Abstract
Signaling downstream of the B cell antigen receptor (BCR) is tightly regulated to allow for cells to gauge the strength and duration of antigen interactions and respond accordingly. In this study, we asked if metabolism of the second messenger diacylglycerol (DAG) by diacylglycerol kinase enzymes (DGKs) played a role in modulating the magnitude of signaling by this second messenger downstream of the BCR. In the absence of DGKζ, the threshold for BCR signaling through the Ras-ERK MAP kinase pathway was markedly reduced in mature follicular B cells, resulting in exaggerated responses to antigen in vitro and in vivo. Inhibition of DAG signaling by DGKζ was especially important for limiting the number of antibody-secreting cells generated early in response to both T-independent type 2 antigens and T cell-dependent antigens. Furthermore, deficiency in DGKζ closely resembled the effects of increasing antigen affinity for the BCR during the T cell-dependent antibody response, strongly indicating that the magnitude of DAG signaling, likely through the degree of ERK activation, is important for translating the affinity of the BCR for antigen into the amount of antibody produced during early stages of an immune response.
Introduction
Engagement of the B cell antigen receptor (BCR) by specific antigen induces a complex cascade of intracellular signaling events that play critical roles in B cell development, activation, survival, and proliferation (1). Early signaling by the BCR involves the activation of Src and Syk family protein tyrosine kinases, which stimulate a number of downstream signaling events, including activation of phospholipase C-γ2 (PLC-γ2) to generate the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG) (2-4). Whereas IP3 is required for calcium mobilization and activation of the NFAT family of transcription factors, DAG signals through PKCβ and the Ras guanine exchange factor, RasGRP, leading to activation of the NF-κB and Ras-MEK-ERK mitogen-activated protein kinase (MAPK) pathways respectively (5-10). Activation of these signaling pathways downstream of the BCR results in rapid transmission of signals to the nucleus and alterations in gene expression necessary for subsequent B cell functional responses.
The ERK MAPK signaling cascade is critical for a number of aspects of B cell function and fate decisions (11). During early B cell development, ERK signaling is required for proliferative expansion induced by signaling through the pre-BCR, as well as for differentiation of immature transitional B cells to the mature follicular stage in the spleen (12, 13). In mature B cells, pharmacological inhibition of MEK, or genetic deficiency in the key signaling intermediates for Ras activation, RasGRP1 and RasGRP3, severely impairs survival and proliferation in response to BCR stimulation (5, 14). Antigen stimulation of mature B cells in vivo induces antibody production through the rapid formation of extrafollicular plasma cells, as well as a slower germinal center response, which gives rise to plasma cells that secrete higher affinity antibodies. ERK signaling in germinal center B cells is required for terminal differentiation to antibody-secreting plasma cells through induction of the key transcription factor Blimp1 (15), however its role in early formation of plasmablasts has not been examined.
Previous work has shown that B cell maturation from the immature transitional stage to the mature follicular stage in the spleen is accompanied by an attenuation in BCR-induced ERK activation (16), suggesting the possibility that ERK is differentially regulated in a pathway-specific manner during B cell maturation. One possible mechanism of such regulation is by the action of diacylglycerol kinase (DGK) family members, which phosphorylate DAG and convert it to phosphatidic acid, therefore limiting signaling by this second messenger (17). Interesting in this regard, previous studies in T cells found that the degree of ERK activation is controlled at the level of DAG metabolism through the actions of the α and ζ isoforms of DGK (18-21).
Here we report evidence for an important role for DGK-dependent regulation of DAG signaling in mature B cells. We observed that inhibition of DGK enzymatic activity enhanced BCR-mediated activation of ERK selectively in mature follicular B cells, and this correlated with increased mRNA expression of DGKα and DGKζ during B cell maturation in the spleen. Interestingly, while mature follicular B cells from mice deficient in DGKζ exhibited enhanced ERK-MAPK signaling and had a reduced threshold for BCR-induced activation and proliferation, ablation of DGKα showed a lesser effect. In addition, in vivo experiments revealed that DGKζ plays a role in limiting B cell activation during immune responses, and is especially important for limiting the number of antibody-secreting plasma cells generated early in response to both T cell-independent type 2 and T cell-dependent antigen immunization. Strikingly, the effect of enhanced ERK signaling in DGKζ-deficient B cells closely mimics the effect of increasing the affinity of antigen for the BCR, strongly suggesting that the magnitude of ERK signaling in B cells is an important determinant of affinity discrimination during the antibody response.
Results
Activation of diacylglycerol-mediated signaling events is tightly regulated by DGKζ in mature follicular B cells
Previously, it was observed that low amounts of BCR crosslinking are sufficient to induce full activation of ERK in immature transitional B cells, whereas even at high doses of BCR crosslinking, activation of ERK is markedly reduced in the mature follicular B cell population (16). To determine if these differences might be due to differences in negative regulation by DGK enzymes, we examined the effect on BCR-induced activation of ERK1 and ERK2 of the DGK inhibitor, R59949, which is known to block activities of several DGK isoforms (22). Pretreating splenic B cells with this inhibitor resulted in substantially enhanced BCR-induced ERK phosphorylation within the immature T3 and mature follicular B cell populations, as measured by flow cytometry using a phospho-specific antibody against the activation sites of ERK1 and ERK2 (Fig. 1A). In contrast, this inhibitor did not further enhance BCR-induced ERK activation in immature T1 or T2 B cells (Fig. 1A).
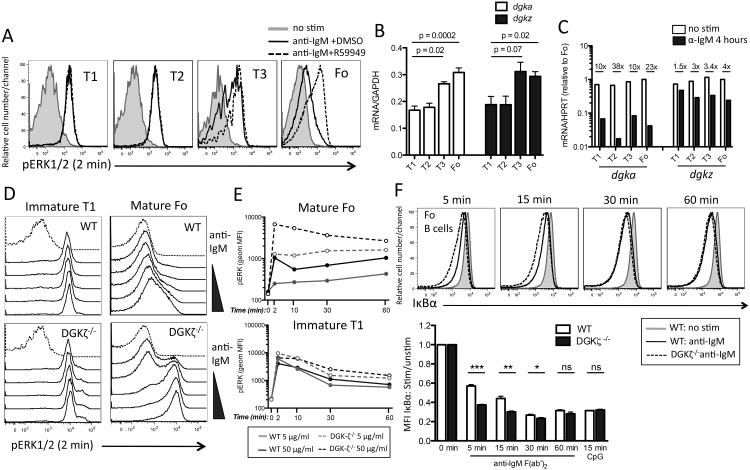
Figure 1. Activation of diacylglycerol-mediated signaling events is tightly regulated by DGKζ in mature follicular B cells.
