome Ablation; American Glaucoma Society Annual Meeting; San Francisco, CA. 2013. [Google Scholar]
- 66.Ahuja Y, Malihi M, Sit AJ. Delayed-onset symptomatic hyphema after ab interno trabeculotomy surgery. Am J Ophthalmol. 2012;154(3):476–80. doi: 10.1016/j.ajo.2012.03.027. e2 doi: 10.1016/j.ajo.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 67.Minckler DS, Baerveldt G, Alfaro MR, et al. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112(6):962–7. doi: 10.1016/j.ophtha.2004.12.043. doi: 10.1016/j.ophtha.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 68.Francis BA, Winarko J. Ab interno Schlemm's canal surgery: trabectome and i-stent. Dev Ophthalmol. 2012;50:125–36. doi: 10.1159/000334794. doi: 10.1159/000334794. [DOI] [PubMed] [Google Scholar]
- 69.Ting JL, Damji KF, Stiles MC, et al. Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38(2):315–23. doi: 10.1016/j.jcrs.2011.08.043. doi: 10.1016/j.jcrs.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 70.Vandewalle E, Zeyen T, Stalmans I. The iStent trabecular micro-bypass stent: a case series. Bull Soc Belge Ophtalmol. 2009;(311):23–9. [PubMed] [Google Scholar]
- 71.Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34(7):1096–103. doi: 10.1016/j.jcrs.2008.03.032. doi: 10.1016/j.jcrs.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Iordanous Y, Kent JS, Hutnik CM, et al. Projected Cost Comparison of Trabectome, iStent, and Endoscopic Cyclophotocoagulation Versus Glaucoma Medication in the Ontario Health Insurance Plan. J Glaucoma. 2013 doi: 10.1097/IJG.0b013e31829d9bc7. doi: 10.1097/IJG.0b013e31829d9bc7. [DOI] [PubMed] [Google Scholar]
- 73.Varma R. Devices in Development: AqueSys Implant. Glaucoma Today. 2012;12(November/December):44–45. [Google Scholar]
- 74.Melamed S, Ben Simon GJ, Goldenfeld M, et al. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: a pilot study. Arch Ophthalmol. 2009;127(3):264–9. doi: 10.1001/archophthalmol.2008.611. doi: 10.1001/archophthalmol.2008.611. [DOI] [PubMed] [Google Scholar]


