Abstract
Introduction
Uniform coordinate systems in neuroimaging research have enabled comprehensive systematic and quantitative meta-analyses. Such approaches are particularly relevant for neuropsychiatric diseases, the understanding of their symptoms, prediction and treatment. Behavioral variant frontotemporal dementia (bvFTD), a common neurodegenerative syndrome, is characterized by deep alterations in behavior and personality. Investigating this ‘nexopathy’ elucidates the healthy social and emotional brain.
Methods
Here, we combine three multimodal meta-analyses approaches – anatomical & activation likelihood estimates and behavioral domain profiles – to identify neural correlates of bvFTD in 417 patients and 406 control subjects and to extract mental functions associated with this disease by meta-analyzing functional activation studies in the comprehensive probabilistic functional brain atlas of the BrainMap database.
Results
The analyses identify the frontomedian cortex, basal ganglia, anterior insulae and thalamus as most relevant hubs, with a regional dissociation between atrophy and hypometabolism. Neural networks affected by bvFTD were associated with emotion and reward processing, empathy and executive functions (mainly inhibition), suggesting these functions as core domains affected by the disease and finally leading to its clinical symptoms. In contrast, changes in theory of mind or mentalizing abilities seem to be secondary phenomena of executive dysfunctions.
Conclusions
The study creates a novel conceptual framework to understand neuropsychiatric diseases by powerful data-driven meta-analytic approaches that shall be extended to the whole neuropsychiatric spectrum in the future.
Keywords: behavioral variant frontotemporal dementia, cognitive neuropsychiatry, FDG-PET, meta-analysis, MRI
1. Introduction
Neurodegenerative disorders are a major public health problem. Frontotemporal lobar degeneration (FTLD) is the second most common diagnosis of dementia in individuals younger than 65 years (Johnson et al., 2005). Clinical criteria divide FTLD into three major subtypes: two subtypes affecting language functions, semantic dementia and progressive non-fluent aphasia, and the behavioral variant frontotemporal dementia (bvFTD) (Neary et al., 1998). bvFTD is the most common subtype and characterized by deep alterations in behavior and personality, namely decline in social interpersonal conduct, impairment in regulation of personal conduct, emotional blunting, and loss of insight (‘diagnostic core features’ according to Neary et al., 1998).
Recently, an international consortium revised bvFTD’s diagnostic criteria by focussing on clinical symptoms in histopathologically confirmed cases (Piguet et al., 2011; Rascovsky et al., 2011). Now, ‘possible’ bvFTD requires three of six clinically discriminating features: disinhibition, apathy/inertia, loss of sympathy/empathy, perseverative/stereotyped/ compulsive/ritualistic behaviors, hyperorality/dietary changes and dysexecutive neuropsychological profile. Interestingly, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) has included also a decline in social cognition to the criteria (American Psychiatric Association, 2013). ‘Probable’ bvFTD adds functional disability and characteristic neuroimaging (frontal and/or anterior temporal atrophy, hypometabolism or hypoperfusion). Finally, bvFTD ‘with definite FTLD’ requires histopathological confirmation or a pathogenic mutation. These revised criteria have a much higher sensitivity in comparison to the earlier criteria (Neary et al., 1998). Although the regional specificity of bvFTD’s imaging markers is still under debate (Schroeter, 2012), incorporating these markers will greatly improve early ante mortem identification of bvFTD, which is particularly relevant for timely treatment – a paradigm shift suggested also for other dementia syndromes (Dubois et al., 2007; Gorno-Tempini et al., 2011).
In the last two decades, neuroimaging studies have revolutionized the understanding of cognitive functions in healthy subjects and in brain diseases (Derrfuss and Mar, 2009; Yarkoni et al., 2011). Uniform coordinate systems enable comprehensive systematic and quantitative meta-analyses that might identify the prototypical neural networks involved in specific neuropsychiatric diseases, such as mood disorders, schizophrenia and dementia syndromes. Recent meta-analyses across imaging studies have applied the likelihood estimate method, the most refined and best-validated approach to coordinate-based voxelwise meta-analyses (Fox et al., 2005; Glahn et al., 2008; Laird et al., 2005; Sacher et al., 2012; Schroeter et al., 2007, 2008, 2009; Schroeter and Neumann, 2011; Turkeltaub et al., 2002). Here, two subtypes exist. The anatomical likelihood estimate (AnLE) method uses coordinates of peaks for atrophy, hypometabolism, or hypoperfusion during rest in patients if compared with control subjects, and determines brain regions that exhibit a higher convergence of these peaks across single studies than would arise by chance. The final AnLE map extracts the prototypical neural correlates of a specific disease based on large cohorts that cannot be investigated in single centre studies. The activation likelihood estimate (AcLE) method, using the same algorithms like the AnLE method, was developed earlier to conduct meta-analyses across functional imaging studies, where subjects are stimulated with psychological stimuli.
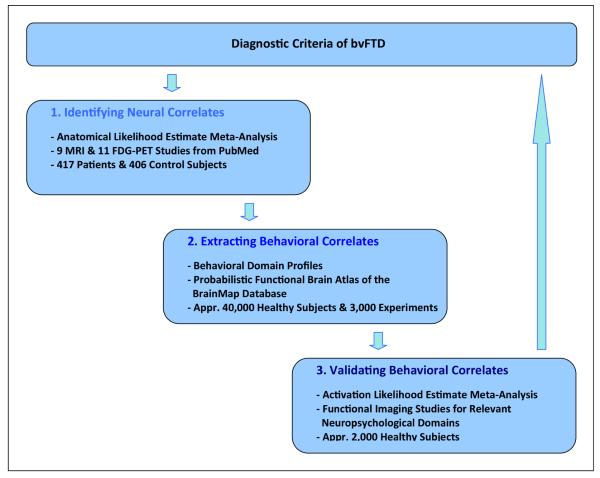
Here, we explore the general potential of combined multimodal imaging meta-analyses with AnLE and AcLE methods to conceptualize – i. e. understand and predict – neuropsychiatric diseases. We chose bvFTD as a model disease, a ‘molecular nexopathy’ (Warren et al., 2012; Zhou et al., 2012) disconnecting the ‘social brain’ (Adolphs, 2010). The rationale of our approach, combing three meta-analytic steps, is illustrated in Figure 1.
Figure 1.
Understanding and validating diagnostic criteria for neuropsychiatric diseases with powerful meta-analyses – Rationale of the study. bvFTD behavioral variant frontotemporal dementia, FDG-PET 18F-fluorodeoxyglucose-positron emission tomography, MRI magnetic resonance imaging.
Identifying bvFTD’s neural correlates
Firstly, we identified all relevant imaging studies of bvFTD from the literature containing 417 patients and 406 control subjects. We conducted an AnLE meta-analysis separately for morphometric studies with magnetic resonance imaging (MRI) and imaging studies applying 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) during rest. This meta-analysis identified the prototypical networks essential for bvFTD, and thereby validated diagnostic criteria as suggested recently by an international consortium (Rascovsky et al., 2011). Extracting bvFTD’s behavioral correlates: Secondly, we wanted to place bvFTD in a framework of cognitive neuropsychiatry by relating these neural changes to clinical and cognitive impairments (Halligan and David, 2001). Former studies discussed results of AnLE meta-analyses simply by reviewing the literature, which may be biased by subjective presumptions and the specificity problem – the fact that specific brain regions might be related to highly diverse brain functions (Schroeter et al., 2008). Overcoming this limitation we now applied a very new data-driven meta-analytic approach by calculating so-called behavioral domain profiles for the neural networks detected in the first AnLE meta-analysis. These behavioral domain profiles extract the behavioral, psychological or mental correlates of the networks affected by bvFTD (Laird et al., 2009a, 2009b; Lancaster et al., 2012). This second meta-analysis was conducted in a probabilistic functional brain atlas, the BrainMap database, containing whole brain functional imaging data from approximately 3000 experiments with 40,000 subjects. Validating bvFTD’s behavioral correlates: Finally, we compared neural correlates of bvFTD with results of AcLE meta-analyses across functional imaging studies investigating empathy and theory of mind (ToM), two typical symptoms of bvFTD, including approximately 2,000 healthy subjects (Adenzato et al., 2010; Rascovsky et al., 2011).
The three meta-analysis methods validate the concept and revised clinical criteria for bvFTD (Rascovsky et al., 2011) with a high reliability by including large numbers of subjects and plenty of cognitive dimensions/categories. The first two meta-analytic approaches (AnLE and behavioral domain profiles) guaranteed a data-driven design. Based on previous studies and the literature, we hypothesized an involvement of mainly frontomedian, insular and thalamic regions in bvFTD that are related to executive functions, emotion processing and social cognition (Rascovsky et al., 2011; Schroeter et al., 2007, 2008).