BCR-induced ERK phosphorylation was measured by flow cytometry in splenic B220+ T1 (CD93+, CD23neg, IgMhi), T2 (CD93+, CD23+, IgMhi), T3 (CD93+, CD23+, IgMlo-int), and follicular (CD93-, CD23+, IgMint-lo) B cells following stimulation with 50 μg/ml anti-IgM in the presence of 20 μM R59949 or DMSO. (B) mRNA transcript expression of DGKα and DGKζ during peripheral B cell maturation was measured by quantitative RT-PCR from sorted WT splenic B cells (T1, T2, T3, and Fo) and normalized to GAPDH. Splenocytes were pooled from n =3 mice prior to cell sorting. Data are representative of 2 independent experiments. (C) Expression of DGKα and DGKζ in response to BCR stimulation was measured in T1, T2, T3, and Fo B cells sorted from spleens of WT mice following in vitro stimulation for 4 hours with 10 μg/ml anti-IgM F(ab′)2. Expression of each isoform was normalized to HPRT and is relative to expression in Fo B cells following BCR stimulation. B cells subsets were sorted from n =3 pooled WT spleens and data are representative of 2 independent experiments. (D) Comparison of BCR-induced ERK activation between WT and DGKζ-/- T1 (left panels) and follicular (right panels) B cells following stimulation with anti-IgM F(ab′)2 for 2 min. Wedge indicates a 50-fold dose response range, starting with 1 μg/ml anti-IgM. (E) Time course (0-60 min) of BCR induced ERK activation in WT (solid lines) and DGKζ-/- (dashed lines) T1 and follicular B cells stimulated with 5μg/ml (grey lines) and 50 μg/ml (black lines) anti-IgM F(ab′)2. Relative amounts of phospho-ERK are derived from the geometric median fluorescence intensity. Data for D-E are representative of the response from stimulated Fo and T1 B cells from n = 3 pooled spleens. Similar results were found in at least 3 independent experiments. (F) Representative histograms showing intracellular IκBα in Fo B cells following BCR stimulation of total splenic B cells from WT (solid black histogram) and DGKζ-/- (dashed black histogram). Degradation of IκBα was quantified (bottom) as the median fluorescence intensity of signal after stimulation with 50 μg/ml anti-IgM F(ab′)2, or 500 ng/ml CpG ODN for the indicated times, and was normalized to unstimulated samples. Data are from n = 3 mice and are representative of 3 independent experiments. * p<0.05, **p<0.01, ***p<0.001 (Student's t-test).
We next examined expression of DGKα and DGKζ mRNAs in B cells at different stages of peripheral maturation in the spleen. Consistent with attenuated BCR-induced ERK signaling in follicular B cells, this population showed nearly 2-fold higher expression of DGKα and DGKζ mRNA than immature transitional T1 and T2 B cells sorted from spleens of WT mice (Fig. 1B). Notably, BCR stimulation of splenic B cells resulted in a substantial decrease in the abundance of DGKα mRNA within 4 hr of stimulation, as well as a smaller reduction in DGKζ mRNA (Fig. 1C). The decrease in levels of DGKα mRNA following BCR stimulation was more pronounced in T2 and follicular B cells compared to T1 and T3 B cells sorted from the spleens of WT mice (Fig. 1C). Follicular B cells showed the largest decrease in DGKζ mRNA following BCR stimulation, however there was less variability in mRNA reduction between the different B cell subsets for this isoform compared to DGKα (Fig. 1C). The decreased expression of DGKζ and DGKα may be important to facilitate prolonged DAG signaling downstream of the BCR at later stages of activation of follicular B cells.
Next, we measured BCR-induced ERK phosphorylation in B cells from mice with targeted deletions of DGKα, DGKζ, or both (19, 21). DGKα-deficient follicular B cells had slightly elevated BCR-induced ERK phosphorylation compared to WT cells (fig. S1A), however this response was enhanced nearly 10 fold in DGKζ-deficient follicular B cells (Fig. 1D and fig. S1A). The increased response of DGKζ-deficient follicular B cells was evident over a wide range of anti-IgM doses used to cluster membrane IgM isoforms of the BCR to trigger downstream signaling (Fig. 1D). Furthermore, this enhanced response was maintained at all time points tested (Fig. 1E). Similar to R59949 treatment, genetic deficiency in DGKζ had only modest effects on BCR-induced ERK activation in T1 B cells (Fig. 1, D and E) indicating that DGKζ-mediated regulation of DAG signaling was primarily a property of mature follicular B cells. Notably, DGKζ-deficiency appeared to shift the phospho-ERK response of follicular B cells from a graded, analogue response seen in WT cells, to a digital or bimodal response, especially at intermediate doses of anti-IgM (Fig. 1D). Follicular B cells from mice deficient in both DGKα and DGKζ exhibited further enhancement of BCR-induced ERK activation, indicating a secondary but significant role for DGKα in restraining BCR-mediated DAG signaling (fig. S1A). However, BCR-induced activation of mature B cells, as measured by surface CD69 induction, as well as antibody responses to the T cell-independent type 2 antigen NP-ficoll were unaffected by loss of DGKα, indicating a minor role for this isoform in restricting downstream responses to BCR stimulation (fig. S1, B and C). Given the dominant role of DGKζ in limiting BCR-dependent DAG signaling in follicular B cells we focused on this isoform in subsequent experiments.
As DAG is also an important second messenger for BCR-induced canonical NFκB activation (6), we examined degradation of the IκBα inhibitory protein, which is required for nuclear translocation of NFκB (23). IκBα protein abundance was measured by intracellular staining and flow cytometry of permeabilized cells. BCR crosslinking of splenic B cells induced a rapid degradation of IκBα in WT follicular B cells, which was clearly accelerated in DGKζ-deficient follicular B cells, although by 30-60 min the amount of intracellular IκBα was indistinguishable (Fig. 1F). Degradation of IκBα in response to the TLR9 ligand CpG, which should be independent of DAG production (24), was not similarly affected (Fig. 1F), indicating specificity of DGKζ for negative regulation of BCR-induced NFκB activation. Similar to results obtained with intracellular staining and flow cytometry, measurement of BCR-induced ERK phosphorylation and IκBα degradation by western blot of follicular B cells purified from lymph nodes of DGKζ-deficient mice, also showed a substantial increase in ERK1/2 phosphorylation compared to WT follicular B cells, whereas the effect on IκBα degradation was less pronounced (fig. S2).
Mice deficient in DGKζ had normal numbers of the various B cell subpopulations in the bone marrow, spleen, and peritoneal cavity (fig. S3A). We did however observe a small but significant decrease in the amount of surface IgM present on all mature and immature splenic B cell subpopulations in the spleen of DGKζ-deficient mice (fig. S3B). As expected, DAG-independent components of BCR signaling including calcium elevation and phosphorylation of the proximal tyrosine kinase Syk were not enhanced, and were even somewhat reduced in DGKζ-deficient follicular B cells (fig. S4, A and B), which may be a result of decreased surface BCR present on the cells.
DGKζ deficiency reduces the threshold for BCR-induced activation and proliferation in vitro
As early BCR-induced ERK activation was strongly enhanced in DGKζ-deficient follicular B cells, we then asked if there was a reduced threshold for BCR induced activation and proliferation of these cells. Indeed, in vitro BCR-induced increase in the early activation marker CD69 was enhanced in DGKζ-deficient B cells (Fig. 2A). Furthermore, in vitro proliferation of anti-IgM stimulated DGKζ-deficient B cells was markedly increased compared to that of WT B cells, representing a roughly five-fold shift in the dose response curve (Fig. 2B). In contrast, proliferation in response to stimulation with the TLR9 ligand, CpG oligonucleotide, or with anti-CD40 and IL-4 were minimally affected (Fig. 2B), as expected given the different signaling mechanisms of these receptors. Correspondingly, enhancement of some early gene inductions in response to BCR stimulation was observed, most prominently of the mRNA encoding the transcription factor Egr-1 (Fig. 2C), which is downstream of Ras and ERK activation (25). The NFκB-induced increase in IκBα, which serves as a feedback inhibitory response for this pathway (26), as well as the survival factor A1, were not noticeably changed, however abundance of the survival factor Bcl-XL, which is also known to be a direct target of NFκB (27) was moderately increased at later times in DGKζ-deficient B cells (Fig. 2C). Taken together, these results demonstrate that DGKζ is a critical negative regulator of DAG signaling downstream of the BCR in mature follicular B cells, which primarily targets the ERK-MAPK signaling pathway and reduces the sensitivity for BCR-induced B cell activation and proliferation.
