Abstract
Lexical orthographic information provides the basis for recovering the meanings of words in reading and for generating correct word spellings in writing. Research has provided evidence that an area of the left ventral temporal cortex, a sub-region of what is often referred to as the Visual Word Form Area (VWFA), plays a significant role specifically in lexical orthographic processing. The current investigation goes beyond this previous work by examining the neurotopography of the interface of lexical orthography with semantics. We apply a novel lesion mapping approach with three individuals with acquired dysgraphia and dyslexia who suffered lesions to left ventral temporal cortex. To map cognitive processes to their neural substrates, this lesion mapping approach applies similar logical constraints as used in cognitive neuropsychological research. Using this approach, this investigation: (1) Identifies a region anterior to the VWFA that is important in the interface of orthographic information with semantics for reading and spelling; (2) Determines that, within this Orthography-Semantics Interface Region (OSIR), access to orthography from semantics (spelling) is topographically distinct from access to semantics from orthography (reading); (3) Provides evidence that, within this region, there is modality-specific access to and from lexical semantics for both spoken and written modalities, in both word production and comprehension. Overall, this study contributes to our understanding of the neural architecture at the lexical orthography-semantic-phonological interface within left ventral temporal cortex.
Keywords: Orthography, Semantics, VWFA, Reading, Spelling
Introduction
Written language is an evolutionarily recent human invention that has dramatically changed how humans are able to communicate and accumulate knowledge. The cognitive and neural processes utilized for fluent reading and writing are likely built upon evolutionarily older systems such as spoken language, visual object recognition, working memory, spatial processing, and manual motor processing. Given this, there has been a great deal of interest in understanding the ways in which the cognitive and neural processes underlying written language interface and interact with these evolutionarily older neural and cognitive systems.
Scientific understanding of the manner in which the brain has responded to the challenge of incorporating written language processes and representations into its repertoire of visual, language, and motor skills has advanced along two research paths. One has involved the cognitive neuropsychological investigation of patterns of impaired and spared behavioral performance directed at identifying the cognitive representations and processes of reading and spelling. The second, more recent path, has involved the application of structural and functional neuroimaging techniques to identify the neural substrates underlying the cognitive representations and processes of reading and spelling. The work reported in this study is specifically directed at furthering our understanding of the neural organization of the orthographic processes used in spelling and reading and the manner by which they interface with the semantic system. To accomplish this we examined the cognitive profiles of individuals with deficits of written language and then mapped the patterns of association and dissociation of their language deficits onto the patterns of intersection and dissociation of the brain lesions. In this way, we were able to consider the neuro-topographic relationships between reading, spelling and related cognitive and language functions. Below, we provide a brief review in order to situate this investigation within the context of our current understanding of orthographic processes and their neural bases.
Functional Architecture of Reading and Spelling
Whereas reading requires the mapping of written symbols to sound and meaning, spelling requires the mapping from sound and meaning to written symbols. Cognitive neuropsychological research over the past 25 years has made very significant contributions to our understanding of the complex cognitive architecture that instantiates these processes (e.g., Coltheart et al. 1993; Rapcsak and Beeson 2002; Rapp and Caramazza 1997; Roeltgen and Heilman 1985). Despite this progress there continues to be considerable debate regarding a number of issues, including the degree to which orthographic, semantic and phonological representations are distributed or local (e.g., Bormann & Weiller, 2012; Coltheart, 2004; Glezer, Jiang, & Riesenhuber, 2009), the extent to which relevant cognitive processes are interactive or discrete, and the circumstances under which semantic processes are involved in word reading and spelling (e.g., Plaut, McClelland, Seidenberg, & Patterson, 1996; Welbourne & Lambon Ralph, 2007). Nonetheless, the basic organization of the cognitive architecture depicted in Figure 1 underlies many current viewpoints and, below, we summarize the key aspects of orthographic processing that we will assume in our work.
Figure 1.
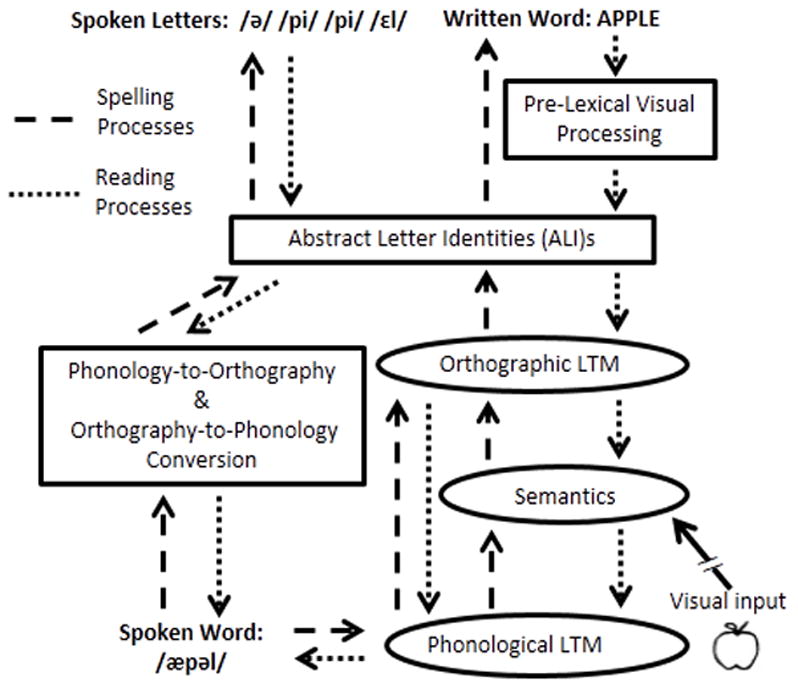
A schematic depiction of the cognitive processes of written language. Dashed lines indicate spelling processing; the short dotted lines indicate reading processes. Ovals indicate lexical processing for orthography, phonology or semantics.
Briefly, in reading, visual processing of letter shapes and their locations results in a representation of the input in terms of abstract letter identities (ALI representations) that are common to letters regardless of case, font or size. Subsequent processing involves searching Orthographic Long-Term Memory (Orthographic LTM; also referred to as the Orthographic Lexicon) to determine if the current stimulus corresponds to an orthographic word form previously encountered and stored in memory. Stimuli identified as known words can then access associated information including their corresponding meanings and phonological forms that have been stored in the Lexical1 Semantic System and Phonological LTM (the Phonological Lexicon), respectively. In spelling, the stimulus for producing a written word form is either a spoken word (e.g., in writing to dictation, taking notes in a lecture, etc.) or a word meaning (in written picture naming, writing down an internally generated message, etc.). These stimuli, which are represented in Phonological LTM and/or the Lexical Semantic System, must interface with the Orthographic LTM to identify their corresponding orthographic word forms. Once identified, the orthographic word forms must be converted to the appropriate letter shapes needed for motor production.
In contrast to these lexical, word-based processes, both reading and spelling can be accomplished using sub-lexical processing of a written (reading) or spoken (spelling) stimulus on the basis of the learned associations between letters/letter groups and sounds/sound groups. In reading, the Orthography-to-Phonology Conversion process provides plausible phonological representations of a written form based on learned associations between sounds and letters; this process is deployed for both familiar words and also unfamiliar strings (e.g., reading FLOPE as /fl ou p/). In spelling, phonology-to-orthography conversion performs the reverse mapping of sounds to letters (spelling /fl ou p/ as FLOPE or FLOAP). Note that, within such an architecture, word knowledge from the Orthographic LTM store is necessary for the correct spelling or reading of a word when the mapping between spelling and sound is not predictable on the basis of learned letter-sound associations (e.g., “eyes”).
Ventral Occipitotemporal Cortex in Reading and Spelling
Functional neuroimaging evidence
Neuroimaging methods have provided a variety of tools for understanding the neural bases of orthographic processing and representations. While a broad network of regions has been implicated in reading and spelling, in this paper we will focus on the role of left ventral occipitotemporal cortex. There are a large number of findings that identify the region surrounding the occipitotemporal sulcus, including areas of the left fusiform (FG) and inferior temporal gyrus (ITG) (caudal BA 37 and rostral BA 20), as important to orthographic processing (e.g., Baker et al., 2007; Cohen et al., 2002; Gros, Boulanouar, Viallard, Cassol, & Celsis, 2001; Kronbichler et al., 2004; Polk & Farah, 2002; Puce, Allison, Asgari, Gore, & McCarthy, 1996). Within this general region, an area in the left mid-fusiform gyrus adjacent to the occipitotemporal sulcus (typically centered on Talairach coordinates x = −43, y = −54, z = −12 (MNI: x = −40, y = −50, z = −12)) that has consistently been identified for its role in reading has been referred to by Dehaene, Cohen and colleagues as the Visual Word Form Area (VWFA) (Baker et al., 2007; Cohen & Dehaene, 2004; Cohen et al., 2000; Dehaene, Cohen, Sigman, & Vinckier, 2005; Dehaene & Cohen, 2011; McCandliss, Cohen, & Dehaene, 2003). Extensive work on the VWFA indicates that it is associated with processing orthographic representations that are independent of size and font and which may range in size from single letters to morphemes (Cohen et al., 2002; Dehaene & Cohen, 2011; Dehaene et al., 2001, 2005; Vinckier et al., 2007).
Additionally, although there has been far less neuroimaging work on the neural substrates of spelling, there are several functional neuroimaging studies of spelling that have consistently found activation for spelling in this same left ventral occipitotemporal region (Purcell, Turkeltaub, Eden, & Rapp, 2011). Furthermore, studies that have examined both reading and spelling in the same individuals have found overlapping activation from reading and spelling in this area (Purcell, Napoliello, & Eden, 2011; Rapp & Dufor, 2011; Rapp & Lipka, 2010). Considerable functional neuroimaging work has been dedicated to the challenge of understanding the mapping of the specific cognitive functions depicted in Figure 1 onto the neural substrates that have been implicated in orthographic processing. In this regard, Dehaene, et al (2005) and Cohen et al (2003) attempted an integration of findings from a number of studies, proposing that letter strings are hierarchically coded in the left fusiform gyrus such that, as processing proceeds in a posterior to anterior direction, it is carried out by neuronal detectors that are increasingly complex, abstract and location-invariant (Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006; Dehaene et al., 2005; Vinckier et al., 2007). Dehaene et al. (2005) specifically proposed that there is a progression from processing in visual areas V1–V4 (MNI: y = −85 to −67 originally reported in (Cohen et al., 2003) that are sensitive to physical characteristics such as word length, visual contrast, rate and duration, through processing by letter detectors located more anteriorly (approximately y = −64), on to local bigram detectors (approximately y = −56) and then, finally, small word and morpheme detectors (approximately y = −48). Within this framework, VWFA processing is considered to range in its posterior to anterior extent from approximately y = −64 to y = −48 (Dehaene et al., 2005); see Figure 2.
Figure 2.
