Abstract
Ephrin B2/EphB4 mediates interactions among osteoblasts (OB), osteoclasts (OCL), and chondrocytes to regulate their differentiation. We investigated the role of ephrin B2/EphB4 signaling in mediating the anabolic effects of IGF1 and PTH on those cells and overall endochondral bone formation.
Immunohistochemistry demonstrated that the expression of ephrin B2 in OBs, OCLs and osteocytes, and the expression of EphB4 in OBs and osteocytes were dramatically decreased in global IGF-I knockout mice. Inactivation of EphB4 by EphB4 siRNA in cultured bone marrow stromal cells significantly decreased the mRNA levels of OB differentiation markers and abolished the stimulatory effects of IGF-I on these markers. Blocking the interaction of EphB4 and ephrin B2 in the OB-OCL co-cultures with the EphB4 specific peptide TNYL-RAW or deletion of ephrin B2 in OCL prior to co-culture led to fewer and smaller TRAP positive cells, decreased expression of OB differentiation markers, and blunted response to IGF-I for both OCL and OB differentiation.
In the growth plate, both ephrin B2 and EphB4 are expressed in late stage proliferating and prehypertrophic chondrocytes, and their expression was decreased in mice lacking the IGF-I receptor specifically in chondrocytes. In vitro, blocking the interaction of EphB4 and ephrin B2 in chondrogenic ATDC5 cells with TNYL-RAW significantly decreased both basal and IGF1-induced expression of type II and type X collagen. In the co-cultures of ATDC5 cells and spleen cells (osteoclast precursors), TNYL-RAW decreased the numbers of TRAP positive cells and the expression of NFATc1 and RANK, and blocked their stimulation by IGF-I. Our data indicate that IGF-I/IGF-IR signaling promotes OB, OCL, and chondrocyte differentiation via ephrin B2/EphB4 mediated cell-cell communication.
Keywords: IGF-I, Ephrin B2, EphB4, cell-cell communication, chondrocyte
Introduction
In mammals, endochondral bone formation and subsequent remodeling involves three specific cell types: chondrocytes, osteoblasts and osteoclasts (1,2). The coordination and communication among these cells are required for the skeletal integrity (3–5).
These cells are derived from different sources, and each has its own differentiation pathway and functions. Chondrocytes derive from condensed mesenchyme formed during early skeletal development. They undergo a well-regulated sequence of proliferation, differentiation, matrix secretion and mineralization, and apoptosis. They subsequently provide a mature matrix to induce blood vessel invasion and support for new bone formation (6–10). Osteoblasts are also mesenchymal in origin. Activated osteoblasts deposit and mineralize matrix proteins to form bone, and eventually become entrapped within the bone matrix as osteocytes, remain quiescently at the surface of the bone as lining cells, or die by apoptosis(11). Bone resorbing osteoclasts are derived from hematopoietic stem cells. Their formation and differentiation depend on the induction by the neighboring osteoblasts or stromal cells. The communication between osteoblasts and osteoclasts involves several signaling pathways. Macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-kB (RANK) ligand (RANKL), produced by osteoblasts and other cells, act on their receptors c-fms and RANK, respectively, to stimulate osteoclast formation and differentiation(12,13). More recent studies have shown that EphB4, a member of the tyrosine kinase receptor Eph family, is expressed in osteoblasts as is its ligand ephrin B2. In osteoclasts ephrin B2 but not EphB4 is expressed. Together they constitute a bidirectional signaling pathway (14).The reverse signaling through ephrinB2 into osteoclast precursors has been reported to suppress osteoclastogenesis by inhibiting the osteoclastogenic c-Fos-NFATc1 cascade, while the forward signaling through EphB4 into osteoblasts has been reported to enhance osteoblast differentiation(15,16). As will be shown, our results are somewhat at variance to this model.
We and others have demonstrated that insulin-like growth factor-I (IGF-I) signaling in chondrocytes (17), osteoblasts (18)and osteoclasts(19), regulates the differentiation of these cells, coordinates osteoblast-osteoclast interactions (19) and is required for parathyroid hormone (PTH) stimulation of bone formation(20,21). However, the molecular mechanisms responsible for these actions remain imperfectly understood. Moreover, the interaction of chondrocytes with adjacent osteoblasts and/or osteoclasts and the role of IGF-I signaling on these interactions have received little attention despite their obvious importance in endochondral bone formation. To address these issues, herein, we used various gene knockout mouse models and in vitro cell culture systems to investigate the role of IGF-I signaling in regulating the interaction between chondrocytes and bone cells with particular attention to the role of ephrin B2/EphB4, and tested whether ephrin B2/EphB4 signaling is involved in mediating the anabolic actions of PTH through IGF-I signaling on bone.
Materials and Methods
Animals
Global IGF-I knockout mice (IGF-IKO, CD1) were developed by Lyn Powell-Braxton and colleagues(22). Their skeletal phenotype has previously been described (17,23).
12 week old control and mice with the IGF-I receptor (IGF-IR) null mutation in mature osteoblasts [OBIGF-IRKO, floxed IGF-IR (C57Bl/6) X osteocalcin driven Cre (FVB-N)] (gift from Dr. Thomas Clemens) (18,21) were treated with either PTH [80 µg/kg body weight; PTH (1–34), rat; Bachem Americas, Inc., Torrance, CA)] or vehicle daily by subcutaneous injection for 2 weeks. Left tibias and femurs were obtained 1 hr after the last PTH injection for mRNA determinations.
Chondrocyte-specific IGF-IR knockout (CartIGF-IRKO) mice (24)were made by breeding floxed IGF-IR mice (FVB-N) (25) with transgenic mice (CreCart) expressing the cre recombinase under the control of a type II collagen [α1(II)] promoter (Mixed background) (26) (The Jackson Laboratory, Bar Harbor, ME). Tibias and growth plate (GP) cartilage from the knee were obtained from the newborn (P0) CartIGF-IRKO mice and control littermates (lacking the cre recombinase) for immunohistochemistry and mRNA measurements, respectively.
Floxed ephrin B2 mice (C57Bl/6) were purchased from The Jackson Laboratory (bar Harbor, ME) (27) for in vitro deletion of ephrin B2 in osteoclast precursors.
These animal studies were approved by the Animal Use Committee of the San Francisco Veterans Affairs Medical Center where the animals were raised and studied.
Histology
Tibias were fixed with 4% paraformaldehyde in PBS (4% PFA/PBS) overnight at 4 C, embedded in paraffin, and cut into 5 µm sections. The sections were stained by immunohistochemisty for ephrin B2 (sc-15397, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Eph B4 (sc-5536, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and counterstained with hematoxylin.
ATDC5 cell cultures
The mouse chondrocyte cell line ATDC5 (gift from Dr. Tamara Alliston) was cultured in a primary ATDC5 medium [a 1:1 mixture of DMEM and Ham’s F-12 medium (DMEM/F-12; Invitrogen, Carlsbad, CA, USA) containing 5% FBS, 50 units/ml penicillin, 50 µg/ml streptomycin, 10µg/ml human transferin, and 3 × 10–8 M sodium selenite (Sigma, St Louis, MO, USA)]. To investigate the role of ephrin B2 and EphB4 in chondrocyte differentiation, ATDC5 cells were plated in the primary ATDC5 medium at 2 × 105 /well in 24 well plates for 3 days before the cultures were switched to a secondary ATDC5 medium (primary medium supplemented with ascorbic acid [0.05 mg/ml] and β-glycerophosphate [10 mM]) according to published protocols (28). To block ephrin B2/EphB4 interactions, 50 µM of TNYL-RAW (TNYLFSPNGPIARAW, synthesized by GenScript, Piscataway, NJ)(29,30), was added in the secondary medium and at every medium change (twice a week). At day 21, RNA levels of chondrocyte differentiation markers were detected by quantitative real-time PCR (Q-PCR).
Deletion of IGF-IR in cultures of osteoblasts and osteoclasts
To knockout the IGF-1R gene in osteoblasts acutely in vitro, bone marrow stromal cells (BMSCs) were cultured from 8 week old floxed IGF-IR mice for 12 days (31) and infected with adenoviruses carrying a Cre recombinase cDNA (Ad-Cre) at 5 plaque forming units (pfu)/cell for 48 hrs. Mock infection with PBS or viruses expressing empty vector (Ad-DNR) was performed as control (32). To knockout IGF-IR in osteoclasts, spleen hematopoietic cells were collected from 8 week old floxed IGF-IR mice (19), immediately infected with Ad-Cre at 5 plaque forming units (pfu)/cell for 72 hrs. Control infections were performed as above. The cells were cultured for an additional 6–7 days in the presence of RANKL (30 ng/ml) and M-CSF (30 ng/ml) before analyses (19).
siRNA transfection
To silence EphB4 in osteoblasts, BMSC previously cultured for 12 days in 6-well plates (31) , were transfected with EphB4 siRNA (100 nM, siGENOME SMART pool) or siGENOME non-targeting controls (Pool of 4 non-targeting siRNA, Dharmacon, Lafayette, CO) using TransIT-siQUEST transfection reagent (Mirus BioLLC, Madison, WI) for 48 hrs. Transfected cells were treated with vehicle or IGF-I 10ng/ml for 24 hrs and the expression of EphB4 mRNA, osteoblast differentiation markers and ephrin B2 receptors were determined by Q-PCR.