Figure 2. DGKζ deficiency reduces the threshold for BCR-induced activation and proliferation in vitro.
(A) Early activation of WT and DGKζ-/- purified splenic B cells measured by surface CD69 induction 6 hours after stimulation with increasing doses (0.5-10 μg/ml) of anti-IgM F(ab′)2 in vitro. Left panels show representative histograms for surface CD69 induction comparing the response of WT (left) and DGKζ-/- (right) B cells to increasing amounts of BCR stimulation. Data are quantified on the right as the median fluorescence intensity of the CD69 signal from n = 3 mice/group, and are representative of 3 independent experiments. (B) In vitro proliferation of WT and DGKζ-/- B cells measured by BrdU incorporation 36-48 hours after stimulation of purified splenic B cells with anti-IgM F(ab′)2 (0.5-10 μg/ml), anti-CD40 plus IL-4 (2.5 μg/ml and 1 ng/ml) or CpG ODN (250 ng/ml). Representative flow cytometry plots are shown on the left, and data from n =3 mice/group are quantified on the right as the percentage of BrdU+ cells for each stimulation condition, and are representative of 2 independent experiments. (C) Induction of ERK and NFκB dependent genes (Egr1, IκBα, A1, and Bcl-xL) was measured by quantitative RT-PCR in purified splenic B cells from WT (solid black) and DGKζ-/- (dashed black) mice (n = 3) stimulated for the indicated times with 2.5 μg/ml anti-IgM F(ab′)2. Data are representative of 2 independent experiments. *p<0.05, **p<0.01, ***p< 0.001 (Student's t-test).
DGKζ-deficient mice have elevated serum IgM and enhanced responses to T cell-independent type 2 antigens
To determine if DGKζ plays a role in limiting B cell activation in vivo, we first compared serum antibody titers between unimmunized WT and DGKζ-deficient mice. DGKζ-deficient mice had normal amounts of class-switched Ig isotypes in the blood, but a roughly 5-fold increase in IgM titers (Fig. 3A). Analysis of the spleen showed that DGKζ-deficient mice have a large increase in the number of IgM-secreting plasma cells, as well as a trend towards increased numbers of IgG-secreting plasma cells as measured by ELISPOT (Fig. 3B). These results suggest that inhibition of DAG signaling by DGKζ limits plasma cell generation or survival in the spleen.
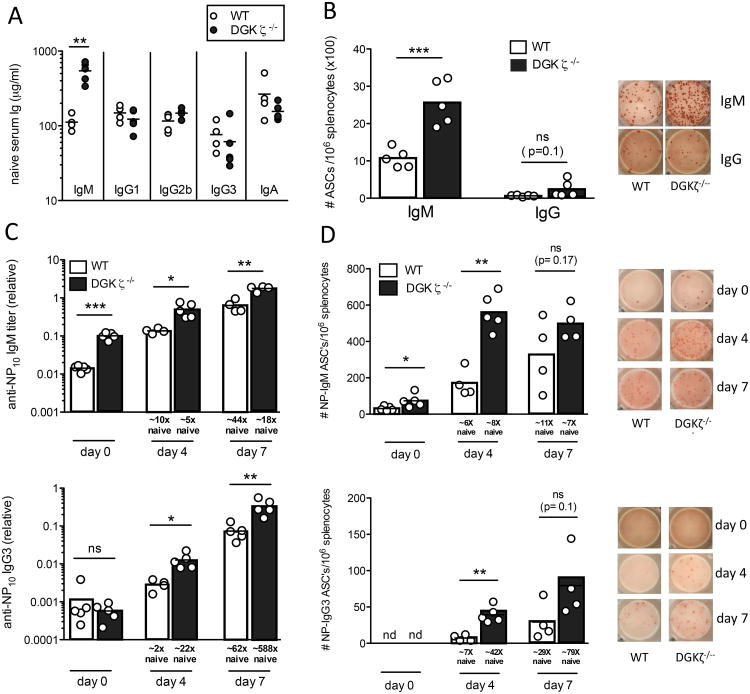
Figure 3. DGKζ-deficient mice have elevated serum IgM and enhanced responses to T cell-independent type 2 antigens.
Serum immunoglobulin titers were measured in unimmunized WT (open circles) and DGKζ-/- (filled circles) mice by ELISA. Data are representative of titers from three separate cohorts of mice containing n= 4-5 mice/group. (B) Total spleen IgM and IgG secreting plasma cells were measured by ELISPOT from unimmunized WT (white bars) and DGKζ-/- (black bars) mice. Representative images of ELISPOT wells are shown in the right panels for total IgM and IgG ASC's. Data are representative of two separate experiments. (C-D) T-independent type 2 antibody response of DGKζ-/- mice. NP-specific serum IgM (top) and IgG3 (bottom) titers were measured by ELISA (C), and numbers of NP-specific IgM (top) and IgG3 (bottom) secreting plasma cells in the spleen were measured by ELISPOT (D), for indicated times before and after NP-Ficoll immunization of WT (white bars) and DGKζ-/- (black bars) mice. Representative images of ELISPOT wells are shown on the right. Data are representative of n =4-5 mice/group, and similar results were found in 2 independent experiments. The fold-increase in NP-specific Ig and ASC's was calculated by normalizing the average value at each time point to unimmunized levels. The fold increase in NP-IgG3 ASC's was determined by assigning a value of 1 for naïve WT and DGKζ-/- mice. Each circle represents an individual mouse for all data shown. *p<0.05, **P<0.01, ***p<0.001 (Student's t-test)
We next looked at the response of DGKζ-deficient mice to the T cell-independent type 2 antigen NP-Ficoll, which triggers vigorous BCR crosslinking on antigen-specific B cells, bypassing their need for T cell help (28). DGKζ-deficient mice generated elevated serum titers of NP-specific IgM and IgG3 on both day 4 and 7 after immunization (Fig. 3C), although the amount of NP-specific IgM was also substantially elevated in unimmunized DGKζ-deficient mice. The increased response was also reflected in the number of plasma cells secreting NP-specific IgM and IgG3 as detected by ELISPOT, especially at day 4 (Fig. 3D), indicating an accelerated early plasma cell response. It must be noted however, that while amounts of NP-specific IgM and NP-IgM secreting plasma cells were elevated at all time points tested in DGKζ-deficient mice, when measured as a fold change over the unimmunized controls, these differences were actually similar or somewhat reduced compared to WT mice. In contrast, the fold change in the amount of NP-specific IgG3 and numbers of NP-IgG3 secreting plasma cells was roughly 3-10× higher in DGKζ-deficient mice compared to WT mice immunized with NP-ficoll. These results indicate that DGKζ functions to limit the early response to antigens with strong BCR crosslinking properties.
DGKζ limits early expansion as well as germinal center and plasma cell formation induced by T cell-dependent antigen immunization
We next wanted to determine whether inhibition of BCR-induced DAG signaling by DGKζ is important for regulating the T cell-dependent antibody response. For this purpose we used an adoptive transfer approach in which small numbers of WT or DGKζ-deficient MD4/IgHEL transgenic B cells, specific for the hen egg lysozyme (HEL) antigen, were transferred into WT recipient mice, followed by immunization with sheep red blood cells (SRBC) coupled to mutant forms of HEL with different affinities for the transgene-encoded IgHEL BCR (29, 30). In vitro stimulation of IgHEL transgenic B cells with intermediate affinity HEL (HEL2×) resulted in robust ERK phosphorylation in WT IgHEL follicular B cells, and this response was enhanced nearly 10-fold in DGKζ-deficient IgHEL B cells (Fig. 4A) similar to what was observed with BCR crosslinking by anti-IgM. Notably, stimulation with the same dose of low affinity HEL (HEL3×), which binds the IgHEL BCR with 50-fold less affinity than HEL2× (30), was unable to induce significant ERK phosphorylation in WT B cells, however it induced a maximal phospho-ERK response in approximately 20% of DGKζ-deficient B cells (Fig. 4A). These results indicate that in the absence of DGKζ, the affinity threshold for antigen-induced BCR signaling to ERK is substantially reduced.
