Abstract
Allergic asthma is a chronic inflammatory airway disease arising from an aberrant immune response following exposure to environmental stimuli in genetically susceptible persons. The complement component 5 (C5)/C5a Receptor (C5aR/CD88) signaling pathway has been implicated in both experimental allergic asthma and human asthmatic disease. Targeting the C5a/C5aR signaling pathway in rodent models has been shown to either enhance or reduce allergic asthma consequences. Treatment with a recombinant humanized monoclonal antibody directed against C5 has shown unclear results in patients with asthma. The objective of this proof-of-concept animal study was to determine whether the low molecular weight C5aR peptidomimetic antagonist, PMX205, would reduce experimental allergic asthma consequences in mice. PMX205 or vehicle control was administered subcutaneously to Balb/c mice prior to and during standard ovalbumin (OVA) allergen sensitization and aerosolized challenge phases. PMX205 substantially reduced OVA-induced total cell (60%), neutrophil (66%) and eosinophil (65%) influx in lavage fluid sampling. There were also significant reductions in OVA-induced lavage fluid IL-13 protein and lung Th2 cytokine gene expression with PMX205 administration. PMX205 treatment also diminished OVA-induced lung parenchyma cellular infiltration. PMX205 administration did not reduce OVA-induced serum IgE levels or epithelial mucous/goblet cell generation. There was no evidence of toxicity observed with PMX205 treatment in saline or OVA-challenged animals. These data provide evidence that pharmacologic blockade of C5aR by a low molecular weight antagonist (PMX205) reduces airway inflammatory cell and cytokine responses in experimental allergic asthma, and suggests that PMX205 might represent a novel therapeutic agent for reducing asthmatic outcomes.
Keywords: allergy, asthma, complement, C5a receptor, therapy, animal, inflammation
Introduction
Allergic asthma is a chronic lung inflammatory disease thought to arise from an aberrant immune response following exposure to environmental stimuli in genetically susceptible persons.(1, 2) Symptoms of asthma include recurrent episodes of wheezing, coughing, chest tightness, and breathlessness with characteristic pathophysiologic changes including airway hyperresponsiveness and airway inflammation marked by influx of eosinophils, lymphocytes, and neutrophils in conjunction with goblet cell hyperplasia and submucosa thickening.(1) Although the mechanisms underlying the initiation, development, and maintenance of asthma is multifactorial, a dysregulated Th2-mediated adaptive immune response has been accepted to play a central role in the major pathophysiologic features of asthma.(1)
The complement system, a major component of the innate immune system, comprises a network of more than 30 proteins that act to protect the host by responding to danger signals and microbial insults (2, 3). Activation of complement occurs through three pathways including the classical, alternative, and lectin pathway leading to downstream proteolytic cleavage of complement factors converging at the level of C3 (3). Cleavage of C3 generates C3a and C3b that further result in the cleavage of C5a and C5b. Allergen-derived proteases can generate the anaphylatoxins, C3a and C5a, from C3 and C5, respectively (4). Diesel exhaust particles can activate complement through the alternative pathway and lead to C3 cleavage in human serum (3, 5). Levels of C3a and C5a following allergen challenge in asthmatics increase, and moreover, eosinophilic and neutrophilic influx correlates with C3a and C5a levels (6, 7). It has also been reported that aluminum hydroxide, which is the most common adjuvant utilized in human vaccines, activates complement and generates the anaphylatoxins C3a and C5a.(8) It has been shown that deficiencies in C3a or the receptor for C3a protect animals from the development of several features of allergic asthma, particularly during the effector phase of the allergic response (9). Prior investigations in rodent models targeting C5 or C5a receptor (C5aR/CD88) in allergic asthma are less consistent. Some studies show a reduction in early and late allergic asthma hyperresponsiveness and inflammatory outcomes (10-12); whereas, others show that blockade of C5aR through use of an anti-C5aR monoclonal antibody is protective against allergic sensitization, but worsens airway inflammation in an established inflammatory environment (13, 14).