OB-OCL or chondrocyte-OCL co-cultures
Co-culture of osteoblasts and spleen hematopoietic cells (stromal cell/OB free OCL precursors) was performed as described (19). To determine the role osteoclast ephrinB2 in OCL-OB communication and response to IGF-I, osteoblasts were co-cultured with osteoclast precursors lacking ephrinB2 or intact controls. For these co-cultures, spleen cells from floxed ephrin B2 mice were collected, red blood cells removed, and the cells infected with Ad-Cre (5 pfu/cell). Mock infection with PBS or Ad-DNR (5 pfu/cell) was performed as controls. After 48 hrs incubation, the infected or control cells were added to osteoblast cultures (BMSC, cultured 9 days before adding OCL). At the same time, the co-cultures were treated by IGF-I (10 ng/ml) or vehicle. The co-cultures were observed under the microscope (up to 10 days) to ensure maximum osteoclast formation. Expression of markers for osteoclast, osteoblast or chondrocyte differentiation and ephrin B receptors was measured by Q-PCR. Osteoclastogenesis in the co-cultures was assessed by tartrate-resistant acid phosphatase (TRAP) staining using a commercial kit (387 A; Sigma).
For chondrocyte and OCL co-cultures, ATDC5 cells were first cultured in 24-well plates (15000/well) for 3 days, then switched to secondary ATDC5 medium and cultured for an additional 14 days. Spleen cells from male FVB-N mice were harvested and cultured overnight (19), then added to ATDC5 cell cultures (1 × 106 /well).
To disrupt the ephrin B2/EphB4 interaction, 50 µM of TNYL-RAW or vehicle was added at the time of co-culture with OCL precursors and at each medium change. The OB-OCL co-cultures were carried for 6 days and then treated with IGF-I 10 ng/ml or vehicle for 24 hrs. The chondrocyte-OCL co-cultures were carried for 8 days. Expression of markers for osteoclast, osteoblast or chondrocyte differentiation and ephrin B receptors was measured by Q-PCR. Osteoclastogenesis in the co-cultures was assessed by tartrate-resistant acid phosphatase (TRAP) staining using a commercial kit (387 A; Sigma).
Quantitative real-time PCR
Total RNA was extracted from epiphyseal growth plates of the knee, the tibial shaft (marrow flushed out) and cultured cells. The RNA was reverse transcribed into cDNA as previously described (20). Expression of IGF-IR, ephrin B2, EphB4 was determined by quantitative real-time PCR using commercial (ephrin B2, EphB4, NFATc1, Applied Biosystems, Foster City, CA) or custom made (IGF-IR, AP, OCN, RUNX2, RANKL, RANK, EphB2, B3, B6 and ephrin B1) primers and probes as described in previous reports (19,21,31).
Statistics
Results were presented as mean ± SD. Data were analyzed by one-way ANOVA or Student’s t-test with GrapPad Prism 4.0 program (GraphPad Software Inc, La Jolla, CA). Significance was assigned for p<0.05. Samples of at least 3 animals were analyzed for each experimental and control group.
Results
Deficiency of IGF-I and IGF-IR decreases the expression of ephrin B2 and EphB4 in OBs and expression of ephrin B2 in OCLs in vivo and in vitro
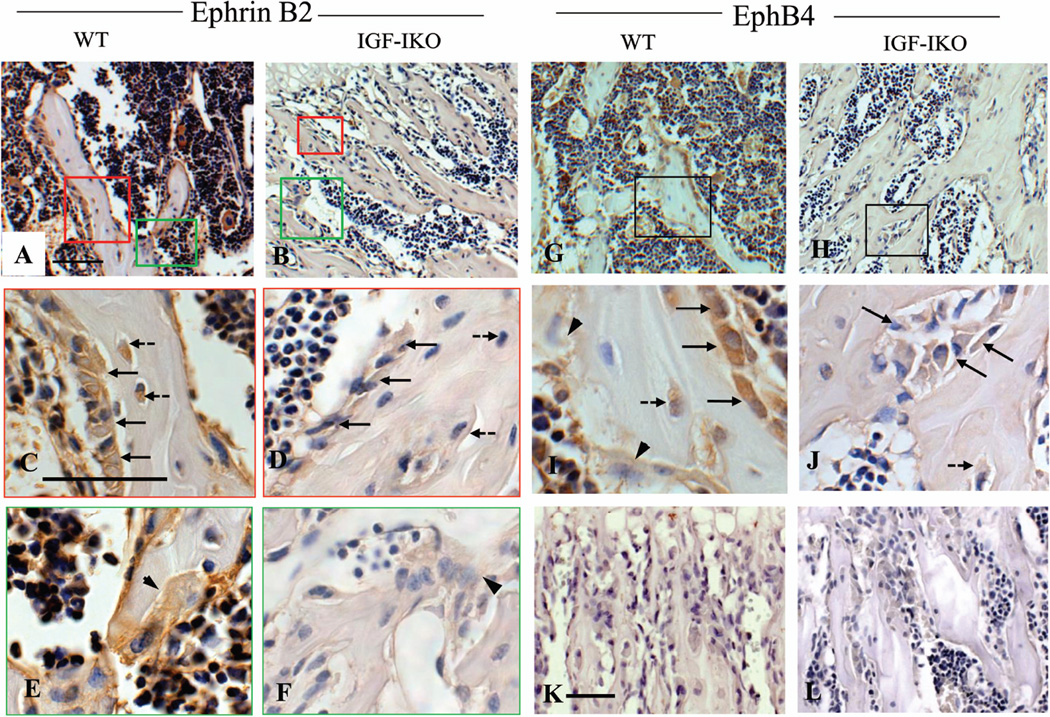
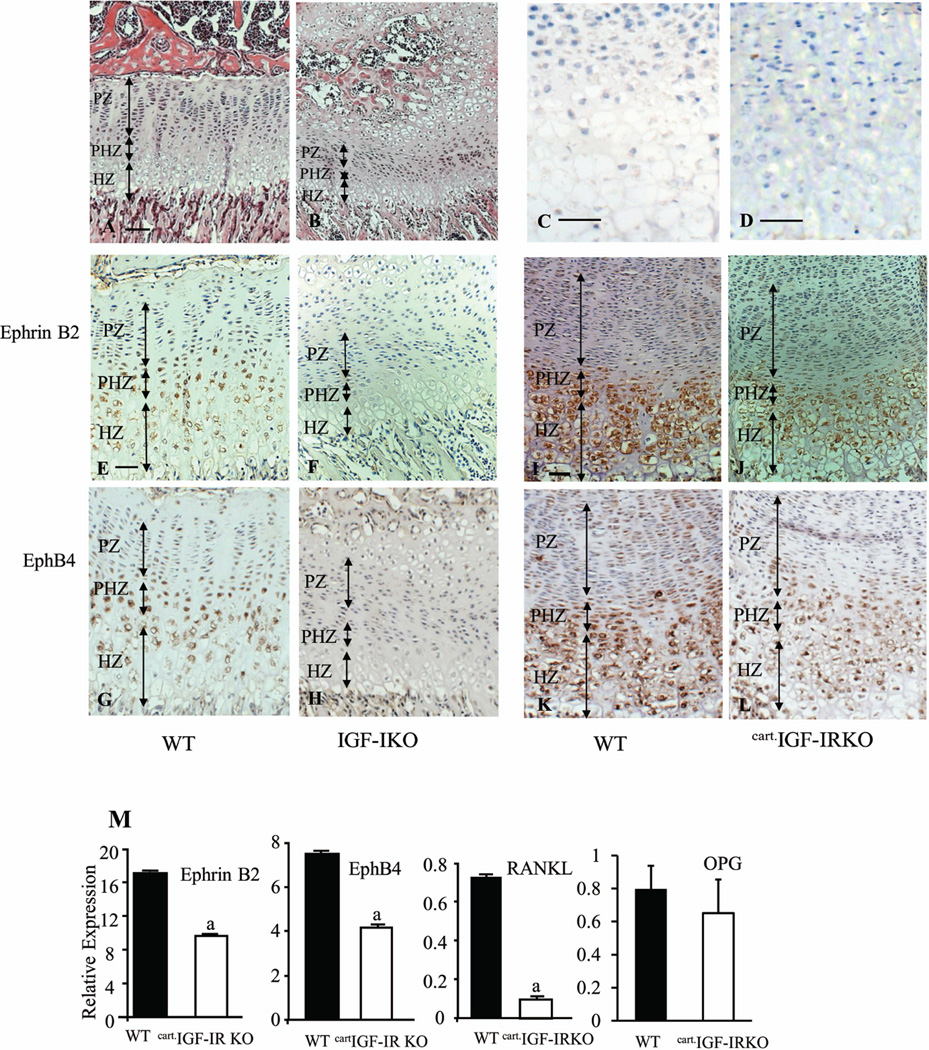
To determine whether IGF-I signaling regulates ephrin B2 and EphB4 expression in bone cells, we performed immunohistochemistry on tibias of 3 week old IGF-IKO and their WT littermates. As shown in Fig. 1, both ephrin B2 (Fig.1A, brown) and EphB4 (Fig.1G, brown) were expressed in the cells located in bone surface, bone matrix and bone marrow. Overall expression of ephrin B2 (Fig.1B vs. A) and EphB4 (Fig.1H vs. G) was remarkably reduced in the IGF-IKO. High magnification photos (HMP) identified that ephrin B2 was expressed in osteoblasts (Fig. 1C, arrows), osteocytes (Fig.1C, dashed arrows) and osteoclasts (Fig. 1E arrow head), while EphB4 was only detectable in the osteoblasts (Fig.1I, arrows) and osteocytes (Fig. 1I dashed arrow), but not in osteoclasts (Fig. 1I, arrow heads). In the IGF-IKO mice, the expression of ephrin B2 was significantly decreased in osteoblasts, osteocytes (Fig.1 D vs. C), and osteoclasts (Fig. 1 F vs. E, arrow head) compared with WT. EphB4 expression in osteoblasts and osteocytes was also markedly decreased in IGF-IKOs (Fig. 1 J vs. I) vs. WTs, suggesting that intact IGF-I signaling is required for the normal expression of ephrin B2 and EphB4 in bone cells. To confirm this observation, we determined the mRNA levels of ephrin B2 and EphB4 in bones isolated from the OBIGF-IRKO mice in which the IGF-IR gene was ablated specifically in mature osteoblasts. In these bones (marrow flushed out), mRNA levels of ephrin B2 and EphB4 were decreased by 44% and 48%, respectively, compared to the controls (Fig.2A).