Figure 4. DGKζ-/- antigen specific B cells undergo enhanced early expansion in response to T cell-dependent antigen immunization.
(A) BCR signaling induced by different affinity variants of HEL. ERK phosphorylation was measured by intracellular staining and flow cytometry as in Fig. 1D in WT/MD4 (solid black histograms) and DGKζ-/-/MD4 (dashed black histograms) splenic Fo B cells stimulated for 2 min with 1μg/ml of soluble HEL2× (left) or HEL3× (right). Data are representative of the response from pooled spleens of n = 2-3 mice, and similar results were found in a second experiment. (B) Experimental set up for measuring in vivo responses of WT/MD4 and DGKζ-/-/MD4 B cells. WT (CD45.2+) mice received an equal mixture (1×105 total) of WT/MD4 (CD45.1+) and DGKζ-/-/MD4 (CD45.2+) purified splenic B cells followed by immunization with HEL2×-SRBC or HEL3×-SRBC. (C) Representative flow cytometry plots show staining of HEL-binding cells on day 3 following HEL2×-SRBC or HEL3×-SRBC immunization (left panels), and frequencies of WT and DGKζ-/-/MD4 B cells (right panel). Frequencies of WT/MD4 (white bars) and DGKζ-/-/MD4 B cells (black bars) on day 3 are quantified in (D) as the percentage of each genotype within the HEL-binding population in response to HEL2×-SRBC and HEL3×-SRBC (n =5 mice/group and representative of 3 experiments). (E) Proliferation of CFSE labeled WT/MD4 (solid black histogram) and DGKζ-/-/MD4 (dashed black histogram) HEL-binding B cells measured by CFSE dilution on day 3 following HEL2×-SRBC immunization. Proliferation is quantified as the percentage of cells that have undergone 4 or more divisions. n=4 mice/group and representative of 2 independent experiments). ***p<0.001 (Student's t-test).
To compare the response of WT and DGKζ-deficient IgHEL B cells to T cell-dependent antigen immunization, WT mice received a 1:1 mixture (1×105 total) of congenically marked WT (CD45.1+) IgHEL and DGKζ-deficient (CD45.2+) IgHEL purified splenic B cells followed by immunization with intermediate affinity HEL2×, or low affinity HEL3×, conjugated to SRBCs (Fig. 4B). As early as day 3 following immunization, small numbers of HEL-binding B cells could be detected by flow cytometry by co-staining with fluorescently labeled HEL and IgMa to distinguish donor-derived B cells from host B cells responding to the HEL-SRBC conjugates (Fig. 4C). Analysis of the allelic expression of these cells revealed a clear but modest enrichment of DGKζ-deficient B cells within the HEL-binding population in response to both intermediate and low affinity HEL-SRBC immunization (Fig. 4, C and D). Furthermore CFSE labeling of transferred cells showed that these differences resulted from more robust proliferation rather than enhanced survival or engraftment of DGKζ-deficient transferred cells (Fig. 4E). These results indicate that DGKζ plays a role in limiting early B cell expansion in vivo in response to T cell-dependent antigens.
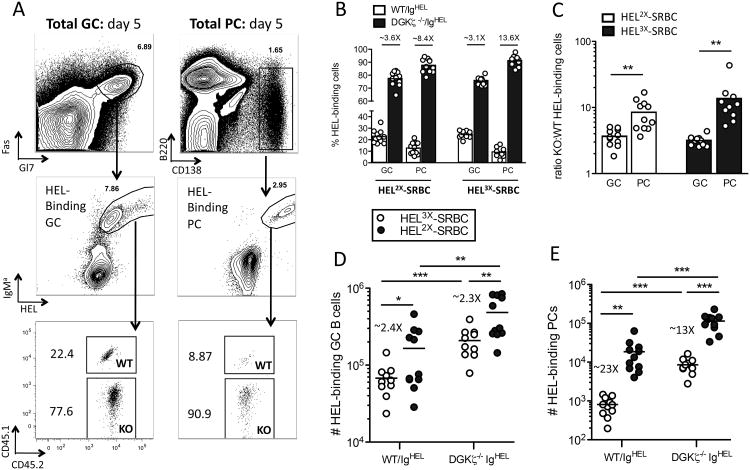
Between days 3 and 5 during the T cell-dependent antibody response, activated cognate B cells partition between a rapid short-lived extrafollicular plasmablast response, analogous to that seen in response to the T-independent antigens described above, and a germinal center (GC) response that involves repeated rounds of somatic mutation of the genes encoding the immunoglobulin heavy and light chains and selection for higher affinity variants (31). Eventually this GC response gives rise to long-lived high affinity antibody-secreting plasma cells, as well as memory B cells. Analysis of the early GC response on day 5 following immunization with HEL2× and HEL3×-SRBC, indicated a further skewing in favor of DGKζ-deficient cells, which comprised roughly 75% of the total HEL-binding GC B cell population (Fig. 5A and B). However, an even more pronounced advantage was seen within the donor-derived HEL-binding plasmablast compartment after immunization with either HEL2×-SRBC or HEL3×-SRBC (Fig. 5, B and C), as indicated by a roughly 8-12 fold enrichment for DGKζ-deficient cells compared to WT cells with the same immunogen (Fig. 5B). The magnitude of the HEL-specific GC and plasmablast response was enhanced following immunization with intermediate affinity HEL2× compared to low affinity HEL3× for both WT and DGKζ-deficient IgHEL transferred cells, although this affinity-dependent difference was much more apparent in the plasmablast population (13-23 fold increase in plasmablasts vs 2.4-2.5 fold increase in GC B cells) (Fig. 5, D and E) in agreement with previous reports (30, 32). Notably, deficiency in DGKζ shifted the magnitude of the GC and plasmablast responses induced by immunization with low affinity HEL3×-SRBC to a degree similar to that of WT cells responding to intermediate affinity antigen (fig 5, D and E) as measured by the number of HEL-binding GC and plasmablast phenotype cell present in the spleen following immunization with the different affinity variants. Importantly, the differences observed were antigen specific, and were not due to an intrinsic ability of DGKζ-deficient B cells to undergo spontaneous activation, as DGKζ-deficient IgHEL B cells did not proliferate or differentiate into GC B cells or plasmablasts in the absence of immunization with HEL-SRBC's (fig. S5, A to C). Thus a critical function of DGKζ is to promote affinity discrimination during the early phases of the T cell-dependent antibody response, and especially to limit early plasmablast formation or maintenance.
Figure 5. Deficiency in DGKζ promotes robust early plasmablast and germinal center responses.
