Abstract
Purpose
To assess the direct effects of prenatal cocaine exposure (PCE) on adolescent internalizing, externalizing and attention problems, controlling for confounding drug and environmental factors.
Method
At 12 and 15 years of age, 371 adolescents (189 PCE, 182 non-cocaine exposed (NCE)), primarily African-American and of low socioeconomic status, participating in a longitudinal, prospective study from birth were assessed for behavioral adjustment using the Youth Self-Report (YSR).
Results
Longitudinal mixed model analyses indicated that PCE was associated with greater externalizing behavioral problems at ages 12 and 15 and more attention problems at age 15, after controlling for confounders. PCE effects were not found for internalizing behaviors. PCE adolescents in adoptive/foster care reported more externalizing and attention problems than PCE adolescents in biological mother/relative care at age 12 or NCE adolescents at both ages. No PCE by gender interaction was found. Prenatal marijuana exposure, home environment, parental attachment and monitoring, family conflict, and violence exposure were also significant predictors of adolescent behavioral adjustment.
Conclusions
Prenatal cocaine exposure is a risk factor for poor behavioral adjustment in adolescence.
Keywords: Prenatal cocaine, behavior, attention, adolescents
Poor behavioral adjustment during adolescence is linked with early onset of substance use and later adult mental health problems. Prenatal cocaine exposure (PCE) may increase the risk for behavioral problems throughout childhood.1–4 PCE disrupts the monoaminergic neurotransmitter system in the prefrontal cortex, affecting emotional and behavioral arousal and regulation, attention, and stress response.5 The neurobehavioral teratology model6 posits that the effects of damage to the developing central nervous system incurred prenatally can extend through later periods of development. Long-term developmental outcome is affected by the timing, duration, and dose of the teratogen in utero, and aspects of the environmental context can modify outcomes, either exacerbating or ameliorating early effects. Additionally, depending on the brain regions affected, some teratogenic effects may not be evident until the cognitive or behavioral domains implicated are emergent.
Several prospective longitudinal studies have documented PCE-related behavioral problems in childhood1,2 and preadolescence.3,4 PCE effects have been found on child-reported symptoms of oppositional defiant disorder and attention-deficit/hyperactivity disorder at 6 years of age,7 on caregiver-reported aggressive behavior at 9 years,8 on child -reported depressive symptoms and teacher-rated anxious/depressed behavior at 10 years,9 and on teacher- and caregiver-rated externalizing behavior problems at 7, 9 and 11 years,3 while other studies have found no such effects.10,11 Further, mixed findings of PCE by gender interaction on behavioral adjustment have been noted, with PCE boys showing more clinically significant externalizing and delinquent behaviors12 and deficits in attention13 than non-cocaine exposed (NCE) boys, while other studies reporting effects of PCE in girls only.2,8,14
Early behavioral problems are likely to persist and intensify given the increasing developmental challenges and demands of adolescence, including puberty, school transitions, changing relationships with parents and peers,15 and further development of the prefrontal cortex and its associated networks. To date, only one study has examined behavioral outcomes in adolescents with cocaine/polydrug exposure,16 although it did not address cocaine-specific effects.
Isolating the effect of PCE on behavioral outcomes is complicated, as multiple biological and environmental confounders may obscure the long-term effects of PCE, including high exposure to other substances,17–19 elevated lead levels (≥ 10 μg/dL),20,21 poor quality of the home environment,22,23 caregiver ongoing substance use and psychological distress,2,8,24 and adoptive/foster care placement.7 Further, family conflict,25 violence exposure,3,26 poor attachment to caregiver,24 and inadequate parental monitoring,27 reflecting the interpersonal developmental contexts in which adolescents transact,28 may heighten the drug exposed adolescent’s vulnerability to behavior problems.
The present study extends previous findings to examine whether negative effects of PCE on behavior persist into adolescence. We hypothesized that adolescents with PCE would report more externalizing, internalizing and attention problems compared to NCE adolescents at 12 and 15 years of age, controlling for the effects of other risk factors. Because a significant proportion of PCE adolescents in this sample were placed in non-kinship adoptive/foster care, we also explored the impact of non-kinship adoptive/foster care placement on behavior. Given previous findings, we also assessed gender as a potential moderator of PCE effects on behavioral outcomes.2,8,12,14
Methods
Sample
This study included 371 (189 PCE, 182 NCE) adolescents recruited at birth from an urban county hospital with a high risk maternal population screened for drug use. Pregnant women who lacked prenatal care, had a history of involvement with the Department of Human Services, exhibited behavior suggesting intoxication, or self-admitted drug use, were considered to be at high risk for drug use and were given drug toxicology screenings at infant birth. Maternal and infant urine samples and infant meconium were obtained shortly before or after infant birth and analyzed for cocaine and other drug metabolites, including benzoylecgonine, meta-hydoxybenzoylecgonine, cocaethylene, cannabinoids, opiates, phencyclidine, amphetamines, and benzodiazepines. Women with a psychiatric history, low intellectual functioning (diagnosis of mental retardation indicated in medical chart review), HIV-positive status, or chronic medical illness were excluded, as were infants with Down Syndrome, Fetal Alcohol Syndrome, or medical illness. A total of 415 newborns and their birth mothers were enrolled at birth, of which 218 infants were identified as cocaine-exposed based on positive screens of maternal and infant urine, infant meconium, or maternal self-report to hospital or research staff. Infants exposed to cocaine were further classified as being either heavier or lighter exposed. The heavier PCE group was defined a priori as >70th percentile for cocaine use, which corresponded to ≥216 ng/g benzoylecgonine in meconium screening or ≥17.5 units (“rocks” of cocaine worth $20 each)/week in maternal self-report.