Figure 1. Ephrin B2 and EphB4 expression in bone cells of the wild-type and global IGF-I KO.
Immunohistochemistry demonstrated that compared with the wild-type mice (WT, A), the global IGF-I KO (KO, B) had lower expression of ephrin B2 (Brown) in the osteoblasts, osteoclasts and bone marrow cells. High magnification photos (HMP, C–F) further confirmed these observations. C- D (red frame, HMP of red frame areas in A and B, respectively): ephrin B2 expression in the osteoblasts (arrows, C and D) and osteocytes (dashed arrows, C and D) in WT (C) and KO (D); E–F (green frame, HMP of green frame areas in A and B, respectively): ephrin B2 expression in the osteoclasts (arrows heads) the WTs (E) and KOs (F). The KO mice (H) also had lower expression of EphB4 (brown) in their osteoblasts and bone marrow than WT (G). I and J (HMP of frame areas in G and H, respectively): showed reduction of EphB4 expression in the osteoblasts (arrows), osteocytes (dashed arrow) in KOs (J) compared with WTs (I). No EphB4 expression was detected in the osteoclasts (I, arrow heads). K–L: Negative controls (IgG controls) of ephrin B2 (K) and EphB4 (L) immunohistochemistry. 10 × in A, B, G and H; 40 × in C–J; 20 × in K–L. Bars = 50 µm. n = 3 in each group.
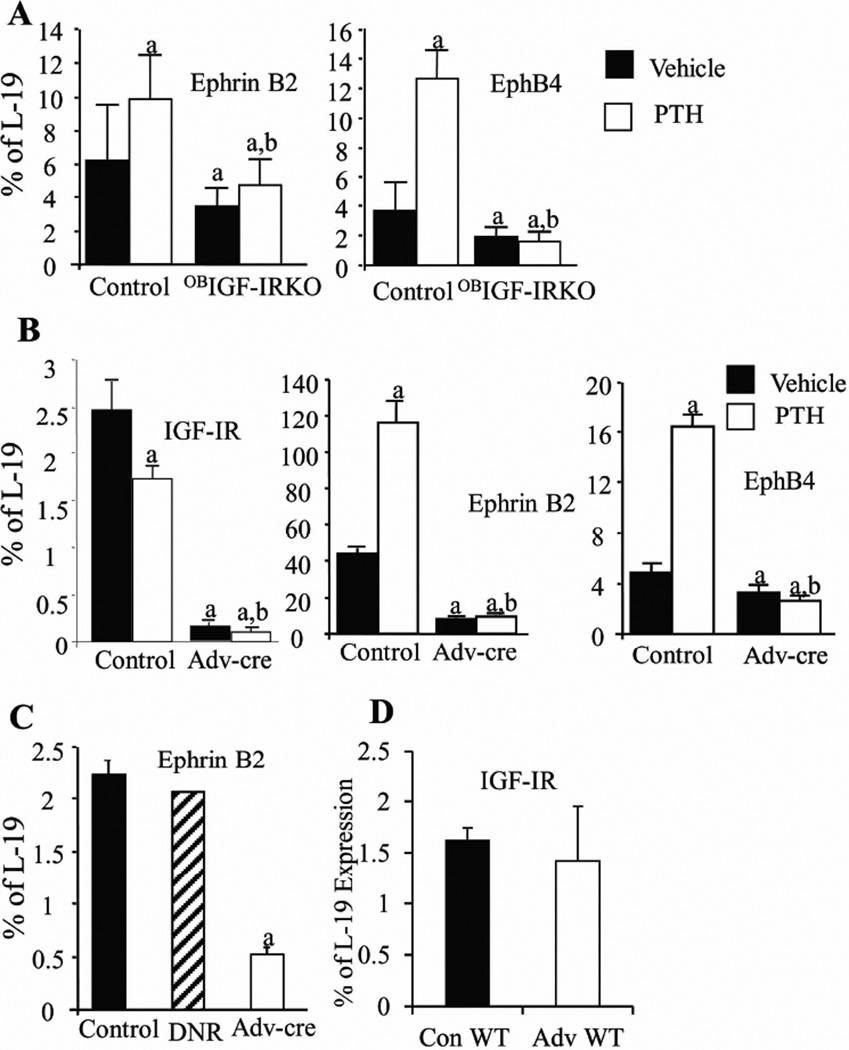
Figure 2. IGF-IR in the osteoblasts or osteoclasts is required for the expression of ephrin B2 and EphB4 in osteoblasts and the expression of ephrin B2 in osteoclasts.
A: Expression of ephrin B2 and EphB4 mRNA levels in bones (marrow flushed out) of osteoblast-specific IGF-IR KO mice (OBIGF-IR KO) and control littermates treated with PTH (open bars) or vehicle (solid bars) for 2 weeks was determined by Q-PCR. n = 4 in vehicle-treated control and OBIGF-IR KO mice and n =5 in PTH-treated control and OBIGF-IR KO mice. B–C: BMSCs (B, OB progenitors) or spleen cells (C, OCL precursors) from the floxed IGF-IR mice were treated with PBS (vehicle control), or infected with empty viruses (DNR, hatched bar, virus control) or adenoviruses carrying a functional cre-recombinase (Adv-cre) to delete IGF-IR in these cells. BMSCs were further treated by PTH (100 ng/ml, open bars) or vehicle (solid bars) for 2 hrs. D: BMSC from the wild-type mice (WT) were treated with PBS (solid bar) or Adv-cre (open bar) to test the effect of Adv-cre infection on WT BMSCs. mRNA levels of ephrin B2, EphB4 and IGF-IR were determined by Q-PCR. In A–B, a: p < 0.05 vs. vehicle treated control; b: p < 0.05 vs. PTH treated controls; in C, a: p < 0.05 vs. PBS control. n = 4 wells in each group. Error bars enclose mean ± SD.
In previous studies, we and others showed that IGF-I signaling is required for the anabolic actions of intermittent PTH treatment in bone, as these actions were blunted in the global IGF-IKO and OBIGF-IRKO mice. To test whether ephrin B2/EphB4 signaling is part of this pathway, we compared the expression of ephrin B2 and EphB4 in bone samples from the control and OBIGF-IRKO mice injected daily with PTH for 14 days. In the control mice, PTH significantly increased the mRNA levels of ephrin B2 and EphB4 by1.6 fold and 3.5 fold, respectively. These PTH effects were, however, absent in bones from the OBIGF-IRKO mice (Fig.2A), confirming that IGF-IR signaling is critical for the expression of ephrin B2 and EphB4 in osteoblasts and their response to PTH.
To confirm that the effects of IGF-I/IGF-IR and PTH signaling on the expression of ephrin B2 and EphB4 in osteoblasts are direct, we performed acute deletion of IGF-IR by viral expression of Cre-recombinase (Adv-cre) in the enriched cultures of BMSCs isolated from the floxed IGF-IR mice and treated the cells with PTH (100 ng/ml, 2hrs). Q-PCR analysis of RNA from the Adv-cre infected BMSCs demonstrated the knockdown of IGF-IR expression by 95% when compared with the cultures infected by the control viral vector (control) (Fig2B), confirming an effective deletion of IGF-IR in these cultures. The deletion of IGF-IR was also confirmed by western blot at the protein level (Fig.S1). In the Adv-Cre infected cultures without PTH, mRNA levels of ephrin B2 and Eph B4 were decreased by 78% and 41%, respectively (Fig.2B). PTH treatment significantly increased the mRNA levels of both ephrin B2 (2.6 fold) and EphB4 (4 fold) in the control cultures, but these effects were abolished in the cultures infected by Adv-cre to delete the IGF-IR (Fig.2B). Moreover, IGF-I treatment increased ephrin B2 expression in the BMSC cultures compared with the vehicle treated cultures (Fig.S2). These in vitro data demonstrate the requirement for IGF-IR in osteoblasts with respect to ephrin B2/EphB4 signaling and its stimulation by PTH and IGF-I in osteoblasts.
