Abstract
Objectives To describe our departmental experience in the surgical repair of tegmen tympani defects using a combined transmastoid/minicraniotomic approach.
Design Retrospective review of videos from surgery and patients' charts.
Setting Tertiary university referral center.
Participants Twenty-two patients who underwent surgical repair of tegmen defects associated with cerebrospinal fluid (CSF) leakage and/or meningocele/meningoencephalocele by a combined transmastoid/minicraniotomic approach.
Main Outcome Measures A retrospective review of videos of surgery and charts of patients with tegmen tympani or tegmen antri defects and CSF leakage, temporal lobe encephalocele, and/or meningoencephalocele.
Results All patients underwent the combined approach and had their defects closed, without significant intraoperative or postoperative complications.
Conclusions Mastoidectomy with temporal minicraniotomy represents an effective approach in patients with tegmen tympani dehiscence; the advantages of this technique are the control of the floor of the middle cranial fossa and the possibility to reach bony defects located anteriorly without manipulation of the ossicular chain and temporal lobe.
Keywords: tegmen tympani, middle cranial fossa approach, meningocele, encephalocele, cerebrospinal fluid leakage
Introduction
The tegmen tympani is a bony plate forming the roof of the tympanic cavity and the antrum. It separates the subarachnoidal space containing cerebrospinal fluid (CSF) from the air of the middle ear.1 2 3 When a dehiscence originates in this particular subsite of the temporal bone, this condition could lead to CSF otorrhea (CSF leakage), with possible herniation of meninges and brain tissue (meningoencephalocele).4 5 Temporal bone CSF leakage can occur as a result of traumatic injuries, congenital malformations, iatrogenic causes, infectious diseases, cholesteatomas, and neoplastic invasion of the skull base, or they may also be spontaneous.6 7 8 9
Clinical symptoms are often nonspecific. Patients may report aural fullness, hearing loss, tinnitus, imbalance, and headaches. Clinical examination may reveal middle ear effusion, otorrhea, rhinorrhea, and pulsatile movement of the tympanic membrane.7 Diagnosis of CSF otorrhea can be performed by β2-transferrin and β-trace protein analysis in the fluid suspected to be liquor.6 Computer tomography and magnetic resonance imaging are the most commonly applied imaging modalities in the evaluation of encephalocele and suspected CSF otorrhea.4 10
The surgical approaches of choice for the repair of tegmen tympani defects depend on the location and size of the bony defect, the status of the ossicular chain, and the experience of the surgeon. Most authors consider a transmastoid (TM) repair as the best surgical approach for the treatment of small tegmental defects. In fact, this minimally invasive surgical approach allows repair of the dehiscence without manipulation and elevation of the dura of the middle cranial fossa (MCF).11 12 13 14 15 16 However, when a large tegmen defect is found in association with CSF leakage, more invasive surgical techniques may be required. In this case, many authors adopt an MCF approach.6 9 13 17 18 19
In our experience over the past 10 years, a combined approach for the repair of large defects of the tegmen has been adopted, performing a mastoidectomy in combination with a temporal minicraniotomy.
The aim of this study was to review our case series, describe our surgical technique, and evaluate the feasibility and results.
Material and Methods
A retrospective chart review of patients with tegmen tympani or tegmen antri defects and CSF leakage, temporal lobe encephalocele, and/or meningoencephalocele operated on between January 2003 and May 2013 was conducted in the Otolaryngology-Head and Neck Surgery Department of Modena University Hospital in August 2013.
Inclusion criteria were all patients who were affected by a tegmental defect associated with CSF otorrhea and requiring dural and tegmen repair, where we performed the combined technique with mastoidectomy and minicraniotomy.
Exclusion criteria were patients who underwent an MCF approach, a classical TM approach, or middle ear obliteration for tegmen and dural defect repair. Patients with iatrogenic CSF leakage following a lateral skull base surgery (retrosigmoid or translabyrinthine craniotomy) and patients who underwent a repair of posterior cranial fossa defects with CSF leakage were also excluded from the study.
All surgical procedures were performed by two experienced surgeons (D.M. and L.P.).
Surgical Approach
A traditional retroauricular incision was performed, extending superiorly to the temporal area. The mastoid bone was widely exposed. A wide mastoidectomy was performed skeletonizing the mastoid tegmen superiorly and the sigmoid sinus posteroinferiorly. The sinodural angle was fully exposed. The site of leakage and, when present, the meningeal herniation were identified along the mastoid or tegmen tympani. When the bony defect was located on the epitympanum (tegmen tympani defect), an anterior epitympanotomy was performed preserving the ossicular chain, whenever possible, and exposing the lateral epitympanum and the whole tegmen tympani. In some cases, an endoscopic procedure was adopted to expose the dural defect and the medial portion of the tegmental dehiscence (Fig. 1).
Fig. 1.
