Abstract
Background and aim
Cardiovascular risk factors in childhood are predictive of adulthood arterial stiffness. However, it is unknown whether this relationship varies by race or sex.
Methods
Six hundred and eighty adults aged 24–43 had been followed for an average of 26.3 years, from the Bogalusa Heart Study. Brachial to ankle pulse wave velocity (baPWV) measured by an automatic oscillometric technique was used as the outcome variable for arterial stiffness during adulthood. Body mass index (BMI), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glucose, and systolic blood pressure (SBP), all measured in childhood, were used as predictors. The average values of childhood measurements at multiple time points were used, standardized to age, race, and sex-specific z-scores.
Results
In the total sample, childhood SBP was the only significant predictor (p<0.001) for adult baPWV. Significant interactions between sex and BMI (p=0.001), between sex and LDL-C (p=0.035), and between race and HDL-C (p=0.002) on adult baPWV were identified. Childhood predictors of adult baPWV were BMI (30.9 cm/second reduction in baPWV per standard deviation increase, 95% confidence interval (CI): −55.0, −6.9 cm/second), LDL-C (30.8 cm/second increase, 95% CI: 2.9, 59.5 cm/second) and HDL-C (46.8 cm/second reduction, 95% CI: −76.2, −17.4 cm/second) in white males, systolic blood pressure (38.2 cm/second increase, 95% CI: 11.0, 65.4 cm/second) in white females, BMI (71.3 cm/second reduction, 95% CI: −119.9, −22.7 cm/second) in black males, and none in black females.
Conclusion
The associations of childhood cardiovascular risk factors with adult arterial stiffness varied by race and sex.
Keywords: Race (black–white), sex, childhood CV risk factors, adult arterial stiffness
Introduction
Previous studies have demonstrated that childhood cardiovascular (CV) risk factors are predictive of subclinical CV structural and functional changes in adults1–5. Among these usually noninvasively measured subclinical changes, pulse wave velocity has been used as an index of arterial stiffness, an independent predictor of CV disease risk and mortality6–9. In the Bogalusa Heart Study and the Young Finns Study, two longitudinal studies beginning in childhood with long follow-up experience, blood pressure and metabolic syndrome have been associated with arterial stiffness in middle-aged adults5,10.
It is well established that there are substantial racial and sex differences in CV risk factors and related CV risk that occurs from childhood to adulthood11–16. In the Bogalusa Heart Study, we have shown that childhood predictors of adult subclinical atherosclerosis vary according to race and sex17. However, it is not known whether associations between childhood risk factors and adulthood arterial stiffness, as observed in the Bogalusa Heart Study and the Young Finns Study5,10, are race- and/or sex-specific. Such information is critical for the understanding of the natural history of CV diseases from childhood in different race and sex groups, and also has implications for early race- and sex-specific prevention and treatment of CV disease. The Bogalusa Heart Study, a black-white, community-based investigation of the natural history of CV disease beginning in childhood18, provides a unique opportunity to examine black-white and sex differences in the association of traditional CV risk factors in children with arterial stiffness in young adults.
Methods
Study population
Between 1973 and 2002, seven cross-sectional surveys of children aged 4–17 years and seven surveys of young adults aged 18–44 years who had participated in earlier surveys as children and remained accessible were conducted in the black-white (65% whites, 35% blacks) community of Bogalusa, LA. This panel design, based on repeated cross-sectional examinations conducted approximately every 3 to 4 years, resulted in multiple observations during childhood and young adulthood. In the 2000–2002 survey of young adults, brachial to ankle pulse wave velocity (baPWV), measured by applanation tonometry, was obtained on 835 participants (aged 24 to 44 years, mean age 35.9 years, 72% white, 45% male). Of these 835 participants, 680 had all risk factor variables considered in this study measured at least once in childhood (on average 4 times). The average follow-up period was 26.3 years (range: 16.9–29.1 years).
Written informed consent was obtained from parents or guardians in childhood and from the participants in adulthood. Protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
Examinations
Relevant to the current analyses, risk factor measurements during childhood included body mass index (BMI), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glucose, or systolic blood pressure (SBP). The outcome measure, brachial-ankle pulse wave velocity (baPWV), was assessed in young adulthood, on average 26.3 years after the first risk factors assessment.