To test whether IGF-I/IGF-IR signaling regulates the production of ephrin B2 in osteoclasts, we deleted the IGF-IR in osteoclast precursors by infecting spleen cells of floxed-IGF-IR mice with the Adv-cre virus. Compared with the control cultures, the mRNA levels of ephrin B2 were decreased by 75% in the cultures infected by Adv-cre (Fig. 2C). These data indicate that IGF-IR in osteoclasts also regulates the expression of ephrin B2 in these cells. As a control, we also infected BMSCs from the wild-type mice by PBS (conWT) and Adv-cre (AdvWT), as shown in Fig 2D. No difference was shown in the mRNA levels of IGF-IR in the conWT and AdvWT cultures, indicating that deletion of IGF-IR by Adv-cre is specific and Adv-cre has no toxic effects on the cells at this dose.
Ablation of EphB4 blocks the anabolic actions of IGF-I in osteoblasts
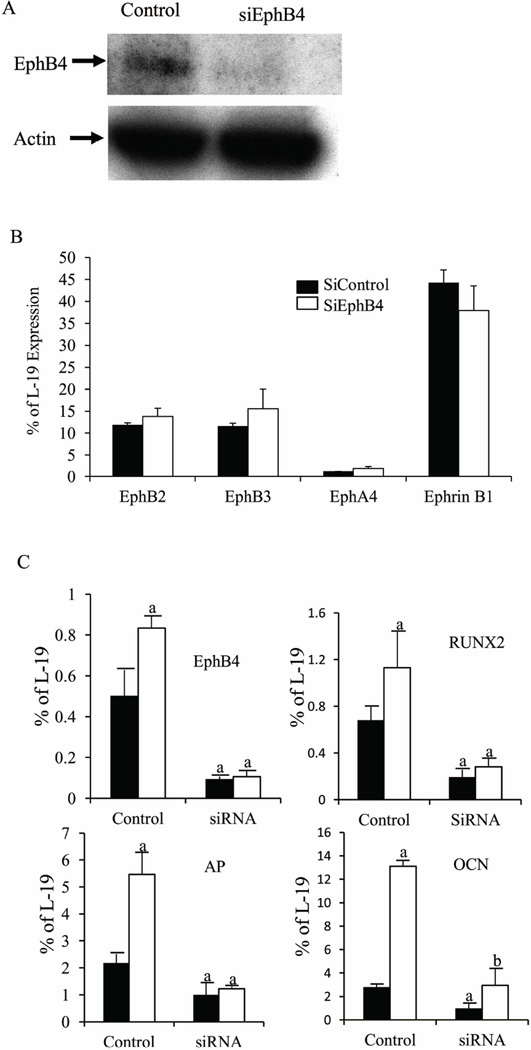
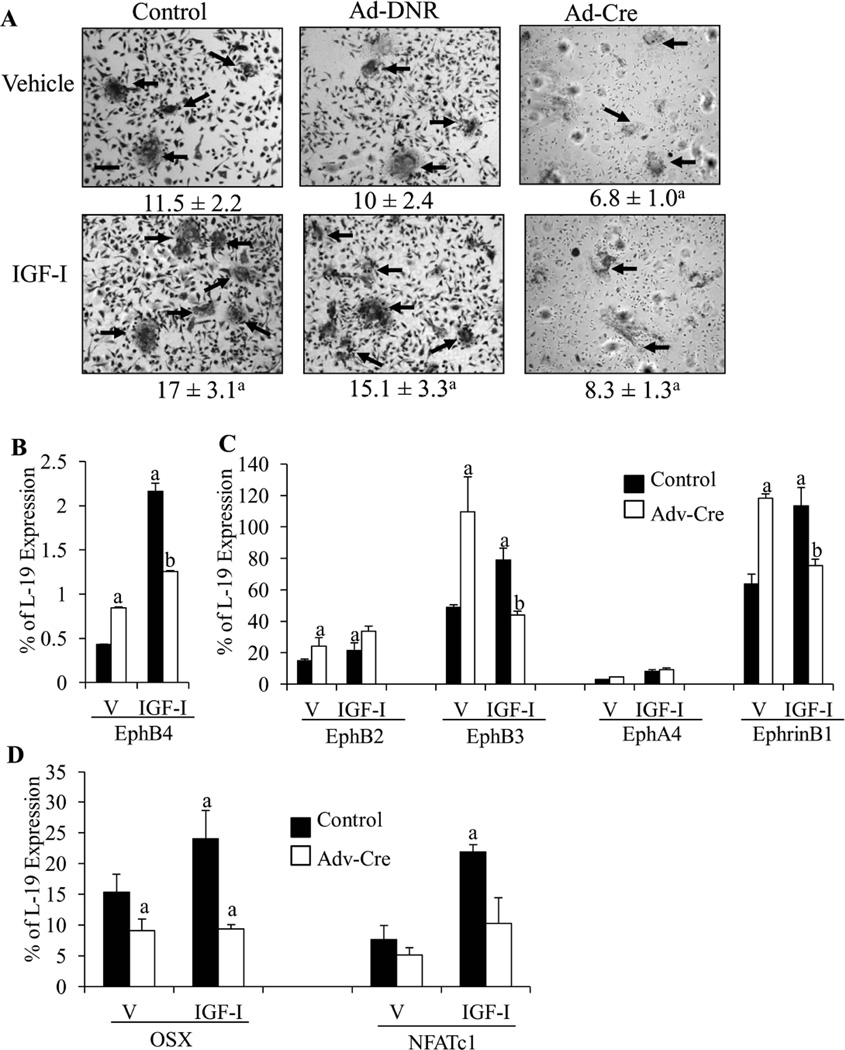
To investigate whether ephrin B2-EphB4 signaling mediates the anabolic actions of IGF-I in osteoblasts, we knocked down EphB4 expression in BMSCs with gene-specific siRNA (siEphB4) and treated the cultures with IGF-I. Western blot demonstrated the deletion of EphB4 protein by siEphB4 (Fig.3A). Silencing of EphB4 did not affect the mRNA levels of osteoblast-expressing ephrin B2 receptors EphB2, B3, EphA4 (33) or other ephrin B ligands (ephrinB1) (Fig.3B). As shown in Fig. 3C, mRNA levels of EphB4 decreased by 82% in the siEphB4 cultures (siRNA), compared with the control cultures transfected with non-targeting controls (control). IGF-I increased the expression of EphB4 in the control cultures by 60%, but not in the siEphB4 cultures, confirming the gene knockdown. IGF-I increased the mRNA levels of RUNX2 and AP in the control cultures by 1.5 fold and 2.5 fold, respectively, when compared to the vehicle-treated controls, supporting the anabolic actions of IGF-I. Knockdown of EphB4 not only suppressed the expression of RUNX2, alkaline phosphatase (AP) and osteocalcin (OCN) by 75%, 53% and 72%, respectively, in the vehicle-treated cultures, but also blocked or blunted the ability of IGF-I to increase the expression of these genes. These data suggest that IGF-I exerts its anabolic actions on osteoblast differentiation at least in part by modulating ephrinB2/EphB4.
Figure 3. IGF-I regulates osteoblast differentiation via the ephrin B2/EphB4 signaling pathway.
A: Western blot confirming the deletion of EphB4 in the siEphB4 cultures. B: The mRNA levels of ephrin B1 and ephrin B2 receptors EphB2, EphB3, EphA4 in the control and siEphB4 cultures were determined by Q-PCR. C: EphB4 was knocked down in the BMSC cultures at day 12 by incubation with gene-specific siRNA for 72 hours, then the cultures were treated by IGF-I (10 ng/ml, open bars) or vehicle (solid bars) for 24 hrs before mRNA levels of EphB4, AP, OCN, and RUNX2 were determined by Q- PCR. Results are expressed as percentage of a housekeeping gene (L-19) expression (mean ± SD). a: p < 0.05 vs. vehicle treated control; b: p < 0.05 vs. vehicle treated siRNA. n = 4 wells in each group.