Since birth, 12 (9 PCE, 3 NCE) enrolled children died. Causes of death for the PCE children included sudden infant death syndrome (SIDS) (4), cardiopulmonary arrest (1), pneumonia (1), accidental asphyxia (1), respiratory distress syndrome (1), and unknown illness (1). For the NCE children, causes of death were SIDS (2) and respiratory distress syndrome (1). The present study utilizes data from 371 adolescents who completed behavioral assessment at ages 12 and/or 15 years, which represents 92% retention of the living participants. Among the 371 participating adolescents, 91.4% (n=339) were assessed at both 12 and 15 years of age. Of the 32 adolescents not seen (19 drop-out, 12 lost contact, 1 low intellectual functioning (IQ <50)), the 20 PCE adolescents were more likely to have birth mothers with lower scores on the Wechsler Adult Intelligence Scale-Revised (WAIS-R)29 Picture Completion subtest, and the 12 NCE adolescents not seen were more likely to be White and to have birth mothers who were older and married. No difference was found by PCE status between the 371 participants and the 32 nonparticipants.
Procedure
Adolescents and their caregivers were seen at the developmental research laboratory for approximately 5 hours at each follow-up visit at ages 6, 12, and 18 months and 2, 4, 6, 9, 10, 11, 12, and 15 years. All participants were given a monetary stipend, lunch and transportation costs. This study was approved by the Institutional Review Board of the participating hospital. Parental written informed consent and child assent were obtained. A Certificate of Confidentiality (DA-98-91) was obtained from the Department of Health and Human Services.
At the newborn visit, birth mothers were asked to recall frequency and amount of drug use for the month prior to and for each trimester of pregnancy. The number of tobacco cigarettes and marijuana joints smoked, and the number of drinks of beer, wine, or hard liquor per week was computed, with each drink equivalent to 0.5 oz. of absolute alcohol. For cocaine, as the majority of women (>90%) in our study primarily used the crack cocaine form, the number of “rocks” consumed and the amount of money spent per day were noted and converted to a standard “unit” or “rock” of cocaine, referring to $20 worth of cocaine. Frequency of use was recorded for each drug on a Likert-type scale ranging from 0 (not at all) to 7 (daily use) and converted to reflect the average number of days per week a drug was used, except for cigarettes, which was collected as the number smoked per day. Frequency was multiplied by the amount used per day to compute an average use score for the month prior to pregnancy and for each trimester. These scores were then averaged to obtain a total average score. The drug assessment was updated with the child’s current caregiver at the 12 and 15 year follow-up visits to obtain a measure of recent (prior 30 day period) caregiver drug use.
Birth, demographic, and medical characteristics extracted from hospital birth records included maternal age and marital status, years of education, number of prenatal care visits, parity, child’s race and gender, and infant head circumference. A Hollingshead score of IV (e.g., skilled manual workers, craftsmen) or V (e.g., clerical and sales workers, high school graduate)30 was used as an indicator of low socioeconomic status. Maternal vocabulary was assessed at birth using the Peabody Picture Vocabulary Test-Revised (PPVT-R),31 and updated using its third edition (PPVT-III)32 at age 6 and later assessments. The Block Design and Picture Completion subtests of the WAIS-R29 were used to estimate maternal non-verbal intelligence at infant birth. Maternal psychological distress was assessed using the Global Severity Index (α=.95), a summary scale of the Brief Symptom Inventory,33 at birth and at each follow-up visit. At each visit, the child’s placement (with either biological mother/relative or adoptive/foster caregiver) and changes (defined by a change in both primary caregiver and physical setting lasting greater than one month) were noted and data on the current caregiver were updated to provide concurrent assessment of caregiver intelligence and psychological distress.
At ages 2 and 4 years, lead exposure was assessed for a subset of children. Venous blood samples could not be obtained from some children due to lack of parental consent, excessive stress related to the blood draw, child sickness or logistical difficulties. Valid hematologic measures were available for 143 two-year and 274 four-year old children. Measures were averaged for the 122 children seen at both assessments. A greater percentage of African-American and married women and a lower percentage of foster parents consented to blood collection.
At 11 years, adolescents’ intelligence was assessed using the Wechsler Intelligence Scales for Children-Fourth Edition.34 At 12 years, parental attachment (α=.80; 5-items on a 4-point Likert scale) and monitoring (α=.74; 6-items on a 4-point Likert scale), family conflict (index of 10-item questionnaire), and violence exposure (α=.75; 8-items on a 5-point Likert scale) were assessed using The Assessment of Liability and Exposure to Substance Use and Antisocial Behavior (ALEXSA),35 an illustration-based, audio, computer-assisted self-report of antisocial behavior, substance involvement and associated risk factors for children ages 9–12.
At 12 and 15 years, adolescents’ behavioral adjustment was assessed using the Youth Self-Report (YSR),36 a 105-item self-rating of emotional, behavioral, and social problems in the last 6 months. T-scores were standardized for gender and age, with higher scores indicating more problem behaviors. For this investigation, externalizing (aggression and rule-breaking behavior; α=.87 at 12 year, .90 at 15 year), internalizing (anxious or depressed, withdrawn, somatic complaints; α=.86, .88), and attention problems (α=.74, .76) were analyzed. The quality of the caregiving environment was assessed via interview using the Home Observation of the Environment-Early Adolescent (HOME; α=.83 at both years).37
Statistical analyses
The effects of PCE were evaluated using a mixed linear model approach with maximum likelihood estimation procedures. Unstructured covariance matrix was used to account for correlated responses within a subject. We tested the homogeneity of PCE effects, as well as the effects of gender and other covariates on adolescents’ behavioral adjustment over time by including an interaction term with time. If the interaction was not significant at p < .10, the interaction terms were removed from the model. Missing data were modeled using full-information maximum likelihood, which utilizes all available information from the observed data.