All surveys followed essentially the same protocol for risk factor measurements18. Participants were instructed to fast for 12 hours before screening, with compliance ascertained by interview on the morning of the examination. Height and weight were measured twice to within 0.1 cm and within 0.1 kg, respectively, and the mean values were used to calculate BMI (calculated as weight in kilograms divided by the square of height in meters) as a measure of body fatness. Information on smoking status (Yes/No) was obtained as part of a health habit questionnaire19.
Replicate blood pressure measurements were obtained on the right arm of the participants while sitting in a relaxed position. Arm measurements, length and circumference, were obtained during the examination to ensure use of a proper cuff size. Systolic and diastolic blood pressure levels were recorded as the first, fourth (in children), and fifth (in adults) Korotkoff phases using mercury sphygmomanometers. Blood pressure levels were reported as the mean of six replicate readings taken by two randomly assigned and trained observers.
Laboratory Analysis
During 1973 to 1986 cholesterol and triglycerides levels were measured with a Technicon AutoAnalyzer II (Technicon Instrument Corp, Tarrytown, NY) according to the Laboratory Manual of the Lipid Research Clinics Program20. These variables were determined by enzymatic procedures21,22 on the Abbott VP instrument (Abbott Laboratories, North Chicago, IL) between 1986 and 1996 and on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN) since then. Both chemical and enzymatic procedures met the performance requirements of the Lipid Standardization Program of the Centers for Disease Control and Prevention (CDC). This standardization monitored the accuracy of measurements of total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol concentrations. Measurements on CDC-assigned quality control samples showed no consistent bias over time within or between surveys. Serum lipoprotein cholesterols were analyzed using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures23. From 1981 to 1991, plasma glucose was measured by a glucose oxidase method using a Beckman Glucose Analyzer (Beckman Instruments, Palo Alto, CA). Since then, it has been measured enzymatically as part of a multichemistry (SMA20) profile.
baPWV Measurements
baPWV was measured using a non-invasive automatic oscillometric device (BP-203RPE, Colin, Komaki, Japan) as described in detail previously24,25. This instrument records baPWV, blood pressure, electrocardiogram, and heart sounds simultaneously. Occlusion and monitoring cuffs were placed snugly around both sides of the upper and lower extremities in subjects in the supine position. After at least 5 minutes of bed rest, 3 waveforms of brachial and tibial arteries were recorded. Electrocardiogram monitoring was performed with electrodes placed on both wrists. The pressure waveforms obtained at the two different sites were simultaneously recorded to determine the time interval between the initial rise in the brachial and tibial pressure waveforms (Ta). The path length from the suprasternal notch to the elbow (Da) and the path length from the suprasternal notch to the ankle (Db) were obtained automatically based on the subject’s height. Finally baPWV was calculated with the equation: (Db-Da)/Ta. To examine the reproducibility of baPWV, 37 randomly selected subjects were reexamined 2–3 hours later, after their first examination. The correlation coefficient between two examinations was 0.84 for the left side (P<0.001) and 0.82 for the right side (P<0.001). The average value of measurements on both left and right sides was used for data analysis.
Statistical methods
Values of risk factor variables were first standardized to survey-, age-, race-, and sex-specific z-scores, and the average values of the z-scores of multiple measurements during childhood were used as childhood values. General linear models were used to examine the childhood predictors of baPWV in adults, adjusted for age, heart rate, and cigarette smoking (and race/sex), where appropriate. Adjustment of baPWV for heart rate was necessary because it is an important confounder of PWV26,27. To examine interactions between childhood risk factor variables and race or sex on baPWV, interaction terms between childhood risk factor variables and race or sex were included in a single model, with the main effects included in the model as well. Having identified significant interactions of certain risk factors with race or sex, we also presented results stratified by race and sex. All data analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
Mean values of risk factor measurements in the study cohort during childhood and adulthood are displayed in Table 1, by race and sex. With some exceptions in particular race, sex, and age groups, blacks vs whites had higher levels of systolic blood pressure and HDL cholesterol and lower triglycerides; males vs females had higher levels of systolic blood pressure, LDL cholesterol, and triglycerides and lower levels of HDL cholesterol; blacks had a higher BMI than whites. Blacks and females had faster pulse rates than whites and males, and blacks and males had higher levels of baPWV than whites and females, respectively, in adulthood.