2. Materials & Methods
2.1. Identifying the Neural Correlates of bvFTD
The first meta-analysis aimed at identifying the neural correlates of bvFTD with AnLE meta-analyses. To ensure a high validity and quality the meta-analysis was conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines (Moher et al., 2009; see also http://www.prisma-statement.org/).
General Study Selection Criteria
The PubMed search engine was used to identify studies on morphometry and glucose utilization in bvFTD. The following search strategy was applied: (frontotemporal AND dementia) AND (PET OR MRI). Studies were included if they fulfilled the following criteria: 1) peer-reviewed, 2) diagnosis according to internationally recognized diagnostic criteria (Clinical and neuropathological criteria for bvFTD by the Lund and Manchester groups, 1994; McKhann et al., 2001; Neary et al., 1998), 3) original studies, 4) comparison with age matched healthy control group, and 5) results normalized to a stereotactic space such as the Talairach or the Montreal Neurological Institute (MNI) reference system, and respective coordinates available. If adequate information was not available authors were contacted to provide more details.
Studies that were based solely on region-of-interest analysis or case studies were excluded to prevent any a priori assumptions with regard to the involved neural networks and enable a data-driven approach. The contrast between patients and control groups was included, reporting either atrophy (MRI) or decreases in glucose utilization (FDG-PET) in the patient group. Since we did not find enough imaging studies applying single photon emission computed tomography (perfusion) or diffusion tensor imaging that reported coordinates in stereotactic space, studies were limited to MRI and FDG-PET. We limited our present meta-analysis to studies published between January 2005 and September 2010 and combined the relevant studies with studies already identified in our previous meta-analysis applying the same search strategy, the same inclusion/exclusion criteria, and covering imaging studies published between January 1980 and October 2005 (Schroeter et al., 2007, 2008).
Data Synthesis – Anatomical Likelihood Estimate Meta-Analysis Method
To extract the prototypical neural networks, we applied the AnLE meta-analysis method (Fox et al., 2005; Laird et al., 2005, 2011; Turkeltaub et al., 2002). Originally, the method was developed for meta-analyses of functional imaging studies including psychological stimulation of subjects (calculation of so called “activation likelihood estimate”, AcLE). Later on, the method has been extended to imaging studies during rest investigating atrophy, changes in metabolism or perfusion (“anatomical likelihood estimate”, AnLE) (Glahn et al., 2008; Sacher et al., 2012; Schroeter et al., 2007, 2008, 2009; Schroeter and Neumann, 2011). The general idea behind this method is to determine brain regions that exhibit a higher density of peak coordinates reported across studies than would arise by chance. Based on peaks of activation, atrophy, hypometabolism or hypoperfusion in comparison with control subjects as reported in the single studies, the algorithm calculates a map that represents the likelihood for each voxel that at least one of the reported peaks is located there. Finally, the analysis reveals brain regions that are most consistently activated during experimental paradigms (AcLE map) or involved in brain diseases (AnLE map).
Recently, an improved version of this method has been published by Eickhoff et al. (2009). The improvements include the adaptive estimation of the width of the Gaussian for each included study and the possibility to perform a random-effects analysis. We used the software GingerALE (http://brainmap.org/ale/index.html) for calculation of AnLE maps, and, where necessary, for the transformation of reported coordinates from Talairach and Tournoux space into stereotactic standard MNI space. The software models each reported maximum by a 3-dimensional Gaussian probability distribution centred at the given coordinate. The width of the Gaussian probability distribution is determined individually for each study based on empirical estimates of between-subject variability taking into account the number of subjects in each study. Probabilities of all maxima are then combined for each voxel, yielding a modeled atrophy or hypometabolism map.
In a random effects analysis, AnLE values in the modeled atrophy/hypometabolism maps are subsequently combined across studies and tested against the null hypothesis of a random spatial association between modeled maps thereby identifying those regions where empirical AnLE values are higher than could be expected by chance. The resulting AnLE map is thresholded at p<0.05 (corrected for multiple comparisons by false discovery rate, FDR). Statistically significant voxels represent the convergence of the investigated effect across the several studies. In our previous meta-analysis we pooled MRI and FDG-PET studies, because only a small number of studies was available for each method alone (Schroeter et al., 2007, 2008). Now, as a higher number of studies was available, we calculated the AnLE map for each imaging method separately, which allows the assessment of commonalities and differences between imaging modalities.
Potential Bias in Individual Studies or Across Studies
Several methods were applied reducing the risk of bias of individual studies and across studies that may affect cumulative evidence. Generally, imaging meta-analyses are likely to be biased toward particular cortical areas, a problem referred to previously as literature or publication bias. We avoided this problem by including only studies that used quantitative automated whole brain analysis, whereas region-of-interest studies were excluded. Consistently, we selected the contrast bvFTD vs. healthy control subjects and expelled comparisons of bvFTD with other dementia syndromes to beware selective reporting within studies. To ensure high reliability the literature search, selection of studies according to the inclusion and exclusion criteria, and compilation of coordinates for the contrasts were performed independently by two investigators (CC & CD). For data synthesis we took into account the number of subjects involved in each individual study enabling a balanced analysis (Eickhoff et al., 2009). Although studies with negative findings might theoretically have been omitted (missing studies), this publication bias is unlikely, because the published bvFTD studies reported strong effects for atrophy or hypometabolism. Some patients present with clinical bvFTD symptoms without positive imaging findings and no further deterioration (Kipps et al., 2009). Because the aetiology of such ‘phenocopy’ cases is still a matter of debate (Piguet et al., 2011), we have excluded these patients if explicitly mentioned in the studies (for instance one subject group in Kipps et al., 2009).
2.2. Extracting the Behavioral Correlates of bvFTD – Analysis of Behavioral Domain Profiles
The second systematic and quantitative meta-analysis aimed at identifying the behavioral, psychological or mental correlates of the networks affected by bvFTD that were characterized in the first AnLE meta-analysis (Laird et al., 2009b). We conducted this meta-analysis in the BrainMap database (Laird et al., 2005, 2009a, 2009b, 2011), which archives peak coordinates of activations and their corresponding meta-data (e.g., number of subjects, analysis technique, paradigm, cognitive domain, etc.) for whole brain functional imaging studies from the literature. At the time of the analysis, BrainMap contained 2114 neuroimaging publications that described analyses from 2994 experiments with 39,672 subjects using 83 unique paradigm classes, yielding 79,577 locations (12th May 2011). Neural clusters of bvFTD as characterized in the AnLE meta-analysis were defined as regions of interest. Thereafter, we identified behavioral or mental processes the regions play a role in executing (for details see Laird et al., 2009b). In BrainMap, meta-data are included on the cognitive, perceptual, or motor process isolated by the statistical contrast in functional imaging studies. The domain is classified according to five main categories – action, cognition, emotion, interoception, and perception – and their related subcategories. The categories contain the following subcategories: action: execution, imagination, inhibition, motor learning, observation, preparation, rest; cognition: attention, language, memory, music, reasoning, social cognition, soma, space, time; emotion: anger, anxiety, disgust, fear, happiness, sadness; interoception: air hunger, baroregulation, bladder, hunger, osmoregulation, sexuality, sleep, thermoregulation, thirst; and perception: audition, gestation, olfaction, somesthesis, vision. Moreover, 83 paradigm classes are systematically tested (for a complete list of BrainMap’s behavioral domains and paradigm classes see http://brainmap.org/scribe/).
We analyzed the behavioral domain meta-data associated with each cluster from the bvFTD AnLE results to determine the frequency of domain “hits” relative to its distribution across the whole brain (i.e., the entire database). This analysis was conducted for each imaging method separately (MRI and FDG-PET). For each region, a X2 test was performed to evaluate the regional distribution as compared to the overall database distribution. If the region’s distribution was significantly different, a binomial test was performed to determine which individual domains were over- or underrepresented. When a significant domain was observed, the test was repeated on the corresponding subcategories of that domain, yielding a hierarchical analysis of behavioral domain profiles that identifies the functional processes associated with the regions affected by bvFTD.