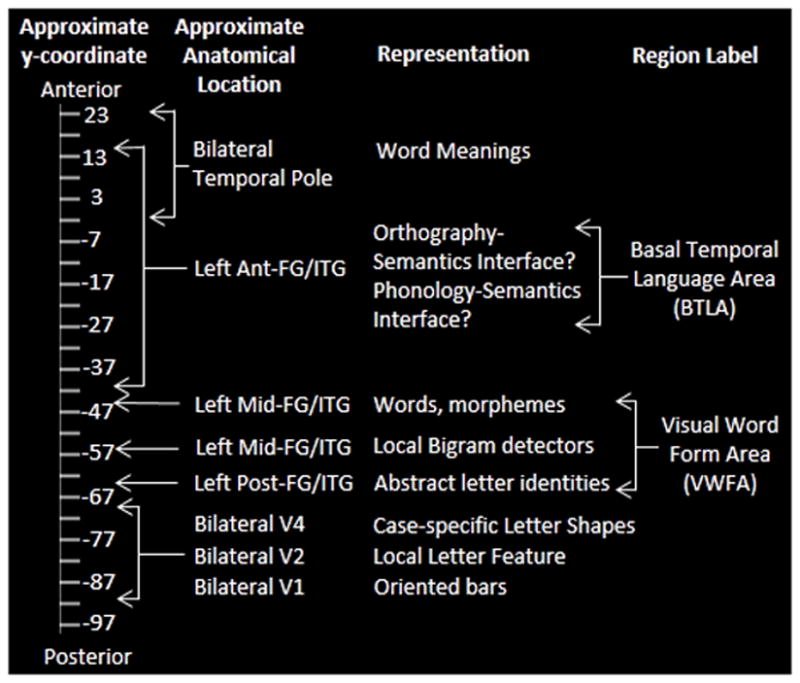
Proposed location of orthographic hierarchical processing scheme along the anterior-posterior axis of the ventral occipitotemporal cortex, extending from V1 to the temporal pole. The y-axis coordinate scale provides approximate locations of the representations in ventral occipitotemporal cortex in MNI coordinate space.
As depicted in Figure 2, in terms of the cognitive processes represented in Figures 1, pre-lexical visual processes critical for recognition of letter identities and their order are situated in the region extending from the left posterior visual areas extending from about −85 to about −66. The representation of abstract letter identities (ALIs) has been specifically identified with a region centered on y = −58 (Rothlein & Rapp, 2013), while the orthographic representations of words and morphemes (Orthographic LTM) are associated with a region centered on y = −48. In terms of the localization of the lexical orthographic processes, evidence comes from a number of sources including Glezer et al, (2009) who argued for whole-word orthographic representations in this area, as well as a number of studies that have found that this region is sensitive to the lexical status of strings (words vs. pseudowords) (Schurz et al., 2010) and the lexical frequency of written words (Graves, Desai, Humphries, Seidenberg, & Binder, 2009; Kronbichler et al., 2004).
Lesion-deficit correlation studies
Lesion-deficit correlation research has also played a key role in our understanding of the cognitive functions of this region. While in some of the earliest work on this topic Dejerine (1892) identified the angular gyrus as key to lexical orthographic representation and processing, subsequent reanalysis of the case suggests that the relevant brain area was actually the left mid-fusiform (Cohen et al., 2003; Epelbaum et al., 2008). Since lesions are often large and accompanied by multiple cognitive deficits, interpretation can be difficult. However, there have been a few studies –reviewed below - of individuals with relatively small lesions or lesion overlap studies that have been especially informative.
In terms of reading, most of the research concerning ventral occipitotemporal cortex has involved cases of “pure alexia” or “letter-by-letter reading”, an impairment most typically characterized by a slowing of reading that is more pronounced for longer versus shorter words and that often occurs in the context of intact spelling and other language functions. In some of these cases, there is severe damage to left visual cortex that requires reliance on right hemisphere visual areas for processing visual input. When coupled with damage to the splenium of the corpus callosum, there is disruption in the transfer of visual information to left hemisphere orthographic processing areas (Cohen et al., 2000; Habib, Ceccaldi, & Poncet, 1990; Suzuki et al., 1998). In other cases, disruption to cortical areas just posterior to the left hemisphere mid-fusiform region can result in a similar behavioral profile. This latter situation was reported by Gaillard et al. (2006) who described an individual who underwent surgical resection of a portion of the left fusiform to treat epilepsy. Behaviorally, this individual’s pre-surgery reading and spelling were normal and an fMRI scan indicated normal activation for reading in the mid-fusiform region. The resection extended from y = −60 to −80 and it was argued that it severed white matter connections entering into the VWFA from posterior visual processing cortex (Epelbaum et al., 2008). Although this individual’s post-surgery spelling, face and object recognition and spoken language skills were intact, he had selective difficulty with reading and exhibited symptoms of letter-by-letter reading. This work provides a very strong case for a critical and causal role of the left-fusiform and the connections to it in normal reading.
To date there have been no cases of focal lesions clearly limited to the VWFA (approximately y = −64 to −48). To be clear, while damage to the VWFA region has been reported in numerous cases, it has occurred in the context of damage to additional cortical areas. In most of the reported cases, the lesions have extended more posteriorly and, not surprisingly, were accompanied by symptoms of pre-lexical processing impairments such as letter-by-letter reading (Cohen et al., 2003; Starrfelt, Habekost, & Leff, 2009). However, Tsapkini and Rapp, (2010; Tsapkini, Vindiola, & Rapp, 2011) reported on the case of an individual with a lesion centered on x = −50, y = −37, z = −26 with posterior-anterior extensions from y = −71 to −12 with (See also, Turkeltaub et al., 2013). This individual (also included in the current investigation), was unlike the case reported by Gaillard et al., (2006) and the various pure alexia cases, in that his cognitive deficits affected both reading and spelling and he had no symptoms of pre-lexical processing deficits in reading or visual processing deficits and was not a letter-by-letter reader. In fact, his reading (and spelling) of nonwords was normal with difficulties affecting only the reading and spelling of words. Furthermore, semantic processing for faces, objects, and auditory words was also intact. If we assume that Orthographic LTM is situated largely posterior to the lesion (a topic we will return to in the General Discussion), then this individual’s pattern of performance can be understood as arising from damage to the interface between Orthographic LTM and more anteriorly located lexical semantics, an area critical for both reading and spelling2.
The results of a lesion overlap study reported by Rapcsak and Beeson (2004) are generally consistent with the findings of Tsapkini and colleagues that a deficit selective to orthographic word processing in both reading and spelling can arise from damage anterior to the VWFA. Rapcsak and Beeson reported the lesion overlap for 8 individuals with lexical deficits that were more pronounced in spelling than reading. In addition to a high level of lesion overlap in the VWFA area (approximately y = −62), the locations of highest lesion overlap corresponded to two more anterior locations at approximately y = −24 and −32, again pointing to the areas anterior to traditional VWFA as playing a key role in lexical orthographic processing.
In sum, the lesion-based evidence suggests that key aspects of lexical orthographic processing for both spelling and reading may be instantiated in neural substrates anterior to the VWFA, extending as far anteriorly as approximately y = −24. In the next section we will discuss other work that has attempted to characterize the language functions of the region anterior to the VWFA (anterior to approximately y = −48).
Beyond the Mid-fusiform Gyrus: The Basal Temporal Language Area
Within the larger anterior ventral temporal region, the area anterior to the anterior commissure (approximately y = 0) is typically referred to the temporal pole and has most often been associated with semantic processing (Mummery et al., 2000; Ralph, Patterson, Garrard, & Hodges, 2003; Yang, Pan, Song, & Shang, 2012), based, in large measure, on findings in the literature on primary progressive aphasia (e.g., Gorno-Tempini et al., 2004; Mesulam et al., 2013; Rogalski et al., 2011). However, the area posterior to the temporal pole and anterior to the VWFA, falling roughly between y = 0 and y = −47, has received considerably less attention although it has been most often associated with multimodal word processing or access to semantics. For example, Luders and colleagues (Lüders et al., 1991; Schäffler, Lüders, Morris, & Wyllie, 1994) proposed a multi-modal Basal Temporal Language Area (BTLA) that extends roughly from y = +17 to −46 and is centered on x = −50, y = −44, z = −10 (See also, Jobard, Crivello, & Tzourio-Mazoyer, 2003). This characterization is generally consistent with the claim of Damasio (1989) and others that the left lateral temporal cortex constitutes a convergence zone supporting the linkage of orthographic, phonemic, and semantic information (e.g., Booth et al., 2002a, 2002b; Buchel, Price, & Friston, 1998; Damasio, 1989; Giraud & Price, 2001; Hillis et al., 2005; Thompson-Schill, Aguirre, D’Esposito, & Farah, 1999). In terms of the posterior-to-anterior orthographic processing pathway proposed by Dehaene and colleagues in which representations/processing increase in abstraction, the region anterior to the VWFA and posterior to the temporal pole could be expected to play a key role in the orthography-semantics interface. Understanding the internal organization of this region and identifying the substrates of modality-specific (orthographic/phonological) and/or amodal processes would be key to understanding the neural mechanisms by which orthographic representations and processes interface and interact with the more “basic” domains of spoken language and meaning representation. The current investigation is specifically focused on examining these issues.
Cognitive Dissociation Lesion Mapping Analysis
Traditional lesion-deficit analyses involve taking individuals that share a cognitive deficit and identifying the intersection of their lesions (Cohen et al. 2003; Rapcsak and Beeson 2004). While generating much useful information, one limitation of this work is that it does not consider other aspects of the participants’ cognitive profiles. Most importantly, although every individual in a lesion overlap map is defined as having the same cognitive deficit, there are likely to be other cognitive deficits that are shared across many or most individuals in the group, potentially confounding the interpretation of the lesion overlap results. Furthermore, there are cognitive deficits that are not shared across subsets of the group members and these might be extremely informative in constraining the cognitive interpretation of the lesion overlap pattern. Therefore, a productive approach to using lesion overlap analysis for understanding cognitive-neural relations may involve examining the inter-subject intersections (associations) and dissociations of both the cognitive deficits and the neural lesions – extending classical cognitive neuropsychological methods into the domain of lesion mapping.
We will do this via what we will call “cognitive dissociation lesion mapping”. Note that this approach not only identifies a common brain area that is damaged in individuals with the same cognitive deficit, but also determines if that same brain area is intact in individuals that do not demonstrate that specific cognitive deficit. The approach, involves first identifying a set of individuals with associations and dissociations of relevant cognitive deficits. For example, take three cognitive components (X–Z) and 3 individuals (A–C) with the following cognitive profiles: all three individuals have an impairment to component X, only A and B have damage to component Y and only C has damage to component Z. These patterns indicate that components X, Y and Z are dissociable since damage to one does not require damage to the others. Presumably, this configuration of patterns can occur only if the neural substrates supporting X, Y and Z are sufficiently distinct that they can be independently damaged. In this case, the area of intersection of lesions A–C is likely to include neural substrates necessary for function X. Of the remaining lesioned areas, the lesion intersection for A and B but not C should correspond to substrates necessary for function Y and, finally, of the remaining lesioned areas, the lesion area for C but not A or B should correspond to substrates necessary for function Z.
In this paper, we report on an investigation that applied this approach to better understand the interface between reading and spelling and the other language functions that are situated within the left ventral occipitotemporal cortex anterior to the mid-fusiform gyrus.
Methods
Participants
DPT Case History
DPT was a right-handed male, who underwent surgical resection of an oligodendroglioma in the left fusiform gyrus. Prior to the surgery DPT worked as a tax attorney and self-reported that he read extensively for his work and that his spelling was comparable to that of other law-school graduates. Immediately after surgery DPT experienced impairments in spoken naming, reading comprehension, spelling and short-term memory. Although he was able to return to work as an attorney after one month due to recovery from many of his original impairments, he continued to experience mild difficulties in reading, moderate difficulties in spelling, and modest impairments in anterograde memory. DPT was 35 years old at the start of the investigation which was carried out 4 to 6 years post-surgery. A high resolution structural T1 weighted MRI scan (Figure 3A) indicates that the resection site primarily involved the anterior and mid left fusiform gyrus and part of the medial portion of the anterior left inferior temporal gyrus. DPT’s T1 scan (and the scans obtained for the other two brain lesioned participants) was normalized in Montreal Neurological Institute (MNI) space via a standard normalization method for lesioned brains (Brett, Leff, Rorden, & Ashburner, 2001); further details of the procedure are provided in the Anatomical Analysis section below. In MNI coordinates, the lesion was centered on coordinates x = −50, y = −37, z = −26 with the furthest extension points approximately as follows: along the medial–lateral axis from x = −25 to −70, along the anterior-posterior axis from y = −13 to −75 and along the superior–inferior axis from z = −32 to −2. Further information regarding DPT is available in Tspakini and Rapp (2010) and Tsapkini, et al. (2011).