In human therapeutics to target C5, eculizumabeclizumab, which is a recombinant humanized monoclonal antibody directed against C5, is effective in treating paroxysmal nocturnal hemoglobinuria (15) and is also utilized for atypical hemolytic-uremic syndrome(16). It has been suggested that eculizumab may attenuate allergen-induced asthma responses in humans, but the clinical benefit with eculizumab for reducing allergic asthma consequences in humans remains unclear (17). Other novel strategies in development for human therapeutic approaches are low molecular weight peptidomimetic antagonists targeting C5aR (18). PMX205 is one low molecular weight C5aR antagonist that has shown promise in rodent models to significantly reduce inflammatory consequences in inflammatory bowel disease (19, 20), Huntington's disease (21), and Alzheimer's disease (22). The aim of this proof-of-concept animal model study was to assess a potential therapeutic approach with PMX205 in asthma by first ascertaining whether PMX205 treatment would reduce murine allergic asthma.
Methods
Animals
BALB/c mice were originally obtained from Jackson Laboratory (Bar Harbor, ME) and were subsequently bred and maintained at the University of Nebraska Medical Center's specific pathogen-free animal facility. Mice were allowed food and water ad libitum and female and male animals were used experimentally at 9-17 weeks of age. All animal procedures were used in accordance with the National Institutes of Health guidelines, and all studies were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center.
C5a receptor antagonist PMX205 preparation
PMX205 was synthesized and prepared by a two-step process in which the linear hexapeptide (hydrocinnamate-ornithine-proline-D-cyclohexylalanine-tryptophan-arginine or HCA-O-P-cha-W-R) was generated by standard solid phase methods followed by a solution-phase intramolecular amide cyclization (ornithine side chain-to-C-terminus) to generate cyclo-HCA-[O-P-cha-W-R] or PMX205. Previously, we described these methods of synthesis, purification, and characterization (23). For all experimental studies, PMX205 was diluted in 1% ethanol. Vehicle control contained equivalent concentration of ethanol.
Murine Model
Mice were sensitized and challenged to ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO) as previously described with modifications (24). On days 1 and 14, 20 μg of OVA mixed with 2 mg of Al(OH)3 (Thermo Fischer Scientific, Waltham, MA) in 0.2 ml saline and was administered intraperitoneally (OVA sensitization). Non-sensitized animals received normal saline injections. On days 21 through 24, mice were challenged with nebulized 1% OVA in saline or saline alone (OVA and saline challenged groups, respectively) for 20 minutes. Animals were administered PMX205 subcutaneously (50μg/100μl/mouse) or vehicle control (100μl/mouse) at 1 and 24 hours prior to OVA or saline sensitization and challenge (Figure 1). Separate dose and frequency studies with PMX205 were conducted to optimize experimental protocol (data not shown).
Figure 1. Protocol for the administration of PMX205 in a murine ovalbumin (OVA) allergic asthma model.
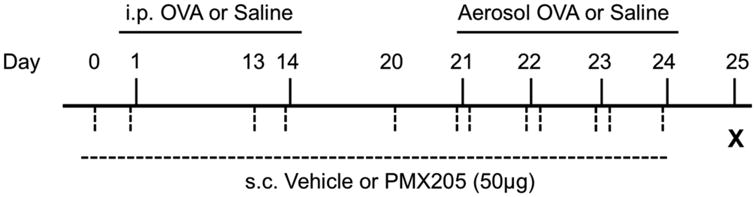
Balb/c mice were either OVA or saline control sensitized on day 1 and 14 followed by OVA or saline aerosolized challenged on days 21-24. PMX205 (2 mg/kg or approximately 50 μg) or vehicle control was administered 24 and 1 hour prior to sensitization and aerosol challenge. i.p., intraperitoneal; s.c., subcutaneous.
Bronchoalveolar lavage fluid cell and cytokine analysis
Bronchoalveolar lavage fluid (BALF) was collected using 3 × 1 ml PBS, and the total cell number recovered from pooled lavages was enumerated and differential cell counts determined using cytospin-prepared slides (Cytopro Cytocentrifuge, Wescor Inc, Logan, UT) stained with DiffQuick (Siemens, Newark, DE). From the cell-free supernatant of the first lavage, interleukin (IL)-4, IL-5 (R&D Systems, Minneapolis, MN), and IL-13 (Invitrogen, Carlsbad, CA) were quantitated by ELISA kits with sensitivities of 7.8, 15.6, and 39 pg/mL, respectively.