2.3. Validating the Behavioral Correlates of bvFTD – Conjunction with Activation Likelihood Estimate Meta-Analyses
Diagnostic criteria and literature data suggest that empathy and ToM are specifically impaired in bvFTD (Adenzato et al., 2010; American Psychiatric Association, 2013; Rankin et al., 2006; Rascovsky et al., 2011; Schroeter, 2012). Accordingly, we investigated in a conjunction analysis whether the neural correlates of bvFTD as identified in the first AnLE meta-analysis coincide with the neural correlates of empathy and ToM or mentalizing as investigated in a recent AcLE meta-analysis across whole brain functional imaging studies in healthy subjects (Bzdok et al., 2012).
3. Results
3.1. Identifying Neural Correlates of bvFTD with Anatomical Likelihood Estimate Meta-Analyses
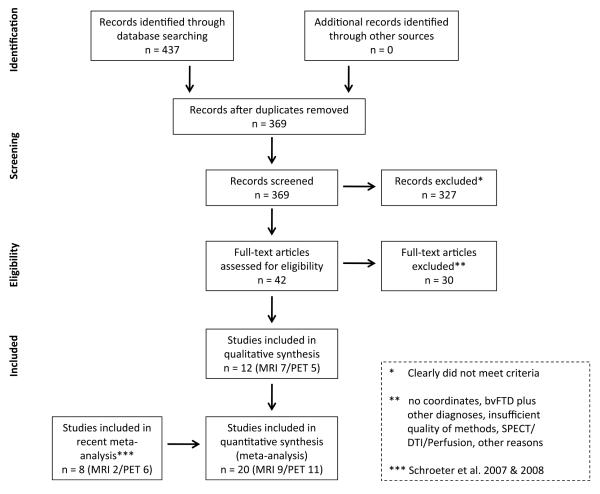
The first (AnLE) meta-analysis aimed at identifying the neural correlates of bvFTD. Figure 2 illustrates the flow of information through the different phases of this systematic review. Table 1 gives details for the imaging studies of bvFTD that were included. The present search identified 12 new studies since 2005 that measured atrophy (7) or reductions in glucose utilization (5) in bvFTD. Note that one study (Kanda et al., 2008) applied both MRI and FDGPET in their cohort and was counted as one MRI and one FDG-PET study. Together with our previous meta-analysis that identified 2/6 studies published between 1980 and 2005 (Schroeter et al., 2007, 2008), the final AnLE meta-analysis included 20 studies applying MRI (9) or FDG-PET (11) and published between 1980 and 2010. In sum, 417 patients with bvFTD were included (MRI 185/FDG-PET 232) and 406 control subjects.
Figure 2.
Flow of information through the different phases of the systematic review identifying the neural correlates of behavioral variant frontotemporal dementia (bvFTD) according to the PRISMA statement (Moher et al., 2009). DTI diffusion tensor imaging, MRI magnetic resonance imaging, PET 18F-fluorodeoxyglucose-positron emission tomography, SPECT single photon emission computed tomography.
Table 1.
Studies included in the meta-analysis identifying bvFTD’s neural correlates
| Study | Subjects (N) bvFTD/Co |
Patients’ sex (f/m) |
Age (years) |
Disease duration (years) |
MMSE | Remarks |
|---|---|---|---|---|---|---|
| MRI | ||||||
| Boccardi et al., 2005 | 9/26 | 2/7 | 62±5 | 2±1 | 14±8 | a |
| Grossman et al., 2004 | 14/12 | NS | 63±12 | 4±3 | 18±6 | |
| Kanda et al., 2008 | 13/20 | NS | 65 | NS | 18 | a/b |
| Kipps et al., 2009 | 11/12 | NS | 62±7 | 5±4 | 25±3 | a/b |
| Pardini et al., 2009 | 22/14 | NS | 60±8 | 5±3 | NS | # |
| Pereira et al., 2009 | 4/25 | 1/3 | 60±8 | 5±5 | 23±4 | a/b |
| Seeley et al., 2008 | 45/45 | 19/26 | 64±9 | 6±4 | 22±6 | a/b |
| Whitwell et al., 2005 | 5/20 | 2/3 | 55±2 | 5±1 | 27±1 | a/b |
| Zamboni et al., 2008 | 62/14 | 33/29 | 61 ± 1 | NS | NS | a/b |
| Total | 185/188 | 61.3±2.9 | 4.6±1.3 | 21.0±4.5 | ||
|
| ||||||
| FDG-PET | ||||||
| Diehl et al., 2004 | 25/15 | 13/12 | 62±8 | 2±2 | 24±4 | |
| Franceschi et al., 2005 | 18/24 | 6/12 | 66 | 2 | 25 | |
| Grimmer et al., 2004 | 10/15 | 3/7 | 60±8 | NS | 25±3 | a |
| Jeong et al., 2005a | 29/11 | 19/10 | 59±11 | 2±1 | 15±10 | a |
| Jeong et al., 2005b | 8/11 | 6/2 | 58±7 | 1 ± 1 | 18±8 | a |
| Kanda et al., 2008 | 13/20 | NS | 65 | NS | 18 | |
| Laws et al., 2007 | 41/16 | 9/32 | 62±11 | 4±2 | 22±5 | a |
| Perneczky et al., 2007 | 29/16 | 9/20 | 62±11 | 3±2 | 22±5 | a |
| Peters et al., 2006 | 23/23 | 10/13 | 64±8 | 3±2 | NS | |
| Raczka et al., 2010 | 7/9 | 2/5 | 60±8 | NS | 27±3 | |
| Salmon et al., 2003 | 29/58 | 14/15 | 62±9 | 3±2 | 22±5 | a |
|
| ||||||
| Total | 232/218 | 61.8±2.5 | 2.5±0.9 | 21.8±3.8 | ||
| Total MRI/FDG-PET | 417/406 | 61.6±2.6 | 3.5±1.5 | 21.5±4.0 | ||
Age, disease duration and MMSE scores (Mini Mental State Examination) are specified for patients (mean±standard deviation). bvFTD behavioral variant frontotemporal dementia, Co controls, f female, FDG-PET 18F-fluorodeoxyglucose positron emission tomography, m male, MNI Montreal Neurological Institute template, MRI magnetic resonance imaging, NS not specified. Values on clinical dementia rating (CDR) scale only reported in six studies. References for the included studies are available in the supplemental information. All MRI studies used 1.5 T, except Kipps et al., 2009, who did not specify field strength.
Correction for multiple comparisons.
Modulated.
The bvFTD patient cohort was characterized by a mean age of 61.6+2.6 years, a disease duration of 3.5+1.5 years and a mean value of the Mini Mental State Examination (MMSE) of 21.5+4.0. Whereas mean age and MMSE as a measure for disease severity did not differ between MRI studies and FDG-PET studies (T=-0.4, =-0.4; df=18, =15; p=0.69, =0.70), the MRI cohort was characterized by a significantly longer disease duration than the FDG-PET cohort (4.6+1.3 vs. 2.5+0.9 years; T=3.6; df=13; p=0.003; two-tailed unpaired Student’s t test).
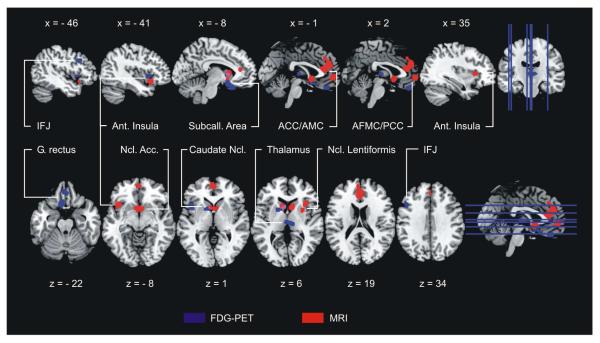
Figure 3 and Table 2 illustrate the results of the AnLE meta-analysis identifying the neural correlates of bvFTD. The analysis across MRI studies revealed regional atrophy mainly in the frontomedian cortex, the anterior insulae and the basal ganglia. More specifically, the disease affected bilaterally the subcallosal area, pregenual anterior cingulate, anterior midcingulate and paracingulate gyri, the anterior frontomedian cortex, the caudate head & body, the nucleus accumbens, both anterior insulae and the right putamen. Glucose metabolism as measured by FDG-PET was reduced in bvFTD in frontal brain regions and the basal ganglia, namely the subcallosal area, caudate head & body, nucleus accumbens, nucleus lentiformis and the left anterior insula. Exceeding atrophy, bvFTD reduced glucose metabolism bilaterally in the gyrus rectus, the middle thalamus and the left posterior inferior frontal sulcus/inferior frontal junction area. Comparing results for both imaging methods revealed only two small overlapping affected brain regions – one in the left ventral striatum and another in the left caudate nucleus (see Figures 3 & 6).
