Abstract
Introduction
Young adults have historically been the least likely to have health insurance in the United States. Previous studies of childhood cancer survivors found lower rates of insurance and less access to medical care compared to siblings; however, no studies have examined continuity of insurance after cancer diagnosis in adolescents and young adults (AYAs).
Methods
Using the AYA Health Outcomes and Patient Experience study, a cohort of 465 15-39 year-olds from participating Surveillance, Epidemiology and End Results registries, we evaluated changes in and sponsors of health insurance coverage after diagnosis, coverage of doctor-recommended tests, and factors associated with lack of insurance post-diagnosis using chi-square tests and multivariable logistic regression.
Results
Over 25% (n=118) of AYA cancer survivors experienced some period without insurance up to 35 months post-diagnosis. Insurance rates were high in the initial year after diagnosis (6-14 months; 93.3%) but decreased substantially at follow-up (15-35 months; 85.2%). The most common sponsor of health insurance was employer/school-coverage (43.7%). Multivariable analysis indicated that older survivors (25-39 vs. 15-19; Odds Ratio (OR): 3.35, p<0.01) and those with less education (high school or less vs. college graduate; OR: 2.80, p<0.01) were more likely to experience a period without insurance after diagnosis. Furthermore, >20% of survivors indicated there were doctor-recommended tests/treatments not covered by insurance, but >80% received them regardless of coverage.
Discussion
Insurance rates decrease with time since diagnosis in AYA cancer survivors. Future studies should examine how new policies under the Affordable Care Act extend access and insurance coverage beyond initial treatment.
Keywords: adolescents and young adults, insurance, SEER, affordable care act
Background
Young adults aged 19-25 have historically been among the most likely to lack health insurance in the United States, with rates ranging from 29.5% to 32.6% in 2008.1 Combined with the already low rates of insurance in this population, previous studies indicate that long-term survivors of childhood cancer have more difficulty obtaining and keeping health insurance compared to their siblings without cancer.2,3 Childhood cancer survivors who were widowed or divorced, with a previous history of cranial radiation or had lower educational and income levels have been more likely to lack health insurance >5 years after diagnosis.3 Furthermore, lack of health insurance in this population has been related to a lower likelihood of receiving recommended survivorship and general medical care.2-6 Previous work among adolescent and young adult (AYA) cancer survivors diagnosed between 15-39 years has shown that this group reports less healthcare access and higher risk of forgoing medical care due to costs compared to insured survivors.7
With insurance rates at their nadir in adolescence and young adulthood,8 identifying risk factors for loss of or gaps in insurance coverage after diagnosis among AYAs is critical for this population’s successful transition to adulthood while meeting survivorship needs.9 However, previous studies have not specifically examined risk factors for lack or loss of insurance after cancer diagnosis in the AYA population or how insurance coverage may change in the period after treatment. Furthermore, little is known about gaps in insurance coverage for doctor-recommended tests and treatments, which can significantly influence receipt of recommended care and long-term outcomes among survivors. As new policies under the Affordable Care Act (ACA) extend insurance coverage options over the coming years, it will be critical to determine which AYAs are most at risk for lacking adequate insurance after diagnosis and treatment.10 We use the National Cancer Institute’s Adolescent and Young Adult Health Outcomes and Patient Experience (AYA HOPE) Study to evaluate changes in and sponsors of health insurance coverage after diagnosis, coverage of doctor-recommended tests, and factors associated with any period of being uninsured after diagnosis. Using one of the largest population-based cohorts of recently-diagnosed AYA cancer survivors in the United States to date, our objective was to identify a broad range of demographic and cancer-related factors associated with being uninsured after diagnosis for the more than 500,000 AYA survivors in the United States today.11
Methods and Materials
Data and Participants
Data from the AYA HOPE study12 were used to evaluate changes in and sponsors of health insurance coverage after diagnosis in AYA survivors. Recruitment methods, characteristics of non-respondents as well as survey development and design have been described in detail elsewhere, with complete versions of surveys available online (http://outcomes.cancer.gov/surveys/aya).12 Briefly, after obtaining Institutional Review Board approval, we recruited AYA patients from seven population-based Surveillance, Epidemiology and End Results (SEER) registries.13 Patients were eligible if they were: 1) diagnosed between July 1, 2007 and October 31, 2008, 2) 15-39 years at diagnosis, 3) diagnosed with primary germ cell cancer, non-Hodgkin lymphoma (NHL), Hodgkin lymphoma (HL), acute lymphocytic leukemia (ALL), Ewing sarcoma, osteosarcoma, or rhabdomyosarcoma, and 4) were able to read and write in English. A baseline survey and medical records release form were mailed (August 2008-November 2009) to eligible participants 6-14 months after diagnosis. The survey queried participants about their demographics, health status, insurance, healthcare delivery and clinical trial participation. A follow-up survey (January-July 2010) was conducted 15-35 months post-diagnosis to examine changes in these items over time. A total of 1,208 patients were eligible for the study and 523 completed baseline surveys. The overall response rate was 43%, which is comparable to other large national samples conducted today that survey new respondents about health behaviors and attitudes.12,14 Of the 523 eligible baseline survey participants, 465 also completed the follow-up questionnaire.12 Previous evaluation of AYA HOPE participants indicated that participants did not vary significantly from non-participants by cancer site, education or time from diagnosis; although males, Hispanics and non-Hispanic blacks were slightly less likely to participate. 12
Measures
Outcomes
Health Insurance Coverage and Insurance Discontinuity
Study participants were asked whether they were currently covered by any type of health insurance in the baseline and follow-up surveys. Additionally, the baseline questionnaire asked participants whether they had health insurance coverage when they first went to see a doctor to get diagnosed and treated. These health insurance variables were used to create a variable indicating insurance continuity/discontinuity at three time points: 1) at diagnosis, 2) at baseline (6-14 months post-diagnosis), and 3) follow-up (15-35 months post diagnosis). If study participants indicated not having insurance coverage at any of these time points, we classified them as not having continuous insurance.