Table 1.
Risk factor variables in childhood and in adulthood in the study cohort by race and sex: The Bogalusa Heart Study
| Risk factor variable | White
|
Black
|
P for difference
|
|||
|---|---|---|---|---|---|---|
| Male (n=222) | Female (n=272) | Male (n=70) | Female (n=116) | Race | Sex | |
| Childhood | ||||||
| Age (years) | 12.3±2.2 | 12.2±2.4 | 12.4±2.0 | 12.2±2.1 | 0.91 | 0.35 |
| BMI (kg/m2) | 19.2±3.6 | 19.0±3.3 | 18.8±3.2 | 19.3±3.7 | 0.92 | 0.93 |
| LDL Cholesterol (mg/dL) | 89.0±22.8 | 92.7±23.3 | 91.6±25.9 | 95.7±25.0 | 0.16 | 0.05 |
| HDL Cholesterol (mg/dL) | 59.5±15.5 | 58.4±14.2 | 61.4±13.0 | 64.1±13.4 | 0.001 | 0.93 |
| Triglycerides (mg/dL) | 68.4±25.8 | 76.5±29.1 | 59.2±19.8 | 62.3±17.4 | <0.001 | <0.001 |
| Glucose (mg/dL) | 86.7±6.6 | 84.7±6.1 | 86.5±8.2 | 83.3±7.9 | 0.10 | <0.001 |
| Systolic BP (mm Hg) | 104.2±8.2 | 103.0±8.1 | 104.3±8.4 | 102.3±7.4 | 0.04 | 0.48 |
| Diastolic BP (mm Hg) | 62.9±6.2 | 64.7±6.8 | 62.9±6.9 | 64.3±6.7 | 0.52 | <0.001 |
| Adulthood | ||||||
| Age (years) | 35.7±4.2 | 35.5±4.6 | 35.6±4.7 | 34.4±5.0 | 0.07 | 0.19 |
| Cigarette smoking (%) | 30.1 | 28.7 | 41.4 | 25.0 | 0.72 | 0.02¶ |
| Heart rate (beats/minute) | 64.3±10.0 | 68.5±9.2 | 66.4±9.8 | 71.2±9.9 | 0.004 | <0.001 |
| BMI (kg/m2) | 28.5±5.8 | 27.9±6.8 | 29.1±6.4 | 30.9±8.2 | 0.001 | 0.97 |
| LDL Cholesterol (mg/dL) | 129.0±32.7 | 123.4±31.4 | 126.0±42.6 | 113.4±30.6 | 0.015 | 0.005 |
| HDL Cholesterol (mg/dL) | 40.7±9.5 | 49.2±11.2 | 46.0±11.3 | 50.4±11.6 | <0.001† | <0.01 |
| Triglycerides (mg/dL) | 149.7±121.6 | 121.0±70.7 | 140.2±124.8 | 84.8±31.8 | <0.001‡ | 0.05 |
| Glucose (mg/dL) | 85.5±20.8 | 81.4±12.1 | 85.8±28.2 | 83.3±18.3 | 0.35 | 0.01 |
| Systolic BP (mm Hg) | 117.1±10.6 | 109.6±10.7 | 124.5±12.8 | 116.3±13.2 | <0.001 | <0.001 |
| Diastolic BP (mm Hg) | 79.2±7.8 | 73.9±8.2 | 83.4±9.7 | 77.6±10.0 | <0.001 | <0.001 |
| baPWV (cm/s) | 1342.3±160.9 | 1183.7±173.5 | 1419.2±169.0 | 1240.0±242.9 | <0.001 | <0.001 |
Mean±SDs are shown, except for cigarette smoking for which proportions are shown.
Average values of multiple measurements for each childhood risk factor are shown.