Figure 3.
Impaired brain regions in behavioral variant frontotemporal dementia in comparison with healthy control subjects – Anatomical likelihood estimates. Atrophy as measured by MRI red, hypometabolism as measured by FDG-PET blue, overlap pink. Nine MRI and 11 FDGPET studies including 417 patients and 406 control subjects. Left is left. MNI coordinates. Acc. accumbens, ACC/AMC anterior cingulate & midcingulate cortex, AFMC/PCC anterior frontomedian/paracingulate cortex, Ant. anterior, FDG-PET 18F-fluorodeoxyglucose positron emission tomography, G. gyrus, IFJ inferior frontal junction, MNI Montreal Neurological Institute, MRI magnetic resonance imaging, Ncl. nucleus, Subcall. subcallosal.
Table 2.
Results of the meta-analysis identifying bvFTD’s neural correlates with anatomical likelihood estimates
| Region | Cluster | BA | Lat. | x | y | z | AnLE | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|
| FDG-PET | ||||||||
| L. subcallosal area, posterior gyrus rectus, caudate head, nucleus accumbens |
F1 | 11-14/25 | L. | −8 −8 |
10 20 |
−14 −24 |
0.0186 0.0164 |
2631 |
| L. caudate head & body, nucleus lentiformis |
F2 | L. | −10 −18 |
10 8 |
2 4 |
0.0155 0.0139 |
1480 | |
| Bl. middle gyrus rectus | F3 | 11/13/14 | Bl. | −2 | 42 | −24 | 0.0165 | 1192 |
| Bl. middle thalamus | F4 | Bl. | 2 10 |
−18 −20 |
4 6 |
0.0152 0.0123 |
1096 | |
| L. superior anterior insula | F5 | 15 | L. | −42 | 12 | 0 | 0.0152 | 816 |
| L. posterior inferior frontal sulcus/inferior frontal junction |
F6 | 6/9/44 | L. | −46 | 18 | 32 | 0.0127 | 568 |
|
| ||||||||
| MRI | ||||||||
| Bl. subcallosal area, caudate head & body, nucleus accumbens |
M1 | 25 | Bl. | 2 8 −8 |
8 12 14 |
−6 10 6 |
0.0292 0.0289 0.0257 |
4832 |
| Bl. pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, anterior frontomedian cortex |
M2 | 9/32/33 | Bl. | −2 0 0 |
36 46 36 |
14 26 38 |
0.0291 0.0271 0.0156 |
4744 |
| Bl. anterior frontomedian cortex | M3 | 10/12 | Bl. | 0 | 54 | −4 | 0.0348 | 1552 |
| L. anterior inferior insula | M4 | 16 | L. | −40 −44 |
18 8 |
−12 −8 |
0.0279 0.0107 |
1488 |
| R. anterior superior insula, putamen |
M5 | 15 | R. | 28 36 |
6 18 |
8 6 |
0.0194 0.0166 |
1264 |
Clusters above an anatomical likelihood estimate (AnLE) threshold p<0.05, false discovery rate (FDR) corrected for multiple comparisons, are listed. Coordinates are in MNI space. BA Brodmann area, Bl. Bilateral, bvFTD behavioral variant frontotemporal dementia, FDG-PET 18F-fluorodeoxyglucose positron emission tomography, L. left, Lat. Lateralization, MNI Montreal Neurological Institute, MRI magnetic resonance imaging, R. right.
Figure 6.
Conceptualizing behavioral variant frontotemporal dementia with combined meta-analyses. The figure illustrates brain regions involved in the disease (light blue), their related cognitive & behavioral correlates (orange, purple and red) and clinical symptoms (dark blue). ACC/AMC anterior cingulate & midcingulate cortex, Ant. anterior, IFJ inferior frontal junction, PCC paracingulate cortex.
Repeating the AnLE meta-analysis with all relevant studies published until April 2012 (04-15-2012) confirmed these disease-specific neural networks involved in bvFTD. The new meta-analysis included only four additionally relevant original studies. Note that the new studies investigated aspects of imaging data in bvFTD cohorts of whom imaging studies with the same methods had already been published in recent years and have been included in the meta-analysis ranging from 1980 to 2010. Accordingly, one might assume substantial overlaps in cohorts with former studies already included in our meta-analysis here, leading to potential bias. The new meta-analysis in 2012 did not identify any other brain region, and, consequently, guaranteed a stable and reliable effect with high validity. For single photon emission computed tomography (SPECT) we identified only one additionally published study between 2010 and 2012, making meta-analyses still not possible. We report meta-analytic results ranging from 1980 to 2010 (i) to avoid the aforementioned bias by ruling out overlapping patient samples, (ii) because the effects were stable and replicated in the 2012 meta-analysis, and (iii) because the following meta-analyses (calculation of behavioral domain profiles and conjunction with AcLE maps) were based on this meta-analysis.
3.2. Extracting Behavioral Correlates of bvFTD – Analysis of Behavioral Domain Profiles
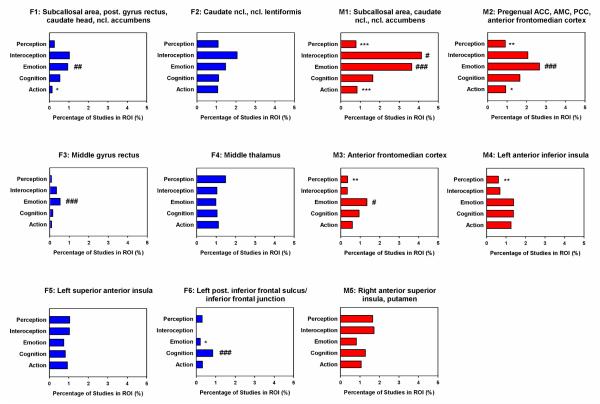
The second meta-analysis characterized the behavioral, psychological or mental correlates of networks affected by bvFTD. It was conducted in the BrainMap database, which archives peak coordinates of statistical contrasts of functional imaging studies across a wide range of cognitive paradigms from the literature. We defined clusters from the anatomical meta-analysis (AnLE) as regions of interest and identified behavioral or mental processes the regions play a role in executing. Results are illustrated in Figure 4 and Table 3.
Figure 4.
Behavioral domain profiles associated with each hypometabolic (blue) or atrophic (red) brain region in behavioral variant frontotemporal dementia. Data are expressed as percentage of specific studies in the region of interest in comparison with the whole brain. Probability higher than expected ###p<0.001, ##p<0.01, #p<0.05; lower than expected ***p<0.001, **p<0.01, *p<0.05. ACC/AMC anterior cingulate & midcingulate cortex, AFMC/PCC anterior frontomedian/paracingulate cortex, Ncl. nucleus, Post. Posterior, ROI region of interest.
Table 3.
Results of the meta-analysis extracting bvFTD’s behavioral correlates by analyzing behavioral domain profiles
| Region | Cluster | BA | Behavioral Domain Profile Subcategory |
Paradigm Class |
|---|---|---|---|---|
| FDG-PET | ||||
| L. subcallosal area, posterior gyrus rectus, caudate head, nucleus accumbens |
F1 | 11-14/25 | n.s. | Reward taska |
| L. caudate head & body, nucleus lentiformis |
F2 | n.s. | Reward taska | |
| Bl. middle gyrus rectus | F3 | 11/13/14 | n.s. | n.s. |
| Bl. middle thalamus | F4 | n.s. | n.s. | |
| L. superior anterior insula | F5 | 15 | n.s. | Word generation (covert)c |
| L. posterior inferior frontal sulcus/inferior frontal junction |
F6 | 6/9/44 | n.s. | Cued explicit recognitionb |
|
| ||||
| MRI | ||||
| Bl. subcallosal area, caudate head & body, nucleus accumbens |
M1 | 25 | Action: imaginationb Perception: olfactiona |
Reward taska |
| Bl. pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, anterior frontomedian cortex |
M2 | 9/32/33 | Action: inhibitionb | Reward taska |
| Bl. anterior frontomedian cortex | M3 | 10/12 | n.s. | Reward taska; chewing/swallowinga |
| L. anterior inferior insula | M4 | 16 | n.s. | n.s. |
| R. anterior superior insula, putamen |
M5 | 15 | n.s. | Pain monitor/ discriminationa recitation/repetition (overt)c |
p<0.001,
p<0.01,
p<0.05 higher than expected. BA Brodmann area, Bl. Bilateral, bvFTD behavioral variant frontotemporal dementia, FDG-PET 18F-fluorodeoxyglucose positron emission tomography, L. left, Lat. lateralization, MRI magnetic resonance imaging, n.s. not significant, R. right.