Figure 3.
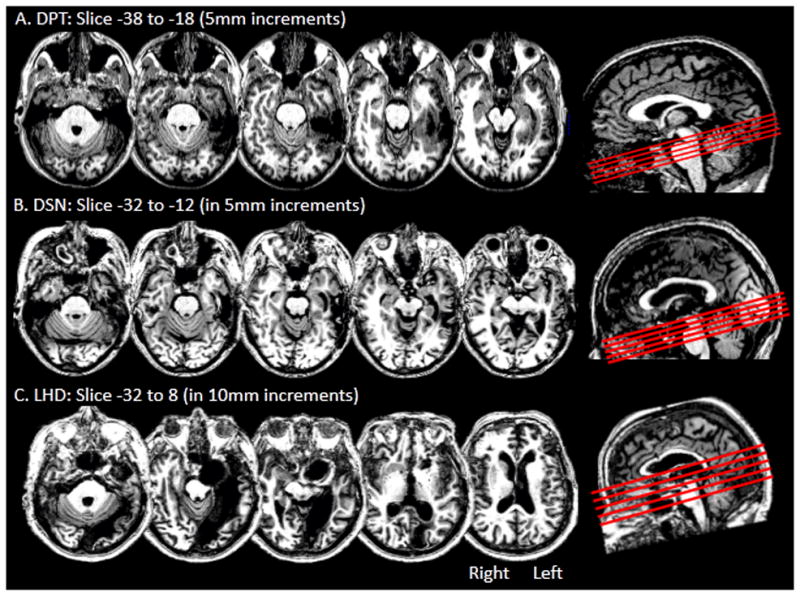
Axial slices depicting the brain lesions in the left ventral occipitotemporal cortex for (A) DPT, (B) DSN, and (C) LHD. The slices were rotated −15 degrees from the AC-PC line and are shown in a sagittal view as red lines in the right side box.
DSN Case History
DSN was a right-handed female, who underwent surgical meningioma resection. DSN held a college degree and prior to the surgery worked as an editor and manager at a government research institute; self-reported premorbid reading and spelling abilities were excellent. After the surgery, DSN experienced difficulties in naming, reading and spelling and in speech comprehension in noisy environments. DSN was 67 years old at the start of the investigation which was carried out 1 to 2 years post-surgery. A high resolution structural T1 weighted MRI scan (Figure 3B) indicates that the resection site primarily involved the anterior and mid left fusiform gyrus and part of the medial aspect of the anterior left inferior temporal gyrus. The lesion was centered on MNI coordinates x = −55, y = −3, z = −20 with the furthest extension points approximately as follows: along the medial–lateral axis from x = −28 to −67, along the anterior-posterior axis from y = −8 to −63 and along the superior–inferior axis from z = −41 to 19.
LHD Case History
LHD was a right-handed female, who suffered a left posterior cerebral artery aneurysm. At the time of the infarct she held an MBA degree and was retired from working as a banking executive; she and her husband reported that she read often and that her spelling abilities were excellent. LHD was 69 years old at the start of the investigation which was carried out 3–6 years post-infarct. A high resolution structural T1 weighted MRI scan (Figure 3C) indicates that the lesion affected an extensive portion of the left ventral occipitotemporal cortex, extending from the occipital pole along the medial aspect of the occipital and temporal lobes into the temporal pole. The lesion extent in the x, y, and z dimensions in MNI coordinates was approximately as follows: along the medial–lateral axis from x = 0 to −58, along the anterior-posterior axis from y = −20 to −102 and along the superior–inferior axis from z = −43 to 36. Visual field testing revealed a complete right homonymous hemianopia without macular sparing. See McCloskey and Schubert (2013, this issue) for further information.
Age-Matched Control Groups
All control participants were recruited from the Johns Hopkins University community. None of the controls had any history of reading or spelling disorders. A spelling screener was used to verify normal spelling ability. The Younger Control Group (YCG) consisted of 11 individuals (6 men and 5 women) who were comparable to DPT with respect to age (age range 31–41) and years of education (2 had BA degrees, 1 had a Ph.D. and the remaining 8 had MA degrees). The Older Control Group (OCG) consisted of 7 female control participants that were comparable to DSN and LHD with respect to age (age range 60–72, median 67) and years of education (median 18 years of education). It was not always possible to test all control participants on every task, so whenever the full set was not tested, the specific number of participants is denoted by a dash after the control group name, such that YCG-6 indicates 6 participants were tested in the younger control group.
Cognitive Assessments
Extensive behavioral testing was carried out to evaluate the following core cognitive domains: Orthographic processing (spelling and reading), semantic processing (from auditory, written and object modalities), spoken language (word production and comprehension) and visual object processing (faces and objects). A number of the tasks used are relevant to evaluating more than one of the core areas, so tasks will be presented grouped thematically, but the overall evaluation of these cognitive domains will draw from all relevant tasks.
All procedures were approved by the Johns Hopkins University Homewood Institutional Review Board and each participant provided informed consent. All computer-based tasks were administered using E-prime 1.2.1 software (Psychological Software Tools, Pittsburgh, PA) for stimulus presentation and data collection. Word frequency counts are from Francis and Kucera (1982), unless noted otherwise. For all timed tasks, the patients and control participants were instructed to respond as quickly and as accurately as possible. For tasks requiring a spoken response, response times (RTs) were collected by voice key. Unless noted otherwise, only correct trial RTs were analyzed. Statistical comparisons of each brain-lesioned participant to their control group were carried out using the Crawford and Garthwaite modified t-test (Crawford & Garthwaite, 2002) or Crawford’s Revised Standardized Difference Test (RSDT) (Crawford & Garthwaite, 2005). For RT analyses, individual participant median RTs were computed and, in comparisons with group data, the means of control participants’ medians were compared with individual participant medians. Results are reported in Table 1.
Table 1.
Summary of Cognitive Testing Results.
| DPT | DSN | LHD | YCG | OCG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| %Errors | RT | %Errors | RT | %Errors | RT | %Error range | Mean RT(SD) | %Error range | Mean RT(SD) | |
| SPELLING | ||||||||||
| Word List 1 | 18%** | - | 15%** | - | 16%** | - | 0–4% | - | 0–3% | - |
| Length List (Long-Short) | 6% | - | 0% | - | 10% | - | - | - | 0% | - |
| Frequency List (LF-HF) | 18%** | - | 22%** | - | 8%* | - | - | - | 0–2% | - |
| Nonword List | 3% | - | 3% | - | 9% | - | 0–3% | - | 0–15% | - |
|
| ||||||||||
| READING | ||||||||||
| Recognition of Oral Spelling (ROS) | - | - | 8% | - | 2% | - | - | - | 0–7% | - |
| Frequency (LF-HF) | - | - | 11%* | - | 2% | - | - | - | - | - |
| Regularity (Irreg.-Reg.) | - | - | 8% | - | - | - | - | - | - | - |
| Oral Reading of ROS words | - | - | 7% | - | 30% | - | - | - | - | - |
| Frequency (LF-HF) | - | - | 13%* | - | 9%* | - | - | - | - | - |
| Regularity (Irreg.-Reg.) | - | - | 15%** | - | - | - | - | - | - | - |
| Visual Lexical Decision | 3% | 793~ | 15%** | 722 | 12%** | 1055** | 2–3% | 642(76) | 1–6% | 753(79) |
| HF words | 2% | 675 | 2% | 636 | 13%** | 1042** | 2–11% | 578(93) | 0–2% | 673(66) |
| LF words | 1% | 807~ | 42%** | 785 | 27%** | 1943** | 0–2% | 640(84) | 0–9% | 747(54) |
| Regular words | 1% | 718 | 10%** | 706 | 20%** | 1266** | 0–17% | 602(89) | 0–3% | 699(63) |
| Irregular words | 1% | 727 | 33%** | 677 | 23%** | 1361** | 0–31% | 597(86) | 0–3% | 703(80) |
| Word reading | 0% | 610~ | 13%** | 691~ | 39%** | 1438** | 0–31% | 477(62) | 0–1% | 515(76) |
| HF words | 0% | 583 | 9%** | 668 | 29%** | 1355** | 0–51% | 470(62) | 0–1% | 514(85) |
| LF words | 0% | 615~ | 19%** | 727* | 50%** | 1620** | 0–51% | 477(65) | 0% | 518(67) |
| Regular words | 0% | 588 | 9%** | 683 | 36%** | 1563** | 0–51% | 475(64) | 0% | 514(81) |
| Irregular words | 0% | 632* | 17%** | 696* | 43%** | 1382** | 0–51% | 477(63) | 0–1% | 516(74) |
| Nonword Reading | 0% | 759 | 11% | 751 | 73%** | 1476** | 0–6% | 582(117) | 0–17% | 640(100) |
|
| ||||||||||
| SEMANTICS | ||||||||||
| Written Synonym Judgment | 9% | 976~ | 7% | 1387~ | 30%* | 15162** | 2–7% | 762(105) | 2–15% | 1035(149) |
| Auditory Synonym Judgment | 2% | 1382 | 0% | 952 | 40%* | 3317** | 0–5% | 1036(248) | 0–5% | 971(140) |
| Pyramids and Palm Trees | 11% | 2269 | 21% | 2000 | 29%* | 10691** | 6–27% | 1765(504) | 4–17% | 2009(340) |
|
| ||||||||||
| SPOKEN WORD PRODUCTION | ||||||||||
| Spoken Picture Naming | 2% | 997 | 48%** | 1635** | 75%** | - | 1–7% | 729(92) | 2–8% | 770(96) |
|
| ||||||||||
| VISUAL OBJECT PROCESSING | ||||||||||
| Faces: Fame Judgment | 8% | 1183 | 23% | 748 | 14% | 1360 | 5–14% | 938(235) | 10–30% | 1033(155) |
| Faces: Profession Judgment | 1% | 838 | 12% | 836 | 12% | 1692* | 5–12% | 823(163) | 5–12% | 1016(181) |
See text for more details. LF = low frequency, HF = high frequency, ROS = Recognition of Oral Spelling, Irreg = irregular, Reg = regular, RT = reaction time. Pyramids and Palm Trees (Howard & Patterson, 1992). SD = standard deviation. Significance notation:
p<0.1;
p<0.05;
p<0.01
1. Spelling Assessment
1.1 Spelling Words to Dictation: Word List 1
DPT, DSN, LHD, YCG-11 and OCG-6 were asked to write a set of mono-morphemic words to dictation. A 68-word list was used with DPT and YCG-11, while a 110-word list was used with DSN, LHD, and OCG-6. Only the first response was scored and there were no time limits for responding. DPT, DSN and LHD all produced well-formed letters indicating that there were no difficulties in motor planning and execution.
Results
DPT made over four times as many errors (18%, 12/67 errors) as the worst performing YCG-11 subject (YCG-11: error range: 0–4%, 0–3/68). Both DSN (15%, 16/110 errors) and LHD (16%, 18/110 errors) performed well outside the OCG-6 error range (0–3%, 0–3/110). All of DPT’s spelling errors were phonologically plausible, as were the majority of DSN’s (14/16) and LHD’s (16/18). Note that DPT, DSN and LHD each exhibited intact repetition of dictated stimuli in the word spelling task.