(A) Representative strategy for flow cytometric measurement of numbers of day 5 HEL-binding plasma cells (B220lo, CD138hi, IgMa-hi, HELhi) and germinal center B cells (Fashi, GL7hi, IgMa-int, HELint) generated after HEL2×-SRBC immunization using the same experimental design as illustrated in Fig. 4B, (B) Frequency of WT/MD4 (white bars) and DGKζ-/-/MD4 (black bars) HEL-binding cells in the plasma cell and germinal center B cell compartments in response to HEL2×-SRBC (left) and HEL3×-SRBC (right). Data are pooled from 2 individual experiments with n=5-6 mice/group; similar results were obtained in a third independent experiment. (C) Ratio of DGKζ-/-/MD4: WT/MD4 HEL-binding cells within the germinal center and plasma cell compartments on day 5 in response to HEL2×-SRBC and HEL3×-SRBC. (D-E) Total numbers of WT/MD4 and DGKζ-/-/MD4 HEL-binding germinal center B cells (D) and plasma cells (E) generated on day 5 in response to HEL2×-SRBC (open circles) and HEL3×-SRBC (filled circles). * p<0.05, **p<0.01, ***p<0.001 (Student's t-test).
DGKζ-deficient plasmablasts have enhanced proliferative capacity and higher amounts of the survival factor Bcl-XL
We next wanted to address whether there were differences in proliferation or survival of DGKζ-deficient IgHEL B cells that might account for their enrichment in the GC and plasmablast populations. BrdU labeling of HEL-binding GC B cell and plasmablast populations revealed a moderate increase in the percentage of DGKζ-deficient cells undergoing active DNA synthesis on day 5 following immunization with HEL2×-SRBC (Fig 6A). While these results indicate that DGKζ-deficient cells maintain an enhanced proliferative capacity upon differentiation to the GC B cell and plasmablast stages, it was not clear whether this was sufficient to fully explain the roughly 10-fold increase in DGKζ-deficient cells over WT within the plasmablast population. We therefore asked whether enhanced BCR signaling in the absence of DGKζ resulted in differences in the abundance of the pro-survival factor Bcl-xL, as measured by intracellular staining and flow cytometry (Fig 6B). Whereas the amount of Bcl-xL was similar between WT and DGKζ-deficient GC B cells (Fig. 6, B and C), there was a significant increase in Bcl-xL in donor-derived HEL-binding DGKζ-deficient plasmablasts compared to their WT counterparts after HEL2×-SRBC or HEL3×-SRBC immunization (Fig. 6, B and C). Furthermore, consistent with the impaired survival capacity of plasmablasts generated in response to low affinity antigen immunization (32), Bcl-xL was reduced in WT plasmablasts generated from HEL3× compared to HEL2×-SRBC immunization (Fig. 6C). Interestingly, the amount of Bcl-xL present in DGKζ-deficient plasmablasts remained high regardless of the initial antigen affinity (Fig. 6C). The increase in Bcl-xL seen in DGKζ-deficient plasmablasts is in agreement with in vitro data showing that BCR stimulation induced higher mRNA induction of this survival factor in naïve DGKζ-deficient B cells (Fig. 2C). Surface expression of the costimulatory molecule CD86, as well as MHC class II was similar between WT and DGKζ-deficient GC B cells and plasmablasts generated from HEL2×-SRBC immunization (fig. S6, A and B), suggesting that the effects of DGKζ-deficiency on the early plasmablast response were unlikely due to superior antigen presentation to cognate T cells. Instead, these results point to an important role for DGKζ, and the strength of BCR-signaling through DAG-dependent pathways, in mediating early plasmablast proliferation and survival based on the affinity of the antigen-BCR interaction.
Figure 6. DGKζ limits expansion of germinal center B cells and plasmablasts, and survival factor expression in plasmablasts.
(A) Mice receiving equal mixtures of congenically marked WT and DGKζ-/- MD4 B cells were injected with BrdU on day 5 following immunization with HEL2×-SRBC and BrdUincorperation was measured by flow cytometry within the HEL-binding germinal center and plasmablast populations. WT/MD4 and DGKζ-/-/MD4 cells were distinguished by CD45 alleles as in Fig. 5A, and the percentages of BrdU+ cells from each genotype were quantified within the HEL-binding germinal center and plasmablast population (right panel). Data are from n=5 mice and similar results were found in 2 independent experiment. (B) Representative histograms showing intracellular staining for Bcl-xL in day 5 WT/MD4 and DGKζ-/-/MD4 HEL-binding germinal center B cells (left) and plasmablasts (right) generated in response to immunization with HEL2×-SRBC. (C) Quantification of the amount of intracellular Bcl-xL (median fluorescence intensity) in HEL-binding germinal center B cells (left panel) and plasma cells (right panel) generated upon immunization with HEL2×-SRBC (open circles) vs. HEL3×-SRBC (filled circles). Data are pooled from 2 independent experiments with n = 5-6 mice each. **p<0.01, ***p<0.001 (Student's t-tests).
Discussion
Antigen stimulation of the B cell antigen receptor (BCR) induces a variety of intracellular signaling events, including those promoted by the second messenger diacylglycerol (DAG), which is generated through hydrolysis of PIP2 by PLC-γ2. In this work, we have examined the regulation of DAG-dependent signaling events by diacylglycerol kinase (DGK) enzymes, which convert DAG to phosphatidic acid to terminate signaling by this second messenger. Ablation of DGKζ substantially enhanced BCR signaling to ERK in mature follicular B cells and reduced the threshold for BCR-dependent activation and proliferation in vitro and in vivo. The most striking effects of DGKζ-deficiency in B cells related to greatly enhanced plasmablast responses that closely mimicked the effect of increasing antigen affinity for the BCR. These results indicate that DAG signaling is likely an important determinant of affinity-based regulation of the early plasmablast response.
In antigen-stimulated B and T cells, DAG binds to RasGRP and protein kinase C isoforms, leading to activation of Ras and the ERK MAP kinase as well as the transcription factor NF-κB. Whereas enhancement of DAG signaling by ablation of DGKζ led to substantially enhanced ERK signaling in mature follicular B cells, the effect on NF-κB activation was primarily kinetic in that IκBα was degraded more rapidly but not to an obviously greater extent. In agreement with the signaling results, whereas anti-IgM-induction of the early response gene Egr-1, which is mediated by the Ras-ERK pathway (25), was enhanced in DGKζ-deficient B cells, there was not greater induction of IκBα or A1, which are both thought to be a direct targets of NF-κB (26). Thus, the Ras-ERK pathway was highly sensitive to changes in the amount of DAG present following BCR engagement, whereas the NF-κB pathway was relatively insensitive to these changes, and instead exhibited more of an all-or-none response. This differential responsiveness of the two major pathways downstream of DAG in B cells to alterations in the amount of DAG present was unexpected, and future studies to define the mechanism of this differential sensitivity will be of considerable interest.
Deletion of DGKζ enhanced ERK signaling in mature follicular B cells, and this signaling was further enhanced in B cells with combined deletion of DGKζ and DGKα. Thus, although DGKα was largely dispensable for proper regulation of DAG signaling in resting follicular B cells, it is clearly capable of acting on DAG and attenuating signaling. DGKα deficiency enhanced in vitro responses to BCR stimulation to a minimal degree, and had little effect on in vivo responses to NP-Ficoll immunization, however it is possible that DGKα plays a more prominent role in regulation of DAG signaling in situations that were not analyzed in these experiments. Given that DGKα exhibited dramatic changes in mRNA expression during B cell development in the spleen, and following BCR stimulation, we speculate that its expression may be increased to attenuate ERK signaling in some circumstances. In this regard, we note that DGKα is important for antigen-induced anergy in T cells (19, 33, 34).