Firstly, the analysis was conducted for the five main categories action, cognition, emotion, interoception, and perception for each of the bvFTD clusters, separately for MRI and FDG-PET. Figure 4 shows histograms of the behavioral domains associated with each hypometabolic or atrophic region in bvFTD. Data are expressed as percentage of specific studies in the region of interest in comparison with the whole brain. Most consistent results were obtained for emotion processing for both MRI and FDG-PET. Five clusters were associated with higher involvement in emotion processing based on functional studies. These clusters included the caudate head & body, nucleus accumbens, gyrus rectus, subcallosal area, pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, and anterior frontomedian cortex (F1, F3, M1-M3; codes for clusters see Table 2). One cluster (F6), the left inferior frontal junction, was significantly related to cognition according to BrainMap meta-data. Finally, interoception was related to one atrophic cluster in the caudate nucleus, nucleus accumbens and subcallosal area (M1). Although some regions were less likely involved in cognitive/behavioral/emotional processes (Figure 4), we do not describe and discuss these results, because ‘less likely involvement’ does not predict any positive involvement and functional deficits if the region is affected by the disease.
Another analysis investigated the association between the various bvFTD clusters and the subcategories of action, cognition, emotion, interoception, perception and the 83 paradigm classes from the BrainMap database separately for MRI and FDG-PET (Table 3). Results showed only higher probable association with domains/paradigms. Most consistently, meta-analytic data of functional imaging studies in the BrainMap database were related to reward processing in five hypometabolic and atrophic clusters, in particular the caudate head & body, nucleus lentiformis, nucleus accumbens, gyrus rectus, subcallosal area, pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, and anterior frontomedian cortex (F1 & F2, M1-M3). Additionally, bvFTD’s neural networks were associated with the processes of inhibition (pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, anterior frontomedian cortex; M2), cued explicit recognition (inferior frontal junction; F6), covert word generation (left superior anterior insula; F5), overt recitation/repetition and pain monitoring/discrimination (right anterior superior insula, putamen; M5), chewing/swallowing (anterior frontomedian cortex; M3), olfaction and action imagination (subcallosal area, caudate head & body, nucleus accumbens; M1).
3.3. Validating Behavioral Correlates of bvFTD – Conjunction with Activation Likelihood Estimate Meta-Analyses
Because diagnostic criteria and literature data suggest that empathy and ToM are specifically impaired in bvFTD (Adenzato et al., 2010; Rascovsky et al., 2011), we investigated in a conjunction analysis whether the neural correlates of bvFTD as identified in the first AnLE meta-analysis coincide regionally with the neural correlates of empathy and ToM or mentalizing. We used data from Bzdok et al. (2012) that applied AcLE meta-analyses across whole brain functional imaging studies for empathy, ToM and moral cognition in 1,790 healthy subjects. Figure 5 illustrates the results. We detected a large overlap between the neural correlates of bvFTD and empathy networks in both anterior insulae, anterior cingulate, midcingulate and paracingulate cortices, whereas ToM networks and bvFTD networks coincided only in a tiny area in the anterior frontomedian cortex. Quantifying results we calculated absolute (in voxels) and relative (percentage) overlap. Networks for bvFTD and empathy overlapped in 253 voxels for MRI and 96 voxels for FDG-PET, whereas for bvFTD and ToM only in 47 voxels for MRI and 0 voxels for FDG-PET. If related to the whole bvFTD network, overlapping regions with empathy networks were much greater for MRI (14.5%) and FDG-PET (9.9%) than with ToM networks (MRI 2.7%, FDG-PET 0%).
Figure 5.
Conjunction between impaired brain regions in behavioral variant frontotemporal dementia (FDG-PET blue, MRI red) and neural correlates of empathy (green) and theory of mind (cyan). Anatomical and functional activation likelihood estimate meta-analyses. Left is left. MNI coordinates. Overlap yellow and light-blue for empathy and white for theory of mind. ACC/AMC/PCC anterior cingulate & midcingulate cortex/paracingulate cortex, AFMC anterior frontomedian cortex, Ant. anterior, FDG-PET 18F-fluorodeoxyglucose positron emission tomography, MNI Montreal Neurological Institute, MRI magnetic resonance imaging.
4. Discussion
Our study has created a novel conceptual framework to understand neuropsychiatric diseases by powerful data-driven meta-analytic approaches. We chose bvFTD as a model disease. We combined new systematic and quantitative meta-analytic approaches to contribute to the understanding of bvFTD in the framework of cognitive neuropsychiatry aiming at a scientific cognitive psychopathology of this disease (Halligan and David, 2001), to explore the ‘social brain’ with bvFTD as a highly relevant ‘nexopathy’ (Adolphs, 2010; Warren et al., 2012), and to validate diagnostic clinical and imaging markers for bvFTD as suggested recently by an international consortium (Rascovsky et al., 2011). The design enabled a very high validity by including approximately 400 patients with bvFTD – the largest cohort in the literature to our knowledge – and functional imaging data mainly from the BrainMap database including approximately 42,000 healthy subjects. Note that disease and healthy cohorts are independent, going far beyond traditional circular approaches investigating the neural correlates of mental/behavioral deficits in disease groups (Seeley et al., 2012). Additionally, the inclusion criterion of whole brain imaging studies for all meta-analyses guaranteed a data-driven approach. In the following, we discuss results in more detail.
4.1. Neural Correlates of bvFTD
The first meta-analysis identified the neural correlates of bvFTD separately for both imaging approaches, MRI and FDG-PET, measuring either atrophy or reduction of glucose utilization in comparison with healthy control subjects. Most interestingly, this approach dissociated regionally the atrophic and hypometabolic regions in bvFTD with only two partly overlapping regions in the left ventral striatum and caudate nucleus. Figure 6 gives an overview of the regions involved in bvFTD and its functional correlates. The overall network included mainly the frontomedian cortex, anterior insulae and thalamus, regions that were already identified as specific for bvFTD in a previous meta-analysis with the same method that pooled MRI and FDG-PET data due to small numbers of studies (Schroeter et al., 2008). A selective decrease in the density of von Economo neurons has been reported in bvFTD in these areas (Kim et al., 2012; Seeley et al., 2006) – neurons that are related to socio-emotional functions and consciousness (Critchley and Seth, 2012; Evrard et al., 2012). Additionally, our study identified lateral prefrontal areas, the left inferior frontal junction area, and, most interestingly, subcortical regions to be involved in bvFTD, in particular the basal ganglia including the caudate nuclei, the nucleus accumbens (‘ventral striatum’), the putamen and globus pallidus (lentiform nucleus) extending results of one recent MRI effect size signed differential mapping meta-analysis on bvFTD (Pan et al., 2012).
Our results suggest that diagnostic imaging criteria for bvFTD (Rascovsky et al., 2011) shall be specified for each imaging approach separately, measuring atrophy, hypometabolism or hypoperfusion as it has already been suggested for Alzheimer’s disease (Dubois et al., 2007). Generally, we assume the same regions to be involved for hypometabolism of glucose and hypoperfusion as already shown for Alzheimer’s disease (Schroeter and Neumann, 2011). Interestingly, disease duration differed between subjects involved in atrophy (MRI) and metabolism (FDG-PET) studies in our meta-analysis with shorter (almost half) disease duration in metabolism studies in comparison with atrophy studies, and without differences for mean age and disease severity. Although this fact might suggest a chronological process in bvFTD, this could not be confirmed with our data sample, as the investigated MRI and FDG-PET cohorts did only partly overlap. Accordingly, further longitudinal studies have to explore whether the different imaging approaches mirror a topographical and chronological hierarchical ‘nexopathic’ model as suggested for Alzheimer’s disease previously, where regional hypometabolism, presumably due to diaschisis/disconnection effects, is followed by regional atrophy (Chételat et al., 2008; Dukart et al., 2013b; Jack et al., 2010; Villain et al., 2008; Woost et al., 2013). The (meta-analytically) identified prototypical networks might be used as ‘hubs or networks of interest’ to increase individual diagnostic accuracy based on multimodal imaging and machine learning algorithms such as support vector machine classification (Dukart et al., 2011, 2013a), and to further the disease’s understanding with connectivity analyses (Farb et al., 2013; Filippi et al., 2013; Jech et al., 2013; Mueller et al., 2013; Seeley et al., 2009; Warren et al., 2012; Zhou et al., 2012).