1.2 Spelling Words to Dictation: Length and Frequency
Length effects were evaluated by spelling to dictation 28 long (7 and 8 letters) and 28 short (4 and 5 letters) frequency-matched words from the JHU Dysgraphia Battery Length List. Frequency effects were evaluated by combining subsets of words from the JHU Dysgraphia Battery that allowed for a comparison of spelling performance for high vs. low frequency words.
Results
There were clearly no effects of length in spelling performance for DSN as she generated the same number of errors (7% (20/28)) for both the long and short words. Moreover, there were no significant effects of length on the spelling performance in DPT and LHD: (x2(1) = .38, p<.54) and (x2(1) = 1.97, p<.16) respectively. In contrast, DPT, DSN, and LHD all exhibited clear frequency effects in their error rates. For DPT: 2% (2/99) errors for high vs. 20% (27/132) for low frequency words (x2(1) = 15.9, p<.001). For DSN: 4% (2/55) errors for high vs. 26% (14/55) for low frequency words (x2(1) = 15.9, p<.001). For LHD: 8% (11/146) errors for high vs. 16% (23/166) for low frequency words (x2(1) = 4.79, p<.03). Errors were primarily phonologically plausible; examples of DPT’s errors include: “sneeze” → SNEAZE, “type” → TIPE, “chief” → CHEAF. DSN produced errors such as: “urge” → ERDGE, “cloak” → CLOKE, “trade” → TRAID. LHD’s errors included: “myth” → MITH, “sauce” → SAUSE, “keep” → KEAP.
1.3 Spelling Nonwords to Dictation
DPT, DSN, and LHD were administered 34 pseudowords (4 to 8 letters) from the JHU Dysgraphia Battery (Goodman & Caramazza, 1985) for spelling to dictation.
Results
DPT and DSN each only made one error and LHD made 3. DPT’s performance was within the YCG-11 error range 0–3% (0–1/34). DSN and LHD’s performances were also within the normal error range of the OCG-5 0–15% (0–5/34 errors).
Spelling Assessment: Summary
DPT, DSN and LHD exhibited remarkably similar spelling profiles. The significant frequency effects on accuracy combined with the production of phonological plausible spelling errors are the classical symptoms of a lexical orthographic impairment. This type of deficit either disrupts access to orthographic LTM from semantics or reflects damage to the orthographic LTM store itself. For all three participants, the deficits were very selective to lexical orthographic processing as the absence of a length effect and intact pseudoword spelling indicate the following intact processes: letter shape production, orthography-to-phonology mapping conversion processing, and graphemic buffering (see Figure 5B).
Figure 5.
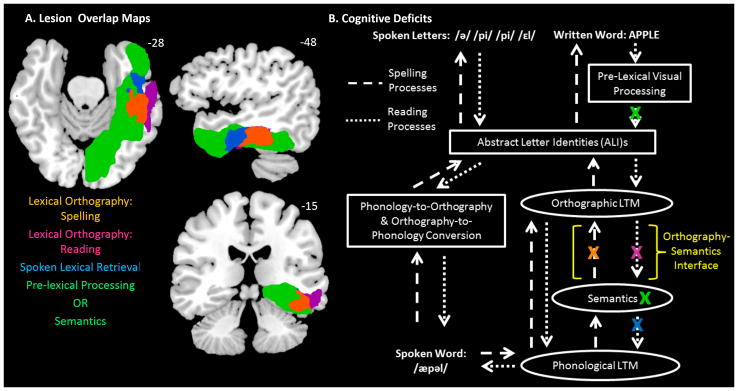
Cognitive deficits and corresponding lesion maps. (A) Depicts the lesion maps associated with each behavioral pattern depicted in Table 2. The lesion maps are projected onto a standard template brain in MNI coordinate space that was rotated −15 degrees from the AC-PC line. The orange area depicts the overlap of DPT’s, DSN’s, and LHD’s lesions, indicating an area involved in lexical orthographic processing in spelling. The violet area depicts the overlap of DPT’s and DSN’s lesions, excluding LHD’s, indicating an area involved in lexical orthographic processing in reading. The blue area depicts the overlap of DSN’s and LHD’s lesion, excluding DPT’s, indicating an area involved in spoken word retrieval. The green area depicts LHD’s lesion excluding DPT and DSN’s, indicating areas involved in either pre-lexical visual processing or semantic processing/representation. The colored text reports the corresponding cognitive processes associated with each (colored) lesion area. (B) Depicts the theory of written language processing including the proposed deficit locations for DPT, DSN and LHD. Lesions are denoted by a colored X. DPT has impairments in lexical orthography for spelling and reading (orange and violet X’s). DSN has an impairment in lexical orthography for spelling and reading and also an in impairment in spoken lexical retrieval (orange, violet and blue X’s). LHD has an impairment in lexical orthography for spelling, pre-lexical processing, and semantic processing (orange and green X’s). The colored X’s correspond to the specific cognitive deficits described in the text, listed in Table 2 and at the bottom of Figure 5A. The yellow text and bracket indicates the Orthography-Semantics Interface.
2. Reading Assessment
2.1 Recognition of Oral Spelling
McCloskey and Schubert (McCloskey & Schubert, 2013, this issue; Schubert & McCloskey,2013) proposed that LHD suffered from a deficit in pre-lexical processing that left intact her ability to process the visual forms of letters but specifically disrupted her ability to map between visual representations of letter shapes and their abstract identities.3 Note that the evidence presented indicates that abstract letter representations are intact, and what is disrupted is access to them from visual input. This type of deficit predicts difficulties in both word and nonword reading with visual stimulus presentation, consequently making it difficult to evaluate the integrity of lexical orthographic processes themselves. Nonetheless, one can evaluate the integrity of lexical orthographic processes in reading by using a task that circumvents the need to analyze visual letter forms. The task of “recognition of oral spelling” is just such a task. In this task, letter names are orally presented and the participant identifies the corresponding word or nonword (e.g., stimulus: /si, ei, ti/ → response: “cat”). This task requires converting heard letter names to ALIs and then searching Orthographic LTM; these latter processes would be equivalent to what is required in reading with visual stimuli (see Figure 1)4. Therefore, an intact ability to recognize a word from its oral spelling reflects intact orthographic LTM. The prediction is that if the only reading difficulty that LHD suffered from is one that affected the translation of visual letter forms to intact abstract letter identities and not orthographic LTM itself, then she should perform normally on the task of recognition of oral spelling. In contrast, an individual who has a deficit at the level of Orthographic LTM (or beyond) should be comparably impaired in the recognition of oral spelling and oral reading.
The tasks of recognition of oral spelling and reading were administered to DSN and LHD and a group of 8 age-matched controls (see McCloskey & Schubert, 2013, this issue; Schubert & McCloskey, 2013). DPT was not tested on recognition of oral spelling but DSN was administered a list of 90 words that varied in frequency and regularity and LHD was administered a list of 92 words varying in frequency (the list was administered 5 times for reading and once for recognition of oral spelling). Controls were administered an 88-word list for recognition of oral spelling.
Results
The control group’s range of error rates on the task of recognition of oral spelling was 0–7%. LHD performed comparably to controls on this task with only 2% (2/92) errors. This contrasted dramatically with her oral reading where her error rate was 30% (140/460). In contrast to LHD, DSN produced abnormal and comparable rates of errors on both tasks: 7% (6/90) in oral reading and 8% (7/90) in recognition of oral spelling. Importantly, on both oral reading and recognition of oral spelling she exhibited significant regularity and frequency effects: 17% vs. 2% and 13% vs. 5% errors for irregular vs. regular words respectively on the two tasks; and 13% vs. 0% and 13% vs. 2% for low vs. high frequency words respectively on the two tasks. Furthermore, for both tasks, the majority of DSN’s errors were regularization errors (e.g., AISLE → “/eIsl/”).
The striking dissociation observed for LHD between her normal performance on recognition of oral spelling and her severely impaired performance for oral reading is predicted by the claim that her reading deficit was pre-lexical and that her orthographic lexical knowledge itself was intact. For DSN, the striking association between both tasks in terms of her impaired performance, effects of frequency and regularity and the production of regularization errors reveal that she suffered a deficit at the level of lexical orthographic processing/representation that impaired her performance on any task that required processing via orthographic LTM.
2.2 Visual Lexical Decision: Frequency, Regularity and Length Effects
Participants were administered a visual lexical decision task with stimuli from Seidenberg, Waters, Barnes, and Tanenhaus (1984). Stimuli were presented on a computer monitor with each trial consisting of a 300 ms centrally located fixation cross, followed by a visual word that remained on the screen until the participant made a key press response to indicate whether the stimulus was a real English word or not. The word list consisted of 90 words, half of high and half of low frequency (mean frequency for HF = 319; LF = 11). In each frequency category, there were three levels of regularity: 15 regular-consistent words, 15 regular-inconsistent words and 15 strange words. There were 90 pronounceable nonwords. To evaluate the effect of length on RTs, the list was divided post-hoc into a set of 62 short words (3–4 letters) and 28 long words (5–6 letters) with the sets matched for frequency. Length effects were investigated by performing a t-test that compared the average RTs for long versus short words in each participant. Both correct and incorrect trials were included in the calculation.
Results
DPT’s overall error rate of 3% (5/180) fell well within the YCG range of 2–3% (3–6/180). His median RT for the LF words was marginally significantly different from the controls (t(9) = 1.90, p<.09). DSN’s overall error rate of 15% (27/180) was significantly different than the OCG range of 1–6% (1–12/180) (t(5) = −5.09, p<.004), although her RT’s were not (t(5) = −.36, p<.731). LHD’s overall error rate of 12% (21/180) was significantly different when compared to the OCG (t(5) = −3.70, p<.014) as was her overall median RT (t(5) = 3.53, p < .017).
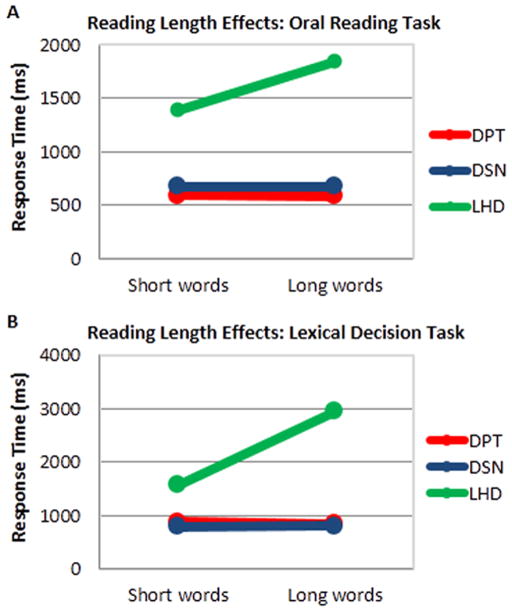
With regard to length effects in the visual lexical decision task (Figure 4A), neither DPT and DSN showed an RT difference in making lexical decisions for long words vs. short words (DPT: t(82) = 1.29, p = .198 and DSN: t(68) = .19, p = .853). In contrast, LHD showed a highly significant length (long, short words) effect of 1375 ms (t(70) = 3.55, p<.001).
Figure 4.
Length effects in reading for DSN, DPT, and LHD. (A) Voice onset response times for short (3 and 4 letter) and long (5 and 6 letter) words in the Oral Word Reading Task. (B) Response times for short (3 and 4 letter) and long (5 and 6 letter) words in the Visual Lexical Decision Task. LHD exhibited slower response times for reading longer vs. shorter words in both tasks, whereas DPT and DSN did not (see text for more details).