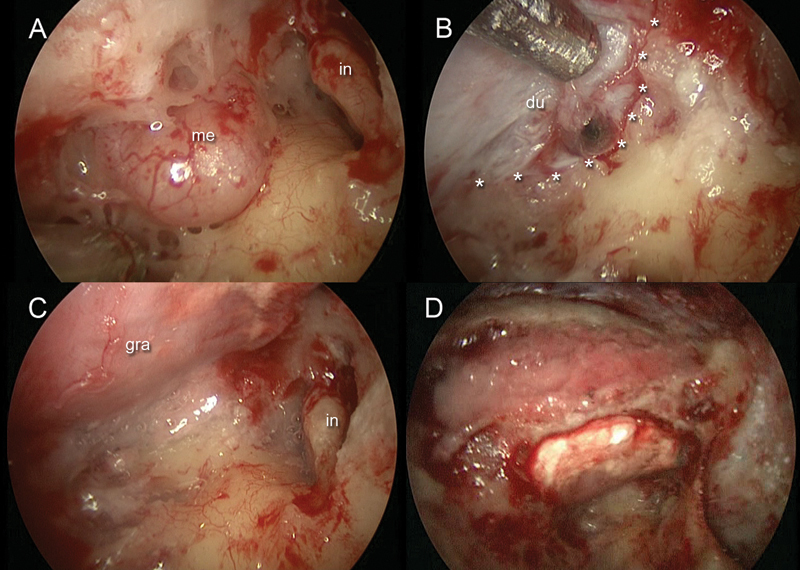
Right ear. (A) Endoscopic view of the meningeal herniation along the mastoid and antral tegmen. (B) After meningeal herniation removal, a 0-degree endoscope was inserted through the mastoid cavity to detect the dural defect and the anterior limit of the tegmental defect (asterisks). (C) A Duragen graft was inserted through the minicraniotomy to repair the defect. (D) Bony dust was placed through the mastoid cavity to cover the tegmental defect and to cover the site of the minicraniotomy. du, dura mater; gra, graft, in, incus; me, meningeal herniation.
When an encephalocele, or meningoencephalocele was found, the cerebral material was gently reduced using bipolar cautery until the site of entrance was located. After this surgical maneuver, the base of the cerebral material was cut with a micro-scissors and the bony wall around the tegmental defect was carefully removed using a diamond burr over the whole entrance area of the tissue herniation.
When a CSF leakage without meningoencephalocele was treated, the site of leakage on the mastoid/tympani tegmen was identified intraoperatively. In this case, the bony wall around the tegmental defect was also gently removed with a diamond burr over the whole dura layer dehiscence in the entrance area.
A minicraniotomy was performed along the temporalis squama until the dura mater of the MCF was exposed (Fig. 2A), the inferior edge of the middle temporal cerebral lobe was detected, and the dura was gently elevated off the floor of the MCF using a small elevator via the minicraniotomy created previously. The extradural and intracranial elevation of the dura allowed us to expose the floor of the mastoid/tympanic tegmen defect intracranially, gently detaching the dura mater circumferentially around the tegmen defect (Fig. 3A, B).
Fig. 2.
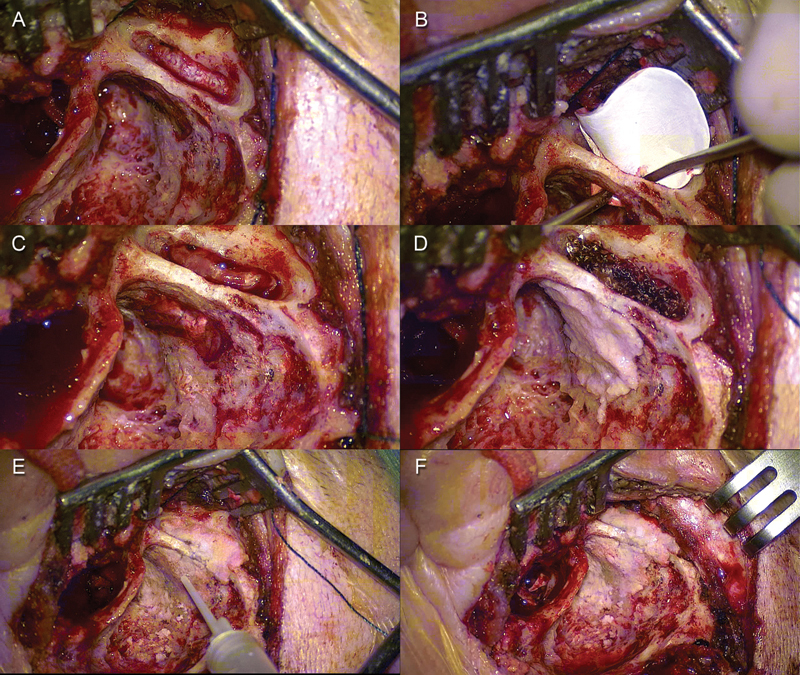
Left ear. (A) A minicraniotomy was performed. (B) Duragen graft was positioned through the minicraniotomy. (C) The graft was placed between the dura mater and the superior surface of the middle cranial fossa covering the tegmental defect. (D) Bony dust was placed over the tegmental defect through the mastoid cavity. (E, F) Final cavity after tegmen repair.
Fig. 3.