Covariates correlated with outcomes at p ≤.20 for at least one time point were entered into the longitudinal regression model stepwise and were retained if, on entry, they were significant at p < .10 or caused substantial (> 10%) change in the PCE coefficient. PCE was entered first followed by socio-demographic covariates, other prenatal substance exposure, parenting, and violence exposure variables. Due to the reduced sample size, blood lead level was entered last. Levels of PCE (NCE, lighter PCE, heavier PCE) and combined effects of PCE and placement (PCE biological/relative, PCE foster/adoptive care, and NCE) at age 12 were evaluated when significant PCE effects were noted. Adjusted least squares mean (Madj) and standard errors (SE) were calculated from the models.
Results
Sample Characteristics
Birth mothers of adolescents with PCE were older, slightly less educated, primarily unmarried, had more children and less prenatal care than birth mothers of NCE adolescents (Table 1). They had lower vocabulary scores and reported more psychological distress. Cocaine-using women on average used more tobacco, alcohol and marijuana over the pregnancy compared to non-cocaine-using women. The average amounts of use of each drug generally declined over the course of pregnancy in both groups. Caregiver and home environment characteristics at age 12 years did not differ except that caregivers of the adolescents with PCE had less education and smoked more cigarettes in the previous month than the current caregivers of NCE adolescents. Adolescents with PCE had a shorter gestational age, lower birth weight, length, and head circumference, and lower blood lead levels during the preschool years compared to NCE adolescents (Table 2). Adolescents with PCE were less likely to be continuously cared for by their birth mothers, with 23% (n=44) of adolescents with PCE, compared to 4.5% (n=8) of their NCE counterparts, living in non-kinship adoptive/foster care (χ2 =27.44, p <.0001) at 12 years. No group difference, however, was noted in placement change from ages 12 to 15. Adolescents with PCE reported a lower level of parental attachment and greater family conflict than their NCE counterparts. No group differences were found in parental monitoring or violence exposure.
Table 1.
Maternal and Caregiver Characteristics
| PCE (n=189)
|
NCE (n=182)
|
P | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Biological maternal | |||||
| Mother’s age at birth | 29.65 | 4.98 | 25.47 | 4.73 | <.0001 |
| Education, years | 11.53 | 1.65 | 11.95 | 1.39 | .01 |
| Married, n (%) | 14 | 7.41 | 29 | 15.93 | .01 |
| Parity | 3.50 | 1.88 | 2.74 | 1.86 | .0001 |
| Number of prenatal visits | 5.27 | 4.62 | 8.69 | 4.92 | <.0001 |
| PPVT-R Standard Score | 73.34 | 14.19 | 77.76 | 14.71 | .004 |
| WAIS-R Block Design Scaled Score | 6.88 | 2.09 | 7.17 | 2.08 | .19 |
| WAIS-R Picture Completion Scaled Score | 6.71 | 2.15 | 6.97 | 2.36 | .28 |
| BSI Global Severity Index | 0.83 | 0.75 | 0.50 | 0.53 | <.0001 |
| Low SES, n (%) | 184 | 97.87 | 178 | 97.80 | .96 |
| African-American, n (%) | 156 | 82.54 | 148 | 81.32 | .76 |
| Substance use during pregnancy a | N (%) | M (SD) | N (%) | M (SD) | p |
|---|---|---|---|---|---|
| Tobacco, cigarettes per day | 160 (87.43) | 11.61 (11.20) | 70 (40.23) | 3.90 (7.18) | <.0001 |
| Month prior | 157 (85.33) | 13.41 (12.43) | 66 (37.93) | 5.36 (9.66) | <.0001 |
| 1st trimester | 155 (84.24) | 12.61 (12.44) | 57 (32.76) | 4.02 (7.88) | <.0001 |
| 2nd trimester | 147 (79.89) | 10.69 (11.72) | 48 (27.59) | 3.20 (6.97) | <.0001 |
| 3rd trimester | 140 (76.09) | 9.49 (11.20) | 49 (28.16) | 3.03 (5.99) | <.0001 |
| Alcohol, drinks per week | 148 (80.87) | 9.81 (17.52) | 75 (43.10) | 1.37 (4.60) | <.0001 |
| Month prior | 128 (69.95) | 12.87 (22.54) | 64 (36.78) | 2.53 (7.81) | <.0001 |
| 1st trimester | 117 (63.93) | 12.09 (23.71) | 35 (20.11) | 1.28 (3.86) | <.0001 |
| 2nd trimester | 89 (48.63) | 8.02 (19.68) | 18 (10.40) | 0.57 (3.03) | <.0001 |
| 3rd trimester | 90 (49.18) | 6.27 (17.07) | 22 (12.64) | 1.11 (7.87) | <.0001 |
| Marijuana, joints per week | 78 (42.62) | 1.33 (3.46) | 16 (9.20) | 0.60 (3.52) | <.0001 |
| Month prior | 61 (33.70) | 1.61 (3.80) | 16 (9.20) | 1.55 (10.10) | .0003 |
| 1st trimester | 50 (27.78) | 1.48 (4.06) | 10 (5.75) | 0.58 (3.78) | <.0001 |
| 2nd trimester | 35 (19.23) | 1.29 (4.26) | 4 (2.30) | 0.19 (1.71) | <.0001 |