Lung tissue for gene expression
Following removal of BALF, lungs were perfused to remove blood from the pulmonary vasculature and whole lung tissues were harvested and stored in RNA later (Applied Biosystems, Foster City, CA) until RNA extraction could be performed by using TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA concentration and purity was determined by NanoDrop spectrophotometer, and samples had A260/A280 ratio of 1.8–2.0. cDNA was synthesized as previously described (24). Real-time PCR reactions were prepared in triplicate using SsoFast Probes Supermix with ROX (Bio-Rad, Hercules, CA) and primers and probed for IL-4 (Applied Biosystems; Mm00445259_m1), IL-5 (Mm00439646_m1), IL-13 (Mm00434204_m1), and C5a (Mm00439275_m1). Ribosomal RNA was used as an endogenous control. PCR was performed using an ABI PRISM 7700 sequence detection system (Applied Biosystems). Threshold values were normalized to the vehicle+saline to represent a normalized gene expression ratio.
Total IgE and OVA-specific IgE serum levels
Blood samples were collected, and the serum was isolated and stored at −80°C until assayed. A murine IgE (BD Biosciences, San Diego, CA) and an OVA-specific IgE ELISA kit (Biolegend, Inc.,San Diego, CA) were used to assay for total IgE and OVA-specific IgE concentrations according to manufacturer's instructions.
Histopathology
Whole lungs were excised and inflated to 20 cm H2O pressure with 10% formalin (Sigma, St. Louis, MO). Subsequently, by routine histologic processing, the fixed lung tissue was embedded in paraffin. Sections (4-5 μM) were cut and either stained with hematoxylin and eosin (H&E), or utilized later for immunohistochemistry (IHC) for T and B cell infiltrates and periodic acid Schiff (PAS) staining procedure to assess mucous/goblet cells. T and B cell infiltrates were determined by IHC as previously described.(25) Briefly, slides were deparrafinized and antigen unmasking was performed using the heat-induced epitope retrieval method and solution (DIVA Decloaker solution; Biocare Medical, Concord, CA). Endogenous peroxidase activity was quenched with Peroxo-block (Invitrogen, Carlsbad, CA). After washing, slides were blocked before application of primary antibodies: rabbit anti-CD3 (Pan-T cell marker, dilution 1:300; Dako, Carpinteria, CA) and rat anti-CD45R/B220 (Pan-B cell marker, clone RA3-6B2, dilution 1:200; BD Pharmingen, San Jose, CA) for 1 hour in humidity chamber. Next, slides were incubated with biotinylated goat-anti-rabbit IgG (dilution 1:500) or biotinylated anti-rat IgG mouse-absorbed (dilution 1:500) secondary antibody. Primary antibody binding was then detected using the avidin-biotin-immunoperoxidase method (Vectastain Elite ABC ready-to-use kit, Vector). Chromogen substract (IMMPACT DAB, Vector) developer was used and slides were counterstained with 1% Meyer's hematoxylin. For the PAS stain, the slides were deparaffinized and hydrated before oxidizing in 0.5% periodic acid solution (EK Industries, Joliet, IL) for 5 minutes. After a rinse, the slides were placed in Schiff reagent (Poy Scientific R&D Corp, Bay Shore, NY) for 15 minutes. The slides were counterstained in hematoxylin (Fisher Scientific, Fair Lawn, NJ) after being washed in water for 20 minutes. After the counterstain, all slides were washed for 5 minutes, and then dehydrated and coverslip applied using cytoseal XYL (Thermo Scientific, Waltham, MA).
Noninvasive Airway Hyperresponsiveness Measurement
To test whether PMX205 would affect OVA-induced airway hyperresponsiveness, whole body barometric plethysmography system was utilized (Max II; Buxco Electronics, Troy, NY) as previously described.(26) This system operates without the use of anesthesia or restraint to allow real-time recordings of airway responsiveness via a dimensionless parameter known as enhanced pause (Penh) to estimate the total pulmonary resistance. Briefly, following the saline baseline challenge, mice were exposed to methacholine in cumulative doses (1.5–24 mg/ml).
Statistical Methods
Data are presented as the mean and standard error of mean (SEM). Statistical significance was assessed by one-way analysis of variance (ANOVA) and two-tailed, Mann-Whitney test, where appropriate. GraphPad (version 5.02, La Jolla, CA) software was used.