IGF-I regulates OB-OCL communication via ephrin B2/EphB4
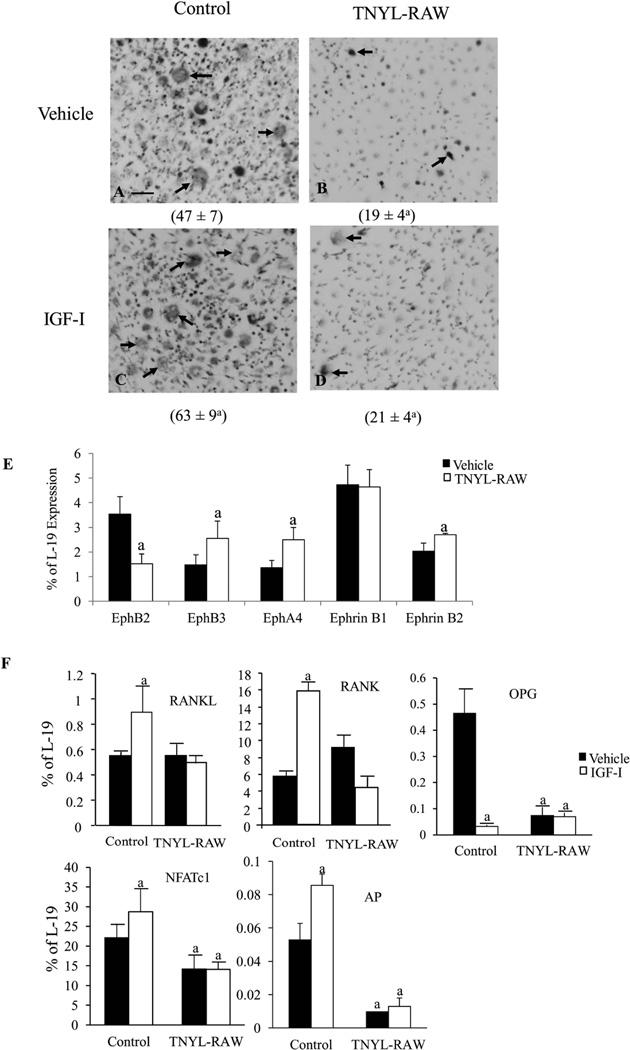
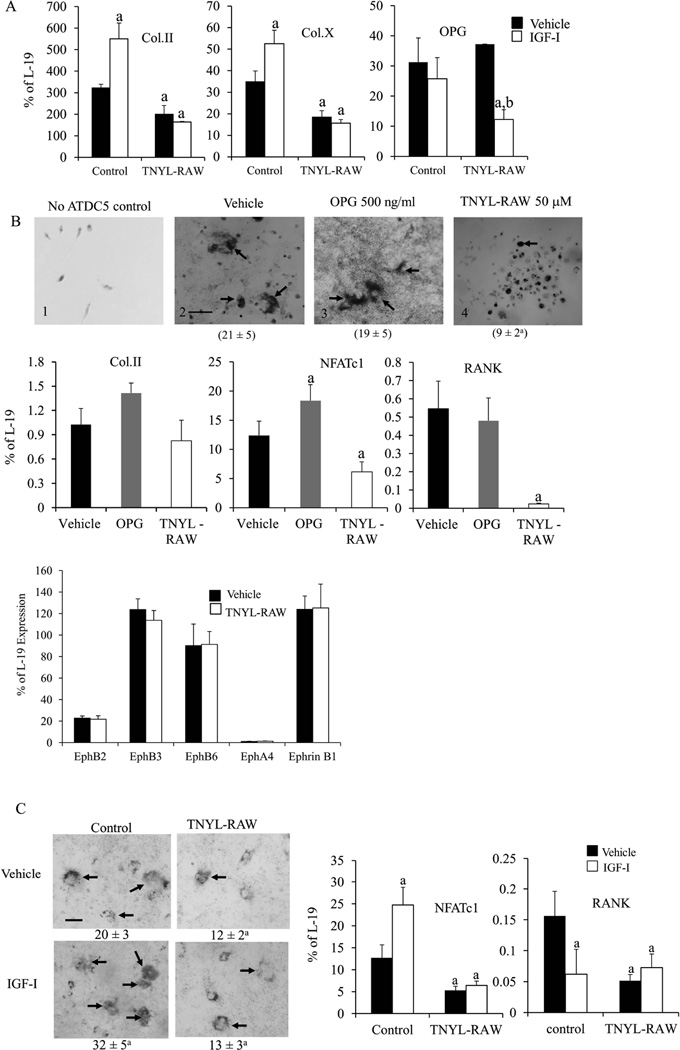
To investigate whether the effects of IGF-I in maintaining osteoblast-osteoclast communication occurred via the ephrin B2-EphB4 signaling pathway, we blocked the interaction between ephrin B2/EphB4 by the addition of TNYL-RAW to the co-culture of osteoblasts and osteoclasts to test the impact on osteoclast formation in these co-cultures. The co-cultures without TNYL-RAW or IGF-I (Fig.4A) formed multinucleated (3 nuclei or more) TRAP positive cells. In the absence of IGF-I, TNYL-RAW treated cultures (Fig.4B) did not form mature osteoclasts containing 3 or more nuclei, and only a small number of mononucleated or binucleated TRAP positive cells (arrows) (47 ± 7 in control vs. 19 ± 4 in TNYL-RAW, p < 0.05). In the absence of TNYL-RAW, IGF-I significantly increased the number of multinucleated osteoclasts in the cultures (47 ± 7 in vehicle treated vs. 63 ±9 in IGF-I treated control cultures, p < 0.05) (Fig.4C). These effects of IGF-I were, however, abolished with the TNYL-RAW treatment (Fig.4D). Thus, disrupting the interaction between ephrin B2 and EphB4 reduced osteoblast stimulated osteoclastogenesis in vitro, and blunted the stimulatory effects of IGF-I on this process. These results are contrary to the concept that ephrin B2/EphB4 signaling blocks osteoclastogenesis. To confirm these results, we first treated osteoclast cultures (spleen cells stimulated with RANKL and M-CSF) by TNYL-RAW 50 µM (the dose we used in the co-cultures) or vehicle to test whether TNYL-RAW itself blocks osteoclastogenesis. At day 9 of the osteoclast culture, TRAP staining showed that the number of mature osteoclasts was comparable in the two types of cultures [12 ± 2 /low magnification field (LMF) in vehicle treated cultures vs. 14± 4/LMF in the TNYL-RAW treated cultures, n=4 wells in each group)]. There were no differences in the morphology of osteoclasts in these cultures, These results indicate that TNYL-RAW did not affect osteoclastogenesis per se. We did find that TNYL-RAW altered the expression of EphB family receptors and ligands other than ephrinB2 and EphB4 in the co-cultures. As shown in Fig.4E, compared to the vehicle treated co-cultures, the mRNA levels of EphB2 were down-regulated by 57%, while the mRNA levels of EphB3 (72%), EphA4 (78%) and ephrin B2 (40 %) were up-regulated in the TNYL-RAW treated co- cultures. TNYL-RAW did not change the mRNA levels of ephrin B1. The role of these other ephrin/Eph family members in osteoblast/osteoclast interactions is unclear. To further investigate the molecular basis for the effects of TNYL-RAW and IGF-I on osteoclastogenesis, we determined the expression of osteoclast markers by Q-PCR. In the co-cultures without TNYL-RAW, IGF-I significantly increased the mRNA levels of RANKL (49%), RANK (171%), and NFATc1 (30%), while decreasing the mRNA levels of OPG (80%). These effects were blunted in the cultures treated by TNYL-RAW (Fig.4F). On the other hand, TNYL-RAW also decreased the mRNA levels of AP by > 80% in the presence or absence of IGF-I (Fig.4F), suggesting that ephrin B2/EphB4 also regulates osteoblast differentiation in the co-cultures and their response to the anabolic actions of IGF-I.
Figure 4. IGF-I maintains osteoblast-osteoclast communication via ephrinB2/EphB4 signaling pathway.
Co-cultures of spleen cells and BMSCs treated with TNYL-RAW (50 µM) or controls. These cultures were further treated by vehicle or IGF-I 10 ng/ml 24 hrs before analyses. A–D: Number of osteoclasts (arrows) formed in the cultures was counted and shown under the corresponding pictures. mRNA levels of ephrin B1 and ephrin B2 receptors EphB2, EphB3, EphA4 (E) and RANKL, RANK, OPG, AP and NFATc1 (F) in the co-cultures were determined by Q-PCR. Results are expressed as mean ± SD. a: P < 0.05 vs. vehicle treated control. n = 4 wells in each group.
