Abstract
Targeting immune inhibitory receptors has brought excitement, innovation and hope to cancer patients. Our recent work revealed the immunological effects of blocking the CTLA4 and PD-1 immune checkpoints on T cell receptor usage among peripheral blood cells, and further uncovers how the expansion of the T cell repertoire matches the immunotoxicity profile of the therapy.
Keywords: CTLA-4, MK-3475, PBMC, PD-1, TCR, Tremelimumab, sequencing
Immune checkpoint blockade continues to transform cancer therapy and outcomes. These new agents can achieve long-lasting responses and potentially cure selected patients with metastatic cancers. Blockade of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) was the first immunotherapy to show durable clinical responses.1,2 Although response occurred in less than 15% of patients, the new therapy showed promise and produced excitement within the medical community. More recently, Phase I clinical trials with antibodies blocking the programmed cell death-1 (PDCD1, better known as PD-1) receptor has produced responses in 20–40% of patients with melanoma, renal, and non-small cell lung cancer.3,4 Many patients even experience continued regression after discontinuation of treatment.5 This is a significant improvement in response rate and durability of response when compared with other common therapies such as targeted therapies and chemotherapy.
Our group has been engaged in exploring these immune checkpoint blockade mechanisms since the first agents were developed.6,7 In our most recent article, CTLA4 blockade broadens the peripheral T cell receptor repertoire,8 we used high-throughput deep sequencing of the TCR V-β CDR3 region to better characterize the expansion and clonality of the T-cell repertoire.9 Our approach was to study the effects of CTLA4 blockade in peripheral blood mononuclear cells (PBMCs). Previously, we had intended to use this technology to further study the tumoral infiltrating lymphocytes (TILs) from formalin-fixed paraffin-embedded (FFPE) tissue blocks. However, this was technically challenging because whole genome amplification (WGA) was required after microdissection of the tumoral area due to low yield of genomic DNA. Unfortunately, this technique introduced artifacts into our results rendering them unreliable for interpretation. We next switched to peripheral blood to extract genomic DNA from patients with metastatic melanoma to comparatively examine the TCR repertoire at baseline (day 0) and after 30 to 60 d of treatment, including 21 patients treated with tremelimumab. At the same time, we extracted genomic DNA from 4 healthy donors at baseline and after 3 mo to use them as controls for background changes in TCR usage among circulating T cells in the blood.
More recently, we have been able to get data for a new pool of 9 paired (before and after treatment) peripheral blood samples from melanoma patients treated with PD-1 blockade (MK-3475, pembrolizumab). These results were presented at the 2014 annual meeting of the American Association for Cancer Research (AACR), San Diego, CA, USA, and are included in this brief report. The in-frame resulting sequences were analyzed in terms of richness (number of unique productive sequences) and Shannon index (diversity index). For the healthy donors, results showed random changes without significance (P = 0.93 and P = 0.68 for richness and Shannon index, respectively). On the other hand, as shown in Figure 1, we found that patients treated with CTLA4 blockade experienced a significant increase in the number and complexity of TCR variants, attributable to drug treatment (P = 0.001 and P = 0.04 respectively). This increase was not reproduced in the 9 paired samples from patients treated with MK-3475 (P = 0.15 and P = 0.56 respectively; Figure 1). Four clinical responders out of 21 patients treated with CTLA4 blockade exhibited an increase in TCR richness. In the case of PD-1 blockade (MK-3475), responders showed both increase in richness (one out of nine) and decrease in richness (two out of nine) indifferently.
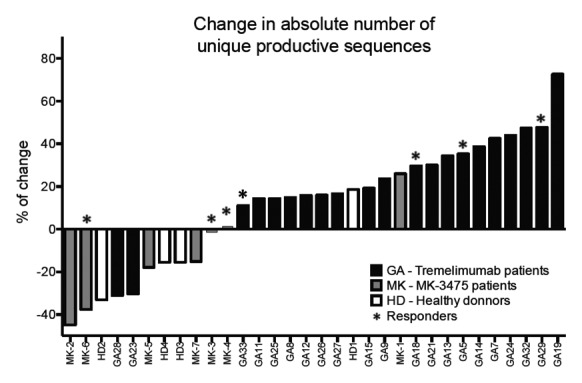
Figure 1. Immunotherapy-specific changes in the absolute number of unique T cell receptor sequences in cancer patient blood lymphocytes. Changes in the T cell receptor (TCR) usage among circulating T cells in the peripheral blood reported between baseline (day 0) and 30 to 60 d following treatment. Data shown are from 21 patients with metastatic melanoma treated with the CTLA-4 blocking antibody tremelimumab (GA, in black), nine with the PD-1 blockade agent MK-3475 (MK, in gray) and four healthy donors (HD, in white).
Our results are suggestive of differing immunological effects of these two agents on circulating T cells. The expansion of TCR usage in samples treated with anti-CTLA4 immunotherapy goes together with the priming encounter that takes place between T cells and antigen presenting cells (APCs) in the lymph node. CTLA4 blockade allows this interaction to occur without coinhibitory signals and can lead to T-cell proliferation and non-specific T-cell activation. For PD-1 blockade the context is different, since this coinhibitory signal occurs between previously primed T cells resident in peripheral tissues, in this case in tumor lesions.10 Targeting this restricted subtype of T cells enriched for tumor antigen specificity, also explains why an immunotoxicity profile is less frequent with PD-1 blockade in comparison to CTLA4 blockade. As we move toward the initiation of combination therapies, and, as new immune checkpoint inhibiting agents are being tested in the clinic, it is important to continue elucidating their mechanism of action in the peripheral blood and intratumoral environments.
References
- 1.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–22. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A, Comin-Anduix B, Chmielowski B, Jalil J, de la Rocha P, McCannel TA, Ochoa MT, Seja E, Villanueva A, Oseguera DK, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–76. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, Bozon VA, Bulanhagui CA, Seja E, Villanueva A, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–32. doi: 10.1158/1078-0432.CCR-13-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–9. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]