Results
Administration of the C5aR antagonist PMX205 reduces OVA allergen-induced inflammatory cell influx
PMX205 has been previously shown to reduce inflammation in disorders in which C5a generated from complement activation is implicated including inflammatory bowel disease and neurologic disorders in rodent models (19-21, 23). C5a generation also occurs in murine (27) and human asthma (7), and we demonstrated that C5a gene expression occurs in the murine model of allergic asthma utilized in these studies. Specifically, mean ± SEM of C5a mRNA expression normalized to ribosomal RNA was saline control animals: 30.1 ± 5.1 as compared to OVA-challenged animals: 81.0 ± 17.0 (p=0.009, N =4 mice/group). Utilizing a standard murine model of allergic asthma,(24) Balb/c mice were s.c. treated with PMX205 or vehicle control prior to and during the allergen (OVA) sensitization and challenge phase (Figure 1). Consistent with other studies using PMX205 (23), there was no weight loss or distress noted in any of these animals (data not shown). We found that PMX205 significantly reduced OVA allergen-induced total cell (p<0.01), neutrophil (p<0.01), and eosinophil (p<0.05) influx as compared to vehicle control animals (Figure 2).
Figure 2. PMX205 administration reduces ovalbumin (OVA) allergen-induced inflammatory cell influx.
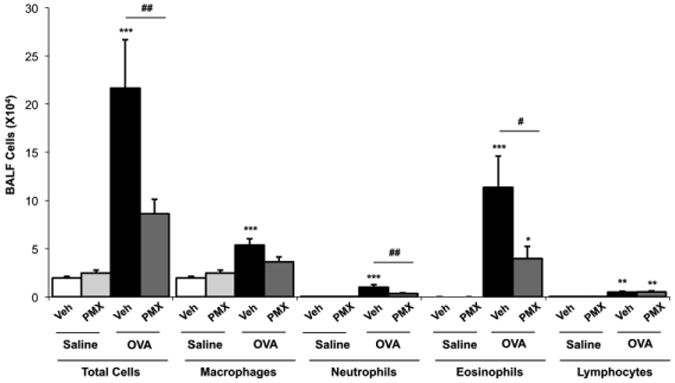
Bronchoalveolar lavage fluid (BALF) was collected 24 hours following final OVA or saline aerosol exposure and total cells and cell differential are shown (mean ± SEM, N=7-10 mice/group from 2 separate studies). Statistical significance determined by ANOVA with Tukey's post- test and denoted by asterisks (*p<0.05, **p<0.01, ***p<0.001) as compared to saline control; hatchmarks (#p<0.05, ##p<0.01) is vehicle-OVA compared to PMX205-OVA. Veh, vehicle; PMX, PMX205.
PMX205 reduces OVA allergen-induced Th2 inflammatory cytokines
In these experiments, IL-4, IL-5, and IL-13 protein levels were quantitated from the BALF from first lavage. OVA treated animals demonstrated increased IL-4, IL-5, and IL-13 as compared to saline control mice (Figure 3A). PMX205 significantly reduced OVA allergen-induced IL-13 levels (p<0.05, Figure 3A) compared to vehicle + OVA treatment. Although there was a slight decrease in OVA-induced IL-5 levels with PMX205 administration as compared to vehicle, this did not reach the level of statistical significance (p>0.05). PMX205 did not reduce OVA-induced IL-4 production. Following BALF removal, lungs were perfused and investigated for Th2 cytokine gene expression as described in the methods section. OVA-treated animals demonstrated increase gene expression of lung tissue IL-4, IL-5, and IL-13 as compared to saline control mice (Figure 3B) as determined by real-time PCR. Whereas the expression of lung tissue IL-4, IL-5, and IL-13 in PMX205 treated-OVA allergen exposed animals increased, this difference did not reach statistical difference as compared to saline-treated mice (p>0.05, Figure 3B). These studies suggest that PMX205 might reduce allergen-induced Th2 cytokine production.
Figure 3. PMX205 reduces ovalbumin (OVA) allergen-induced inflammatory cytokines.