LDL: low-density lipoprotein; HDL: high-density lipoprotein; BP: Blood pressure; baPWV: brachial-ankle pulse wave velocity
only in blacks;
only in males;
only in females
P values were adjusted for age and (race/sex) where appropriate.
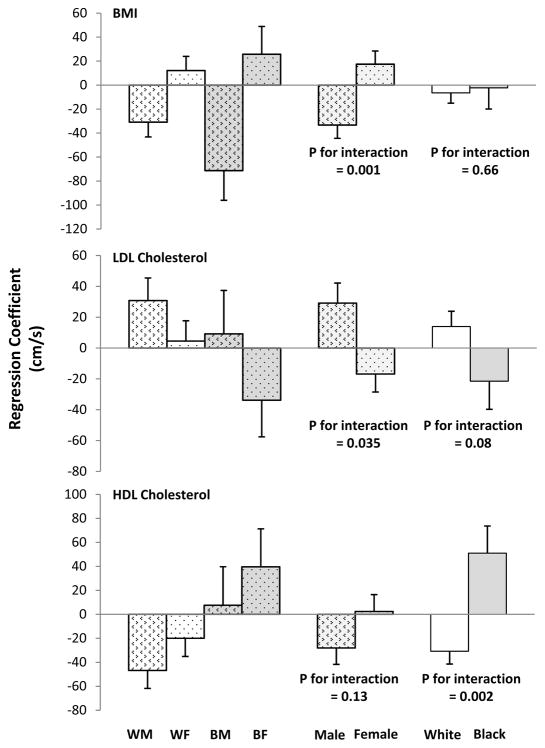
In the total sample, systolic blood pressure measured in childhood was the only significant predictor (P<0.001) of adult baPWV, adjusted for age, race, sex, cigarette smoking, and heart rate (Table 2). Similar results were observed for childhood diastolic blood pressure. However, a model that included interaction terms between race/sex and childhood risk factor variables revealed significant interactions between race and HDL cholesterol in childhood, between sex and BMI in childhood, and between sex and LDL cholesterol in childhood. In Figure 1, childhood BMI tended to be inversely associated with adulthood baPWV in males and positively in females (p for interaction = 0.001), and childhood LDL cholesterol tended to be positively associated with adulthood baPWV in males and inversely in females (p for interaction = 0.035); HDL cholesterol was inversely associated with adulthood baPWV in whites but positively in blacks (p for interaction = 0.002).
Table 2.
Associations of adulthood brachial-ankle pulse wave velocity (outcome variable) with childhood risk factor variables (predictor variables) in the total sample: The Bogalusa Heart Study (n=680)
| Independent Variable | Regression coefficient | Standard error of regression coefficient | p |
|---|---|---|---|
| Age (years) | 11.53 | 1.44 | <0.0001 |
| Race (1=white, 2=black) | 66.22 | 14.87 | <0.0001 |
| Sex (1=male, 2=female) | −169.99 | 13.58 | <0.0001 |
| Cigarette smoking (1=no, 2=yes) | 45.09 | 14.26 | 0.002 |
| Heart rate (beats/minute) | 3.32 | 0.68 | <0.0001 |
| BMI in childhood | −3.13 | 7.76 | 0.69 |
| LDL-C in childhood | −2.08 | 8.56 | 0.81 |
| HDL-C in childhood | −9.59 | 9.71 | 0.32 |
| TG in childhood | 3.95 | 11.73 | 0.74 |
| Glucose in childhood | −7.46 | 8.38 | 0.37 |
| Systolic BP in childhood | 34.27 | 9.28 | <0.001 |
LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglycerides; BP: blood pressure
All variables were included in a single general linear model.
Means of multiple age-, sex-, race-, and survey-standardized z-scores during childhood were used as childhood values.
Figure 1.
Associations of Adult Brachial-Ankle Pulse Wave Velocity with Childhood BMI, Low-Density Lipoprotein (LDL) Cholesterol, and High-Density Lipoprotein (HDL) Cholesterol by Race and/or Sex: The Bogalusa Heart Study
WM: white male; WF: white female; BM: black male; BF: black female
P values were derived from a single general linear model that included adulthood age, cigarette smoking, heart rate, childhood risk factor variables, race, sex, and the interaction terms between childhood risk factor variables and race/sex.