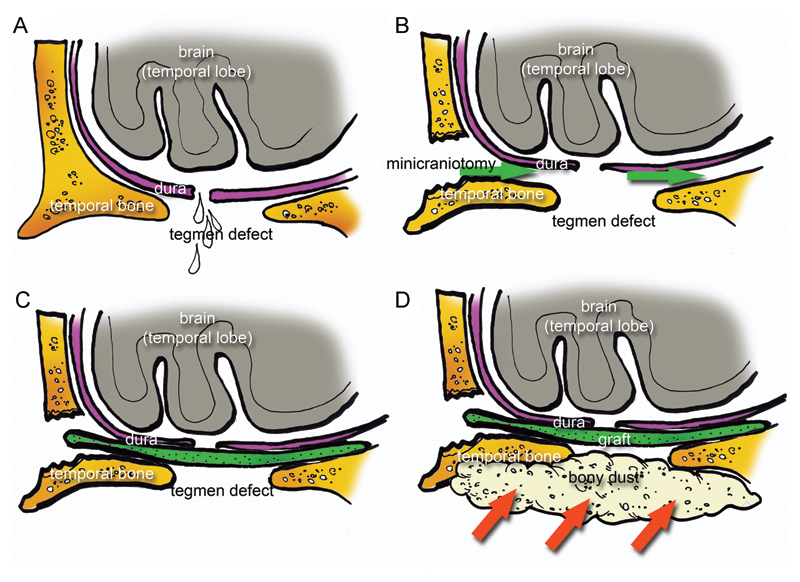
Schematic drawing representing the surgical repair with a single layer method. (A) A tegmental defect was detected in association with dura laceration. (B) A minicraniotomy was performed and the dura mater was gently elevated through the minicraniotomy (green arrows) from the superior surface of the middle cranial fossa to create a site for placement of the graft. (C) A graft. (D) Bony dust was placed over the tegmental defect through the mastoid cavity (red arrows).
The repair of the defect was performed using different materials, depending on the size of the defect; a Duragen graft (Integra, Plainsboro, NJ, USA) or temporal fascia graft were used in a single layer in most cases (Fig. 3C). When an extremely large defect was found, a multilayer repair was required; in these cases, we used a Duragen graft or temporal fascia graft as the first layer in association with a piece of cortical mastoid graft used as the second layer to reconstruct the whole tegmental defect (Fig. 4). The whole graft was adequately shaped and inserted through the minicraniotomy between the floor of the MCF (superior surface of the tegmen) and the dura of the temporal cerebral lobe, covering the whole tegmen defect into the epidural space (Figs. 2B, C, 3C). Bone paté, obtained from the patient during drilling of the mastoid or craniotomy, was used over the site of repair via mastoidectomy to cover the tegmental defect, and fibrin glue was used to fix and stabilize the repair (Figs. 2D–E, 3D).The mastoid was filled with Gelfoam, and the closure was completed in a multilayered fashion.
Fig. 4.
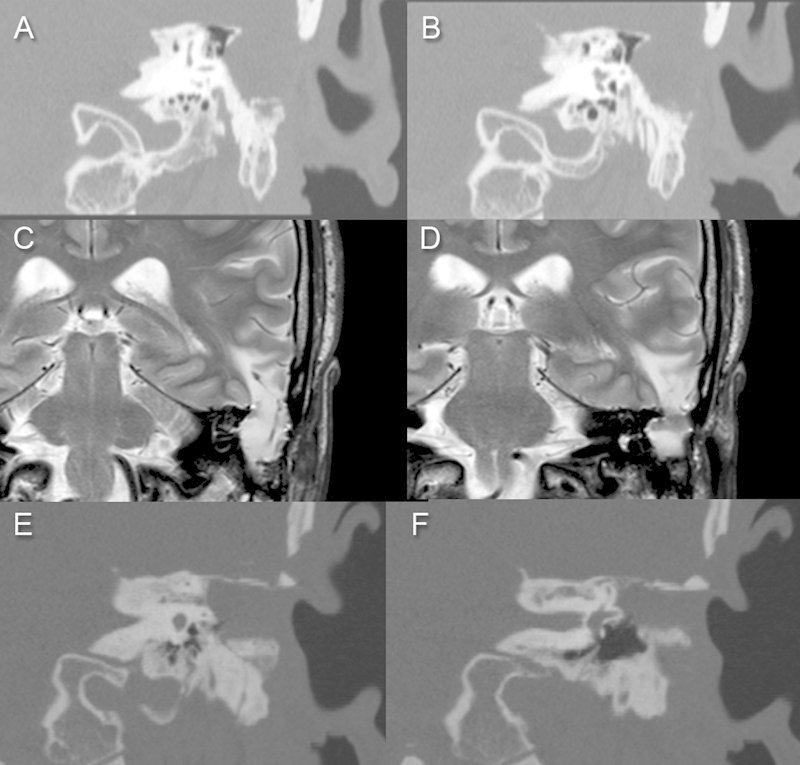
Patient with a large iatrogenic defect of the tegmen in association with a meningoencephalocele. (A,B) Computed tomography (CT) scan, coronal view. (C,D) Magnetic resonance imaging, coronal view. (E,F) CT scan in coronal view 1 month after the surgical repair. In this case, a multilayer repair was adopted, using a Duragen layer over the dural defect and a cortical mastoid graft between the Duragen and the superior surface of the middle cranial fossa to reconstruct the wide tegmental defect.