Bronchoalveolar lavage fluid (BALF; panel A) and lung tissues (panel B) were collected 24 hours following final OVA or saline aerosol challenge (N=7-10 mice/group from 2 separate studies). A, Mean (± SEM) BALF protein cytokine levels for interleukin (IL)-4, IL-5, and IL-13 are shown. Gene expression (mRNA) for IL-4, IL-5, and IL-13 was normalized to endogenous ribosomal RNA for each animal and then normalized to vehicle+saline to represent normalized ratio are shown (mean ± SEM). Statistical significance determined by ANOVA with Tukey's post- test and Mann-Whitney and denoted by asterisks (*p<0.05, **p<0.01, ***p<0.001) as compared to saline control; hatchmarks (#p<0.05) is Vehicle-OVA compared to PMX205-OVA. Veh, vehicle; PMX, PMX205
PMX205 does not alter OVA-induced total IgE levels or OVA-specific IgE serum levels
Serum for IgE and OVA-specific IgE levels were collected to determine whether PMX205 might reduce systemic allergic IgE sensitization. Whereas OVA allergen treatment induced increased levels of serum total IgE as compared to saline control animals, there was no reduction in OVA-induced total IgE with PMX205 administration (Figure 4). OVA allergen treatment also increased levels of OVA-specific IgE levels, and there was a non-significant (p=0.15) decrease in OVA-specific IgE levels with PMX205 administration (Figure 4).
Figure 4. PMX205 does not alter ovalbumin (OVA)-induced serum IgE or OVA-specific IgE levels.

Blood for serum was collected 24 hours following final OVA or saline aerosolized challenge and mean (±SEM) total IgE and OVA-specific IgE levels are shown (N=7-10 mice/group from 2 separate experimental studies). Statistical significance denoted by asterisks (***p<0.001) as compared to respective saline control. Veh, vehicle; PMX, PMX205.
The effect of PMX205 on OVA-induced lung inflammatory histopathology
The effect of PMX205 administration on the lung parenchyma was ascertained following saline and OVA exposure from formalin-fixed, paraffin-embedded whole lungs that were sectioned and stained with H&E (Figure 5A-D). PMX205 alone did not result in adverse lung histopathology (Figure 5B). The lung histology was notable for increased inflammatory cells in the lung parenchyma following vehicle/OVA and PMX205/OVA exposure (Figure 5C-D). Microscopic review of the lung tissue demonstrated a modest reduction in the degree and distribution of the lung inflammation in the PMX205/OVA treated animals as compared to vehicle/OVA treatment. To determine the composition of these mononuclear cellular infiltrates observed with OVA treatment, lung section slides were analyzed for CD3 (pan-T cell marker) and CD45R/B220 (pan-B cell murine marker) by immunohistochemistry. As shown in Figure 6, mononuclear cellular infiltrates were admixture of T and B lymphocytes, and there was no discernible difference in the admixture of T and B lymphocytes with PMX205 treatment.
Figure 5. Histology of the effect of PMX205 with ovalbumin (OVA)-induced lung inflammation.

A representative 4- to 5-μm-thick lung section, H&E-stained, from vehicle/saline (A), PMX205/saline (B), vehicle/OVA (C), and PMX205/OVA (D) treated mice shown at 10× magnification. All lung specimens were inflated to 20 cm H2O during fixation to avoid atelectasis artifact. Line scale represents 100μm.
Figure 6. Immunohistochemistry staining of mononuclear cellular infiltrates in vehicle control and PMX205 OVA-treated mice.

A representative 4- to 5-μm-thick lung section stained with anti-CD3 antibody for T lymphocytes and anti-CD45R/B220 antibody for B lymphocytes shown at 20× magnification. Line scale represents 100μm.
To determine whether PMX205 would affect OVA-induced mucous/goblet cells, PAS staining was conducted and representative image shown in for all treatment groups in Figure 6A-D. OVA treatment induced positive PAS staining that was not observed with saline treatment. However, there was no notable difference in PAS staining between vehicle/OVA and PMX205/OVA animal treatment groups.
Noninvasive Airway Hyperresponsiveness Measurement
To test whether PMX205 would affect OVA-induced airway hyperresponsiveness, whole body barometric plethysmography was conducted. PMX205 treatment did not reduce OVA-induced airway hyperresponsiveness (data not shown).
Discussion
We report that treatment with the low molecular weight C5aR antagonist PMX205 during OVA allergen sensitization and challenge resulted in reduction in eosinophil and neutrophil influx and pro-inflammatory cytokine production. Whereas PMX205 did not modulate serum IgE levels induced by OVA allergen treatment, there was evidence that PMX205 administration reduced OVA-induced lung parenchymal cellular infiltration. However, PMX205 did not reduce OVA-induced mucous/goblet cell generation. PMX205 alone did not result in any detectable adverse lung consequences. Collectively, these animal studies suggest that blockading the C5a receptor by low molecular peptide antagonists might have a potential immunomodulatory role in allergic asthma therapy.