Means of age-, sex-, race-, and survey-standardized z-scores from multiple measurements during childhood were used as childhood values in the general linear model
Error bars show one standard error.
Since there were significant interactions, we performed race- and sex-specific analyses. Significant childhood predictors of adult baPWV were BMI (30.9 cm/s reduction in baPWV per standard deviation increase, 95% confidence interval (CI): −55.0, −6.9 cm/s), LDL-C (30.8 cm/s increase, 95% CI: 2.9, 59.5 cm/s) and HDL-C (46.8 cm/s reduction, 95% CI: −76.2, −17.4 cm/s) in white males, systolic blood pressure (38.2 cm/s increase, 95% CI: 11.0, 65.4 cm/s) in white females, BMI (71.3 cm/s reduction, 95% CI: −119.9, −22.7 cm/s) in black males, and none in black females (Table 3).
Table 3.
Association of adulthood brachial-ankle pulse wave velocity (outcome variable) with childhood risk factor variables (predictor variables) by race and sex: The Bogalusa Heart Study
| Independent variable | White Male (n=222) | White Female (n=272) | Black Male (n=70) | Black Female (n=116) | P for difference* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||
| β | SEβ | p | β | SEβ | p | β | SEβ | p | β | SEβ | p | Race | Sex | |
| Age (years) | 8.11 | 2.42 | 0.001 | 11.29 | 2.14 | <.0001 | 4.74 | 4.46 | 0.29 | 18.01 | 4.18 | <.0001 | - | - |
| Cigarette smoking (1=no, 2=yes) | 0.29 | 22.03 | 0.99 | 54.03 | 21.59 | 0.01 | 40.74 | 39.99 | 0.31 | 63.58 | 48.14 | 0.19 | - | - |
| Hear rate (beats/minute) | 3.50 | 1.03 | 0.001 | 3.63 | 1.08 | 0.001 | 5.16 | 2.20 | 0.02 | 3.90 | 2.12 | 0.07 | - | - |
| BMI in childhood | −30.92 | 12.27 | 0.01 | 12.17 | 11.79 | 0.30 | −71.28 | 24.80 | 0.006 | 25.75 | 23.20 | 0.27 | 0.66 | 0.001 |
| LDL cholesterol in childhood | 30.78 | 14.64 | 0.04 | 4.53 | 13.16 | 0.73 | 9.21 | 28.14 | 0.74 | −33.84 | 23.76 | 0.16 | 0.08 | 0.035 |
| HDL cholesterol in childhood | −46.78 | 15.00 | 0.002 | −19.99 | 15.17 | 0.19 | 7.61 | 32.07 | 0.81 | 39.64 | 31.64 | 0.21 | 0.002 | 0.13 |
| Triglycerides in childhood | −14.92 | 20.54 | 0.48 | −10.94 | 18.24 | 0.55 | −12.60 | 33.13 | 0.70 | 30.81 | 32.15 | 0.34 | 0.19 | 0.38 |
| Glucose in childhood | −6.74 | 13.47 | 0.62 | −7.51 | 12.73 | 0.56 | 3.94 | 23.66 | 0.87 | −11.85 | 25.33 | 0.64 | 0.96 | 0.73 |
| Systolic BP in childhood | 27.36 | 13.97 | 0.051 | 38.18 | 13.89 | 0.006 | 22.58 | 29.20 | 0.44 | 51.71 | 30.67 | 0.09 | 0.59 | 0.49 |
β: regression coefficient; SE: standard error; LDL: low-density lipoprotein; HDL: high-density lipoprotein; BP: Blood pressure
All variables were included in a single general linear model for each race and sex group.
Means of multiple age-, sex-, race-, and survey-standardized z-scores during childhood were used as childhood values.
P values were derived from a single general linear model that included all main effect terms and interaction terms between race/sex and childhood risk factors.
-: Race and sex differences were not examined.