Postoperative Care
A postoperative computed tomography (CT) scan was performed 1 day after surgery (Fig. 5). The patients had to maintain a supine position for 2 days, and antibiotic therapy associated with corticosteroids was administered to prevent infections.
Fig. 5.
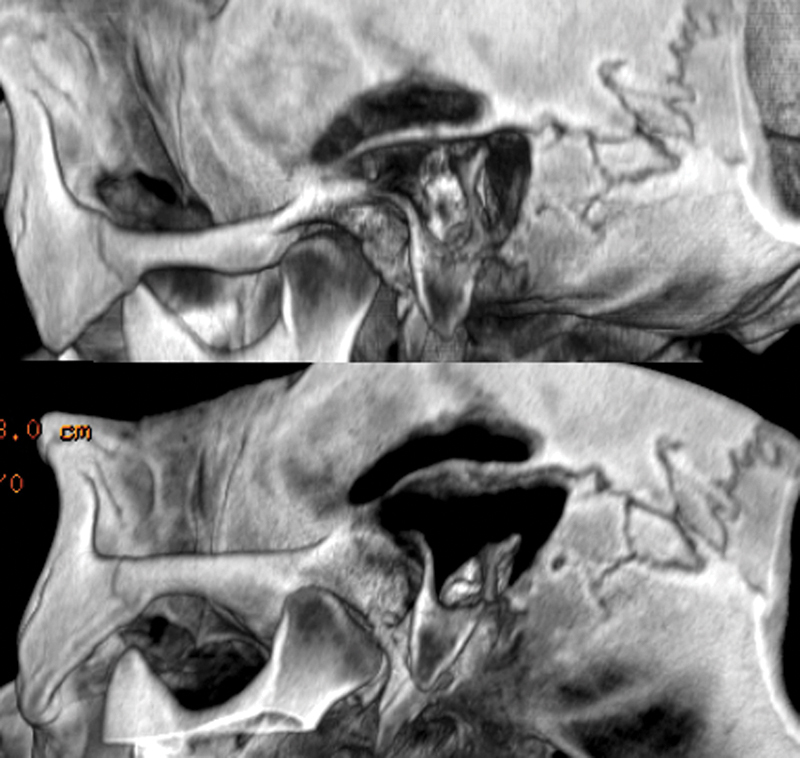
Computed tomography scan with three-dimensional reconstructions. Note the site of the minicraniotomy related to the mastoid cavity.
Results
A total of 42 patients underwent surgical repair of tegmental defects associated with CSF leakage and/or meningocele/meningoencephalocele (Table 1). From this group, patients who underwent middle ear obliteration, an exclusive TM approach, an MCF approach, or patients affected by CSF leakage from the posterior fossa were excluded. Twenty-two patients underwent a combined approach, and these patients were included in the study (12 of 22 patients were male, 10 of 22 patients were female). The mean age was 57 years (range: 18–89 years).
Table 1. Site of defect and etiology.
| Case no. | Sex | Age, y | Site of the tegmen defect (tegmen tympani, mastoideum, antri) | First signs and symptoms | Etiology |
|---|---|---|---|---|---|
| 1 | M | 42 | Tegmen antri and mastoideum | Headache | Iatrogenic (TPL) |
| 2 | M | 74 | Tegmen antri and mastoideum | Hearing loss | Cholesteatoma |
| 3 | M | 74 | Tegmen antri, mastoideum, and tympani | Otorrhea, hearing loss | Spontaneous |
| 4 | F | 58 | Tegmen tympani and antri | Noted occasionally (CT scan) | Spontaneous |
| 5 | F | 67 | Tegmen tympani and antri | Otorhinoliquorrhea | Spontaneous |
| 6 | F | 60 | Tegmen timpani and mastoideum | Parietal headache, otorrhea | Iatrogenic (TPL) |
| 7 | M | 18 | Tegmen antri and mastoideum | Hearing loss | Posttraumatic |
| 8 | F | 74 | Tegmen tympani | Ear fullness, otoliquorrhea | Spontaneous |
| 9 | M | 42 | Tegmen antri and mastoideum | Otalgia, frontal headache, agitation, falling blood pressure, hyperpyrexia | Otogenic meningitis |
| 10 | M | 69 | Tegmen tympani | Otalgia, otorrhea, hyperpyrexia | Otogenic meningitis |
| 11 | M | 60 | Tegmen tympani | Confusional state | Otogenic meningitis |
| 12 | F | 67 | Tegmen tympani, antri, and mastoideum | Otorrhea, hearing loss dizziness, headache | Spontaneous |
| 13 | F | 82 | Tegmen tympani, antri, and mastoideum | Hearing loss, tinnitus otorrhea, ear fullness | Cholesteatoma |
| 14 | F | 65 | Tegmen mastoideum | Altered mental status, vomiting, hyperpyrexia | Otogenic meningitis |
| 15 | F | 27 | Tegmen tympani and mastoideum | Otorrhea, otalgia | Spontaneous |
| 16 | M | 53 | Tegmen mastoideum | Otorrhea, ear fullness, otoliquorrhea | Cholesteatoma |
| 17 | F | 59 | Tegmen mastoideum | Hyperpyrexia, loss of consciousness | Otogenic meningitis |
| 18 | M | 89 | Tegmen antri | Otorrhea, headache hyperpyrexia, nuchal rigidity | Otogenic meningitis |
| 19 | M | 31 | Tegmen antri | Confusional state, tonic-clonic convulsive crisis | Otogenic meningitis |
| 20 | F | 65 | Tegmen antri and tympani | Hearing loss, tinnitus | Cholesteatoma |
| 21 | M | 28 | Tegmen tympani | Otalgia | Otogenic meningitis |
| 22 | M | 43 | Tegmen antri and tympani | Rhinoliquorrea | Posttraumatic |
Abbreviations: CT, computed tomography; TPL, tympanoplasty.