Our data support the idea that bvFTD is a mainly (medio)frontal and basal ganglia disease, where the core network of the disease does not include temporal regions (Schroeter, 2012). As imaging criteria for bvFTD seem to be regionally ‘underspecified’ to date (Rascovsky et al., 2011; Schroeter, 2012) one might define its anatomical correlates more in detail such as already suggested for language subtypes of FTLD (Gorno-Tempini et al., 2011).
4.2. Behavioral Correlates of bvFTD – Emotion Processing & Empathy
The identified neural correlates of bvFTD have been related to various emotional and cognitive functions in the literature (Schroeter et al., 2007, 2008). The anterior cingulate cortex is well known to be related to the perception of emotion and pain (Frith and Frith, 2003; Vogt, 2005), the midline and intralaminar thalamic nuclei (particularly the parafascicular nucleus) are involved in pain processing (Vogt, 2005). Indeed, our second data-driven meta-analytic approach (behavioral domain profiles) confirmed that bvFTD affects mainly and most consistently the emotional network including the caudate head & body, nucleus accumbens, gyrus rectus, subcallosal area, pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, and anterior frontomedian cortex. All of these regions were highly associated with functional activations during emotional paradigms in healthy subjects. Moreover, the second meta-analysis showed with behavioral domain profiles that the right anterior superior insula and putamen are associated with pain monitoring/discrimination. These findings are mirrored in the clinical deficits of subjects with bvFTD showing an inappropriate emotional shallowness with unconcern, a loss of emotional warmth, an indifference to others, impairments in recognizing both facial and vocal expressions of emotions and a loss of pain awareness (reviewed in Schroeter et al., 2007, 2008).
Proper emotional abilities are a prerequisite to empathy, the ability to share another’s emotional state. It is well known that patients with bvFTD are impaired in empathy (Rankin et al., 2006), which is even contained in the new revised bvFTD criteria (Rascovsky et al., 2011). Indeed, the comprehensive AcLE meta-analysis on empathy across functional imaging studies revealed a close overlap with the impaired networks of bvFTD in both anterior insulae, anterior cingulate, midcingulate and paracingulate cortices. The negative finding in the BrainMap database is just related to the fact that empathy is not contained as a category there.
4.3. Behavioral Correlates of bvFTD – Executive Functions
Deficits in executive functions are a well known feature of bvFTD and already included in the old and revised criteria (Neary et al., 1998; Raczka et al., 2010; Rascovsky et al., 2011; Schroeter et al., 2012). Based on literature data and comprehensive AcLE meta-analyses in healthy subjects, executive or control functions depend mainly on the left lateral prefrontal cortex, in particular the inferior frontal junction (IFJ), and on the anterior cingulate cortex (Derrfuss et al., 2005; Niendam et al., 2012; Ridderinkhof et al., 2004; Vogt, 2005). Our second data-driven meta-analysis confirmed this assumption as the left inferior frontal junction was significantly related to the domain ‘cognition’ and another cluster containing the pregenual anterior cingulate, anterior midcingulate/paracingulate gyrus, and anterior frontomedian cortex to ‘inhibition’ according to the functional BrainMap meta-data.
4.4. Behavioral Correlates of bvFTD – Reward Processing
Reward is a central component for driving incentive-based learning, appropriate responses to stimuli and the development of goal-directed behaviors (Haber and Knutson, 2010). Adaptive behaviors as essential for human beings require a combination of reward evaluation, associative learning, developing appropriate action plans and inhibiting inappropriate choices on the basis of earlier experience. Hence, integrating reward processing and cognition is essential for successful behavior.
According to our first AnLE meta-analysis bvFTD affects the most important and highly interconnected regions for reward processing in the ‘reward circuit’ (Haber and Knutson, 2010) – the anterior cingulate cortex, the orbitofrontal/ventromedial prefrontal cortex, the ventral striatum, containing the nucleus accumbens and one subcomponent, the shell of the ventral striatum, and the midline thalamus. These areas have been discussed as relevant for both primary (pleasant tastes, food and sexual stimuli, drugs) and secondary rewards (monetary gains, gambling) (Haber and Knutson, 2010). Recently, a functional AcLE meta-analysis added the anterior insula to the reward circuit (Liu et al., 2011). The behavioral domain profile meta-analysis confirmed this assumption by identifying five relevant reward clusters that contained the caudate head & body, nucleus lentiformis, nucleus accumbens, gyrus rectus, subcallosal area, pregenual anterior cingulate, anterior midcingulate and paracingulate gyrus, and anterior frontomedian cortex.
4.5. Behavioral Correlates of bvFTD – Theory of Mind or Mentalizing Abilities
Based on literature and meta-analytic data, we expected a strong association between bvFTD’s alterations in the anterior medial frontal cortex (Brodmann area 9/32) and ToM or ‘mentalizing’ abilities, where mental states have to be attributed to self and other people and which constitutes one aspect of social cognition (Amodio and Frith, 2006; Frith and Frith, 2003). Although empathy is another prerequisite for social cognition it follows another concept. Whereas empathy represents an emotional sharing of another’s state, ToM affords only an understanding of this state (Bzdok et al., 2012; Hein and Singer, 2008). Surprisingly, behavioral domain profiles did not confirm our hypothesis for mentalizing abilities. On the one hand, this missing finding might be related to a small number of such studies in the BrainMap database. On the other hand, one might assume that the well known clinical impairments in ToM tasks in bvFTD are epiphenomena and are caused by deficits in other cognitive domains (Adenzato et al., 2010; Gregory et al., 2002; Rankin et al., 2006; Schroeter et al., 2007, 2008). This assumption is supported by a generally more anterior frontomedian localization of ToM networks according to AcLE meta-analyses than regions affected by bvFTD according to our study (beside one tiny regional overlap).
4.6. Towards a Concept of bvFTD
Figure 6 summarizes the results of our multimodal meta-analytic approach by suggesting a concept for bvFTD. Frontomedian structures, containing the anterior cingulate & midcingulate cortex, the paracingulate and anterior frontomedian cortex, are obviously the core hubs affected by bvFTD, because all clinical core dimensions (emotion and reward processing, empathy and executive functions, in particular inhibition) are associated with these structures. The impairment of frontomedian areas might further lead to a loss of self-awareness, self-knowledge and, finally, the ‘self’ in bvFTD as discussed recently (Miller et al., 2001; Schroeter et al., 2007, 2008). One might consider, accordingly, bvFTD as a prototypical frontomedian disease (Schroeter, 2012). Whether frontomedian structures are the first impaired in bvFTD has to be explored in longitudinal studies (Seeley et al., 2012). Beside the more general cognitive/emotional involvement of frontomedian brain areas, other hubs are related to specific dysfunctions in bvFTD – the left inferior frontal junction to executive deficits, the basal ganglia, subcallosal area and gyrus rectus to changes in emotion and reward processing, and the anterior insula to a loss of empathy/sympathy and changes in pain monitoring and discrimination.
How can the other specific clinical symptoms be integrated in this concept of bvFTD? Here, apathy, disinhibition and hyperorality/dietary changes are the most important clinical phenomena of interest (Rascovsky et al., 2011). All of them have been related to atrophy or hypometabolism in frontomedian regions (Rosen et al., 2005; Schroeter et al., 2011). Although still an issue of debate, one might hypothesize that frontomedian alterations might disturb the interplay and integration between reward and cognitive control functions, finally leading to disinhibition and hyperorality/dietary changes (Schroeter et al., 2011).
Of note, inhibitory control, one of the three core executive abilities beside working memory and task switching (Miyake et al., 2000), was the only specific executive function related to the neural (frontomedian) networks of bvFTD in our second meta-analysis with behavioral domain profiles. This result is in agreement with AcLE meta-analyses of functional imaging studies in healthy subjects that identified the frontomedian cortex and inferior frontal junction area as central for these executive core components (Christ et al., 2009; Derrfuss et al., 2005). Accordingly, inhibitory malfunction seems to support disinhibition and hyperorality/dietary changes, beside perseverative, stereotyped, compulsive and ritualistic behaviors in bvFTD. Interestingly, failure of inhibitory control is an early and specific feature in bvFTD (O’Callaghan et al., 2013). Beyond that, impairments in inhibition might represent the ‘missing link’ to ToM deficits (‘mind blindness’) that occur very early in bvFTD (Pardini et al., 2013). Our meta-analyses did not find a substantial overlap between networks involved in bvFTD and in ToM or mentalizing processes. Most interestingly, a recent study (Le Bouc et al., 2012) showed that bvFTD patients are selectively impaired in inhibiting their own mental perspective leading to ToM deficits. This kind of inhibition was closely correlated with inhibition abilities in a classical Stroop task.