2.3 Oral Word Reading: Frequency, Regularity and Length Effects
Participants were asked to read out loud single 160 monosyllabic words from Jared (2002; Experiment 2). Half of the words were high frequency (mean frequency = 321) and half were low frequency (mean frequency = 6). Furthermore, half of the words in each frequency group were exception words and half were regular. Stimuli were presented on a computer monitor; each trial consisting of a 1000 ms centrally located fixation cross, followed by a 500 ms blank screen, and then the stimulus appeared and remained on the screen until the participant responded orally. To evaluate the effect of length, the list was divided post-hoc into a set of 40 short words (3–4 letters) and 40 long words (5–6 letters) with the sets matched for frequency and regularity. Length effects were investigated by performing a t-test that compared the average RTs for long versus short words in each participant. Both correct and incorrect trials were included to calculate RTs.
Results
DPT’s accuracy for all word categories was within normal limits, but his median RT of 632 ms for exception words was significantly slower than the YCG-11 (t(10) = 2.36, p<.04). DSN’s overall error rate of 13/% (21/160) was significantly different than the OCG-6 (t(5) = −38.33, p<.001) and her median RTs were significantly different than those of the OCG-6 for the LF category only t(5) = 2.88, p <.03). LHD’s overall accuracy and RTs were significantly different than those of the OCG-6 (t(5) = −116.1, p<.001 and t(5) = 11.26, p<.001, respectively). DPT made no reading errors. The majority of DSN’s word reading errors were regularizations such as PLAID → “played”, SOOT → “suit” and PINT → “/pInt/”. In contrast, the majority of LHD’s reading errors were involved letter substitutions such as BORN → “horn” MINK → “mint”, and RISK → “/rIsp/”.
With regard to length effects in oral reading (Figure 4B), as was the case the visual lexical decision task, neither DPT and DSN showed an RT difference in making lexical decisions for long words vs. short words (DPT: t(80) = .16, p = .87 and DSN: t(68) = .98, p = .98). In contrast, LHD demonstrated a significant length effect of 461 ms (t(46) = 1.99, p = .05). The length effect exhibited by LHD, with a slope of approximately 500 ms/letter, is consistent with McCloskey and Schubert’s (2013, this issue) report of a significant effect of length on LHD’s oral reading RTs,.
2.4 Nonword Reading
Participants were asked to read out loud 64 pronounceable nonword stimuli from Andrews and Scarratt (1998-Experiment 2). Stimuli were presented on a computer monitor; each trial consisted of a 500 ms. centrally located fixation cross, followed by a lowercase nonword that remained on the screen until the participant responded orally.
Results
DPT made 0 errors, and his median RT of 759 ms was not significantly different from the YCG-11’s average median of 582 ms (t = 1.45, p = .18). DSN’s 11% (7/64) error rate and median RT of 751 ms were not significantly different from the OCG-5’s error rate (0–17%, 0–11/64 errors) and the OCG’s average median RT of 640 ms (t = 1.02, p = .18). In contrast, LHD’s error rate of 73% (47/64) was outside the OCG-5’s accuracy range and her median RT of 1476 ms was significantly different from the OCG-5’s (t = 7.36, p<.002).
Reading Assessment: Summary
A very clear pattern is evident from this set of tasks. Both DPT and DSN showed clear evidence of lexical orthographic deficits in reading, with abnormal performance with low frequency and/or irregular words in terms of accuracy and/or RTs (also see next section for additional evidence). The deficits were selective to lexical orthographic processes as nonword reading was perfectly normal, indicating fully intact pre-lexical processes as well as orthography-to-phonology conversion processing. As would be expected given this deficit locus, DSN exhibited the signs of the lexical orthographic impairment in the task of recognition of oral spelling in which she showed abnormal performance with low frequency and irregular words and produced regularization errors, just as she did in oral reading of the same words when presented visually. LHD, in contrast, other than the pre-lexical difficulties that have been documented by McCloskey & Schubert (McCloskey & Schubert, 2013, this issue; Schubert & McCloskey, 2013), showed no evidence of additional disruption to the reading system. Her pre-lexical difficulties quite naturally disrupted reading of words and nonwords presented visually but her recognition of oral spelling was strikingly intact and provided clear evidence of the integrity of the lexical orthographic processes and representations used for reading.
3. Semantic Processing: From Orthographic, Auditory or Picture Input
3.1 Orthographic Input: Written Synonym Judgment
Participants were administered a computerized version of the synonym judgment task from the Johns Hopkins University Dyslexia Battery (Goodman & Caramazza, 1985). The task consisted of 54 pairs of high frequency words (mean = 154), half of which were synonyms. Word pairs were presented simultaneously on the computer monitor and participants were instructed press one key when the words were related and another when they were not. The stimuli remained on the screen until a response was recorded.
Results
DPT’s error rate of 9% (5/54) fell outside of the normal YCG-10 range of 2–7% (1–4/54) errors and his RTs were marginally significantly slower than those of the YCG-10 (t(10) = 1.9; p<.08). Although, DSN’s error rate of 7% (4/54 errors) was not different than the OCG-6 2–15% (1–8/54) errors, she exhibited marginally significantly slower RTs (t(6) = 2.18; p<.08). LHD’s error rate of 22% (12/54) was outside the OCG-6 range, as were her RTs (t(6) = 87.8; p<.001).
3.2 Auditory Input: Spoken Synonym Judgment
Participants were administered the synonym judgment task from the PALPA (Task #49) (Kay, Coltheart, & Lesser, 1992). The stimuli for this task consisted of 60 pairs of high frequency words (mean = 48), half were synonyms and half were unrelated. Single spoken words were presented auditorily while the participant fixated on a computer screen. Trials proceeded as follows: a centrally located fixation point appeared for 800ms, followed immediately by a pair of related or unrelated words with 100ms silence between each word. Participants were asked to press one of two response keys depending on whether the word pairs were semantically related or not. Response times were recorded from the onset of the second auditory word.
Results
DPT’s one error and DSN’s zero errors were within the range of their respective control groups. Furthermore, with regard to RTs, neither DPT or DSN’s RTs were significantly different from their respective control groups (t(10) = 1.33, p = .216 and(t(6) = −.126, p = .91, respectively). LHD on the other hand demonstrated significantly higher error rates (24/60, 40% errors) and slower RTs than the OCG-6 (t(6) = 15.51, p<.001).
Task 3.3 Visual Object Input
Non-verbal semantic processing based on visual object stimuli was evaluated using a computerized version of the Pyramids and Palm Trees task (Howard & Patterson, 1992). Stimuli were presented on a computer screen. There were 55 stimuli each of which consisted of a set of three line-drawings with one displayed above the other two. Participants were asked to indicate by button press which of the two lower line-drawings was semantically related to the upper line-drawing. Participants were asked to respond as quickly and as accurately as possible. Stimuli remained on the screen until a response was recorded.
Results
Both DPT’s and DSN’s error rates of 6/55 (11%) and 12/52 (21%) fell well within YCG-8 error range (6–27%, 3–15/55) and the OCG-5 error range (4–17%, 2–9/52). Furthermore, in terms of RTs, both DPT and DSN responded no differently than their respective control groups YCG-8 (DPT: t(8) = .94, p = .38) and OCG-4 (DSN t(4) = −.03, p = .98). LHD, on the other hand, demonstrated abnormal performance both in number of errors (15/52, 29%), and median RT (10691ms, t(4) = 23.3, p<.001).
Semantic Processing: Summary
These findings indicate modality-specific difficulties in accessing semantics from orthographic input for both DPT and DSN. They both exhibited abnormal performance in terms of accuracy and RTs in the written synonym judgment task, but entirely normal performance (accuracy and RTs) with both auditory and object stimuli, indicating that semantic processing was generally intact. In contrast, LHD exhibited some difficulty in semantic judgments regardless of the input modality, indicating a more general impairment in semantic processing.
4. Spoken Word Production
4.1 Spoken Picture Naming
The set of images to be named were colored line drawings from Rossion and Pourtois (2004) that had been adapted from Snodgrass and Vanderwart (1980). Each picture was presented individually on a computer monitor, and remained until the participant responded.
Results
Response accuracy was determined by comparison to previously published norms (Bates et al., 2003; Rossion & Pourtois, 2004; Snodgrass & Vanderwart, 1980). DPT’s spoken naming error rate of 2% (5/260) fell well within the YCG-6 error range of 1–7% (2–19/260) as did his median RT of 997ms (t(5) = 1.844, p = .13). Unlike DPT, both DSN and LHD exhibited impairments in spoken naming. DSN and LHD elected to discontinue the spoken naming task before completion of the full 260 trials; DSN completed 130 trials and LHD 65, producing 48% (62/130) and 75% (49/65) errors, respectively. Both DSN and LHD’s error rates fell clearly outside of the normal range of the OCG (2–8%, 2–11/130 errors). Furthermore, DSN’s median RT of 1635ms was significantly slower when compared to the OCG-6 (t(5) = 8.432, p<.001). Reliable response time data could not be obtained for LHD due to the number of her circumlocution responses. In terms of error types, DSN failed to produce a response on 46/130 spoken naming trials and her other errors included semantically related words such as COMB → “brush” and GRAPES → “berries”. LHD also produced semantically related errors e.g. BUS → “ship”, but most of LHD’s errors were descriptions of the target e.g. CAKE → “food”, WHISTLE → “something to blow on that makes a noise”.
Spoken Production: Summary
DPT’s lexical selection and production in the spoken modality were within normal range, while both DSN and LHD suffered disruption to these processes. DSN’s normal semantic performance from the auditory and visual object modalities precludes a semantic basis for the naming difficulties. For LHD, while a semantic basis could not be discounted for some of her naming errors, many of her errors and naming difficulties were accompanied with explanations and/or gestures that indicated good semantic understanding (e.g., MITTEN → “something to cover your hand and keep it warm”, BUS → “that is a ship, not really a ship, it’s to be placed on the floor, 4 wheels, big thing in the upper part and inside go people. It has a motor”), indicating that at least some of her spoken naming difficulties originated, like DSN’s, in lexical retrieval of spoken word forms.
5. Visual Object Processing
5.1 Faces: Fame Judgment
Participants were administered a famous face judgment task that consisted of a total of 210 faces, half of which were famous and half were not; the famous persons had professions that ranged from sports, politics, business and entertainment. Face stimuli were presented on a computer screen. Participants were asked to press one of two keys if the face corresponded to a famous person or not. Each face remained on the screen until the participant responded.
Results
All three brain-lesioned participants performed normally on this task. DPT’s error rate of 8% (17/210) was well within the YCG-7 range of 5–14% (8–31/210) as were his RTs (t(7) = .98, p = .4). Both DSN’s error rate of 23% (48/209) and her RTs were no different than the OCG range of 10–30% (20–63/209) (t(5) = −.17, p = .87 and t(5) = −1.7 p = .15, respectively for accuracy and RT). Similarly, LHD’s error rate of 14% (30/209) was within the normal OCG-5 range as were her RTs (t(5) = .86, p = .42 and t(5) = 1.95, p = .11 respectively).
5.2 Faces: Profession Judgment
Participants were tested on a forced choice categorization task in which a total of 266 famous faces appeared sequentially on a computer screen and the participant was required to press one of two buttons depending on whether a face belonged to a person that corresponded to one of two specific professions. For different sets of items the profession categories varied as follows: sports-politics, politics-entertainment, sports-entertainment, business-sports, and business-politics. Faces remained on the screen until the participant responded.