Etiology
The etiology of the CSF leakage requiring repair by a combined approach was as follows: spontaneous in 6 of 22 patients, chronic otitis media with a history of meningitis in 8 of 22, posttraumatic in 2 of 22, due to cholesteatoma in 4 of 22, and iatrogenic in 2 of 22.
Surgical Technique and Intraoperative Findings
Regarding the size of the defect, in all patients, a large tegmental defect was found with dural laceration and consequent CSF leakage (Table 2). Analyzing the site of the tegmental defect, in 12 of 22 patients, the defect was found in the tegmen at the level of the mastoid, in 13 of 22, the defect was present in the tegmen antri, in 13 of 22 the defect occurred in the tegmen tympani.
Table 2. Surgical technique and intraoperative findings.
| Case, no. | Ossicular chain preservation | Follow-up, mo | Postoperative complications | Material used for tegmen repair |
|---|---|---|---|---|
| 1 | Removed (previous TPL) | 34 | None | Duragen and cortical mastoid |
| 2 | Eroded (cholesteatoma) | 27 | None | Duragen graft |
| 3 | Removed (previous ossiculoplasty) | 34 | None | Duragen graft |
| 4 | Preserved | 8 | None | Duragen graft |
| 5 | Preserved | 8 | None | Duragen graft |
| 6 | Preserved | 14 | None | Duragen graft |
| 7 | Dislocated (meningoencephalocele) | 28 | None | Temporal fascia |
| 8 | Preserved | 22 | None | Duragen graft |
| 9 | Preserved | 22 | None | Duragen graft |
| 10 | Preserved | 14 | Dead (other diseases related) | Duragen graft |
| 11 | Preserved | 4 | None | Duragen graft |
| 12 | Dislocation (meningoencephalocele) | 31 | None | Duragen graft |
| 13 | Eroded (chronic middle otitis) | 64 | None | Temporal fascia |
| 14 | Preserved | 32 | None | Duragen graft |
| 15 | Preserved | 15 | None | Duragen graft |
| 16 | Removed (previous TPL) | 31 | Cerebral infection required only antibiotic therapy | Duragen graft |
| 17 | Preserved | 34 | None | Duragen graft |
| 18 | Preserved | 96 | None | Temporal fascia |
| 19 | Removed | 87 | None | Duragen graft |
| 20 | Removed (previous PORP) | 69 | None | Duragen graft |
| 21 | Preserved | 96 | None | Temporal fascia |
| 22 | Removed | 75 | None | Duragen graft |
Abbreviations: TPL, tympanoplasty; PORP, prosthetic ossicular reconstruction procedure.
Overall, 14 of 22 patients intraoperatively presented integrity of the ossicular chain. In 12 of 14 patients, the combined approach allowed us to preserve the integrity of the ossicular chain, whereas in 2 of 14 patients, it was necessary to remove the incus and the head of the malleus to perform the repair of the tegmental defect. Among the remaining patients, 4 of 22 patients presented an erosion of the ossicular chain and 4 of 22 patients presented ossiculoplasties because they had undergone previous surgical procedures.
In all patients, it was possible to perform the combined technique without intraoperative problems, inserting the graft through the miniocraniotomy. In 17 of 22 patients, a Duragen graft in association with bony dust was used for defect repair. In 4 of 22 patients, a large temporal fascia in association with bone paté was used for repair of the tegmental defect. In all cases, the graft was inserted intracranially via minicraniotomy and the bony dust was placed through the mastoid cavity to cover the tegmen defect.
In 1 of 22 patients, owing to the large size of the defect, we used a multilayer reconstruction, using Duragen graft for reconstruction of the dura mater and a piece of specially shaped cortical mastoid for reconstruction of the wide tegmental defect.
Outcome and Complications
The mean follow-up was 38 months (range: 4–96 months). None of the patients in our case series required a lumbar drain. All 22 patients had resolution of their CSF leakage, and no clinical recurrences were seen during the follow-up period. No intraoperative complications were observed.
After the surgery, one patient developed a cerebral infection requiring medical treatment by antibiotic therapy and was discharged from hospital without any revision surgery after 20 days. One patient with several comorbidities and affected by meningitis in association with CSF leakage before surgery developed a systemic septicemia despite surgical treatment and eventually died 2 months after surgery.