| 3rd trimester | 32 (17.68) | 0.97 (3.83) | 4 (2.30) | 0.09 (0.76) | .0002 |
| Cocaine, units per week | 189 (100) | 22.55 (37.88) | --- | --- | --- |
| Month prior | 156 (82.54) | 30.06 (57.77) | --- | --- | --- |
| 1st trimester | 150 (79.37) | 31.94 (64.68) | --- | --- | --- |
| 2nd trimester | 128 (67.72) | 24.55 (62.73) | --- | --- | --- |
| 3rd trimester | 138 (73.02) | 12.32 (27.34) | --- | --- | --- |
| Caregiver at age 12 | M | SD | M | SD | p |
|---|---|---|---|---|---|
| Education, years | 12.13 | 2.26 | 12.80 | 1.93 | .003 |
| PPVT-III Standard Score | 79.33 | 14.66 | 79.57 | 15.68 | .88 |
| WAIS-R Block Design Scaled Score | 7.09 | 2.13 | 7.33 | 1.98 | .28 |
| WAIS-R Picture Completion Scaled Score | 7.45 | 2.64 | 7.17 | 2.33 | .29 |
| BSI Global Severity Index | 0.37 | 0.46 | 0.36 | 0.49 | .77 |
| Amount of substance use in the past 30 daysb | |||||
| Tobacco, cigarettes per day | 5.34 | 7.53 | 3.77 | 6.66 | .01 |
| Alcohol, dose per week | 1.54 | 4.75 | 1.73 | 5.50 | .97 |
| Marijuana, dose per week | 0.86 | 7.01 | 0.10 | 1.07 | .16 |
| HOME environment | 47.84 | 7.00 | 49.04 | 6.21 | .08 |
PPVT= Peabody Picture Vocabulary Test, PPVT-R (Revised) used at birth; PPVT-III (Third edition) was used at 6 and later years; WAIS-R = Wechsler Adult Intelligence Scale-Revised; BSI=Brief Symptom Inventory; WISC-IV= Wechsler Intelligence Scales for Children-Fourth Edition.
Data were normalized using a log transformation; p-value based on M(SD).
All caregivers reported no use of cocaine in the past 30 days.
Table 2.
Adolescents Characteristics
| PCE (n=189)
|
NCE (n=182)
|
P | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| At birth | |||||
| Gestational age, weeks | 37.81 | 2.86 | 38.47 | 2.87 | .03 |
| Hobel Neonatal Risk score | 7.49 | 16.55 | 5.83 | 15.83 | .33 |
| Birth weight, gramsa | 2712 | 650 | 3102 | 698 | <.0001 |
| Birth length, cma | 47.32 | 3.97 | 49.13 | 3.74 | <.0001 |
| Head circumference, cma | 32.29 | 2.15 | 33.48 | 2.38 | <.0001 |
| Male, n (%) | 85 | 44.97 | 89 | 48.90 | .45 |
| African-American, n (%) | 155 | 82.01 | 147 | 80.77 | .76 |
| Postnatal | |||||
| Blood lead level at 2 or/and 4 yearb | 7.00 | 4.13 | 8.02 | 4.62 | .04 |
| Elevated blood lead level (≥ 10 μg/dLb), n (%) | 26 | 17.69 | 37 | 26.06 | .08 |
| Age at assessment, years | |||||
| 12 year | 12.08 | 0.25 | 12.10 | 0.24 | .36 |
| 15 year | 15.69 | 0.27 | 15.67 | 0.28 | .53 |
| WISC-IV Full Scale IQ at age 11 | 84.70 | 11.79 | 86.41 | 14.70 | .22 |
| Parental attachmentc | 2.09 | 0.68 | 2.27 | 0.61 | .01 |
| Parental monitoringc | 2.42 | 0.64 | 2.48 | 0.59 | .39 |
| Family conflictc | 3.22 | 2.55 | 2.60 | 2.37 | .02 |
| Violence exposurec | 0.63 | 0.76 | 0.57 | 0.80 | .43 |
| Always in birth parents’ care up to age 12, n (%) | 67 | 35.45 | 155 | 85.16 | <.0001 |
| Placement at 12, n (%) | <.0001 | ||||
| Birth parents’ care | 96 | 50.79 | 168 | 92.31 | |
| Relative care | 49 | 25.93 | 6 | 3.30 | |
| Non-kinship adoptive care | 40 | 21.16 | 5 | 2.75 | |
| Non-kinship foster care | 4 | 2.12 | 3 | 1.65 | |
| Any placement change between ages 12 to 15, n (%) | 36 | 19.05 | 26 | 14.29 | .22 |
| Self-reported lifetime substance use by age 12, n (%)d | 58 | 32.95 | 55 | 31.98 | .85 |
WISC-IV=Wechsler Intelligence Scales for Children-Fourth Edition
Adjusted for gestational age
Sub-sample of 147 PCE and 142 NCE
Assessed at 12 year
Lifetime any substance use (Yes/No). Summarized from ALEXSA at 9, 10, 11, and 12 year and the Youth Risk Behavior Surveillance System (YRBSS) at 12 year
Behavioral Adjustment at 12 and 15 Years
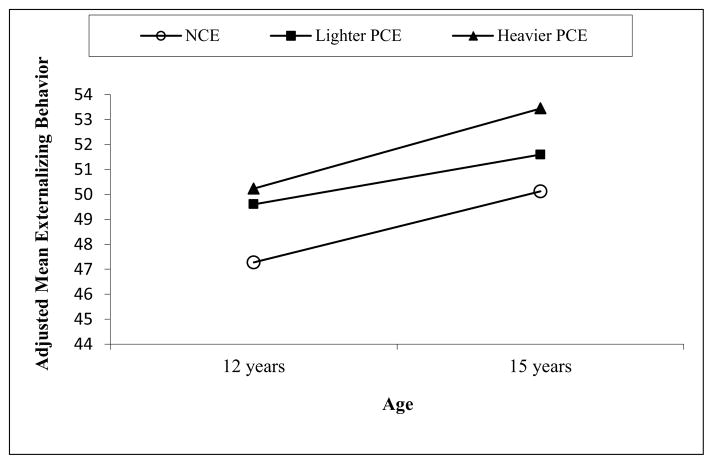
After controlling for covariates, PCE was associated with more externalizing behaviors at both 12 and 15 years and with greater attention problems at 15 years (Table 3). Adolescents with PCE reported 2.54 higher externalizing scores on average than NCE youth at both time points. When the PCE adolescents were classified into heavier and lighter exposure groups, greater effects were seen in the heavier exposure group (Figure 1). Also, the PCE group (Madj=59.34, SE=0.68) had an estimated 2.05 higher mean inattention score than the NCE group (Madj=57.26, SE=0.67) at age 15, despite no significant difference at age 12 between the PCE (Madj=56.36, SE=0.62) and NCE (Madj=56.33, SE=0.61) groups. No PCE effect was found on internalizing behavior. No gender by PCE interaction was found. Prenatal marijuana exposure was related to more attention problems.