Apathy or inertia, a syndrome of diminished motivation, is frequently observed in bvFTD. This syndrome might be discussed in the context of generally diminished reward abilities as it has been related to a dopaminergic neural network consisting of the ventral tegmental area, nucleus accumbens, ventral pallidum, and the anterior cingulate/prefrontal cortex (Der-Avakian and Markou, 2012; Huey et al., 2006; Schroeter et al., 2011). Our results for the reward system might support the idea that dopaminergic agents may improve behavioral symptoms in bvFTD (Huey et al., 2006) as shown also for decision-making behavior in this disease, namely less risk taking (Rahman et al., 2006).
4.7. Validation of Diagnostic Criteria for bvFTD
Our meta-analytic findings support the new clinical diagnostic criteria for bvFTD as developed by Rascovsky et al. (2011) and the American Psychiatric Association (2013) in the new DSM-5. The exclusion of ToM deficits in Rascovsky et al.’s criteria (2011) seems to be justified, because impairments in social cognition, as suggested solely in the DSM-5 criteria, might be related mainly to changes in empathy, emotional processing and inhibition in this disease. The new diagnostic imaging criteria, on the other hand, might be specified for region ((medio)frontal lobe and basal ganglia) and parameter (atrophy and glucose metabolism).
4.8. Study’s Limitations
Finally, one has to discuss the study’s limitations. Because AnLE/AcLE meta-analyses and behavioral domain profiles generally include maxima and not cluster sizes of the various imaging studies, they extract the prototypical, most characteristic neural networks representing the brain regions that are consistently involved in specific dementia syndromes or paradigms. Accordingly, single studies might have shown that dementia diseases may affect and functional imaging studies might involve other brain structures and may be regionally more unspecific than the present meta-analyses suggest. This argument is particularly relevant for the regional dissociation between the neural correlates of bvFTD and ToM. One might even argue that temporal clusters have been missed in bvFTD due to our meta-analytic method that considered only coordinates of maxima of atrophy/hypometabolism and not respective cluster sizes. However, one would expect also maxima of alterations in temporal regions in the original studies if highly relevant for this disease. Although for diagnostic and interpretative purposes, the most consistent prototypical or ‘essential’ brain networks are of highest interest, our results and hypotheses have generally to be validated in original patient studies.
Beyond FDG-PET and structural MRI, a plethora of new imaging studies have been published in recent years that focus on connectivity changes in bvFTD, including diffusion tensor imaging and resting-state fMRI. Although highly relevant for the field, methodological problems make conducting quantitative likelihood estimate meta-analyses across such studies impossible to date. Diffusion tensor imaging studies do not report coordinates of the changes’ maxima, rather they show regional maps for fractional anisotropy or radial/axial diffusivity. Resting state fMRI studies, on the other hand, use a wide range of methods including seed based and data-driven approaches, such as (fractional) amplitude of low-frequency fluctuations (FALFF & ALFF), independent or principal component analysis (PCA & ICA), or Eigenvector centrality mapping (ECM), each addressing different aspects of functional connectivity. Results of these different analysis approaches are thus not directly comparable, and so far no standard analysis technique for functional connectivity data has been established. These issues hamper likelihood estimate meta-analysis approaches for these imaging methods to date.
Another limitation might be related to the ‘ontology’ of the BrainMap database. This database contains specific paradigm classes depending on the studies conducted in the literature and their classification into behavioral domains. Accordingly, negative findings might be just related to the fact that categories are not represented here as shown for empathy. Furthermore, one might generally criticize validating diagnostic imaging criteria with studies that used imaging data for diagnosis. Here, we avoided such a circular argumentation by including only studies applying old diagnostic criteria and not revised criteria as suggested by Rascovsky et al. (2011). However, if enough studies are available a meta-analysis with the new criteria seems to be recommendable, because these criteria have a much higher sensitivity for bvFTD, and because they are based on histopathologically confirmed cases. Hence, this future analysis might yield neural networks more specifically related to bvFTD.
To further characterize the sample of bvFTD studies involved in our meta-analysis we checked how many subjects suffered from histopathologically or genetically confirmed bvFTD. Only two MRI (atrophy) studies fulfilled these criteria. From Whitwell et al. (2005) we included five cases with a positive family history and tau exon 10+16 C-to-T (cytosine-tothymine) splice site mutations (microtubule-associated protein tau). All of these five subjects presented clinically with bvFTD, justifying an inclusion in our meta-analysis. We excluded the other patients reported by Whitwell et al. (2005) with histopathologically proven ubiquitin-positive (tau- and alpha-synuclein-negative) inclusions or with Pick disease, because these patients presented clinically with different subtypes of FTLD (bvFTD and language variants, in particular semantic dementia and progressive non-fluent aphasia). The other relevant study by Pereira et al. (2009) included only histopathologically confirmed cases with FTLD. Of the four bvFTD patients, which were involved in our study, three had ubiquitin inclusions, and one had tau inclusions. In sum, just nine (9) cases of bvFTD in our study had either a genetically or histopathologically confirmed bvFTD, which is 2.16% of the whole (417) patients sample. Consequently, a sub-meta-analysis was not possible due to the very small number of histopathologically or genetically proven bvFTD patients and even their high histopathological/genetic variability. One can assume that these data had no impact on the overall results of our meta-analyses. However, one cannot rule out that further genetically positive or possibly not histopathologically verifiable cases were involved in our sample. More clinical information on patients involved in the single studies would further have helped to interpret the results of the current meta-analysis. In particular, rating of clinical impairment with the MMSE seems to be not optimal as it represents an instrument developed primarily for Alzheimer’s disease and not bvFTD. Accordingly, the MMSE might rather be unimpaired in early bvFTD. Although clinical instruments more appropriate for bvFTD exist, such as the Neuropsychiatric Inventory (NPI), the Clinical Dementia Rating Scale for FTLD (CDR FTLD) or the Frontal Systems Behavior Scale (FrSBe), no consensus has been achieved for the most valid instrument yet. Hence, these instruments have not been applied consistently in the original studies, and we could not include more clinical information for the analysis.
4.9. Conclusions
Our study has created a novel conceptual framework to understand neuropsychiatric diseases by powerful data-driven meta-analytic approaches. We combined three new systematic and quantitative meta-analytic methods to understand bvFTD in the framework of cognitive neuropsychiatry, and to validate its diagnostic clinical and imaging markers as suggested recently by an international consortium. The study identified the frontomedian cortex as the central hub beside the basal ganglia, anterior insulae and thalamus – with a regional dissociation between atrophy and hypometabolism. These neural networks were related to emotion processing, empathy, executive functions and reward processing suggesting these impairments as most relevant for bvFTD, whereas changes in mentalizing abilities seem to be secondary phenomena. Results suggest specifying diagnostic imaging criteria for the disease.
Supplementary Material
Highlights.
comprehensive meta-analyses enable understanding of neuropsychiatric diseases
we identify neural correlates of behavioral variant frontotemporal dementia
and extract mental functions associated with this disease
frontomedian cortex, basal ganglia, and insulae are most relevant brain regions
emotion/reward processing, empathy & executive functions are cognitive core domains
Acknowledgements
This work was supported by the Parkinson’s Disease Foundation (Grant No. PDF-IRG-1307), and by LIFE – Leipzig Research Center for Civilization Diseases at the University of Leipzig to Matthias L. Schroeter. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERFD) and by means of the Free State of Saxony within the framework of the excellence initiative. Furthermore, Matthias L. Schroeter and Jane Neumann are supported by the German Federal Ministry of Education and Research (BMBF; German FTLD Consortium – Grant No. FKZ 01GI1007A & FKZ 01EO1001). Jane Neumann further acknowledges funding by the German Research Foundation (SFB 1052). Angela Laird has been supported by the National Institutes of Health (Grant No. R01-MH074457). Simon B. Eickhoff acknowledges funding by the Human Brain Project (Grant No. R01-MH074457-01A1) and the Helmholtz Initiative on Systems Biology (Human Brain Model).