The use of DGKζ-deficient B cells allowed us to probe the effects of different amounts of DAG signaling on B cell activation. These B cells were more sensitive to anti-IgM stimulation in vitro for CD69 induction and for proliferation. Taken together with previous evidence that CD69 is a Ras-regulated gene in T cells (35), and that MEK inhibitors block BCR-induced B cell proliferation and surface CD69 induction in vitro (14), these results are in agreement with the hypothesis that enhanced ERK signaling in DGKζ-deficient B cells makes these cells more sensitive to BCR engagement for activation. We also observed enhanced proliferation of HEL-specific DGKζ-deficient B cells in vivo during the first three days following immunization with HEL2×-SRBC, indicating that a similar regulation occurs during immune responses.
Among the most striking effects of DGKζ-deficiency in B cells for in vivo antibody responses was the enhancement of plasmablast numbers seen in response to immunization with both T-independent type 2 antigens and T-cell dependent antigens. In the case of immunization with NP-Ficoll, there appeared to be a more rapid early plasmablast response, which correlated with increased serum antibody titers. Interestingly, previous studies have implicated the amount of BCR signaling as well as the ERK pathway in regulation of plasma cell differentiation. For example, in an in vitro system, the degree of BCR signaling translated into the amount IRF4 induction, which is required to promote expression of the transcriptional regulator of plasma cell differentiation, Blimp1 (36). Other studies have directly linked ERK signaling to plasma cell differentiation. For example, ERK activation downstream of the BCR has been shown to promote proteosomal degradation of the Blimp1 transcriptional repressor Bcl6 (37), and also was found to inhibit Pax5-dependent repression of Blimp1 transcription (38). Moreover, deletion of ERK1 and ERK2 in B cells after they reach the germinal center stage prevented plasma cell differentiation from germinal center B cells, apparently by preventing ERK dependent phosphorylation of ELK1 and indirect induction of Blimp1 (12). Whether any of these mechanisms are responsible, in part, for the more rapid plasmablast response of DGKζ-deficient mice to NP-Ficoll will require additional study.
We also saw a very large increase in early plasmablast numbers in DGKζ-deficient HEL-specific B cells on day 5 after immunization with mutant forms of HEL that have different affinities for the anti-HEL MD4 BCR (29). In this system, increasing the affinity of antigen for the BCR dramatically increases the magnitude of the early antibody response, and this has been shown to be largely mediated by enhancing proliferation and survival of plasmablasts (30, 32). It was not clear from the previous studies, however, whether this effect of affinity was mediated by the magnitude of BCR signaling in plasmablasts, or whether it was related to the amount or quality of T cell help that had been received by B cells of different affinities. In the experiments described here, the effects of DGKζ-deficiency were strikingly similar to the effects of higher affinity. These results point to the magnitude of BCR signaling, and especially DAG signaling, as a primary determinant of the magnitude of the early plasmablast response. Although we cannot fully rule out higher quality T cell help as a contributor to the effect of antigen affinity on plasmablast numbers, we observed that in vitro responses to CD40 were normal in DGKζ-deficient B cells, and we also did not observe any difference in the amount of surface MHC class II or the T cell costimulatory molecule CD86 on DGKζ-deficient germinal center B cells or plasmablasts from HEL-SRBC immunized mice. These observations strongly suggest that the mechanism by which affinity is translated into plasmablast proliferation and survival is largely due to the magnitude of DAG signaling, likely through the ERK pathway.
The findings of this study illustrate the virtue of modulating signaling reactions, as was done here with DGKζ-deficiency, versus the more common approach of completely ablating signaling reactions, for example by deletion of ERK1 and ERK2 (12, 15). Whereas ablation of a signaling pathway demonstrates when that pathway is required, and this is highly informative, modulation of the magnitude of a signaling pathway makes it possible to assess whether regulation of biological responses takes advantage of changes in the amount of signaling, as was the case for affinity regulation of B cells during the early plasmablast response. Of course, each approach to perturbing signaling reactions has its advantages and disadvantages. Ablation of DGKζ increases the elevation of DAG that occurs upon BCR engagement, but it may attenuate somewhat the amount of phosphatidic acid (PA), and this molecule has been reported to modulate the activity of some signaling pathways. For example, PA has been shown to promote mTOR signaling in HEK293 cells (39). However, we think this signaling function of PA is unlikely to be a major factor in our studies given that DGKs have been shown to limit mTOR signaling in T cells through a mechanism involving Ras and MEK1 (40), which is opposite of what would be expected if DGK-dependent PA production was positively regulating this signaling pathway downstream of antigen receptor stimulation.
Whereas the canonical pathway of ERK activation in lymphocytes involves DAG-mediated activation of RasGRP, which activates Ras, leading through Raf1 and MEK1/2 to ERK1/2, recent studies have identified several alternative pathways of ERK activation in B cells stimulated by antigen. For example, Limnander et al (41) showed that in immature B cells in the bone marrow, BCR signaling to ERK is mediated primarily through a mechanism involving store-operated calcium entry rather than DAG, which may explain why there was not an obvious effect of DGKζ-deficiency on early B cell development. In addition, Guo et al (42) have demonstrated an alternative pathway by which the BCR activates ERK in IL-4-pretreated B cells, and this pathway seems to operate in addition to the canonical pathway. Furthermore, the adaptor protein Bam32 has been shown to important for a significant proportion of ERK activation downstream of the BCR in naïve resting B cells, apparently by a pathway involving MEKK1 rather than Raf1 as the kinase upstream of MEK1/2 (43-45). To what extent these alternate pathways for ERK activation downstream of the BCR are sensitive to changes in the amount of DAG present in the cell is not entirely clear. Mice lacking Bam32 have a substantial defect in T-independent type II antibody responses (43, 45), and have impaired germinal center maintenance in response to T cell dependent antigens (43, 45). These observations are in good agreement with the results of enhancing ERK signaling in the experiments presented here, however it is possible that additional effects of Bam32 deficiency beyond reduced ERK signaling contribute to impaired antibody responses in these mice.
In conclusion, we have demonstrated a critical role for DGKζ in B cells for regulating BCR signaling through the second messenger DAG to the ERK MAP kinase pathway. We have shown that attenuation of DAG signaling by DGKζ is an important mechanism for limiting the numbers of short-lived plasmablasts generated that secrete low affinity antibody against the immunizing antigen. We propose that this inhibitory mechanism is important to maintain a higher affinity initial antibody response, which may be more protective against pathogens during the early stages of the infection before high affinity class switched antibody is produced by the germinal center response. Alternatively or additionally, more stringent regulation of B cell activation and antibody responses by the presence of DGKζ in B cells may protect against autoimmunity (46) or lymphoma development (47).
Materials and Methods
Mice
Mice were used between the ages of 7-12 weeks for most experiments. B6 (000664; C57BL/6J) and CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) were purchased from Jackson laboratory. DGKα-/- (Dgkatm1Xpz) and DGKζ-/- (Dgkztm1Gak) mice were generated as previously described (19, 21) and were backcrossed to the C57BL/6J background for 8 generations. MD4 transgenic (IgHEL) mice were obtained from J. Cyster (University of California, San Francisco) and bred to the DGKζ-/- background for some experiments. All animals were housed in a specific pathogen-free facility at the University of California San Francisco, according to University and National Institutes of Health guidelines. Animal use was approved by the UCSF Institutional Animal Care and Use Committee.