Discussion
The present study demonstrates that there are substantial race and sex differences in the relationships between risk factor variables in childhood and adult arterial stiffness as measured by baPWV. This extends our earlier observation that among measured risk factors during childhood (BMI, blood pressure, LDL cholesterol, HDL cholesterol, and triglycerides), blood pressure was the only predictor for adult arterial stiffness in the total cohort5, although there are divergences of central nervous system control of heart rate from blood pressure in the two race groups28. BMI, LDL cholesterol, and HDL cholesterol in white males, systolic blood pressure in white females, and BMI in black males, all measured in childhood, are associated with adult arterial stiffness measured decades later (on average 26.3 years). These observations from a community-based cohort indicate that childhood risk factors play an important role for adult vascular diseases and gradual structural changes of the CV system. Thus, risk factors early in life are predictive of adult CV risk, varying according to race and sex, and provide guidelines for early preventive measures.
The current study builds upon and enhances our earlier observations that the associations between childhood risk factors and adult subclinical atherosclerosis vary by race and sex17, and suggests that the associations between childhood risk factors and adult arterial stiffness also vary by race and sex. We found that individual childhood (mean age12–13 years) risk factor variables had varying degrees of associations with adult (mean age 34–36 years) arterial stiffness in terms of the absolute value of regression coefficients and their directions. Childhood BMI was associated with reduced arterial stiffness in adults in both white and black males; in females, it tended to be associated with increased arterial stiffness in adults, albeit not significantly. The inverse association between childhood BMI and adult arterial stiffness in males is consistent with previous observations that adiposity is associated with reduced arterial stiffness until middle age29. The participants in our study were 24–44 years old at their last examination. It has also been shown that obesity is inversely associated with arterial stiffness in children and youth30–33. The seemingly positive association of childhood BMI with adult arterial stiffness in women may be due to the relatively small artery diameters in women34 and thus reduced adaption capacity in the context of obesity. Further, the positive association suggests that women may be more vulnerable to the adverse effect of obesity, probably even more so when they get older, as has been suggested by Zebekakis et al35. Similar sex difference has been reported previously in morbidly obese patients36. In a most recent report, Weng et al. reported that women were more sensitive to the adverse effects of metabolic syndrome and its components including obesity, though the participants were older (average 48 years old) Chinese adults37, compared to participants in the current study. Some studies have shown that obesity is positively correlated with arterial stiffness in adolescents38 and young adults39. Reasons for this discrepancy in research findings are not clear and should be addressed in future studies. Sex differences in the relationship between childhood LDL cholesterol and adult arterial stiffness suggest that males, particularly white males, are more vulnerable to the adverse effect of increased LDL cholesterol on the arterial stiffening process. The overall inverse association between childhood HDL cholesterol and adult arterial stiffness in whites is consistent with the protective effect of high levels of HDL cholesterol in this group. The fact that LDL cholesterol and HDL cholesterol in childhood were associated with arterial stiffness only in white male adults underscores the importance of lipids metabolism in arterial stiffening in white males. The positive association between childhood HDL cholesterol and adult arterial stiffness in blacks may partly explain why blacks have higher levels of HDL cholesterol but still have higher CV mortality than whites40.
There may be more race and sex differences in the relationships of childhood risk factors with adult baPWV than what the current study has identified. The power to identify interactions in our study may have been limited for certain risk factor variables as a result of the available sample size (n=680). Further, risk factors not considered here may have differential effects on the development of arterial stiffening from childhood to adulthood in different race and sex groups. More studies are needed to confirm and to extend our observations in the current study.
Our observations have important implications for future research and for targeted early prevention and treatment of CV diseases in different race and sex groups. Although the underlying mechanisms for the observed race and sex differences in the relationship between childhood risk factor variables and adult arterial stiffness cannot be addressed by the current observational study, our observations should serve as a starting point for future mechanistic studies. The fact that childhood risk factor profiles are different in different race and sex groups argues for tailored prevention/intervention programs in diverse race and sex groups. Future translational research is needed to address the feasibility and usefulness of such programs.