Results
Both DPT and DSN demonstrated normal performance on this task when compared to their respective control groups. DPT’s error rate of 1% (3/266) was actually better than the YCG-7 (error range: 5–12%, 5–25/266) and his RTs were no different from those of the YCG-7 (t(7) = −0.38, p = .79). DSN’s error rate (12%, 231/263) was also within the range of the OCG-6 (error range: 5–12%, 12–32/263), and her RTs were not different from the OCG-5 (t(5) = −.92, p = .40). In contrast, although, LHD’s error rate of 9% (240/263) was within the OCG-5 range (t(5) = −.35, p = .73), she took significantly longer to respond (t(5) = 3.45, p = .018).
Section Summary: Object Processing
Overall, these findings indicate intact face processing for both DPT and DSN, providing further evidence that their difficulties in orthographic processing were category specific, in other words, they were limited to orthographic lexical processing and did not affect other visual (faces) or orthographic (pseudowords) categories. LHD’s performance on these tasks was somewhat mixed as not all indices were normal. This may be consistent with other evidence of a mild general impairment in semantic processing across modality and category.
Cognitive Profiles
The findings from the behavioral tests revealed three key patterns which provide the cross-participant cognitive intersections and dissociations that can be examined further via lesion mapping analysis (see Figure 5). (1) DPT, DSN, and LHD all demonstrated clear impairments in lexical orthographic processing for spelling. This was evidenced by the significant effects of frequency on their spelling accuracy and the production of phonological plausible errors. This deficit reflects an impaired ability to gain access, from semantic representations, to word spellings in orthographic long-term memory (see Figures 1 and 5B). (2) Both DPT and DSN, but not LHD, exhibited impairments in lexical orthographic processing for reading. This was evidenced for DPT and DSN by the abnormal effects of frequency and/or regularity in their response times and/or accuracy in oral reading and lexical decision. For DSN the lexical deficit was also evident in the regularization errors she produced both in oral reading and in recognition of oral spelling. Orthographic lexical processes in reading are especially dependent on access to semantics from orthographic input. For both DPT and DSN, low performance in written synonym judgment indicated modality-specific impairment at the orthography-semantics interface. For both of them, access to semantics from spoken words and pictures was intact. Critically, LHD provided a key dissociation as she was intact with regard to lexical orthographic processing in reading, with completely normal performance in recognition of oral spelling. To be clear, LHD’s reading of words and pseudowords and her written synonym judgment performance were severely impaired, but this was clearly attributable to her impaired pre-lexical processing deficit and a mild, general semantic impairment. (3) Both DSN and LHD, but not DPT exhibited impairments in lexical retrieval for spoken word production. As indicated earlier, while some of LHD’s naming difficulties could be related to her semantic difficulties, specific difficulties in phonological lexical retrieval were clearly evident for both DSN and LHD.
Lesion Mapping Analysis
Imaging Parameters
High resolution T1-weighted scans were acquired for DPT, DSN, and LHD. Slightly different imaging parameters were employed for each participant. For DPT the following parameters were used: TR = 8.06 ms, TE = 3.8 ms, matrix = 256 × 256, FOV = 256 × 200, and 200 slices with 1mm thickness. For DSN and LHD the following parameters were used: TR = 8.28 ms, TE = 3.8 ms, flip angle = 8°, matrix = 256 × 256, FOV = 256 × 180, and 200 slices with 1mm thickness.
Anatomical Analysis
Each of the participants’ MRI scans were pre-processed and normalized in the same manner in SPM8 (Wellcome Institute of Cognitive Neurology, London). All scans were initially resliced to a final voxel size of 1 mm3. Next, in order to facilitate the normalization procedure, each of the patients’ structural images were reoriented and aligned along the anterior and posterior commissure (AC-PC) plane to match the template orientation; these were then spatial spatially normalized to the standard Montreal Neurological Institute (MNI). Due to the age difference of the participants, different normalization templates were employed for DPT as compared to DSN and LHD. DPT was normalized to the standard “younger template” which based on the average of 152 individuals (86 male) with a mean age of 25 (median = 24, stdev = 4.9) (Collins, Neelin, Peters, & Evans, 1994). Both DSN and LHD, on the other hand, were normalized to the standard “older template,” which was based on the average of 50 individuals (18 male) with a mean age of 72.9 (median = 74; stdev = 7.63) (Christopher Rorden, Bonilha, Fridriksson, Bender, & Karnath, 2012). Critically, this method of spatial normalization can be influenced by the presence of a brain lesion, therefore a “Cost Function Masking” procedure is required in order to accurately normalize each brain and corresponding lesion (Andersen, Rapcsak, & Beeson, 2010). This was carried out by first tracing out the signal abnormalities due to the brain lesion in each brain in MRIcron (Chris Rorden & Brett, 2000). This lesion mask was then smoothed by an 8mm FWHM Gaussian and then incorporated into the normalization procedure such that the non-linear normalization transformation parameters were derived only from the intact brain tissue, not the lesion area (Brett et al., 2001).
Lesion Mapping
In order to display each of the lesion volumes in the same standard MNI space, we performed further processing on the lesion images. In order to obtain a lesion mask in normalized MNI space, the normalization parameters acquired from the Anatomical Analysis were then applied to the unsmoothed lesion mask. For display purposes the lesion volumes were rotated −15 degrees along the axial axis from the AC-PC line plane.
Cognitive Dissociation Lesion Mapping: Results
The brain lesion images that had been normalized to a common space were then overlaid revealing areas of intersection. The cognitive roles of the areas of lesion intersection were characterized on the basis of the cross-participant cognitive associations and dissociations that were described above and listed in Table 2.
Table 2.
Summary of the cognitive profiles for DPT, DSN and LHD. Cognitive processes are in the left column. X denotes a deficit, and a √ denotes an intact function. Colors orange, violet, and blue depict the unique and dissociable cognitive processes. Green depicts patterns that are not dissociated across this set of participants.
| DPT | DSN | LHD | |
|---|---|---|---|
| Lexical Orthography: Spelling | X | X | X |
| Lexical Orthography: Reading | X | X | √ |
| Spoken Lexical Retrieval | √ | X | X |
| Semantics | √ | √ | X |
| Pre-lexical Processing | √ | √ | X |
First, given that all three participants suffered from a disruption to lexical orthographic processing for spelling, the inference can be drawn that at least some portion of the neural area that was lesioned in all three participants is necessary for lexical orthographic processing in spelling. This area is depicted in orange in Figure 5A and corresponds to the mid-anterior portion of the fusiform gyrus (BA 37). Specifically, the lesion overlap for the three participants is centered on x = −48, y = −32, z = −27.
Second, given that DPT and DSN shared a lexical orthographic processing deficit in reading but LHD did not, we can assume that, within the remaining lesioned area, the specific region of overlap between DPT and DSN’s lesion that did not include LHD’s lesion should correspond to neural substrates necessary for lexical orthographic processing in reading. This area, depicted in purple in Figure 5A, is centered on x = −60, y = −31, z = −23 and corresponds to an anterior portion of the inferior temporal gyrus that is lateral to the area identified just above as corresponding to orthographic lexical processing in spelling.
Third, given that DSN and LHD, but not DPT, shared a deficit in spoken word lexical retrieval that disrupted access to the phonological lexicon from the semantic system, we can assume that, within the remaining lesioned area, the specific region of lesion overlap between DSN and LHD that did not include DPT’s lesion should include neural substrates necessary for spoken word lexical retrieval. This area, depicted in blue in Figure 5A corresponds to the most anterior portion of the fusiform gyrus just abutting the temporal pole, centered on x = −48, y = −12, z = −27.
Finally, the remaining lesioned areas that were not accounted for should correspond to additional deficits. For DSN and DPT, there is very little lesion volume that is unaccounted for: 16.82 cm3 for DPT and 17.15 cm3 for DSN. We did not identify any additional deficits for either DPT or DSN. For LHD, on the hand, there were large areas of lesion unaccounted for (depicted in green): 88.97 cm3. In her case we identified at least two additional deficits: pre-lexical processing of letter forms and semantic processing. Given the distribution of the remaining lesion area, it is likely that the pre-lexical deficit/s originate in the posterior portion of the lesion. The anterior portion of the lesion lies largely within the temporal pole. LHD’s mild, general semantic impairment is consistent with traditional accounts of the functionality of this region.
General Discussion
In this study we applied a novel lesion mapping approach to identify the neural substrates of the specific cognitive processes involved in reading and spelling. The approach relies on identifying patterns of impaired and spared cognitive functions that vary across participants, with the cross-participant cognitive dissociations providing constraints on the interpretation of the patterns of intersection and dissociation of the lesioned neural substrates. The findings obtained from this analysis further our understanding of the functional organization of an area anterior to the mid-fusiform gyrus and posterior to the temporal pole –a region sometimes referred to as the Basal Temporal Language Area or BTLA (Lüders et al., 1991; Schäffler et al., 1994). The key findings from this study are as follows: (1) The orthography-semantics interface for spelling and reading relies on neural substrates in the anterior fusiform and (medial) inferior temporal gyrus; (2) This orthography-semantic interface region distinguishes between substrates required for the processing of lexical orthographic input (word reading) vs. output (word spelling); (3) A further distinction within this region exists between word selection processes for written vs. spoken production. We discuss these findings and their implications in more detail in the following sections.
Reading and Spelling Deficits and Ventral Temporal Cortex
Research on pure alexia has made it abundantly clear that lesions to occipitotemporal cortex can give rise to acquired reading deficits. Much of the current focus of the pure alexia research, as can be seen by the articles in this Special Issue, is on understanding the nature of the underlying cognitive impairments that give rise to the behavioral symptoms associated with pure alexia with particular interest in the question of whether any of these impairments are specific to orthographic symbol processing or if, alternatively, they reflect more general visual impairments.
In contrast, deficits in written language processing affecting higher-level, “central” components of the reading (or spelling) systems are not usually associated with ventral temporal lobe lesions. To be clear, while researchers have reported impaired performance in tasks such as oral reading or written word comprehension subsequent to disruption/damage within ventral temporal cortex (Hillis et al., 2005; Lüders et al., 1991; Schäffler et al., 1994), these task difficulties have typically been ascribed to underlying impairments of spoken language or semantic processes. However, as reviewed in the Introduction, Rapcsak & Beeson (2004) and Tsapkini and Rapp (2010; Tsapkini et al, 2011) have reported on individuals who, subsequent to a lesion to ventral temporal cortex, suffered deficits that specifically and selectively affected lexical orthographic processing in both reading and spelling.
The current investigation adds to this small literature of cases of lexical orthographic impairment subsequent to ventral temporal lesion with a case (DSN) that is remarkably similar in both cognitive deficits and distribution of neural damage to the case (DPT) first reported on by Tsapkini and Rapp (2010). These reports of specific orthographic deficits resulting from damage to this brain region are consistent with the functional neuroimaging findings reviewed in the Introduction indicating that higher level orthographic processes for reading and spelling rely on neural substrates anterior to classical VWFA. The finding that neither DPT nor DSN exhibited length effects in their oral reading or lexical decision (Figure 4) further supports the claim that although damage to the left FG/ITG can lead to characteristic symptoms of pure alexia, it does not necessarily do so.
The current investigation also provides a clear example that, in addition to the explorations of pre-lexical processing in reading that are afforded by the cases of pure alexia, there are a host of issues related to higher level orthographic processing and representation that can be profitably examined with individuals suffering lexical orthographic deficits arising from ventral temporal lesions.
The Orthography-Semantics Interface Region (OSIR)
The clearest and most significant finding of this study is that there is a region in the anterior fusiform/medial inferior temporal gyrus that is involved in lexical orthographic processing/representation in reading and spelling.