Discussion
Temporal bone CSF leakage is a challenging condition for the otolaryngologists in terms of diagnosis and management. It can be secondary to trauma, chronic ear disease, congenital malformations, infections, tumors, or surgical procedures that extend through or into the temporal bone.6 8 18 20
Spontaneous CSF otorrhea is much less common. Some authors associate this condition with erosion of the posterior or MCF by abnormal arachnoid granulations,21 encephaloceles,22 and increased intracranial pressure.23
Patients with temporal bone dehiscence often present with middle ear pathology, and in particular, serous otitis media, otorrhea, headaches, imbalance, tinnitus, or conductive hearing loss.7 24 In other cases, diagnosis can be made at the time of surgery, particularly when a mastoidectomy is required to treat infectious diseases or cholesteatomas.22
Generally, diagnosis is on the basis of the association of clinical signs and symptoms, fluid analysis in cases of frank CSF leakage, CT, and magnetic resonance imaging (MRI). In particular, MRI with T2-weighted sequences can help reveal the presence of CSF leakage.
The most significant complication of persistent CSF otorrhea is meningitis, which may also be the initial presentation in some patients. Other serious complications include intracranial abscesses and seizures. Repair of the temporal bone dehiscence is the primary treatment to prevent these potentially devastating complications.4 6
Surgical treatment of the mastoid/tegmen tympani defects associated with CSF leakage depends on the location and size of the bony defect, the status of the ossicular chain related to the hearing function of the patient, the association of middle ear/mastoid infection, and the experience of the surgeons.
Several approaches for tegmen repair have been proposed by different authors.6 9 11 12 13 14 15 16 17 18 19 25 26 27 28 The TM approach, the MCF approach, the combined approach, and the middle ear obliteration approach are the main ones described.
The Transmastoid Approach
The TM approach is widely used, consisting of a traditional postauricular incision with a subsequent mastoidectomy. During this procedure, the tegmen is exposed until the bony defect is detected; when present, herniated brain tissue is gently removed using a bipolar forceps and sectioning the hernia waist. After location of the bony defect on the tegmen and after herniation removal, a graft (cartilage, bone, fascia, or synthetic fascia) is inserted through the defect using the underlay technique, interposing the graft between the dura mater and the superior edge of the tegmen. The reconstruction may be reinforced with bone paté.
The TM technique is widely adopted especially for small mastoid tegmental defects because this is a simple technique with good results,10 11 14 15 but when a tegmen tympani defect is found at the level of the anterior epitympanum, ossicular chain removal is necessary to expose the defect area. Only in this way is it possible to obtain sufficient access for the repair. Moreover, when a large defect is found, the approach cannot guarantee enough space for successful repair of the defect.
For these reasons, for the repair of large tegmental defects, other surgical procedures have been described, usually the middle cranial approach, the combined approach, or middle ear obliteration.
The Middle Cranial Fossa Approach
The MCF approach permits tegmen defects located anteriorly (on the anterior attic tegmen) to be reached, allowing preservation of the ossicular chain. This surgical technique is especially indicated in patients with normal hearing function without middle ear/mastoid infection. To perform this approach, incision of the temporalis muscle in the pretragal area is required to expose the bone of the temporalis squama. The zygomatic process is detected and a 4 cm × 4 cm craniotomy is required until the dura of the temporal lobe is exposed; then a temporal lobe retraction is necessary with a dissector to elevate the temporal lobe, exposing the superior surface of the tegmen until the tegmental defect is detected; a cartilage graft is then placed between the bony defect and the dura. Many authors advocate an MCF approach as the initial approach for all defects.6 9 17 18 19 However, this may lead to significantly greater morbidity due to the large craniotomy and temporal lobe retraction.
Middle Ear Obliteration
Some authors consider middle ear obliteration to be the treatment of choice,13 consisting of a TM subtotal petrosectomy, with removal of the external auditory canal skin, the tympanic membrane, the mastoid and the tympanic cavity mucosa with malleus and incus, and creation of an open cavity. This cavity is then obliterated with abdominal fat, and the eustachian tube is packed with a piece of muscle or cartilage. A blind sac closure of the external auditory canal is required in this operation; in this way, the middle ear and mastoid cavities are completely isolated from the external environment. Because of the nature of this approach, it should be reserved for patients with poor hearing function or in cases where no other solution is possible because a large defect with brain herniation is present.
The Combined Approach (Transmastoid and Temporal Minicraniotomy)
The combined approach consists of a combination of the TM approach with a minicraniotomy performed at the level of the temporalis squama. The mastoidectomy allows brain tissue to be removed, detecting the site of entrance and the site of the dural lesion in the same fashion as with a TM approach, but instead, the minicraniotomy allows access to the temporal fossa. This permits management of the repair from above, working intracranially and extradurally. Once the dura of the temporal lobe is exposed by the minicraniotomy, using an elevator and with a gentle surgical maneuver, it is carefully elevated until the site of the tegmen defect is identified from above and the dura mater around the defect is elevated, allowing the creation of a wide bed for placement of the graft. A piece of Duragen or temporal fascia is placed between the bony defect and the dura. The defect is further reinforced through the mastoidectomy by placing bone paté over the tegmental defect.