Table 3.
Effects of Prenatal Cocaine Exposure on Adolescent Self-Reported Behavior Problem at 12 & 15 Years
| Externalizing behavior
|
Internalizing behavior
|
Attention
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Prenatal cocaine exposure | 2.54 | 1.10 | .02 | −0.70 | 1.06 | .51 | 0.03 | 0.91 | .97 |
| Time, 15 year | 3.82 | 0.76 | <.0001 | 2.65 | 0.79 | .0009 | 0.93 | 0.66 | .16 |
| PCE*Time | 2.05 | 0.94 | .03 | ||||||
| Sex, male | 0.07 | 0.99 | .95 | 2.21 | 1.03 | .03 | −1.54 | 0.75 | .04 |
| Sex*Time | −2.25 | 1.11 | .04 | −3.22 | 1.15 | .005 | |||
| Adolescent race, African American | −3.30 | 1.25 | .009 | −1.63 | 1.04 | .12 | |||
| Maternal age at birth | −0.17 | 0.09 | .06 | −0.10 | 0.09 | .25 | −0.13 | 0.08 | .09 |
| Maternal GSI at birth | 1.14 | 1.34 | .39 | 2.94 | 1.34 | .03 | 0.56 | 1.15 | .63 |
| Prenatal alcohol exposure, average | 0.14 | 0.43 | .75 | 0.73a | 0.42 | .08 | |||
| Prenatal cigarette exposure, average | −0.30 | 0.42 | .48 | −0.21 | 0.38 | .59 | |||
| Prenatal marijuana exposure, 3rd trimester | 1.81 | 0.74 | .015 | ||||||
| Total HOME score | −0.12 | 0.06 | .049 | ||||||
| Parental attachmentb | −1.45 | 0.68 | .03 | ||||||
| Parental monitoringb | −2.55 | 0.75 | .008 | ||||||
| Family conflictb | 0.68 | 0.19 | .0005 | 0.56 | 0.16 | .0004 | |||
| Violence exposureb | 2.36 | 0.43 | < .0001 | 1.83 | 0.39 | < .0001 | 0.67 | 0.36 | .06 |
Note. The outcome is the YSR. Blank spaces indicate that the variable did not meet the criteria (eg. not significant at the bivariate level) and therefore not included in the model.
prenatal alcohol exposure during the 2nd trimester
Assessed at 12 year
Figure 1.
Externalizing behavior (YSR) by level of PCE at 12 and 15 years with significant mean difference between the NCE group and the heavier PCE group at both 12 and 15 year (p’s<.04). The mean scores were adjusted for covariates listed in Table 3. Significant time effect for NCE (p=.0003) and Heavier PCE (p=.003).
Girls reported an increase in externalizing and internalizing behavior problems from 12 to 15 years and more attention problems than boys at both assessments. African American youth reported fewer externalizing behaviors. Greater maternal psychological distress at the child’s birth was associated with more internalizing behavior problems. Better HOME scores and parental monitoring were related to fewer externalizing behaviors, while better parental attachment was associated with fewer internalizing behavior problems. Greater family conflict was related to more externalizing behavior and attention problems. Greater violence exposure was related to more externalizing and internalizing behavior problems. Blood lead level was not associated with any behavioral outcome. Self-reported substance use between the ages of 9–12 years was not related to any outcome.
Effects Adoptive/Foster Care Placement
PCE adolescents in adoptive/foster care differed from those in biological/relative care in that they lived in better caregiving environments and their caregivers had better vocabulary and higher educational attainment, and reported lower alcohol and tobacco use (Table 4). PCE adolescents in adoptive/foster care had lower blood lead levels than NCE adolescents. They also experienced 2.25 (SD=2.11) placement changes on average by age 12 compared to 1.02 (SD=1.19) in PCE adolescents in biological/relative care and 0.38 (SD=0.86) in NCE adolescents. Of those 44 adolescents with PCE in adoptive/foster care at age 12, 48% (n=21) had only one placement change and 27% (n=12) had two, indicating three quarters of them had been in relatively stable living arrangements. No group difference in placement change between ages 12 and 15 was found.