Under asthmatic conditions, it is well-recognized that the anaphylatoxins, C3a and C5a, are produced in the lung environment and are associated with proinflammatory events (7, 9, 10, 13, 14, 28) Thus, interest in targeting complement pathways such as the C5a/C5aR signaling pathway for potential therapeutic options in asthma have been considered, but the benefits of this strategy are unclear. Prior work by others have shown a potential dual role for the C5a/C5aR receptor pathway in animals whereby allergen-induced airway inflammation increased in C5-deficient mice and also worsened when C5aR signaling was ablated prior to initial allergen sensitization (13, 29, 30). In contrast, pharmacologic blockade of C5aR signaling by utilizing an anti-C5aR monoclonal antibody in already sensitized mice was protective against allergen-induced airway inflammation (13). In humans, we are aware of only one clinical trial utilizing the humanized antibody to C5, eculizumab, in allergic asthma subjects (17) The results of this study were summarized in a review article that reported a significant period effect with a single infusion of eculizumab 24 hours before allergen challenge. There was inhibition of the late asthmatic response in subjects who received placebo treatment first, but no effect in the group of subjects receiving eculizumab in the first treatment period of the crossover design study (17). In comparison to these prior studies, we found that the low molecular weight C5aR antagonist (PMX205) reduced allergen-induced airway inflammation when administered prior to and during allergen sensitization and allergen challenge phases in mice. We found a reduction in OVA-induced neutrophil and eosinophil influx (Figure 2), and evidence to support a role for PMX205 in reducing OVA-induced Th2 cytokine production (Figure 3). In studies not shown, we found no effect (no increase or decrease) when PMX205 was given after allergen sensitization and only during allergen challenge (aerosolized) phase. Future studies could investigate the use of PMX205 only during allergen sensitization phase.
It is possible that these differing observations with the effects of blocking C5aR and allergic asthma might be related to the drug utilized. Although protein inhibitors/anti-monoclonal antibody drugs are largely efficacious, they do have disadvantages owing to their tissue penetration and potential immunogenicity as well as productivity cost (18, 31). The low molecular weight compounds do not have these disadvantages, but instead, concern has been raised regarding low potency, short half-life, and potential toxicity (1618) Our studies found no evidence for toxicity with utilizing PMX205 alone or in the experimental OVA-allergen asthma model. A beneficial role for PMX205 was found for reducing allergen-induced airway inflammation; however, PMX205 did not completely abrogate these OVA-induced inflammatory consequences, and there was no effect observed for its ability to reduce systemic allergy sensitization, mucous/goblet cell production, or airway hyperresponsiveness. There are several potential explanations for these observations. The concentration and frequency of the drug utilized may be one explanation. The concentration of PMX205 utilized (∼2 mg/kg) for the data shown was approximately double the concentration utilized in other studies that demonstrated efficacy of PMX205 including murine model studies of Alzheimer's disease whereby animals were dosed at 1 mg/kg twice weekly (23). Of note, we found no role for PMX205 at lower concentrations or less frequent dosing schedules (data not shown). Because no toxicity was observed, future studies might consider further increasing the frequency of the dosing schedule.
The finding that PMX205 exerted its greatest effect on reducing airway inflammatory cell influx and cytokine production and did not affect systemic IgE sensitization or mucous/goblet cell generation is most likely explained by the known biologic function of the C5/C5aR signaling pathway. C5a is a strong chemoattractant for neutrophils, but it is also important in recruiting eosinophils, monocytes, and T lymphocytes (28). In addition, work centered on neutrophils has demonstrated that C5a/C5aR signaling appears to provide survival or anti-apoptotic signals (28). Moreover, anti-C5a/C5aR signaling strategies to reduce neutrophil-associated inflammation have been attractive for therapeutic options for sepsis, systemic inflammatory responses, and acute lung injury process (28). Thus, it may be warranted to focus future studies with PMX205 on neutrophilic-associated asthmatic phenotypes. Alternatively, future investigations could consider utilizing PMX205 as an immunomodulator in combination with other standard drug therapies such as corticosteroid therapy because systemic or inhaled corticosteroid therapy does not ameliorate the neutrophil response associated with asthmatic inflammation. It is also warranted to highlight that this therapy did not target C3a, which is also an important player in asthma, and moreover, there has been described a second C5a receptor, C5L2. Whereas the biological/pathophysiological and function of C5L2 remains unclear, (32) there is evidence for its role in experimental allergic asthma through its ability to affect Th1/Th17 cell development (33). PMX205 does not block C5a binding to C5L2.