Our study has important strengths, including the consistency and quality of the observations, the long duration of follow-up, and the community-based nature of the study sample. The latter enhances the generalizability of our findings to other populations with a similar demographic composition. However, the limited nature of our sample sizes, particularly in the analyses in our black participants may have limited our ability to identify significant associations between childhood risk factor variables and adult arterial stiffness. Future studies would benefit from use of datasets with a larger sample size. This could be achieved by combining similar cohorts, as has been done by the international childhood cardiovascular cohort consortium41. As mentioned earlier, our study was observational in nature, which did not allow us to address the underlying mechanisms for the observed race and sex divergences.
In conclusion, the associations between childhood CV risk factors and arterial stiffness in young adults are divergent in different race and sex groups. These findings, along with our previous observations on subclinical atherosclerosis, suggest that CV risk assessment and prevention in early life should take race and sex into account. Further, more studies are needed to examine the differential impact of CV risk factors on the development of CV diseases from childhood in different race and sex groups.
Acknowledgments
The Bogalusa Heart Study is supported by grants 5R01ES021724 from National Institute of Environmental Health Science, and 2R01AG016592 from the National Institute on Aging. Shengxu Li is a scholar of the Building Interdisciplinary Research in Women’s Health program, supported by Award Number K12HD043451 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. Shengxu Li is partly supported by Grant 13SDG14650068 from American Heart Association.
The Bogalusa Heart Study is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. We especially thank the Bogalusa, LA school system, and most importantly, the children and young adults who have participated in this study over many years. The authors further express appreciation to staff from Colin Medical Instruments Corporation for the support and training in use of the Colin instrument.
References
- 1.Hartiala O, Magnussen CG, Kajander S, et al. Adolescence risk factors are predictive of coronary artery calcification at middle age: the cardiovascular risk in young Finns study. J Am Coll Cardiol. 2012;60:1364–70. doi: 10.1016/j.jacc.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 2.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation. 2004;110:3488–92. doi: 10.1161/01.CIR.0000149713.48317.27. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the bogalusa heart study. Hypertension. 2004;43:541–6. doi: 10.1161/01.HYP.0000115922.98155.23. [DOI] [PubMed] [Google Scholar]
- 6.Dolan E, Thijs L, Li Y, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006;47:365–70. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- 7.Kikuya M, Staessen JA, Ohkubo T, et al. Ambulatory arterial stiffness index and 24-hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke. 2007;38:1161–6. doi: 10.1161/01.STR.0000259604.67283.69. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 10.Koivistoinen T, Hutri-Kahonen N, Juonala M, et al. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk In Young Finns Study. Ann Med. 2011;43:312–9. doi: 10.3109/07853890.2010.549145. [DOI] [PubMed] [Google Scholar]
- 11.Greenlund KJ, Kiefe CI, Gidding SS, et al. Differences in cardiovascular disease risk factors in black and white young adults: comparisons among five communities of the CARDIA and the Bogalusa heart studies. Coronary Artery Risk Development In Young Adults. Ann Epidemiol. 1998;8:22–30. doi: 10.1016/s1047-2797(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 12.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study. Am J Cardiol. 2002;90:3L–7L. doi: 10.1016/s0002-9149(02)02953-3. [DOI] [PubMed] [Google Scholar]
- 13.Freedman DS, Newman WP, 3rd, Tracy RE, et al. Black-white differences in aortic fatty streaks in adolescence and early adulthood: the Bogalusa Heart Study. Circulation. 1988;77:856–64. doi: 10.1161/01.cir.77.4.856. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi N, Bulpitt CJ, Leggetter S, et al. Ethnic differences in vascular stiffness and relations to hypertensive target organ damage. J Hypertens. 2004;22:1731–7. doi: 10.1097/00004872-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Strain WD, Chaturvedi N, Leggetter S, et al. Ethnic differences in skin microvascular function and their relation to cardiac target-organ damage. J Hypertens. 