Table 4.
Comparisons of Key Characteristics and Adolescent Behavioral Outcomes by Cocaine Status and Placement at Age 12
| PCE | NCE | F/χ2 | p | Pair-wise difference | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Group 1: Biological/relative (n=145) | Group 2: non-kinship adoptive/foster (n=44) | Group 3 (n=182) | ||||
|
| ||||||
| M (SD) | M (SD) | M (SD) | ||||
| PCE, units per week | 20.97 (36.08) | 27.75 (43.35) | --- | 1.45 | .23 | |
| WISC-IV Full Scale IQ at age 11 | 84.92 (11.94) | 83.98 (11.39) | 86.41 (14.70) | 0.85 | .43 | |
| Key covariates | ||||||
| Birth maternal age | 29.43 (5.05) | 30.39 (4.69) | 25.47 (4.73) | 35.11 | < .0001 | 3≠1,2 |
| Biological mother’s GSI | 0.82 (0.77) | 0.86 (0.66) | 0.50 (0.53) | 13.45 | < .0001 | 3≠1,2 |
| Prenatal alcohol exposure, average | 8.77 (15.01) | 13.32 (24.03) | 1.37 (4.60) | 57.09 | < .0001 | 3≠1,2 |
| Prenatal cigarette exposure, average, | 10.82 (10.42) | 14.28 (13.29) | 3.90 (7.18) | 58.22 | < .0001 | 3≠1,2 |
| Prenatal marijuana exposure, 3rd trimester | 0.84 (3.59) | 1.42 (4.55) | 0.09 (0.76) | 7.29 | .0008 | 3≠1,2 |
| HOME scorea | 46.93 (6.90) | 50.86 (6.32) | 49.04 (6.21) | 7.66 | .0005 | 1≠2,3 |
| Caregiver PPVT-III standard scorea | 76.47 (13.36) | 90.66 (14.26) | 79.57 (15.68) | 12.70 | < .0001 | 2≠1,3 |
| Caregiver Educationa | 11.72 (2.05) | 13.51 (2.43) | 12.80 (1.93) | 17.19 | < .0001 | 1≠2,3 |
| Caregiver alcohol, dose per weeka | 1.89 (5.31) | 0.36 (1.27) | 1.73 (5.50) | 3.44 | .03 | 1≠2 |
| Caregiver tobacco, cigarettes per daya | 6.60 (7.90) | 1.00 (3.62) | 3.77 (6.66) | 20.20 | < .0001 | 1≠2, 1≠3, 2≠3 |
| Parental attachmenta | 2.09 (0.66) | 2.12 (0.75) | 2.27 (0.61) | 3.36 | .04 | 1≠3 |
| Parental monitoringa | 2.46 (0.61) | 2.29 (0.74) | 2.48 (0.59) | 1.30 | .27 | |
| Family conflicta | 3.18 (2.54) | 3.36 (2.61) | 2.60 (2.37) | 2.71 | .07 | |
| Violence exposurea | 0.66 (0.76) | 0.50 (0.78) | 0.57 (0.80) | 1.36 | .26 | |
| Lead exposureb | 7.35 (4.21) | 5.42 (3.41) | 8.02 (4.62) | 5.63 | .004 | 2≠1,3 |
| Elevated lead (≥ 10 μg/dL)b, n (%) | 25 (20.83) | 1 (3.70) | 37 (26.06) | 6.76 | .03 | 2≠3 |
| Number of placement changes up to 12 year | 1.02 (1.19) | 2.25 (2.11) | 0.38 (0.86) | 45.24 | < .0001 | 1≠2, 1≠3, 2≠3 |
| Range (5%tile – 95%tile) | 0–6 (0–3) | 1–11 (1–7) | 0–4 (0–2) | |||
| Placement change between ages 12 to 15, n (%) | 27 (18.62) | 9 (20.45) | 26 (14.29) | 1.59 | .45 | |
| Adolescent outcomes (YSR), adjusted mean (SE)c | ||||||
| Externalizing behavior | 6.24 | .002 | ||||
| 12 year | 48.99 (0.82) | 54.54 (1.65) | 47.22 (0.74) | 2≠1,3 | ||
| 15 year | 52.13 (0.92) | 54.72 (1.76) | 50.05 (0.83) | 2≠3 | ||
| Attention problems | 4.75 | .009 | ||||
| 12 year | 55.51 (0.67) | 60.16 (1.34) | 56.31 (0.60) | 2≠1,3 | ||
| 15 year | 58.83 (0.76) | 61.31 (1.43) | 57.23 (0.67) | 2≠3 | ||
Assessed at 12 year.
Sub-sample of 120 PCE biological/relative, 27 PCE adoptive/foster, and 142 NCE.
Adjusted for the same covariates of the model from Table 3.
PCE adolescents in adoptive/foster care reported more externalizing and attention problems than NCE adolescents at both 12 and 15 years, while no significant difference was found between PCE adolescents in biological/relative care and NCE adolescents. The elevated scores on attention problems even in NCE adolescents suggest a global effect of socioeconomic stressors pervasive in this study sample.