To confirm the results with TNYL-RAW indicating that inhibition of ephrin B2/EphB4 blocked osteoblast induced osteoclastogenesis, we co-cultured osteoblasts with osteoclast precursors from mice expressing the floxed ephrinB2 in which ephrinB2 was deleted by Ad-Cre prior to co-culture. After 48 hrs of Ad-Cre infection, ephrin B2 was deleted by 85% in the osteoclast precursors as indicated by Q-PCR (data not shown). After 10 days of co-culture, the number of osteoclasts (Fig 5A, arrows) formed was 39% less (p <0.05) and smaller in size in the co-cultures of OB and Ad-Cre infected OCL (Ad-Cre) co-cultures, compared with the co-cultures of osteoblast and control (control) or Ad-DNR infected (Ad-DNR) OCL (Fig.5A). IGF-I treatment significantly increased osteoclast number and size in the control (50%) or Ad-DNR (55%) co-cultures, but not in the Ad-Cre co-cultures (Fig.5A), suggesting that ephrin B2 in the osteoclasts is required for osteoblast induced osteoclastogenesis and its stimulation by IGF-I. These results confirm the observations with TNYL-RAW. Compared with the control cultures, the mRNA levels of EphB4, the specific receptor for ephrin B2, was increased by 95% in the Ad-Cre co-cultures perhaps reflecting the lack of ligand. IGF-I treatment increased EphB4 in both control (5 fold) and Ad-Cre (1.5 fold) cultures (Fig.5B). In the Ad-Cre co-cultures, the expression of EphB2, Eph B3 and ephrin B1 were also up-regulated by 1 fold, 2.2 fold and 1.9 fold, respectively, compared with the control co-cultures (Fig.5C). In the control co-cultures, IGF-I increased EphB2 (50%), EphB3 (65%) and ephrin B1 (79%) expression, but in the Ad-Cre co-cultures IGF-I decreased EphB3 and ephrin B1 expression (59%, 40% respectively), but did not alter EphB2 expression (Fig.5C). There were no significant differences in expression of EphA4 in the control or Ad-Cre co-cultures or following IGF-I (Fig. 5C). The implications of these changes in other members of the ephrin/Eph family will require further study. But the key point is disruption of ephrinB2/EphB4 by deleting ephrin B2 from osteoclasts blocks osteoblast stimulated osteoclastogenesis similar to the effects of TNYL-RAW in these co-cultures. In the Ad-Cre co-cultures, the mRNA levels of osterix (OSX) and NFATc1 were decreased by 40% (p<0.05) and 23% (p > 0.05), respectively, compared with the control co-cultures. IGF-I increased OSX (60%) and NFATc1 (110%) expression in the control-cultures, but these effects were blocked in the Ad-Cre co-cultures. These data indicate that deletion of ephrin B2 in osteoclasts not only inhibits osteoblast stimulated osteoclastogenesis but osteoclast stimulated osteoblast differentiation and the stimulation of such differentiation by IGF-I.
Figure 5. Ephrin B2 in osteoclasts promotes osteoblast and osteoclast differentiation.
Osteoblasts were co-cultured with spleen cells (osteoclast precursors) from floxed ephrinB2 mice infected with control (control), empty viruses (Ad-DNR, virus control) or adenoviruses carrying a functional cre-recombinase (Adv-cre) prior to co-culture. These co-cultures were further treated by vehicle or IGF-I. A: TRAP staining. The number of osteoclasts (arrows) per low magnification field (LMF) formed in the cultures was counted and shown under the corresponding pictures. The mRNA levels of ephrin B1 and ephrin B2 receptors (B) and osteoblast (osx) and osteoclast (NFATc1) differentiation markers (C) in the co-cultures were determined by Q-PCR. V: vehicle treated co-cultures, IGF-I: IGF-I treated co-cultures. Results are expressed as mean ± SD. a: p < 0.05 vs. vehicle treated control; b: p < .0.5 vs. vehicle treated Ad-Cre. n = 4 wells in each group
Deficiency of IGF-I and IGF-IR decreases expression of ephrin B2 and EphB4 in growth plate (GP) chondrocytes in vivo
H&E staining showed that at 3 weeks WT mice (Fig.6A) formed well-organized GPs in their long bones (tibia). Compared to the WTs, the IGF-IKOs (Fig.6B) had smaller GPs with shorter proliferating zones (PZ) (194.96 ± 11.11 in WT vs. 118.47 ± 37.58 in KO), prehypertrophic zones (PHZ) (73.76 ± 6.82in WT vs. 38.71± 5.6 in KO) and hypertrophic zones (HZ) (152.01± 7.52 in WT vs. 90.25 ± 9.35 in KO) (n = 3 in each group, p < 0.05 for each zone). The cells in each zone were also disorganized. To test whether ephrin B2/EphB4 signaling is also involved in the regulation of GP development and whether these molecules interact with IGF-IR signaling in this tissue, we compared the expression of ephrin B2 and EphB4 by immunohistochemistry in the GPs of global IGF-IKO and chondrocyte-specific IGF-IRKO (CartIGF-IRKO) mice. Negative controls for the immunohistochemistry of ephrin B2 (Fig.6C) and EphB4 (Fig.6D) showed no non specificity. In the GPs of WT mice, the expression of ephrin B2 and EphB4 was predominantly found in chondrocytes in the prehypertrophic/maturation zone and to a lesser extent in cells in the proliferating zone and upper hypertrophic zone (Fig.6E & G). Little or no expression of these molecules was found in the lower HZ (Fig.6E & G). The IGF-IKO mice (littermates) had profound reductions in the expression of ephrin B2 (Fig. 6F) and EphB4 (Fig. 6H) protein in all subpopulations of chondrocytes in the GPs of IGF-IKO compared to WT mice.
Figure 6. IGF-I/IGF-IR signaling is required for the expression of ephrin B2, EphB4 and RANKL in the growth plate.
A–B: H&E staining of the growth plates (GP) from the WTs (A) and global IGF-IKOs (B). C–D: Negative controls (IgG controls) of ephrin B2 (C) and EphB4 (D) immunohistochemistry. E–L: Immunohistochemical detection of ephrin B2 (E, F, I and J) and EphB4 (G, H, K and L) in the GPs of IGF-IKO (E–H) and cart.IGF-IRKO (I–L) mice and corresponding WT littermates (E, G for IGF-IKO; I, K for cart.IGF-IRKO). Compared with the wild-types (WT), the expression of ephrin B2 and EphB4 was markedly reduced in the chondrocytes in each zone of GPs from both KO models. PZ: proliferating zone; PHZ: prehypertrophic zone; HZ: hypertrophic zone. Bars = 50 µm in A–L. n = 3 in each group. M: Expression of ephrin B2, EphB4, RANKL and OPG RNA in the GP from the knees of cart.IGF-IRKO (open bars) and control mice (solid bars) was determined by Q-PCR. Results are expressed as percentage of L-19 expression (means ± SD of triplicate determinations). a: p < 0.05 cart.IGF-IRKO vs. control.
We next examined the expression of ephrin B2 and EphB4 in the GP of neonatal CartIGF-IRKO mice and their control littermates. As in the global IGF-IKO, the CartIGF-IRKO showed a reduction in the HZ (Fig.6J, L) compared to littermate controls (Fig. 6I, K), although the PZ and PHZ were comparable. In the control mice, ephrin B2 (Fig.6I) and EphB4 (Fig. 6K) were strongly expressed by chondrocytes in the lower PZ, PHZ, and throughout the HZ. These expression patterns are compatible with the patterns in the 3 week old WT mice as described above. Similarly, the expression of ephrin B2 (Fig.6J) and Eph B4 (Fig. 6L) was significantly decreased in all subpopulations of chondrocytes in the GPs of CartIGF-IRKO mice. Q-PCR analyses of RNA extracted from the GP showed significant reductions in the expression of ephrin B2 and EphB4 RNA, by 40% and 43%, respectively, in the CartIGF-IRKO mice, when compared with WT controls (Fig.6M). The mRNA levels of RANKL and OPG were low in the WT mice. Compared with controls, RANKL expression was further decreased by more than 80% in the CartIGF-IRKOs. No significant difference in OPG expression between WT and CartIGF-IRKO was observed (Fig.6M). Thus the expression of ephrin B2 and EphB4 in chondrocytes like that in osteoblasts is regulated by IGF-I/IGF-IR signaling.