Abbreviations
- AcLE
Activation Likelihood Estimate
- AnLE
Anatomical Likelihood Estimate
- bvFTD
Behavioral variant Frontotemporal Dementia
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- FDG-PET
18F-Fluorodeoxyglucose Positron Emission Tomography
- FTLD
Frontotemporal Lobar Degeneration
- MMSE
Mini Mental State Examination
- MNI
Montreal Neurological Institute
- MRI
Magnetic Resonance Imaging
- ToM
Theory of Mind
Footnotes
Appendix – Supplemental Information
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adenzato M, Cavallo M, Enrici I. Theory of mind ability in the behavioural variant of frontotemporal dementia: An analysis of the neural, cognitive, and social levels. Neuropsychologia. 2010;48:2–12. doi: 10.1016/j.neuropsychologia.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, fifth edition. DSM-5. American Psychiatric Publishing, DC; Washington: 2013. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, Landeau B, Mézenge F, Poline JB, de la Sayette V, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer’s disease. Brain. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- Christ SE, Van Essen DC, Watson JM, Brubaker LE, McDermott KB. The contributions of prefrontal cortex and executive control to deception: evidence from activation likelihood estimate meta-analyses. Cerebral Cortex. 2009;19:1557–1566. doi: 10.1093/cercor/bhn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups Journal of Neurology Neurosurgery and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H, Seth A. Will studies of macaque insula reveal the neural mechanisms of self-awareness? Neuron. 2012;74:423–426. doi: 10.1016/j.neuron.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Human Brain Mapping. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Mar RA. Lost in localization: The need for a universal coordinate database. NeuroImage. 2009;48:1–7. doi: 10.1016/j.neuroimage.2009.01.053. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dukart J, Mueller K, Horstmann A, Barthel H, Möller HE, Villringer A, et al. Combined evaluation of FDG-PET and MRI improves detection and differentiation of dementia. PLoS One. 2011;6:e18111. doi: 10.1371/journal.pone.0018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J, Mueller K, Barthel H, Villringer A, Sabri O, Schroeter ML. Meta-analyses-based SVM classification enables accurate detection of Alzheimer’s disease across different clinical centers using FDG-PET and MRI. Psychiatry Research Neuroimaging. 2013a;212:230–236. doi: 10.1016/j.pscychresns.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Dukart J, Mueller K, Villringer A, Kherif F, Draganski B, Frackowiak R, et al. Relationship between imaging biomarkers, age, progression and symptom severity in Alzheimer’s disease. NeuroImage Clinical. 2013b;3:84–94. doi: 10.1016/j.nicl.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC, Forro T, Logothetis NK. Von Economo neurons in the anterior insula of the macaque monkey. Neuron. 2012;74:482–489. doi: 10.1016/j.neuron.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Grady CL, Strother S, Tang-Wai DF, Masellis M, Black S, et al. Abnormal network connectivity in frontotemporal dementia: Evidence for prefrontal isolation. Cortex. 2013;49:1856–1873. doi: 10.1016/j.cortex.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Filippi M, Agosta F, Scola E, Canu E, Magnani G, Marcone A, et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex. 2013;49:2389–2401. doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Lancaster JL. Coordinate-based voxel-wise meta-analysis: dividends of spatial normalization. Report of a virtual workshop. Human Brain Mapping. 2005;25:1–5. doi: 10.1002/hbm.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society London B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. A quantitative meta-analysis of voxel-based morphometry studies in schizophrenia: Application of anatomical likelihood estimation. Biological Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan PW, David AS. Cognitive neuropsychiatry: Towards a scientific psychopathology. Nature Reviews Neuroscience. 2001;2:209–215. doi: 10.1038/35058586. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: The empathic brain and its modulation. Current Opinion in Neurobiology. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jech R, Mueller K, Schroeter ML, Ruzicka E. Levodopa increases functional connectivity in the cerebellum and brainstem in Parkinson’s disease. Brain. 2013;136(Pt 7):e234. doi: 10.1093/brain/awt015. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration. Demographic characteristics of 353 patients. Archives of Neurology. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Sidhu M, Gaus SE, Huang EJ, Hof PR, Miller BL, et al. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cerebral Cortex. 2012;22:251–259. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: The role of emotion and sarcasm processing. Brain. 2009;132:592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, et al. ALE meta-analysis workflows via the Brainmap database: Progress towards a probabilistic functional brain atlas. Frontiers in Neuroinformatics. 2009a;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. Journal of Neuroscience. 2009b;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, et al. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Research Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Laird AR, Eickhoff SB, Martinez MJ, Fox PM, Fox PT. Automated regional behavioral analysis for human brain images. Frontiers in Neuroinformatics. 2012;6:23. doi: 10.3389/fninf.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouc R, Lenfant P, Delbeuck X, Ravasi L, Lebert F, Semah F, et al. My belief or yours? Differential theory of mind deficits in frontotemporal dementia and Alzheimer’s disease. Brain. 2012;135:3026–3038. doi: 10.1093/brain/aws237. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ, Work Group on Frontotemporal Dementia and Pick’s Disease Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K. Neuroanatomy of the Self. Evidence from patients with frontotemporal dementia. Neurology. 2001;57:817–821. doi: 10.1212/wnl.57.5.817. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Mueller K, Jech R, Schroeter ML. Identifying central hubs affected by deep-brain stimulation in Parkinson’s disease. New England Journal of Medicine. 2013;368:482–483. doi: 10.1056/NEJMc1214078. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan C, Hodges JR, Hornberger M. Inhibitory dysfunction in frontotemporal dementia. Alzheimer Disease & Associated Disorders. 2013;27:102–108. doi: 10.1097/WAD.0b013e318265bbc9. [DOI] [PubMed] [Google Scholar]
- Pan PL, Song W, Yang J, Huang R, Chen K, Gong QY, et al. Gray matter atrophy in behavioral variant frontotemporal dementia: a meta-analysis of voxel-based morphometry studies. Dementia and Geriatric Cognitive Disorders. 2012;33:141–148. doi: 10.1159/000338176. [DOI] [PubMed] [Google Scholar]
- Pardini M, Emberti Gialloreti L, Mascolo M, Benassi F, Abate L, et al. Isolated theory of mind deficits and risk for frontotemporal dementia: A longitudinal pilot study. Journal of Neurology, Neurosurgery & Psychiatry. 2013;84:818–821. doi: 10.1136/jnnp-2012-303684. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurology. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Raczka KA, Becker G, Seese A, Frisch S, Heiner S, Marschhauser A, et al. Executive and behavioral deficits share common neural substrates in frontotemporal lobar degeneration. Psychiatry Research Neuroimaging. 2010;182:274–280. doi: 10.1016/j.pscychresns.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, et al. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: A meta-analysis of structural and functional alterations in major depressive disorder. Journal of Affective Disorders. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Schroeter ML. Considering the frontomedian cortex in revised criteria for behavioural variant frontotemporal dementia. Brain. 2012;135:e213. doi: 10.1093/brain/aws030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Neumann J. Combined imaging markers dissociate Alzheimer’s disease and frontotemporal lobar degeneration - An ALE meta-analysis. Frontiers in Aging Neuroscience. 2011;3:10. doi: 10.3389/fnagi.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, von Cramon DY. Towards a nosology for frontotemporal lobar degenerations - A meta-analysis involving 267 subjects. NeuroImage. 2007;36:497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, von Cramon DY. Neural networks in frontotemporal dementia - A meta-analysis. Neurobiology of Aging. 2008;29:418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer’s disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1351 patients. NeuroImage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Vogt B, Frisch S, Becker G, Seese A, Barthel H, et al. Dissociating behavioral disorders in early dementia – An FDG-PET study. Psychiatry Research Neuroimaging. 2011;194:235–244. doi: 10.1016/j.pscychresns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Vogt B, Frisch S, Becker G, Barthel H, Müller K, et al. Executive deficits are related to the inferior frontal junction – An FDG-PET study in early dementia. Brain. 2012;135:201–215. doi: 10.1093/brain/awr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Annals of Neurology. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Zhou J, Kim EJ. Frontotemporal dementia: What can the behavioral variant teach us about human brain organization? Neuroscientist. 2012;18:373–385. doi: 10.1177/1073858411410354. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, de la Sayette V, Mézenge F, Landeau B, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. Journal of Neuroscience. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Rohrer JD, Hardy J. Disintegrating brain networks: From syndromes to molecular nexopathies. Neuron. 2012;73:1060–1062. doi: 10.1016/j.neuron.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woost TB, Dukart J, Frisch S, Barthel H, Sabri O, Mueller K, et al. Neural correlates of the DemTect in Alzheimer’s disease and frontotemporal lobar degeneration – A combined MRI & FDG-PET study. NeuroImage Clinical. 2013;2:746–758. doi: 10.1016/j.nicl.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.