Discussion
PCE adolescents reported more externalizing behavior at 12 and 15 years and more attention problems at 15 years, which may reflect long-lasting PCE-related impairments. Additionally, a dose-response relationship was present, with heavier PCE related to more externalizing behavior problems, consistent with the neurobehavioral teratology model. More attention problems also were reported among PCE adolescents compared to their NCE counterparts at age 15, suggesting that some of the effects of PCE on the developing central nervous system may become apparent only with increased developmental challenges and demands of adolescence. Our observed PCE effect sizes, 2.54 points for externalizing and 2.05 points for attention problems, are quite similar to the effect sizes reported by Bada et al. (2007),1 despite different informants (caregiver) and assessed ages (ages 3, 5, and 7). Differential gender effects of PCE on externalizing behavior were not found in this study however. Differences in informant, developmental stage, and confounders affecting the outcome may account for the discrepancy.
PCE adolescents in adoptive/foster care reported more externalizing behavior and attention problems than PCE adolescents in biological/relative care at age 12, consistent with our previous findings based on caregiver report.2,7,8 These findings on behavioral outcomes in relation to adoptive/foster care placement contrast with our previous findings, in which better cognitive and language development were shown for PCE children in adoptive/foster care compared to those in biological/relative care.22,23 Adoptive/foster care placement did not have the same protective impact on the behavioral domain as was shown on cognitive and language outcomes.38 There was no difference in externalizing and attention problems between PCE adolescents in biological/relative care and NCE adolescents at both 12 and 15 years.
Independent of PCE and other biological risk factors, perceived parent-adolescent relationships and the quality of the family/home environment also additively contributed to adolescent behavioral adjustment, underscoring the importance of family environment in shaping behavioral adjustment in adolescence. Parent-adolescent relationships not only directly impact adolescent behavioral adjustment but also moderate and mediate the impact of the stress within and beyond the family (e.g., peer influence, school hassles).39 Our findings demonstrate that improving caregiver functioning and parent-child relationships are likely to be effective in mitigating behavioral problems among cocaine and poly-drug exposed adolescents.
The present study focused on examining direct effects of PCE, suggested by preclinical and human studies demonstrating PCE-related brain alteration, rather than considering indirect effects through environmental/sociological mediators. Parenting related risk/protective factors (parental attachment and family conflict) might operate as mediators linking PCE effects with behavioral problems. Also, individual characteristics such as difficult temperament, impulsivity, and disinhibition may be early markers of externalizing behaviors. Future studies examining the role of these precursors linking PCE and externalizing behavior will expand the understanding of PCE effects on the transactional developmental pathways of behavioral adjustment.
Several limitations in our study should be noted. Without a comparable number of NCE adolescents living in non-kinship adoptive/foster care, our study is limited in separating the effect of PCE from the effects of placement changes among PCE adolescents. However, the significant proportion of PCE adolescents in non-kinship care allowed some separation of PCE effects from the effects of postnatal negative environmental factors (e.g., quality of home environments, lead exposure) often confounded with PCE. There is a potential for recall bias in the prenatal drug use assessment as we used retrospective data collected by asking mothers to recall frequency and amount of drug use for the month prior to and for each trimester of pregnancy. Also, relying on adolescents’ self-report might be subject to adolescents’ ability to accurately self-assess their behaviors. Our limited data on fathers is another limitation as paternal substance use and psychopathology are associated with adolescent adjustment. Finally, the sample composition and sample screening criteria limit the generalizability of the findings to low income, urban, predominantly African American adolescents.
This study has multiple strengths including the prospective design, assessing a large number of adolescents and their caregivers since birth with a high follow-up rate (92%). PCE was determined through both biological and clinical means, enhancing the reliability of the classification.40 A comprehensive list of covariates and confounders were evaluated and controlled statistically when necessary. By examining non-kinship adoptive/foster care placement, our study assessed the effects of both protective (better quality home environment) and/or negative (placement changes) environmental factors related to non-kinship placement among adolescents with PCE.
The present study extends previous studies of PCE by demonstrating that behavioral problems, especially externalizing behaviors noted in childhood and preadolescence, continue into adolescence. Our study indicates that PCE is a risk factor for poor behavioral adjustment in adolescence.
Implications and Contribution.
Adolescents prenatally exposed to cocaine reported more problems in attention and externalizing behaviors than non-cocaine exposed adolescents, controlling for confounding drug and environmental factors. Findings from this prospective, longitudinal sample are consistent with neuroimaging studies suggesting that PCE leads to alterations in behavioral domains of the prefrontal cortex.