Antibodies, flow cytometry analysis, B cell purification, and cell sorting
Fluorophore-conjugated Abs directed against the following molecules were used: B220, CD23, CD69, CD95 (Fas), CD4, GL7, IgD, IgMa, IgMb, CD45.1, CD45.2, CD138, (syndecan), CD86, I-Ab (MHCII) and CD19 (all from BD Pharmingen); CD24 from Biolegend; CD93 (AA4.1) from eBioscience; IgM (goat polyclonal F(ab) monomer, μ chain specific) from Jackson Immunoresearch. For most experiments RBC lysed splenocytes were surface stained on ice following FcR blocking with anti-CD16/32 (UCSF Hybridoma core) and analyzed on an LSR-II (BD Pharmingen). Dead cells were excluded by propidium iodide (BioChemika) uptake. For cell sorting, splenic T1, T2, T3, and follicular B cell subpopulations were identified using the method described by Allman et al. (48) (B220, CD93, CD23, IgM), and sorting was performed with a MoFlo sorter (DakoCytomation). Purified B cells were isolated from spleens of 7-12 week old mice by negative selection using CD43 microbeads (Miltenyi Biotech) according to the manufacturer's instructions, and passage through MACS LS separation columns (Miltenyi Biotech). All FACS data were analyzed with FlowJo version 9.3.3 (Tree Star software).
Adoptive transfers, HEL-SRBC conjugation, and immunizations
Splenic B cells were purified from WT/MD4 (IgHEL) (CD45.1+ or CD45.1+.2+) and DGKζ-/-/MD4 (IgHEL) (CD45.2+ or CD45.1+.2+) mice by negative selection as described above. Purified WT/MD4 and DGKζ-/-/MD4 B cells were mixed at a 50:50 ratio and injected intravenously (1×105 total) into WT C57BL/6J CD45.2+ hosts. To track cell proliferation in vivo, cells were pre-labeled with CFSE and prepared as described above except mice received 1×106 cells prior to immunization. SRBCs (Colorado Serum Company) were labeled with HEL2× or HEL3× (20 μg/ml final concentration) on the day of immunization as described (30). HEL labeling efficiency was measured by flow cytometry by staining the conjugated SRBC with an anti-Hy9HEL-PE-Cy5.5 antibody (Gift from Dr. Jason Cyster UCSF). The following day after adoptive transfer, mice were immunized by intraperitoneal injection with 2×108 freshly conjugated HEL2×- or HEL3×-SRBC. Spleens were harvested and analyzed on day 3 and day 5 following immunization, and donor derived cells were distinguished from host cells by costaining for IgMa and with hen egg lysozyme (Sigma) conjugated to Alexa-647 (Invitrogen) fluorescent dye. WT and DGKζ-/- HEL-binding cells were distinguished by CD45.1 and CD45.2 surface staining combinations.
Analysis of HEL-binding germinal center B cells and plasma cells generated from HEL-SRBC immunization
HEL-binding germinal center B cells and plasma cells were analyzed in the spleens of day 5 HEL-SRBC immunized mice. For improved plasma cell recovery spleens were injected with type 4 collagenase (Worthington) and incubated at 37°C for 45 min prior to isolating cells for staining. HEL-binding germinal center B cells were defined as CD4-, IgMa+ HEL-A647+, B220+, Fas+, GL7+. HEL-binding plasma cells were identified from total CD4-, B220lo, CD138hi cells, and further distinguished from germinal center B cells by intracellular staining for IgMa and HEL using cytofix and cytoperm (BD Biosciences).
In vivo and in vitro BrdU labeling
For in vivo BrdU labeling experiments, mice were injected IP with 2.5 mg BrdU (in 250 μl PBS) on day 5 following HEL-SRBC immunization and sacrificed 60 min later. For in vitro proliferation analysis, purified WT and DGKζ-/- splenic B cells were suspended in complete Iscove's medium with 10% FBS (1×106 cell/ml) and stimulated at 37°C with 0.25-10 μg/ml anti-IgM F(ab′)2 (Jackson immunoresearch), 250 ng/ml CpG (Integrated DNA Technologies), or 2.5 μg/ml anti-CD40 (BD Pharmingen) + 2.5 ng/ml IL-4 (Roche) for 48 h. BrdU (BD Pharmingen) was added to cells at a final concentration of 10 μM at 36 h after stimulation, and cells were harvested for analysis 48 h after stimulation. For in vitro and in vivo analysis, cells were stained for appropriate surface markers, and BrdU incorporation was measured by intracellular staining and flow cytometry using a BrdU Flow kit (BD Pharmingen) according to manufacturer's instructions.
Intracellular flow cytometry
Intracellular measurement of phospho-ERK, phospho-Syk, and total IκBα were performed as described (49). Briefly, 4×106 splenocytes (2×107/ml) were pre-warmed at 37°C for 30 min and stimulated with anti-IgM F(ab′)2 followed by immediate fixation and permeabilization with 2% PFA and ice cold methanol respectively (both from Electron Microscopy Services). For some experiments, cells were pretreated for 20 min with 20 μM of the DGK inhibitor II R59949 (Alexis biochemicals) or vehicle (% DMSO). Cells were labeled with anti-phospho-Syk-Alexa-647 (BD Pharmingen), anti-phospho-ERK1/2 (thr202/Tyr204) rabbit mAB, or anti-IκBα rabbit Ab (both from Cell Signaling Technology), followed by staining with anti-rabbit IgG-APC (Jackson Immunoresearch) (for p-ERK and IκBα) and surface staining to distinguish T1, T2, T3, and Fo B cells. Cells were analyzed by flow cytometry, and for most experiments the analysis was performed on the T1 (B220+, CD24hi, CD23neg, IgMhi) and follicular (B220+, CD24lo, CD23+, IgM lo-int) populations. Measurement of intracellular free calcium levels was performed as described (16).
ELISA and ELISPOT
To assess T cell-independent antibody production, mice were injected intraperitoneally with 10 μg NP-AECM-Ficoll (Biosearch Technologies Inc.). Pre-immune and immune sera (day 4 and 7) were collected, and NP-specific IgM and IgG3 titers were measured by ELISA as described (49). Total naïve serum immunoglobulin was captured with goat anti-mouse antibodies against IgM, IgA, or total IgG (Southern Biotech), and detected with isotype-specific HRP-conjugated anti-mouse antibodies. Serum concentration was extrapolated from a standard curve generated for each isotype.
Numbers of NP-specific and total IgM and IgG secreting plasma cells were determined by ELISPOT. Briefly, splenocytes from naïve or NP-Ficoll immunized mice were resuspended in complete Iscove's medium containing 10% FBS, and incubated overnight in wells of multiscreen HTS 96 well filter plates (Millipore) that were previously coated with NP10-BSA or with anti-mouse capture antibodies against IgM and IgG. The following day, plates were washed of cells and spots were detected with HRP-conjugated anti-mouse IgM, total IgG, or IgG3 (Southern Biotech) and developed with a 3-amino-9-ethylcarbazole (AEC) chromogen kit (Sigma), according to manufacturer's instructions. Spots were counted with an ELISPOT reader.
Supplementary Material
fig. S1. DGKα plays a minor role in negative regulation of BCR-induced ERK activation, but does not limit B cell activation or T independent type II antibody responses
fig. S2. DGKζ-/- deficiency in mature follicular B cells largely enhances BCR-induced ERK activation but has minimal effects on PKCβ dependent IκBα degradation.
fig. S3. B cells from DGKζ-/- mice develop normally but have reduced amounts of surface BCR.
fig. S4. DAG-independent proximal BCR signaling is mostly normal in DGKζ-/- follicular B cells.
fig. S5. DGKζ deficient IgHEL B cells do no undergo spontaneous proliferation and differentiation into GC B cells or plasmablasts in the absence of antigen.
fig. S6. DGKζ deficiency does not alter the amount of surface CD86 or MHC II on HEL-binding plasmablasts and GC B cells.