2005;23:133–40. doi: 10.1097/00004872-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Kimm SY, Barton BA, Obarzanek E, et al. Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics. 2001;107:E34. doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Chen W, Srinivasan SR, Tang R, Bond MG, Berenson GS. Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima-media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis. 2007;194:421–5. doi: 10.1016/j.atherosclerosis.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Berenson GS, Wattigney WA, Bao W, Srinivasan SR, Radhakrishnamurthy B. Rationale to study the early natural history of heart disease: the Bogalusa Heart Study. Am J Med Sci. 1995;310:S22–8. doi: 10.1097/00000441-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hunter SM, Webber LS, Berenson GS. Cigarette smoking and tobacco usage behavior in children with adolescents: Bogalusa Heart Study. Prev Med. 1980;9:701–12. doi: 10.1016/0091-7435(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 20.Lipid Research Clinics Program. Lipid and Lipoprotein Analysis. Washington, DC: National Institutes of Health; 1974. Manual of Laboratory Operations. DHEW Publication No (NIH) 75–628. [Google Scholar]
- 21.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 22.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 23.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In: Lewis LA, editor. CRC Handbook of Electrophoresis v III Lipoprotein Methodology and Human Studies. Boca Raton, FL: CRC Press; 1983. pp. 185–204. [Google Scholar]
- 24.Suzuki E, Kashiwagi A, Nishio Y, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care. 2001;24:2107–14. doi: 10.2337/diacare.24.12.2107. [DOI] [PubMed] [Google Scholar]
- 25.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–64. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 26.Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–7. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson IB, Mohammad NH, Tyrrell S, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 28.Berenson GS. Evolution of cardio-metabolic risk from birth to middle age: the Bogalusa Heart Study. Dordrecht: Springer; 2011. [Google Scholar]
- 29.Corden B, Keenan NG, de Marvao AS, et al. Body fat is associated with reduced aortic stiffness until middle age. Hypertension. 2013;61:1322–7. doi: 10.1161/HYPERTENSIONAHA.113.01177. [DOI] [PubMed] [Google Scholar]
- 30.Park BJ, Lee HR, Shim JY, Lee JH, Jung DH, Lee YJ. Association between resting heart rate and arterial stiffness in Korean adults. Arch Cardiovasc Dis. 2010;103:246–52. doi: 10.1016/j.acvd.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008;28:287–93. doi: 10.1111/j.1475-097X.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 32.Lurbe E, Torro I, Garcia-Vicent C, Alvarez J, Fernandez-Fornoso JA, Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60:550–5. doi: 10.1161/HYPERTENSIONAHA.112.194746. [DOI] [PubMed] [Google Scholar]
- 33.Chalmers LJ, Copeland KC, Hester CN, Fields DA, Gardner AW. Paradoxical increase in arterial compliance in obese pubertal children. Angiology. 2011;62:565–70. doi: 10.1177/0003319711399117. [DOI] [PubMed] [Google Scholar]
- 34.Krejza J, Arkuszewski M, Kasner SE, et al. Carotid artery diameter in men and women and the relation to body and neck size. Stroke. 2006;37:1103–5. doi: 10.1161/01.STR.0000206440.48756.f7. [DOI] [PubMed] [Google Scholar]
- 35.Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–46. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 36.Nordstrand N, Gjevestad E, Dinh KN, et al. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord. 2011;11:7. doi: 10.1186/1471-2261-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng C, Yuan H, Yang K, et al. Sex-Specific Association Between the Metabolic Syndrome and Arterial Stiffness in 8300 Subjects. The American journal of the medical sciences. 2013;14:14. doi: 10.1097/MAJ.0b013e3182732e97. [DOI] [PubMed] [Google Scholar]
- 38.Miyai N, Arita M, Miyashita K, Morioka I, Takeda S. The influence of obesity and metabolic risk variables on brachial-ankle pulse wave velocity in healthy adolescents. J Hum Hypertens. 2009;23:444–50. doi: 10.1038/jhh.2008.143. [DOI] [PubMed] [Google Scholar]
- 39.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–73. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 40.Ferdinand KC. Coronary artery disease in minority racial and ethnic groups in the United States. Am J Cardiol. 2006;97:12A–9A. doi: 10.1016/j.amjcard.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Dwyer T, Sun C, Magnussen CG, et al. Cohort Profile: the international childhood cardiovascular cohort (i3C) consortium. Int J Epidemiol. 2013;42:86–96. doi: 10.1093/ije/dys004. [DOI] [PMC free article] [PubMed] [Google Scholar]