The role of ephrin B2/EphB4 in chondrocyte differentiation and chondrocyte-OCL interaction
To determine the role of ephrin B2/EphB4 signaling in regulating the differentiation of chondrocytes and mediating the anabolic effects of IGF-I, we examined the impact of TNYL-RAW on the differentiation of vehicle or IGF-I treated chondrogenic ATDC5 cells. First, Q-PCR determined that ATDC5 cells expressed both ephrin B2 and EphB4 and that IGF-I treatment significantly increased the mRNA levels of ephrin B2 and EphB4 when compared with the vehicle treatment (Fig.S3). Other than EphB4 and ephrin B2, ATDC5 cells and growth plate cartilage also expressed ephrin B1 and receptors EphB2, B3, B6 and lower levels of EphA4, but no EphB1 (FigS4). As shown in Fig. 7, TNYL-RAW treatment significantly decreased mRNA levels of type II collagen (55% of vehicle treated control) and type X collagen (52% of vehicle treated control), respectively, compared with the vehicle treated cultures (Fig.7A). IGF-I treatment increased the mRNA levels of type II collagen and type X collagen in the ATDC5 cells without TNYL-RAW treatment, but these effects were blunted in the ATDC5 cells treated by TNYL-RAW (Fig. 7A). OPG was also expressed in ATDC5 cells, but neither TNYL-RAW nor IGF-I treatment alone significantly affected the mRNA level of OPG in the ATDC5 cells. However, surprisingly, IGF-I decreased the mRNA level of OPG in the TNYL-RAW treated ATDC5 cells suggesting a difference with the role of ephrin B2-EphB4 in these cells compared to osteoblasts with respect to IGF-I signaling. Nevertheless, these data suggest that ephrin B2/EphB4 stimulates chondrocyte differentiation, and at least in part, mediates the anabolic effects of IGF-I. The ability of ephrin B2/EphB4 to regulate RANKL expression and promote osteoclast differentiation (Fig. 4) in BMSCs led us to hypothesize that ephrin B2/EphB4 signaling may also mediate osteoclastogenesis by altering the RANKL/OPG pathway in chondrocytes. To test this, we established co-cultures of ATDC5 cells and spleen cells (osteoclast precursors) and examined the impact of TNYL-RAW on the differentiation of osteoclasts in these cultures. In support of our hypothesis, we observed osteoclast formation in the co-cultures of ATDC5 cells and spleen cells (Fig. 7B2) but not in cultures of spleen cells alone (Fig. 7B1). However, to our surprise treatment of these co-cultures with OPG failed to prevent ATDC5 induction of OCL formation (TRAP positive cell number: 21 ± 5 cells/well in vehicle-treated vs. 19 ± 5 cells/well in OPG-treated) (Fig. 7B3), suggesting that RANKL/OPG pathway was not involved in inducing osteoclast differentiation in the co-cultures with chondrocytes at least under the conditions of these experiments. Furthermore, the expression of RANKL by Q-PCR in the ATDC5 cell cultures or in the co-cultures of ATDC5 cells and osteoclast precursors was below the limits of detection (data not shown). On the other hand, TNYL-RAW treatment significantly decreased the formation of TRAP positive cells (3 or more nuclei) by 58% in these co-cultures (TRAP positive cell number: 21 ± 5 in vehicle vs. 9 ± 2 in TNYL-RAW, p < 0.05) (Fig.7B4). mRNA levels of chondrocyte and osteoclast differentiation markers from these cultures were determined by Q-PCR (Fig. 7B bar graph). Neither OPG nor TNYL-RAW treatment affected the mRNA levels of type II collagen (Col.II). OPG treatment did not significantly affect NFATc1 or RANK expression, but TNYL-RAW treatment substantially reduced the mRNA levels of NFATc1 (50% of control) and RANK (9% of control) in the co-cultures. In addition, compared with the vehicle treated co-cultures, TNYL-RAW induced no changes in the mRNA levels of ephrin B1 or ephrin B2 receptors (EphB2, B3, B6 and EphA4) (Fig.7B, bar graph), suggesting that in the case of chondrocyte/osteoclast co-cultures ephrinB2/EphB4 signaling has less regulation of other ephrin/Eph family members than is the case for osteoblast/osteoclast interactions. The significance of these differences will require further investigation. To determine the effects of IGF-I in these co-cultures, control and TNYL-RAW treated co-cultures were further treated by vehicle or IGF-I (Fig. 7C). IGF-I significantly increased the osteoclast formation in the co-cultures without TNYL-RAW treatment (20 ± 3 in control vs. 32 ± 5 in IGF-I, p < 0.05), but these effects were blunted in the TNYL-RAW treated co-cultures (12 ±2 in vehicle treated TNYL-RAW cultures vs. 13 ±3 in IGF-I treated TNYL-RAW cultures) (Fig.7C). IGF-I increased mRNA levels of NFATc1 (2.5 fold) but decreased mRNA levels of RANK in the co-cultures. These effects were blocked by TNYL-RAW (Fig.7C bar graph), further evidence that chondrocyte regulation of osteoclastogenesis differs from that of osteoblast regulation of osteoclastogenesis at least with respect to IGF-I. Regardless, these results indicate that in the co-culture of ATDC5 cells and osteoclast precursors, ephrin B2-EphB4 mediates the induction of osteoclastogenesis by chondrocytes independent of RANKL.
Figure 7. Ephrin B2/EphB4 signaling pathway is important for chondrocyte differentiation and chondrocyte-OCL communication.
A: ATDC5 cells were treated with vehicle or TNYL-RAW, then further treated with vehicle or IGF-I for 24 hrs. The mRNA levels of Col.II, Col. X and OPG were determined by Q-PCR. B: Spleen cell culture alone (no ATDC5 cells) (B1). Co-culture of ATDC5 cells and spleen cells were treated with vehicle (B2), OPG (B3) or TNYL-RAW (B4). TRAP positive cells (arrows, 3 or more nuclei) formed in the cultures were counted, and the numbers are shown below the relevant pictures. The mRNA levels of Col.II, NFATc1, RANK and ephrin B2 receptors in the co-cultures were determined by Q-PCR. C: Co-cultures of ATDC5 cells and spleen cells were treated with vehicle or TNYL-RAW and subsequently with IGF-I or vehicle. TRAP positive cells formed in the cultures were counted, and the numbers are shown below the relevant pictures. Bar graph: The mRNA levels of NFATc1 and RANKL in the co-cultures were determined by Q-PCR. D: The mRNA levels of ephrin B1 and ephrin B2 receptors in the above co-cultures were determined by Q-PCR. V: vehicle treated co-cultures, IGF-I treated co-cultures. Results are expressed as mean ± SD, a: p < 0.05 TNYL-RAW vs. control; b: p < 0.05 IGF-I/TNYL-RAW treatment vs. vehicle/TNYL-RAW treatment. n = 4 wells in each group.
Discussion
In this study we have demonstrated that IGF-I signaling induces ephrin B2/EphB4 expression in osteoblasts and chondrocytes and ephrin B2 expression in osteoclasts. This up-regulation of ephrin B2/EphB4 signaling is necessary for IGF-I stimulation of osteoblast, osteoclast and chondrocyte differentiation required for endochondral bone formation and skeletal remodeling.
Our data are compatible with prior reports demonstrating the expression of ephrin B2 and/or EphB4 in BMSCs/osteoblasts/osteocytes, osteoclasts (16,34,35) and bone marrow cells (36), and their regulation by both anabolic and catabolic regulators of bone remodeling. We observed that the expression of ephrin B2 in osteoblasts and osteoclasts and the expression of EphB4 in osteoblasts were impaired by global IGF-I deficiency or ablation of IGF-IR in these cells. Moreover, IGF-I treatment increased the mRNA levels of EphB4 in BMSC cultures and mRNA levels of ephrin B2 in BMSC and osteoclast precursor cultures. These data indicate that IGF-I signaling is required for ephrin B2 and EphB4 gene expression in osteoblasts and ephrin B2 expression in osteoclasts, and as such is a critical regulator of ephrin B2/ EphB4 signaling in these bone cell populations.
The anabolic effects of IGF-I on bone can be attributed to the increased differentiation and function of osteoblasts(18,21,37). Recent studies reported that ephrin B2 produced by osteoblasts acts as a paracrine and/or autocrine factor to stimulate their differentiation (38). In our study, inactivation of EphB4 in osteoblasts not only decreased the expression of osteoblast differentiation markers RUNX2, AP and OCN, but abolished the stimulatory effects of IGF-I on these markers, suggesting that IGF-I stimulates osteoblast differentiation via ephrin B2/EphB4 signaling. Moreover, deletion of IGF-IR in osteoblasts in vivo or in vitro not only decreased ephrin B2 and EphB4 expression, but blunted the anabolic actions of PTH on osteoblast differentiation. These results indicate that in osteoblasts, PTH activates IGF-I signaling(20,21), which increases the production of ephrin B2 and EphB4 and promotes osteoblast differentiation and bone formation (38,39).
Previous studies have demonstrated that IGF-I is required for maintaining the interaction between osteoblasts and osteoclasts to support osteoclast formation through its regulation of RANKL/RANK and M-CSF/c-fms expression(19). In the current study TNYL-RAW, a specific antagonist of ephrinB2 interactions with EphB4 (40), blocked osteoblast induced osteoclastogenesis, decreased osteoblast differentiation (as indicated by lower mRNA levels of AP) and blocked the ability of IGF-I to promote osteoblast differentiation and osteoclastogenesis in these co-cultures. Our data do not support a role for ephrin B2/EphB4 in osteoblast suppression of osteoclast differentiation as suggested by earlier studies (16); rather our results indicate the opposite—ephrin B2/EphB4 is required for osteoblasts to promote osteoclastogenesis. To confirm these results we performed the following. First, the inhibition of osteoclastogenesis is not due to TNYL-RAW itself as demonstrated by the inability of TNYL-RAW to block osteoclastogenesis induced by M-CSF and RANKL. TNYL-RAW resulted in increased expression of other ephrin B2 receptors, EphB3, EphB4 and ephrin B2 itself with no change in ephrin B1 indicating that the suppression of osteoclastogenesis was not due to failure of other potentially compensating changes in ephrin signaling(41). Likewise, TNYL-RAW led to an increase in RANKL, a trend toward an increase in RANK and a decrease in OPG expression, which should have increased osteoclastogenesis, but did not. Most compelling, however, is that deletion of ephrin B2 from osteoclast precursors prior to co-culture with osteoblasts blocked osteoclastogenesis and blocked its stimulation by IGF-I. Of note, when ephrin B2 was deleted, the expression of ephrin B1, EphB2, EphB3, and EphB4 was increased. In other studies deletion of ephrin B1 from the myeloid lineage led to increase osteoclast activity(42). Conceivably, the deletion of ephrin B2 from the osteoclast precursor in our studies resulted in decreased osteoclastogenesis in part by the increase in ephrin B1. Furthermore, deletion of ephrin B2 from osteoclasts not only decreased osteoclastogenesis but blocked osteoblast differentiation, as indicated by decreased OSX expression, and blocked its stimulation by IGF-I. Thus, disruption of ephrinB2/EphB4 signaling between osteoblasts and osteoclasts whether by a specific inhibitor, TNYL-RAW, or deletion of ephrin B2 from osteoclasts results in both decreased osteoblast and osteoclast differentiation, and blocks the stimulation of these events by IGF-I.