Acknowledgments
We thank Jason Cyster, Arthur Weiss, Andre Limnander, Linda Lee, Irina Proekt, Derek Rookhuizen, and Shan-shan Zhang for helpful comments on the manuscript, and Tangsheng Yi for expert advice with the HEL-SRBC experiments. We also thank Jason Cyster for providing the MD4/IgHEL mice and anti-Hy9HEL-PE-Cy5.5 antibody. This work was supported by the National Institutes of Health grants R01A1-20038, R01A1-072058, and P01A1-078869 (A.L.D.)
References and Notes
- 1.Kurosaki T, Shinohara H, Baba Y. B Cell Signaling and Fate Decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto A, Takeda K, Inaba M, Sekimata M, Kaisho T, Ikehara S, Homma Y, Akira S, Kurosaki T. Cutting edge: essential role of phospholipase C-gamma 2 in B cell development and function. J Immunol. 2000;165:1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunological reviews. 2009;228:132–148. doi: 10.1111/j.1600-065X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 4.Saijo K, Schmedt C, Su I, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nature Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 6.Guo B, Su TT, Rawlings DJ. Protein kinase C family functions in B-cell activation. Current opinion in immunology. 2004;16:367–373. doi: 10.1016/j.coi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Newton AC. Regulation of protein kinase C. Current opinion in cell biology. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 8.Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, Kato RM, Kang S, Patrone L, Wall R, Teitell M, Leitges M, Kawakami T, Rawlings DJ. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nature immunology. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 9.Tognon CE, Kirk HE, Passmore LA, Whitehead IP, Der CJ, Kay RJ. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Molecular and cellular biology. 1998;18:6995–7008. doi: 10.1128/mcb.18.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vig M, Kinet JP. Calcium signaling in immune cells. Nature immunology. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda T, Kurosaki T. Regulation of lymphocyte fate by Ras/ERK signals. Cell Cycle. 2008;7:3634–3640. doi: 10.4161/cc.7.23.7103. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, Ogata M, Kurosaki T. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Rowland SL, DePersis CL, Torres RM, Pelanda R. Ras activation of Erk restores impaired tonic BCR signaling and rescues immature B cell differentiation. The Journal of experimental medicine. 2010;207:607–621. doi: 10.1084/jem.20091673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards JD, Dave SH, Chou CH, Mamchak AA, DeFranco AL. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J Immunol. 2001;166:3855–3864. doi: 10.4049/jimmunol.166.6.3855. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda T, Kometani K, Takahashi N, Imai Y, Aiba Y, Kurosaki T. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Science signaling. 2011;4:ra25. doi: 10.1126/scisignal.2001592. [DOI] [PubMed] [Google Scholar]
- 16.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunological reviews. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nature immunology. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 20.Shin J, O'Brien TF, Grayson JM, Zhong XP. Differential regulation of primary and memory CD8 T cell immune responses by diacylglycerol kinases. J Immunol. 2012;188:2111–2117. doi: 10.4049/jimmunol.1102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nature immunology. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Sakane F, Kanoh H, Walsh JP. Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2, 3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochemical pharmacology. 2000;59:763–772. doi: 10.1016/s0006-2952(99)00395-0. [DOI] [PubMed] [Google Scholar]
- 23.Ruland J, Mak TW. Transducing signals from antigen receptors to nuclear factor kappaB. Immunological reviews. 2003;193:93–100. doi: 10.1034/j.1600-065x.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature reviews Immunology. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 25.McMahon SB, Monroe JG. Activation of the p21ras pathway couples antigen receptor stimulation to induction of the primary response gene egr-1 in B lymphocytes. The Journal of experimental medicine. 1995;181:417–422. doi: 10.1084/jem.181.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Molecular and cellular biology. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annual review of immunology. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 29.Brink R, Phan TG, Paus D, Chan TD. Visualizing the effects of antigen affinity on T-dependent B-cell differentiation. Immunology and cell biology. 2008;86:31–39. doi: 10.1038/sj.icb.7100143. [DOI] [PubMed] [Google Scholar]
- 30.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. The Journal of experimental medicine. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nature immunology. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 32.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 33.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nature immunology. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. The Journal of experimental medicine. 2012;209:2157–2163. doi: 10.1084/jem.20120342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Ambrosio D, Cantrell DA, Frati L, Santoni A, Testi R. Involvement of p21ras activation in T cell CD69 expression. European journal of immunology. 1994;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- 36.Sciammas R, Li Y, Warmflash A, Song Y, Dinner AR, Singh H. An incoherent regulatory network architecture that orchestrates B cell diversification in response to antigen signaling. Molecular systems biology. 2011;7:495. doi: 10.1038/msb.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu H, Ye BH, Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes & development. 1998;12:1953–1961. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda T, Hayakawa F, Kurahashi S, Sugimoto K, Minami Y, Tomita A, Naoe T. B cell receptor-ERK1/2 signal cancels PAX5-dependent repression of BLIMP1 through PAX5 phosphorylation: a mechanism of antigen-triggering plasma cell differentiation. J Immunol. 2012;188:6127–6134. doi: 10.4049/jimmunol.1103039. [DOI] [PubMed] [Google Scholar]
- 39.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 40.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, Roose JP, Weiss A. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nature immunology. 2011;12:425–433. doi: 10.1038/ni.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo B, Blair D, Chiles TC, Lowell CA, Rothstein TL. Cutting Edge: B cell receptor (BCR) cross-talk: the IL-4-induced alternate pathway for BCR signaling operates in parallel with the classical pathway, is sensitive to Rottlerin, and depends on Lyn. J Immunol. 2007;178:4726–4730. doi: 10.4049/jimmunol.178.8.4726. [DOI] [PubMed] [Google Scholar]
- 43.Han A, Saijo K, Mecklenbrauker I, Tarakhovsky A, Nussenzweig MC. Bam32 links the B cell receptor to ERK and JNK and mediates B cell proliferation but not survival. Immunity. 2003;19:621–632. doi: 10.1016/s1074-7613(03)00275-9. [DOI] [PubMed] [Google Scholar]
- 44.Niiro H, Maeda A, Kurosaki T, Clark EA. The B lymphocyte adaptor molecule of 32 kD (Bam32) regulates B cell antigen receptor signaling and cell survival. The Journal of experimental medicine. 2002;195:143–149. doi: 10.1084/jem.20011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fournier E, Isakoff SJ, Ko K, Cardinale CJ, Inghirami GG, Li Z, Curotto de Lafaille MA, Skolnik EY. The B cell SH2/PH domain-containing adaptor Bam32/DAPP1 is required for T cell-independent II antigen responses. Current biology : CB. 2003;13:1858–1866. doi: 10.1016/j.cub.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annual review of immunology. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler ML, Defranco AL. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J Immunol. 2012;189:4405–4416. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fig. S1. DGKα plays a minor role in negative regulation of BCR-induced ERK activation, but does not limit B cell activation or T independent type II antibody responses
fig. S2. DGKζ-/- deficiency in mature follicular B cells largely enhances BCR-induced ERK activation but has minimal effects on PKCβ dependent IκBα degradation.
fig. S3. B cells from DGKζ-/- mice develop normally but have reduced amounts of surface BCR.
fig. S4. DAG-independent proximal BCR signaling is mostly normal in DGKζ-/- follicular B cells.
fig. S5. DGKζ deficient IgHEL B cells do no undergo spontaneous proliferation and differentiation into GC B cells or plasmablasts in the absence of antigen.
fig. S6. DGKζ deficiency does not alter the amount of surface CD86 or MHC II on HEL-binding plasmablasts and GC B cells.