In our opinion, when a small defect of the mastoid tegmen is expected from the CT scan, a TM repair should be attempted because this is a minimally invasive procedure without manipulation of the MCF dura. During this surgical procedure, when necessary, it is possible to adapt the TM procedure into the combined procedure, by adding the minicraniotomy.
For larger defects, most authors consider the MCF approach as the surgery of choice, but it should be noted that an extensive craniotomy is required in association with significant temporal lobe retraction to reach the bony defects and to attain a sufficient view of the tegmental defect area. For this reason, the risk of complications during the postoperative period is too high with respect to the other surgical techniques.10 However, some authors13 prefer to perform a middle ear obliteration because it is a safer and simple technique, but this approach requires the closure of the external auditory canal with fat obliteration of the mastoid cavity after ossicular chain removal, resulting in conductive hearing loss. Moreover, this approach requires MRI follow-up to detect the presence of an iatrogenic cholesteatoma.
From our experience, the combined approach allowed us to repair in a safe manner even large defects of the tegmen, permitting exposure of the dehiscence through the mastoid and through the MCF. We believe that when a large defect of the mastoid tegmen is found and the patient presents with normal hearing function, a combined approach could be attempted because this combines the advantages of the MCF approach with the TM approach, requiring just a minicraniotomy, and minimizing the risk of temporal lobe retraction with a great view and control of the site of repair of the tegmental defect through the mastoid cavity. Because the combined approach allowed us to preserve the integrity of the ossicular chain in 12 of 14 patients, this kind of approach can also be considered a minimally invasive procedure, preserving the ossicular chain and with good postoperative hearing. Both Duragen (used in 17 of 22 patients) and temporal muscle fascia (used in 4 of 22 patients), associated with bone paté, proved to be adequate and safe materials for underlay placement and repair. Only in the case of very large defects, such as in 1 of 22 patients in our case series, a multilayer reconstruction is performed, using Duragen graft for the reconstruction of the dura mater and a piece of cortical mastoid that could guarantee sufficient stiffness to hold the brain tissue in place during the healing process. As mentioned earlier, in all patients, the overlay procedure included the use of bone paté that allowed further isolation of the middle ear from the MCF and finally, after reossification, giving a hard and permanent support to prevent further herniation.
Conclusion
In our experience, a combined approach (mastoidectomy and temporal minicraniotomy) represents the treatment of choice even in patients with large tegmental defects. The advantages of this technique are the control of the floor of the MCF and the possibility to reach bony defects located anteriorly without manipulation of the ossicular chain and temporal lobe.
Financial Disclosure
None of the authors have any financial relationship to disclose.
Footnotes
Conflict of Interest None.
References
- 1.Tóth M, Helling K, Baksa G, Mann W. Localization of congenital tegmen tympani defects. Otol Neurotol. 2007;28(8):1120–1123. doi: 10.1097/MAO.0b013e31815aee0c. [DOI] [PubMed] [Google Scholar]
- 2.Tóth M. Nagykovácsi, Hungary: Remedium; 2006. Tegmen; pp. 233–242. [Google Scholar]
- 3.Stenzel M, Preuss S, Orloff L, Jecker P, Mann W. Cerebrospinal fluid leaks of temporal bone origin: etiology and management. ORL J Otorhinolaryngol Relat Spec. 2005;67(1):51–55. doi: 10.1159/000084306. [DOI] [PubMed] [Google Scholar]
- 4.Stucken E Z, Selesnick S H, Brown K D. The role of obesity in spontaneous temporal bone encephaloceles and CSF leak. Otol Neurotol. 2012;33(8):1412–1417. doi: 10.1097/MAO.0b013e318268d350. [DOI] [PubMed] [Google Scholar]
- 5.Connor S EJ. Imaging of skull-base cephalocoeles and cerebrospinal fluid leaks. Clin Radiol. 2010;65(10):832–841. doi: 10.1016/j.crad.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kutz J W Jr, Husain I A, Isaacson B, Roland P S. Management of spontaneous cerebrospinal fluid otorrhea. Laryngoscope. 2008;118(12):2195–2199. doi: 10.1097/MLG.0b013e318182f833. [DOI] [PubMed] [Google Scholar]
- 7.Leonetti J P Marzo S Anderson D Origitano T Vukas D D Spontaneous transtemporal CSF leakage: a study of 51 cases Ear Nose Throat J 20058411700, 702–704, 706 [PubMed] [Google Scholar]
- 8.Gacek R R, Gacek M R, Tart R. Adult spontaneous cerebrospinal fluid otorrhea: diagnosis and management. Am J Otol. 1999;20(6):770–776. [PubMed] [Google Scholar]