First, all three participants exhibited remarkably similar deficits in word spelling in the face of intact pseudoword spelling. In word spelling, they all exhibited significant effects of lexical frequency in the absence of effects of word length and they all produced almost exclusively phonologically plausible errors. Of the various cognitive skills that were evaluated, lexical orthographic processing in spelling was this only one disrupted in all three participants. It stands to reason, therefore, that the lesioned neural substrates that were shared by all three individuals should include neural tissue that is necessary for successful lexical orthographic processing in spelling. This overlap region, depicted in orange in Figures 5 and 6 and centered on x = −48, y = −32, z = −27, lies just anterior to the anterior edge of the typical coordinates for the VWFA (approximately y = −64 to y = −48).
Figure 6.
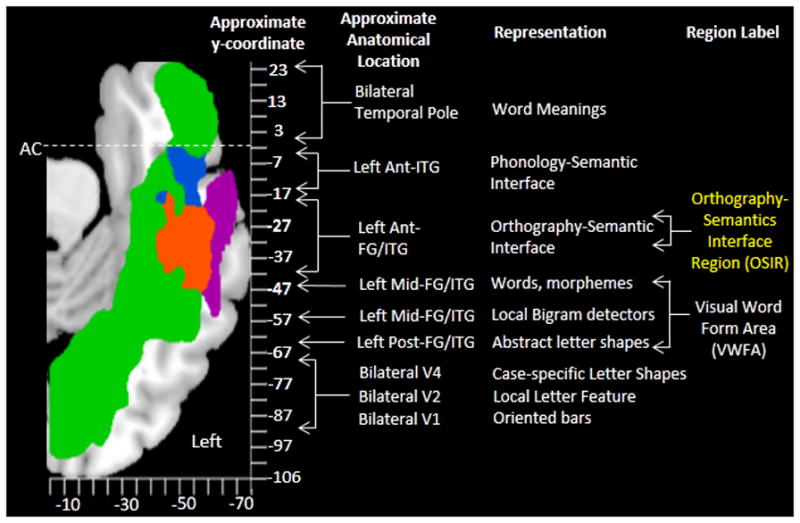
A summary of the lesion maps in MNI coordinate space in relation to an orthographic hierarchical processing scheme. This figure is an adaptation of the Visual Word Form System hierarchy schematic discussed in (Dehaene et al., 2005). The Orthography-Semantics Interface Region (OSIR) is depicted in yellow and is positioned in the left anterior fusiform gyrus (FG) and inferior temporal gyrus (ITG).
Second, the dissociation, in the case of LHD but not DSN and DPT, between lexical orthographic processing in spelling vs. reading, allows for the identification of neural substrates specifically associated with lexical orthographic processing in reading. DPT and DSN had reading deficits that mirrored their spelling impairments –intact pseudoword reading, effects of word frequency and/or regularity and, in the case of DSN, the production of regularization errors. LHD, on the other hand, exhibited a remarkably intact ability to process orthographic word forms in a recognition of oral spelling task regardless of word length or frequency. In this task, LHD was essentially able to “read” words by utilizing the spoken letters to access abstract letter identities and, on that basis, identify the corresponding orthographic word forms in Orthographic LTM (see Figure 5B). This task allowed her to circumvent her pre-lexical letter processing deficit and revealed the integrity of her lexical orthographic processing in reading (see Footnote 3). Based on the cognitive dissociation lesion mapping logic, we would expect that the area of lesion overlap for DPT and DSN that is unlesioned for LHD should include neural substrates specifically involved in lexical orthographic processing for reading but not spelling (nor the other cognitive functions preserved in the cases of DSN and DPT). This region, depicted in violet in Figures 5 and 6, lies just lateral to the area implicated in lexical orthographic processing in spelling, described just above. It lies largely within the inferior temporal gyrus and is centered on x = −60, y = −31, and z = −23.
Together these two regions involved in lexical orthographic processing in reading and spelling extend from approximately y = −53 to y = −7. The key question is how best to characterize the cognitive role of this region given that it is clearly necessary for the normal processing of orthographic word forms. There are two candidate interpretations: one is that the region corresponds to Orthographic LTM another is that it corresponds to the interface between Orthographic LTM and Lexical Semantics. The primary argument against the interpretation that the region corresponds to Orthographic LTM itself comes largely from the neuroimaging data. As reviewed in the Introduction, the neuroimaging evidence to date has supported the representation of orthographic word form knowledge in more posterior areas in the vicinity of y = −48. This proposal has been based on findings such as lexicality effects, sensitivity to lexical frequency, etc. that have typically been centered in areas posterior to the lesion overlap areas identified in this study. If we take seriously the proposal that a portion of the VWFA is required for Orthographic LTM, then the alternative is that the region identified in the current study plays a critical role in the subsequent processing that takes place at the interface between Orthographic LTM and semantics. This would be consistent with this region’s topographically intermediate location between the VWFA and the temporal pole (the latter being traditionally associated with semantic processing and representation) (but see, Tsapkini, Frangakis, & Hillis, 2011). This characterization would also be consistent with the finding that the lesions to this area resulted, as we have documented, in disrupted access to stored orthographic word forms both to and from intact semantic representations of the words to be spelled or read.
An Orthographic LTM store shared by reading and spelling at this site would predict comparable deficits in word reading and spelling and not the dissociation that was observed for LHD who exhibited a lexical orthographic deficit in spelling but not in reading. On the other hand, if we assume that this region represents the orthography-semantics interface, then we would expect it to be internally organized into separate and neurally distinct computations involved in the mapping of semantics to orthography (spelling) vs. those involved in the mapping of orthography to semantics (reading). From these data it is clear that, at a minimum, there is impairment in access to and from orthographic LTM. Still, it is entirely possible that there is an additional a partial impairment in orthographic LTM itself for DSN and DPT. This is difficult to determine with these data, and further work will be required to distinguish impairments to the interface between semantics-orthography and orthographic LTM itself.
Although, the current state of both the neuroimaging and lesion evidence precludes definitive conclusions, the overall weight of the evidence and arguments would seem to favor the position that this area anterior to the VWFA specifically supports processes that negotiate the orthography-semantic interface, with an internal organization of the area that distinguishes between process involved in reading and spelling. On this basis we will refer to the region as the OSIR (Orthography-Semantics Interface Region). Although further research will be required to characterize the specific computations involved in this interface. One possibility is that the learned associative links between orthographic and semantic representations in this area correspond to the “hidden units” that, in certain architectures, serve to map learned orthographic patterns to lexical semantic representations. Another possibility is that the region includes processes that control the dynamic aspects of activation, selection and inhibition that play a key role in navigating the relations between orthography and semantics. Although, further study is most certainly required to better understand the specific operations instantiated in the OSIR,, the research reported in this paper serves to highlight the importance of this region for the reading and spelling of words.
Language Processing Beyond the VWFA: The Question of Modality Specificity
As indicated in the Introduction, one of the key questions regarding the left hemisphere region anterior to and “beyond” the mid-fusiform area concerns whether it is comprised of amodal language processes or of dissociable, modality-specific regions that each facilitate the interface of semantics with different components of language, i.e. orthography and phonology. The results of this investigation would seem to be quite clear in this regard, both in terms of modality-specificity defined in terms of perception vs. production (input vs. output) as well as modality-specificity defined in terms of written vs. spoken language (see Figure 6). As indicated in the previous section, in terms of orthographic perception vs. production, we find clear evidence that lesions within this region can result in deficits that specifically affect lexical orthographic processing in production (spelling) but not perception (reading). LHD was able to easily and correctly recognize high and low frequency, long and short words when letter names were presented to her orally, although her accuracy in producing word spellings was affected by word frequency and she produced phonologically plausible errors. On this basis, the lesion mapping analysis indicates that orthographic input and output (perception and production) processes are differentiated in the violet vs. orange areas respectively depicted in Figures 5 and 6. Importantly, we can further infer that the (violet) area involved in the linking orthographic input and semantics, is limited to the orthographic and not spoken language modality because both DSN and DPT were normal in their access to semantics from either auditorily presented words of picture stimuli, exhibiting difficulties only when semantic access was based on written stimuli.
This study also provides evidence regarding the neural differentiation of lexical retrieval processes for spoken vs. written word production. Both DSN and LHD had difficulties in spoken lexical retrieval, producing either semantic errors or descriptions of the meanings of intended words, indicating a deficit at the interface of semantics and Phonological LTM. In contrast, DPT was normal in both accuracy and RT in spoken word naming, despite his lexical orthographic deficit in written word production. These cognitive dissociations allows us to interpret the area of lesion overlap for DSN and LHD (but not DPT) as corresponding to neural substrates implicated in spoken (but not written) lexical retrieval. This area is depicted in blue in Figures 5 and 6; it is centered on x = −48, y = −12, z = −27 anterior to the OSIR and extending anteriorly until the posterior border of the temporal pole (y = 0); see Figure 6. Furthermore, we can infer that this region is involved in lexical selection for spoken word production and not spoken word perception because DSN was intact with regard to both accuracy and RTs in spoken word comprehension (auditory synonym judgment task, Table 1).
In sum, the behavioral profiles reported in this investigation provide evidence for a number of modality-specific operations, revealing that access to and from semantics for word comprehension and production is differentiated by language modality. While there may very well also be amodal processes instantiated in neural substrates within this general region (Hillis et al., 2005; Newhart, Ken, Kleinman, Heidler-Gary, & Hillis, 2007), this study provides positive evidence for considerable modality-specific organization within this region.
Reading and Spelling: Visual Category-Specific Deficits?
As discussed earlier, there has been great interest in understanding whether some of the symptoms of pure alexia arise from general visual processing impairments or impairments that are specific to the category of letters (and/or numbers; see McCloskey & Schubert, 2013, this issue). A similar question also arises at higher levels of the processing system, with various researchers positing that orthographic knowledge and processing does not represent a knowledge category distinct from any other visual category (Behrmann & Plaut, 2013; Price & Devlin, 2011). However, DPT and DSN present evidence that contradicts this claim (see Tsapkini & Rapp, 2010 for more detailed review and arguments). While clearly suffering impairments that affected written word processing in reading (but not in spoken language) they were normal in both accuracy and RT across a number of tasks that involved visual pictures and faces. This pattern of performance is consistent with previous work demonstrating deficits selectively affecting written words, faces and objects (for discussion see: Kanwisher & Yovel, 2006) and is also in line with previous neuroimaging work demonstrating that that the activation patterns for words or letter strings are dissociable from those for visual objects and faces (Gauthier et al., 2000; Malach, Levy, & Hasson, 2002; Puce et al., 1996).
Conclusions
This investigation of the patterns of intersection and dissociation of cognitive functions and brain lesions within the ventral temporal cortex revealed a region within the left anterior fusiform gyrus that plays a key role in the interface between the orthographic representations of words and their meanings for reading and spelling, a region that we have referred to as the OSIR. Further, this work revealed that access to semantics is supported by procedures that are specific to modality (input/output; spoken/written) and category (orthography/visual objects). Overall, these findings support the view that, in the course of learning a written language, the brain allocates neural resources specifically to the orthographic “newcomer”, with the result that written language processes are instantiated with considerable independence from the evolutionarily older skills of visual object processing and spoken language.
Acknowledgments
This research was made possible through the support of NIH grants DC006740 and 1-P50-DC012283-01A1. We would like to thank DPT, DSN, and LHD for their dedication and good humor through many hours of testing. We are grateful to Michael McCloskey and Teresa Schubert for providing information and data regarding LHD and to Kyrana Tsapkini for her contributions to data collection and analysis for DPT and control participants.
Footnotes
We use the term “lexical” to modify “semantic system” in the way in which the term “verbal” is sometimes used. We use “lexical” to distinguish the semantic representations of concepts that correspond to specific words of the language from those which do not. In our opinion, the term “lexical” more precisely denotes this distinction than does the term “verbal”.