In summary, in this proof-of-concept animal study, we demonstrated that administration of the low molecular weight C5aR antagonist PMX205 during OVA allergen sensitization and challenge phase resulted in reduction in airway eosinophil and neutrophil influx and inflammatory cytokine production. The findings were not global because PMX205 treatment did not reduce systemic IgE sensitization or reduce epithelial cell mucous/goblet cell generation. There was also no evidence of toxicity with PMX205 in saline or OVA-treated animals. We suggest that blocking C5a/C5aR signaling with the C5aR antagonist PMX205 might represent a novel therapeutic approach strategy to reduce asthma disease burden.
Figure 7. PMX205 does not alter ovalbumin (OVA)-induced mucous/goblet cells.

A representative periodic acid-Schiff (PAS) stained lung section from vehicle/saline (A), PMX205/saline (B), vehicle/OVA (C), and PMX205/OVA (D) treated mice shown at 40× magnification. Line scale represents 100μm.
Highlights.
The C5aR antagonist PMX205 is a potential novel agent for reducing asthma outcomes.
PMX205 reduced OVA-induced airway eosinophil and neutrophil influx.
PMX205 reduced inflammatory Th2 cytokine production and histopathology.
There was no evidence of toxicity observed with PMX205 administration.
Acknowledgments
The authors wish to thank Angela Gleason for technical assistance, and David Wert, Facility Supervisor, Pathology and Microbiology Department in the UNMC Tissue Science Facility for assistance with lung tissue processing, sectioning, H&E staining, and PAS staining. We also thank Lisa Chudomelka for manuscript preparation assistance.
Declaration of all sources of funding for the research reported in the manuscript: Study supported by grants from the National Institute of Allergy and Infectious Diseases (R41: #AI094710 to SDS, SMW, JAP).
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- C5
Complement component 5
- C5aR
C5a Receptor
- HPLC
High-performance liquid chromatography
- IL
Interleukin
- OVA
Ovalbumin
- PAS
Periodic acid Schiff
- PMX205
C5a Receptor Antagonist
- sc
subcutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kauffmann F, Demenais F. Gene-environment interactions in asthma and allergic diseases: Challenges and perspectives. J Allergy Clin Immunol. 2012 Dec;130(6):1229–40. doi: 10.1016/j.jaci.2012.10.038. quiz 1241-2. [DOI] [PubMed] [Google Scholar]
- 2.Schmudde I, Laumonnier Y, Kohl J. Anaphylatoxins coordinate innate and adaptive immune responses in allergic asthma. Semin Immunol. 2013 Feb;25(1):2–11. doi: 10.1016/j.smim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Kohl J. A complex role for complement in allergic asthma. Expert Rev Clin Immunol. 2010 Mar;6(2):269–77. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J Allergy Clin Immunol. 1997 Aug;100(2):253–60. doi: 10.1016/s0091-6749(97)70233-1. [DOI] [PubMed] [Google Scholar]
- 5.Kanemitsu H, Nagasawa S, Sagai M, Mori Y. Complement activation by diesel exhaust particles (DEP) Biol Pharm Bull. 1998 Feb;21(2):129–32. doi: 10.1248/bpb.21.129. [DOI] [PubMed] [Google Scholar]
- 6.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000 Aug 31;406(6799):998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 7.Krug N, Tschernig T, Erpenbeck VJ, Hohlfeld JM, Kohl J. Complement factors C3a and C5a are increased in bronchoalveolar lavage fluid after segmental allergen provocation in subjects with asthma. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1841–3. doi: 10.1164/ajrccm.164.10.2010096. [DOI] [PubMed] [Google Scholar]
- 8.Guven E, Duus K, Laursen I, Hojrup P, Houen G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS One. 2013 Sep 9;8(9):e74445. doi: 10.1371/journal.pone.0074445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawlisch H, Wills-Karp M, Karp CL, Kohl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol Immunol. 2004 Jun;41(2-3):123–31. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Baelder R, Fuchs B, Bautsch W, Zwirner J, Kohl J, Hoymann HG, et al. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J Immunol. 2005 Jan 15;174(2):783–9. doi: 10.4049/jimmunol.174.2.783. [DOI] [PubMed] [Google Scholar]