- 9.Markou K, Goudakos J, Franco-Vidal V, Vergnolles V, Vignes J R, Darrouzet V. Spontaneous osteodural defects of the temporal bone: diagnosis and management of 12 cases. Am J Otolaryngol. 2011;32(2):135–140. doi: 10.1016/j.amjoto.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Oliaei S, Mahboubi H, Djalilian H R. Transmastoid approach to temporal bone cerebrospinal fluid leaks. Am J Otolaryngol. 2012;33(5):556–561. doi: 10.1016/j.amjoto.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Rao A K, Merenda D M, Wetmore S J. Diagnosis and management of spontaneous cerebrospinal fluid otorrhea. Otol Neurotol. 2005;26(6):1171–1175. doi: 10.1097/01.mao.0000179526.17285.cc. [DOI] [PubMed] [Google Scholar]
- 12.Ramalingam K K, Ramalingam R, SreenivasaMurthy T M, Chandrakala G R. Management of temporal bone meningo-encephalocoele. J Laryngol Otol. 2008;122(11):1168–1174. doi: 10.1017/S0022215108001990. [DOI] [PubMed] [Google Scholar]
- 13.Sanna M, Fois P, Russo A, Falcioni M. Management of meningoencephalic herniation of the temporal bone: personal experience and literature review. Laryngoscope. 2009;119(8):1579–1585. doi: 10.1002/lary.20510. [DOI] [PubMed] [Google Scholar]
- 14.Semaan M T, Gilpin D A, Hsu D P, Wasman J K, Megerian C A. Transmastoid extradural-intracranial approach for repair of transtemporal meningoencephalocele: a review of 31 consecutive cases. Laryngoscope. 2011;121(8):1765–1772. doi: 10.1002/lary.21887. [DOI] [PubMed] [Google Scholar]
- 15.Pappas D G, Hoffman R A, Holliday R A, Hammerschlag P E, Pappas D G, Swaid S N. Evaluation and management of spontaneous temporal bone cerebrospinal fluid leaks. Skull Base Surg. 1995;5(1):1–7. doi: 10.1055/s-2008-1058944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kari E, Mattox D E. Transtemporal management of temporal bone encephaloceles and CSF leaks: review of 56 consecutive patients. Acta Otolaryngol. 2011;131(4):391–394. doi: 10.3109/00016489.2011.557836. [DOI] [PubMed] [Google Scholar]
- 17.Gubbels S P, Selden N R, Delashaw J B Jr, McMenomey S O. Spontaneous middle fossa encephalocele and cerebrospinal fluid leakage: diagnosis and management. Otol Neurotol. 2007;28(8):1131–1139. doi: 10.1097/MAO.0b013e318157f7b6. [DOI] [PubMed] [Google Scholar]
- 18.Brown N E, Grundfast K M, Jabre A, Megerian C A, O'Malley B W Jr, Rosenberg S I. Diagnosis and management of spontaneous cerebrospinal fluid-middle ear effusion and otorrhea. Laryngoscope. 2004;114(5):800–805. doi: 10.1097/00005537-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Dutt S N, Mirza S, Irving R M. Middle cranial fossa approach for the repair of spontaneous cerebrospinal fluid otorrhoea using autologous bone pate. Clin Otolaryngol Allied Sci. 2001;26(2):117–123. doi: 10.1046/j.1365-2273.2001.00438.x. [DOI] [PubMed] [Google Scholar]
- 20.Glasscock M E III, Dickins J RE, Jackson C G, Wiet R J, Feenstra L. Surgical management of brain tissue herniation into the middle ear and mastoid. Laryngoscope. 1979;89(11):1743–1754. doi: 10.1288/00005537-197911000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Gacek R R. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol. 1990;99(11):854–862. doi: 10.1177/000348949009901102. [DOI] [PubMed] [Google Scholar]
- 22.Lundy L B, Graham M D, Kartush J M, LaRouere M J. Temporal bone encephalocele and cerebrospinal fluid leaks. Am J Otol. 1996;17(3):461–469. [PubMed] [Google Scholar]
- 23.Prichard C N, Isaacson B, Oghalai J S, Coker N J, Vrabec J T. Adult spontaneous CSF otorrhea: correlation with radiographic empty sella. Otolaryngol Head Neck Surg. 2006;134(5):767–771. doi: 10.1016/j.otohns.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Patel R B Kwartler J A Hodosh R M Baredes S Spontaneous cerebrospinal fluid leakage and middle ear encephalocele in seven patients Ear Nose Throat J 2000795372–373., 376–378 [PubMed] [Google Scholar]
- 25.Jackson C G Pappas D G Jr Manolidis S et al. Brain herniation into the middle ear and mastoid: concepts in diagnosis and surgical management Am J Otol 1997182198–205.; discussion 205–206 [PubMed] [Google Scholar]
- 26.Mosnier I, Fiky L EL, Shahidi A, Sterkers O. Brain herniation and chronic otitis media: diagnosis and surgical management. Clin Otolaryngol Allied Sci. 2000;25(5):385–391. doi: 10.1046/j.1365-2273.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 27.Nahas Z, Tatlipinar A, Limb C J, Francis H W. Spontaneous meningoencephalocele of the temporal bone: clinical spectrum and presentation. Arch Otolaryngol Head Neck Surg. 2008;134(5):509–518. doi: 10.1001/archotol.134.5.509. [DOI] [PubMed] [Google Scholar]
- 28.Scurry W C Jr, Ort S A, Peterson W M, Sheehan J M, Isaacson J E. Idiopathic temporal bone encephaloceles in the obese patient. Otolaryngol Head Neck Surg. 2007;136(6):961–965. doi: 10.1016/j.otohns.2006.11.036. [DOI] [PubMed] [Google Scholar]