A recent study reported on an individual with damage to the left ventral occipitotemporal cortex that extended anterior to the VWFA (y = −88 to −30) (Seghier et al., 2012). While lexical orthographic deficits were not specifically reported by the authors, the presence of frequency and regularity effects in reading indicate some degree of lexical deficit in reading (in addition to the clear pre-lexical impairment) and some of the errors reported in the spelling sample (see Seghier et al., 2012 Supplementary materials) are consistent with some degree of lexical orthographic deficit also in spelling.
The work of McCloskey and Schubert (2013) and Schubert and McCloskey (2013) documents that LHD’s reading impairment is based on impairment in pre-lexical processing of letters, specifically in the mapping between intact representations of letter shapes to abstract letter identities. This conclusion was based, among other things, on three key findings: 1) Intact letter form processing as evidenced by intact ability to discriminate between real letters and pseudoletters; 2) Impaired access to abstract letter identities from visual input as evidenced by impaired letter naming and impaired identification of lower case letters with their upper case counterparts; and 3) Intact orthographic LTM processing for reading as evidenced by the ability to recognize words from oral spelling.
Two points worth making regarding this task. First, for irregular/exception words, access to orthographic LTM will be as necessary as it is in reading. Second, if we assume two different Orthographic LTM stores for reading and spelling, one could imagine (a rather cumbersome strategy) by which one uses a subset of the letter names to generate various candidate spellings from the orthographic LTM used for spelling which are then checked against the letter names held in phonological working memory. In that case, the Orthographic LTM store used to generate candidate spellings would be the one used for spelling. Critically, LHD’s spelling performance (described in the previous section) rules out this interpretation as it indicates that access to Orthographic LTM for word spelling is clearly impaired and marked by the production of phonologically plausible errors. In contrast, as the results of recognition of oral spelling task show—she is fully intact in accessing Orthographic LTM when she can bypass visual processing of letter shapes.
References
- Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? Neuro Image. 2010;53(1):78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S, Scarratt DR. Rule and Analogy Mechanisms in Reading Non words: Hough Dou Peapel Rede Gnew Wirds? Journal of Experimental Psychology: Human Perception and Performance. 1998;24(4):1052–1086. [Google Scholar]
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(21):9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, D’Amico S, Jacobsen T, Székely A, Andonova E, Devescovi A, Tzeng O. Timed picture naming in seven languages. Psychonomic Bulletin & Review. 2003;10(2):344–380. doi: 10.3758/bf03196494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Plaut DC. Distributed circuits, not circumscribed centers, mediate visual recognition. Trends in Cognitive Sciences. 2013;17(5):210–219. doi: 10.1016/j.tics.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33(2):739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002a;16(1):7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Hum Brain Mapp. 2002b;16(4):251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann T, Weiller C. “Are there lexicons?” A study of lexical and semantic processing in word-meaning deafness suggests “yes”. Cortex. 2012;48(3):294–307. doi: 10.1016/j.cortex.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuro Image. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394(6690):274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22(1):466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, Dehaene S. Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cerebral Cortex. 2003;13(12):1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Coltheart M. Are there lexicons? The Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology. 2004;57(7):1153–1171. doi: 10.1080/02724980443000007. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: Dual-route and parallel-distributed-processing approaches. Psychological Review. 1993;100(4):589–608. doi: 10.1037/0033-295X.100.4.589. [DOI] [Google Scholar]
- Crawford JR, Garthwaite PH. Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40(8):1196–1208. doi: 10.1016/s0028-3932(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Testing for suspected impairments and dissociations in single-case studies in neuropsychology: evaluation of alternatives using monte carlo simulations and revised tests for dissociations. Neuropsychology. 2005;19(3):318–331. doi: 10.1037/0894-4105.19.3.318. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Brain Binds Entities and Events by Multiregional Activation from Convergence Zones. Neural Computation. 1989;1(1):123–132. doi: 10.1162/neco.1989.1.1.123. [DOI] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4(7):752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution a l’étude anatomopathologique et clinique des différentes variétés de cécité verbale. Mem Soc Biol. 1892:61–90. [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Cohen L. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50(2):191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience. 2000;12(3):495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Price CJ. The constraints functional neuroimaging places on classical models of auditory word processing. J Cogn Neurosci. 2001;13(6):754–765. doi: 10.1162/08989290152541421. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area. Neuron. 2009;62(2):199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, Caramazza A. The Johns Hopkins University Dysgraphia Battery. The Johns Hopkins University; Baltimore, MD: 1985. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural Systems for Reading Aloud: A Multiparametric Approach. Cereb Cortex. 2009;20(8):1799–815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros H, Boulanouar K, Viallard G, Cassol E, Celsis P. Event-related functional magnetic resonance imaging study of the extrastriate cortex response to a categorically ambiguous stimulus primed by letters and familiar geometric figures. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21(11):1330–1341. doi: 10.1097/00004647-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Habib M, Ceccaldi M, Poncet M. Syndrome de déconnexion calleuse par infarctus jonctionnel hémisphérique gauche. Revue neurologique. 1990;146(1):19–24. [PubMed] [Google Scholar]
- Hillis A, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. Neuroimage. 2005;24(2):548–559. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson KE. The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Pictures3: [manual] Thames Valley Test Company; 1992. [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J, Coltheart M, Lesser R. Psychololinguistic Assessments of Language Processing in Aphasia (PALPA) Hove, East Sussex: Lawrence Erlbaum; 1992. [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21(3):946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, Godoy J. Basal temporal language area. Brain: A Journal of Neurology. 1991;114(Pt 2):743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends in Cognitive Sciences. 2002;6(4):176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCloskey M, Schubert T. Shared versus separate processes for letter and digit identification. Cognitive Neuropsychology. 2013:1–24. doi: 10.1080/02643294.2013.869202. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136(2):601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47(1):36–45. [PubMed] [Google Scholar]
- Newhart M, Ken L, Kleinman JT, Heidler-Gary J, Hillis A. Neural networks essential for naming and word comprehension. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2007;20(1):25–30. doi: 10.1097/WNN.0b013e31802dc4a7. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: computational principles in quasi-regular domains. Psychological Review. 1996;103(1):56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. Functional MRI evidence for an abstract, not perceptual, word-form area. Journal of Experimental Psychology. General. 2002;131(1):65–72. doi: 10.1037//0096-3445.131.1.65. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential Sensitivity of Human Visual Cortex to Faces, Letterstrings, and Textures: A Functional Magnetic Resonance Imaging Study. The Journal of Neuroscience. 1996;16(16):5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Napoliello EM, Eden GF. A combined fMRI study of typed spelling and reading. Neuroimage. 2011;55(2):750–762. doi: 10.1016/j.neuroimage.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, Rapp B. Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology. 2011;2(October):239. doi: 10.3389/fpsyg.2011.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MAL, Patterson K, Garrard P, Hodges JR. Semantic dementia with category specificity: a comparative case-series study. Cognitive Neuropsychology. 2003;20(3):307–326. doi: 10.1080/02643290244000301. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. Neuroanatomical correlates of spelling and writing. In: Hillis A, editor. The Handbook of Adult Language Disorders. New York: Psychology Press; 2002. pp. 71–100. [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62(12):2221–2229. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rapp B, Caramazza A. From graphemes to abstract letter shapes: levels of representation in written spelling. J Exp Psychol Hum Percept Perform. 1997;23(4):1130–1152. doi: 10.1037//0096-1523.23.4.1130. [DOI] [PubMed] [Google Scholar]
- Rapp B, Dufor O. The neurotopography of written word production: an FMRI investigation of the distribution of sensitivity to length and frequency. Journal of Cognitive Neuroscience. 2011;23(12):4067–81. doi: 10.1162/jocn_a_00109. [DOI] [PubMed] [Google Scholar]
- Rapp B, Lipka K. The literate brain: The relationship between spelling and reading. Journal of Cognitive Neuroscience. 2010;23(5):1180–97. doi: 10.1162/jocn.2010.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeltgen D, Heilman K. Review of Agraphia and a Proposal for an Anatomically-Based Neuropsychological Model of Writing. Applied Psycholinguistics. 1985;6(3):205–29. [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam MM. Anatomy of Language Impairments in Primary Progressive Aphasia. The Journal of Neuroscience. 2011;31(9):3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: The role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Rothlein D, Rapp B. The similarity structure of distributed neural responses reveals the multiple representations of letters. Neuro Image. 2013 doi: 10.1016/j.neuroimage.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffler L, Lüders HO, Morris HH, 3rd, Wyllie E. Anatomic distribution of cortical language sites in the basal temporal language area in patients with left temporal lobe epilepsy. Epilepsia. 1994;35(3):525–528. doi: 10.1111/j.1528-1157.1994.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Schubert T, McCloskey M. Pre-Lexical Representations and Processes in Reading: Evidence from Acquired Dyslexia. Cognitive Neuropsychology. 2013 doi: 10.1080/02643294.2014.880677. [DOI] [PubMed] [Google Scholar]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. A dual-route perspective on brain activation in response to visual words: evidence for a length by lexicality interaction in the visual word form area (VWFA) Neuroimage. 2010;49(3):2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Neufeld NH, Zeidman P, Leff AP, Mechelli A, Nagendran A, Price CJ. Reading without the left ventral occipito-temporal cortex. Neuropsychologia. 2012;50(14):3621–3635. doi: 10.1016/j.neuropsychologia.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, Waters GS, Barns MA, Tanenhaus MK. When Does Irregular Spelling or Pronunciation Influence Word Recognition? Journal of Verbal Learning and Verbal Behavior. 1984;23:383–404. [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037/0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Habekost T, Leff AP. Too Little, Too Late: Reduced Visual Span and Speed Characterize Pure Alexia. Cerebral Cortex. 2009;19(12):2880–2890. doi: 10.1093/cercor/bhp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yamadori A, Endo K, Fujii T, Ezura M, Takahashi A. Dissociation of letter and picture naming resulting from callosal disconnection. Neurology. 1998;51(5):1390–1394. doi: 10.1212/wnl.51.5.1390. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Aguirre GK, D’Esposito M, Farah MJ. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia. 1999;37(6):671–676. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis C, Hillis A. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain: A Journal of Neurology. 2011;134(Pt 10):3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Rapp B. The orthography-specific functions of the left fusiform gyrus: Evidence of modality and category specificity. Cortex. 2010;46(2):185–205. doi: 10.1016/j.cortex.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Vindiola M, Rapp B. Patterns of brain reorganization subsequent to left fusiform damage: fMRI evidence from visual processing of words and pseudowords, faces and objects. Neuro Image. 2011;55(3):1357–1372. doi: 10.1016/j.neuroimage.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Goldberg EM, Postman-Caucheteux WA, Palovcak M, Quinn C, Cantor C, Coslett HB. Alexia due to ischemic stroke of the visual word form area. Neurocase. 2013 doi: 10.1080/13554794.2013.770873. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical Coding of Letter Strings in the Ventral Stream: Dissecting the Inner Organization of the Visual Word-Form System. Neuron. 2007;55(1):143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Welbourne SR, Lambon Ralph MA. Using Parallel Distributed Processing Models to Simulate Phonological Dyslexia: The Key Role of Plasticity-related Recovery. Journal of Cognitive Neuroscience. 2007;19(7):1125–1139. doi: 10.1162/jocn.2007.19.7.1125. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan P, Song W, Shang HF. Quantitative Meta-Analysis of Gray Matter Abnormalities in Semantic Dementia. Journal of Alzheimer’s Disease. 2012;31(4):827–833. doi: 10.3233/JAD-2012-120736. [DOI] [PubMed] [Google Scholar]