- 11.Peng T, Hao L, Madri JA, Su X, Elias JA, Stahl GL, et al. Role of C5 in the development of airway inflammation, airway hyperresponsiveness, and ongoing airway response. J Clin Invest. 2005 Jun;115(6):1590–600. doi: 10.1172/JCI22906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe M, Shibata K, Akatsu H, Shimizu N, Sakata N, Katsuragi T, et al. Contribution of anaphylatoxin C5a to late airway responses after repeated exposure of antigen to allergic rats. J Immunol. 2001 Oct 15;167(8):4651–60. doi: 10.4049/jimmunol.167.8.4651. [DOI] [PubMed] [Google Scholar]
- 13.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006 Mar;116(3):783–96. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karp CL, Grupe A, Schadt E, Ewart SL, Keane-Moore M, Cuomo PJ, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000 Sep;1(3):221–6. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 15.Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: Recent insights and novel anticomplement strategies. Adv Exp Med Biol. 2013;735:155–72. doi: 10.1007/978-1-4614-4118-2_10. [DOI] [PubMed] [Google Scholar]
- 16.Tanimoto T, Oshima Y, Kami M. Eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013 Oct 3;369(14):1378–9. doi: 10.1056/NEJMc1308826. [DOI] [PubMed] [Google Scholar]
- 17.Smith SG, Watson B, Clark G, Gauvreau GM. Eculizumab for treatment of asthma. Expert Opin Biol Ther. 2012 Apr;12(4):529–37. doi: 10.1517/14712598.2012.668517. [DOI] [PubMed] [Google Scholar]
- 18.Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol Immunol. 2009 Dec;47(2-3):185–95. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodruff TM, Pollitt S, Proctor LM, Stocks SZ, Manthey HD, Williams HM, et al. Increased potency of a novel complement factor 5a receptor antagonist in a rat model of inflammatory bowel disease. J Pharmacol Exp Ther. 2005 Aug;314(2):811–7. doi: 10.1124/jpet.105.086835. [DOI] [PubMed] [Google Scholar]
- 20.Jain U, Woodruff TM, Stadnyk AW. The C5a receptor antagonist PMX205 ameliorates experimentally induced colitis associated with increased IL-4 and IL-10. Br J Pharmacol. 2013 Jan;168(2):488–501. doi: 10.1111/j.1476-5381.2012.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff TM, Crane JW, Proctor LM, Buller KM, Shek AB, de Vos K, et al. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006 Jul;20(9):1407–17. doi: 10.1096/fj.05-5814com. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of alzheimer's disease. J Immunol. 2009 Jul 15;183(2):1375–83. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of alzheimer's disease. J Immunol. 2009 Jul 15;183(2):1375–83. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein E, Weigel J, Buford MC, Holian A, Wells SM. Asymmetric dimethylarginine potentiates lung inflammation in a mouse model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2010 Dec;299(6):L816–25. doi: 10.1152/ajplung.00188.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, et al. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol. 2009 Apr 24;296(6):L1085–95. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldenburg PJ, Wyatt TA, Factor PH, Sisson JH. Alcohol feeding blocks methacholine-induced airway responsiveness in mice. Am J Physiol Lung Cell Mol Physiol. 2009 Jan;296(1):L109–14. doi: 10.1152/ajplung.00487.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer JD, Van't Veer C, Stroo I, van der Meer AJ, de Vos AF, van der Zee JS, et al. Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 2013 Sep 18; doi: 10.1177/1753425913503387. [DOI] [PubMed] [Google Scholar]
- 28.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 29.Drouin SM, Sinha M, Sfyroera G, Lambris JD, Wetsel RA. A protective role for the fifth complement component (c5) in allergic airway disease. Am J Respir Crit Care Med. 2006 Apr 15;173(8):852–7. doi: 10.1164/rccm.200503-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. Allergens induce enhanced bronchoconstriction and leukotriene production in C5 deficient mice. Respir Res. 2006 Oct 17;7:129. doi: 10.1186/1465-9921-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin Immunol. 2013 Dec;149(3):534–55. doi: 10.1016/j.clim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Coulthard LG, Wu MC, Taylor SM, Woodruff TM. C5L2: A controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013 Mar;27(3):855–64. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, et al. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol. 2010 Dec 1;185(11):6741–52. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]