Previous studies indicate that ephrin B2 treatment of osteoarthritic chondrocytes increases gene expression levels of type II collagen in these cells(43), suggesting that ephrin B2 also plays a role in regulating chondrocyte differentiation. In our current study, blocking ephrin B2/EphB4 interaction in ATDC5 cells reduced not only the mRNA levels of type II and type X collagen, but their stimulation by IGF-I, indicating that ephrin B2/EphB4 signaling also promotes chondrocyte differentiation as well as that of osteoblasts and osteoclasts. Deficiency of IGF-I in the IGF-IKO mouse or deletion of IGF-IR in chondrocytes decreased the mRNA and protein levels of ephrin B2 and EphB4 in these cells, indicating that IGF-I signaling is required for the expression of ephrin B2 and EphB4 in chondrocytes. Ephrin B2/EphB4 mediates IGF-I stimulated chondrocyte differentiation and function as it does in IGF-I stimulated osteoblast and osteoclast differentiation and function.
At the chondro-ossesous junction in the growth plates, osteoclast activity follows vascular invasion and is required for the conversion of cartilage to bone. The communication between chondrocytes and adjacent osteoclasts is critical for this process but has received little attention. Two previous studies showed that chondrocytes support osteoclastogenesis via RANKL/RANK signaling(44,45). In our hands, ATDC5 cells express very low levels of RANKL mRNA as does neonatal growth plate cartilage (Fig. 6), but multinucleated osteoclasts formed spontaneously in the co-cultures of ATDC5 and spleen cells but not in spleen cell or ATDC5 cultures alone. OPG failed to inhibit osteoclastogenesis in these co-cultures, indicating that a signaling pathway other than RANKL/RANK was involved. This alternative pathway likely involves ephrin B2/EphB4 as osteoclasts failed to form in co-cultures of chondrocytes and osteoclast precursors when the ephrin B2/EphB4 interaction was blocked by TNYL-RAW even in the presence of IGF-I. As in osteoblasts, deletion of IGF-IR in chondrocytes decreased the mRNA levels of ephrin B2, EphB4 and RANKL. We previously showed that IGF-IKO mice have a high trabecular bone volume with more cartilage remnants due to decreased osteoclast numbers in the metaphysis(19). Our results, herein, suggest that IGF-I/IGF-IR in chondrocytes regulates the ephrin B2/EphB4 interaction between chondrocytes and osteoclast precursors that if impaired results in increased trabecular bone volume despite reduced bone formation.
In summary, our data indicate that IGF-I/IGF-IR promotes osteoblast and osteoclast differentiation via ephrin B2/EphB4 by stimulating ephrin B2 and EphB4 production in osteoblasts and ephrin B2 in osteoclasts, thus regulating communication between these two cell types. In addition chondrocyte expression of ephrin B2 and EphB4 is regulated by IGF-I/IGF-IR, and ephrin B2/EphB4 mediates the communication between chondrocytes and osteoclasts. Our results indicate that IGF-I/IGF-IR signaling controls the interaction between chondrocytes, osteoblasts, and osteoclasts via its regulation of ephrin B2/EphB4 expression, and so controls key processes required for endochondral bone formation and remodeling .
Supplementary Material
Acknowledgments
We thank for Dr. Tamara Alliston for providing us ATDC5 cell line, and Dr. Thomas Clemens for providing us OBIGF-IRKO. This work was supported by NIH grants RO1 AR055924, DK 054793 (D.B.) and AG021353 (W.C.), and by the Department of Veteran Affairs Research Enhancement Award Program in Bone Disease and Merit Review Grants (D.B., W.C.).
Funding: NIH grants RO1 AR055924 , DK054793, and AG21353 and, Department of Veteran Affairs Research Enhancement Award Program in, Bone Disease and Merit Review Awards to DDB and WC
Footnotes
Conflict of Interest:
All authors have no conflict of interest.
Yongmei Wang
Alicia Menendez
Frankie Fong
Hashem Z. ElAlieh
Wenhan Chang
Daniel D. Bikle
Authors’ roles: Study design: YW, WC, DB, Experimental conduct: YW, Data analysis: YW, DB, Animal work: AM, HEZ, CF, Manuscript preparation: YW, WC, DB
References
- 1.Karsenty G. The complexities of skeletal biology. Nature. 2003;423(6937):316–318. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K. Cross-talk among bone cells. Curr Opin Nephrol Hypertens. 2009;18(4):292–297. doi: 10.1097/MNH.0b013e32832b75f1. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44(6):1026–1033. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 7.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 8.Karsenty G. Genetic control of skeletal development. Novartis Found Symp. 2001;232:6–17. discussion 17–22. [PubMed] [Google Scholar]
- 9.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328(3):658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 10.Zuscik MJ, Hilton MJ, Zhang X, Chen D, O'Keefe RJ. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 2008;118(2):429–438. doi: 10.1172/JCI34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maes C, Kobayashi T, Kronenberg HM. A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann N Y Acad Sci. 2007;1116:149–164. doi: 10.1196/annals.1402.060. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto T, Suda T. Differentiation and function of osteoclasts. Keio J Med. 2003;52(1):1–7. doi: 10.2302/kjm.52.1. [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 14.Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci. 2008;5(5):263–272. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126(3):441–443. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Nishida S, Sakata T, Elalieh HZ, Chang W, Halloran BP, Doty SB, Bikle DD. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology. 2006;147(10):4753–4761. doi: 10.1210/en.2006-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21(9):1350–1358. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17(9):1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22(9):1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12B):2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 23.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16(12):2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen MT, Mackem S, Clemens TL, Bikle DD, Chang W. rIGF-1R Signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res. 2011 doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 2000;11(3):196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- 26.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26(2):145–146. [PubMed] [Google Scholar]
- 27.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129(6):1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- 28.Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133(2):457–468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280(17):17301–17311. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- 30.Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14(2):321–330. doi: 10.1016/j.str.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Sakata T, Elalieh HZ, Munson SJ, Burghardt A, Majumdar S, Halloran BP, Bikle DD. Gender differences in the response of CD-1 mouse bone to parathyroid hormone: potential role of IGF-I. J Endocrinol. 2006;189(2):279–287. doi: 10.1677/joe.1.06351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez L, Cheng Z, Chen TH, Tu C, Chang W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology. 2005;146(11):4597–4608. doi: 10.1210/en.2005-0437. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012;6(2):148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson MD, Opperman LA, Westerlund J, Fernandez CR, San Miguel S, Henkemeyer M, Chenaux G. Ephrin-B stimulation of calvarial bone formation. Dev Dyn. 2013;241(12):1901–1910. doi: 10.1002/dvdy.23874. [DOI] [PubMed] [Google Scholar]
- 35.Arthur A, Panagopoulos RA, Cooper L, Menicanin D, Parkinson IH, Codrington JD, Vandyke K, Zannettino AC, Koblar SA, Sims NA, Matsuo K, Gronthos S. EphB4 enhances the process of endochondral ossification and inhibits remodeling during bone fracture repair. J Bone Miner Res. 2013;28(4):926–935. doi: 10.1002/jbmr.1821. [DOI] [PubMed] [Google Scholar]
- 36.Arthur A, Zannettino A, Panagopoulos R, Koblar SA, Sims NA, Stylianou C, Matsuo K, Gronthos S. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48(3):533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 37.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141(7):2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 38.Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE, Martin TJ. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23(8):1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 39.Takyar FM, Tonna S, Ho PW, Crimeen-Irwin B, Baker EK, Martin TJ, Sims NA. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28(4):912–925. doi: 10.1002/jbmr.1820. [DOI] [PubMed] [Google Scholar]
- 40.Duggineni S, Mitra S, Noberini R, Han X, Lin N, Xu Y, Tian W, An J, Pasquale EB, Huang Z. Design, synthesis and characterization of novel small molecular inhibitors of ephrin-B2 binding to EphB4. Biochem Pharmacol. 2013;85(4):507–513. doi: 10.1016/j.bcp.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen PL. Eph receptors and ephrins. Stem Cells. 2000;18(1):63–64. doi: 10.1634/stemcells.18-1-63. [DOI] [PubMed] [Google Scholar]
- 42.Cheng S, Zhao SL, Nelson B, Kesavan C, Qin X, Wergedal J, Mohan S, Xing W. Targeted disruption of ephrin B1 in cells of myeloid lineage increases osteoclast differentiation and bone resorption in mice. PLoS One. 2012;7(3):e32887. doi: 10.1371/journal.pone.0032887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lavigne M, Martel-Pelletier J. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Res Ther. 2009;11(4):R119. doi: 10.1186/ar2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116(12):3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usui M, Xing L, Drissi H, Zuscik M, O'Keefe R, Chen D, Boyce BF. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2008;23(3):314–325. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.