Acknowledgments
This research was supported by a National Institute on Drug Abuse Grant R01-07957. Portions of this paper were presented at the 75th Annual Meeting of College on Problems of Drug Dependence (CPDD) in June 2013. Thanks are extended to Adelaide Lang, PhD for reviewing early drafts, and Laurie Ellison, LISW, and Paul Weishampel, MA for research assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bada HS, Das A, Bauer CR, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119(2):e348–59. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 2.Minnes S, Singer LT, Kirchner HL, et al. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicol Teratol. 2010;32(4):443–451. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bada HS, Bann CM, Bauer CR, et al. Preadolescent behavior problems after prenatal cocaine exposure: Relationship between teacher and caretaker ratings (maternal lifestyle study) Neurotoxicol Teratol. 2011;33(1):78–87. doi: 10.1016/j.ntt.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DS, Marini VA, Berzenski SR, Carmody DP, Lewis M. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J Pediatr Psychol. 2013;38(3):296–308. doi: 10.1093/jpepsy/jss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorhees CV. Concepts in teratology and developmental toxicology derived from animal research. Ann N Y Acad Sci. 1989;562:31–41. doi: 10.1111/j.1749-6632.1989.tb21005.x. [DOI] [PubMed] [Google Scholar]
- 7.Linares TJ, Singer LT, Kirchner HL, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31(1):85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin AA, Minnes S, Singer LT, et al. Caregiver and self-report of mental health symptoms in 9-year old children with prenatal cocaine exposure. Neurotoxicol Teratol. 2011;33(5):582–591. doi: 10.1016/j.ntt.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson GA, Goldschmidt L, Larkby C, Day NL. Effects of prenatal cocaine exposure on child behavior and growth at 10years of age. Neurotoxicol Teratol. 2013;40C:1–8. doi: 10.1016/j.ntt.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerteis J, Chartrand M, Martin B, et al. Are there effects of intrauterine cocaine exposure on delinquency during early adolescence? A preliminary report. J Dev Behav Pediatr. 2011;32(5):393–401. doi: 10.1097/DBP.0b013e318218d9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagasse LL, Hammond J, Liu J, et al. Violence and delinquency, early onset drug use, and psychopathology in drug-exposed youth at 11 years. Ann N Y Acad Sci. 2006;1094:313–318. doi: 10.1196/annals.1376.041. [DOI] [PubMed] [Google Scholar]
- 12.Delaney-Black V, Covington C, Templin T, et al. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106(4):782–791. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- 13.Carmody DP, Bennett DS, Lewis M. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol Teratol. 2011;33(1):61–68. doi: 10.1016/j.ntt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sood BG, Nordstrom Bailey B, Covington C, et al. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol. 2005;27(2):191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Eccles JS. School and family effects on the ontogeny of children’s interests, self-perceptions, and activity choices. Nebr Symp Motiv. 1992;40:145–208. [PubMed] [Google Scholar]
- 16.Bada HS, Bann CM, Whitaker TM, et al. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics. 2012;130(6):e1479–88. doi: 10.1542/peds.2011-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22(3):325–336. doi: 10.1016/S0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 18.Larkby CA, Goldschmidt L, Hanusa BH, Day NL. Prenatal alcohol exposure is associated with conduct disorder in adolescence: Findings from a birth cohort. J Am Acad Child Adolesc Psychiatry. 2011;50(3):262–271. doi: 10.1016/j.jaac.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: Testing genetic and environmental explanations of the association. Arch Gen Psychiatry. 2004;61(8):836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- 20.Bandstra ES, Morrow CE, Vogel AL, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24(3):297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 21.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275(5):363–369. doi: 10.1001/jama.275.5.363. [DOI] [PubMed] [Google Scholar]
- 22.Singer LT, Nelson S, Short E, et al. Prenatal cocaine exposure: Drug and environmental effects at 9 years. J Pediatr. 2008;153(1):105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis BA, Minnes S, Short EJ, et al. The effects of prenatal cocaine on language development at 10 years of age. Neurotoxicol Teratol. 2011;33(1):17–24. doi: 10.1016/j.ntt.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner TD, Behnke M, Eyler FD, Szabo NJ. Early adolescent cocaine use as determined by hair analysis in a prenatal cocaine exposure cohort. Neurotoxicol Teratol. 2011;33(1):88–99. doi: 10.1016/j.ntt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fosco GM, Stormshak EA, Dishion TJ, Winter CE. Family relationships and parental monitoring during middle school as predictors of early adolescent problem behavior. J Clin Child Adolesc Psychol. 2012;41(2):202–213. doi: 10.1080/15374416.2012.651989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank DA, Rose-Jacobs R, Crooks D, et al. Adolescent initiation of licit and illicit substance use: Impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol Teratol. 2011;33(1):100–109. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laird RD, Criss MM, Pettit GS, Dodge KA, Bates JE. Parents’ monitoring knowledge attenuates the link between antisocial friends and adolescent delinquent behavior. J Abnorm Child Psychol. 2008;36(3):299–310. doi: 10.1007/s10802-007-9178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. J Consult Clin Psychol. 2002;70(1):6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler adult intelligence scale-revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- 30.Hollingshead AB. Two factor index of social position. New Haven, CT: Yale University; 1957. [Google Scholar]
- 31.Dunn L, Dunn L. Peabody picture vocabulary test - revised. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- 32.Dunn L, Dunn L, Williams KT, Wang JJ, Booklets N. Peabody picture vocabulary test, (PPVT-III): Form IIA. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 33.Derogatis LR. The brief symptom inventory (BSI): Administration, scoring, and procedures manual—II. Towson, MD: Clinical Psychometric Research; 1992. [Google Scholar]
- 34.Wechsler D. WISC-IV: Administration and scoring manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 35.Ridenour TA, Clark DB, Cottler LB. The illustration-based Assessment of Liability and EXposure to Substance use and Antisocial behavior for children. Am J Drug Alcohol Abuse. 2009;35(4):242–252. doi: 10.1080/00952990902998715. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach TM, Rescorla LA. Manual for the ASEBA school age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 37.Caldwell BM, Bradley RH. HOME inventory early adolescent version. Little Rock, AR: University of Arkansas for Medical Sciences; 2003. [Google Scholar]
- 38.McNichol T, Tash C. Parental substance abuse and the development of children in family foster care. Child Welfare. 2001;80(2):239–256. [PubMed] [Google Scholar]
- 39.Laursen B, Collins WA. Parent-child relationships during adolescence. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology, Vol2: Contextual influences on adolescent development. 3. Hoboken, NJ: John Wiley & Sons Inc; 2009. pp. 3–42. [Google Scholar]
- 40.Arendt RE, Singer LT, Minnes S, Salvator A. Accuracy in detecting prenatal drug exposure. J Drug Iss. 1999;29(2):203–214. doi: 10.1016/S0163-6383(98)91224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]