We additionally evaluated how insurance was provided at baseline and follow-up among insured respondents. At baseline, response categories included employer/school, spouse’s employer/school, parent, Medicaid or other public assistance program, other state program (e.g. Medi-Cal, SCHIP), Military or veteran’s benefits, Other, and Don’t know. In the follow-up survey, two new categories were included (purchased own policy and Consolidated Omnibus Budget Reconciliation Act (COBRA)- which gives workers and their families who lose their health benefits due to job loss the right to continue group health benefits for limited periods of time). Participants could mark more than one health insurance; therefore we used the following mutually exclusive, hierarchical approach to categorize the insurance sponsor: 1) employer/school; 2) spouse/parent; 3) government program (Medicaid or public assistance/state program); 4) other (veteran’s benefits, self/family pay, COBRA, other/don’t know).
Other insurance measures
We assessed whether participants in the baseline survey reported any doctor-recommended tests and treatments that were not covered by their insurance (yes, no, don’t know), and if they had received the tests/treatments regardless of insurance coverage (yes, no, don’t know). Additionally, we examined whether insurance coverage type had changed between the cancer diagnosis and baseline survey, and if so, how it changed (Table 3 lists non-mutually exclusive responses).
Table 3.
Change in Insurance Coverage between Diagnosis and Baseline Survey 6-14 Months After Diagnosis (N=465)
| N | Percent | |
|---|---|---|
|
Has insurance coverage changed between diagnosis and
baseline survey? |
||
| Yes, it changed | 139 | 29.9% |
| No, it remained unchanged | 312 | 67.1% |
| Don’t know/Missing | 14 | 3.0% |
| How has insurance coverage changed?(n=139) * | ||
| Changed insurance companies | 47 | 33.8% |
| Became eligible for public insurance | 41 | 29.5% |
| Changed to different type of coverage/product with same employer |
32 | 23.0% |
| Other | 28 | 20.1% |
| Lost coverage completely | 21 | 15.1% |
| Became eligible for employer-based insurance | 10 | 7.2% |
| Bought additional insurance | 3 | 2.2% |
| Don’t Know | 3 | 2.2% |
Not mutually exclusive categories
Survivor Characteristics
Patient demographic characteristics, including gender, age at diagnosis, and race/ethnicity from SEER cancer registry records, were included in our analysis (see Table 1 for all categories). From the baseline survey, we assessed educational attainment, marital status, and whether respondents had experienced changes in work/school after their cancer diagnosis. We defined participants as receiving ongoing treatment if they indicated that they were currently receiving treatment at baseline and/or currently scheduled to receive future treatment at follow-up. To account for differences in health, we included a comorbidity index derived from the initial hospitalization records based on previously reported methodology for this age group.15 Furthermore, we included the number of reported symptoms at baseline (nausea/vomiting; frequent or severe stomach pain; diarrhea/constipation; joint pain; weight loss/gain; frequent/severe fevers; tingling, weakness in hands/feet; problems with memory, attention or concentration).16 Finally, we assessed self-reported general health at baseline.17
Table 1.
Characteristics of AYA HOPE Participants (N=465)
| All Survivors | Continuous Insurance* (N=347) |
At least some time uninsured (N=118) |
|||
|---|---|---|---|---|---|
| N | % | % | % | p-value | |
| Demographic Characteristics † | |||||
| Age at Diagnosis | |||||
| 15-19 | 62 | 13.3 | 80.7 | 19.4 | 0.45 |
| 20-24 | 81 | 17.4 | 71.6 | 28.4 | |
| 25-39 | 322 | 69.3 | 74.2 | 25.8 | |
| Sex | |||||
| Male | 287 | 61.7 | 71.8 | 28.2 | 0.07 |
| Female | 178 | 38.3 | 79.2 | 20.8 | |
| Race | |||||
| white | 375 | 80.7 | 74.7 | 25.3 | 0.97 |
| Non-white | 90 | 19.4 | 74.4 | 25.6 | |
| Education | |||||
| ≤High School | 130 | 28.0 | 64.6 | 35.4 | <0.01 |
| Some College/Vocational School | 123 | 26.5 | 72.4 | 27.6 | |
| ≥College Graduate* | 212 | 45.6 | 82.1 | 17.9 | |
| Marital Status | |||||
| Married/Living as married | 198 | 42.6 | 78.3 | 21.7 | 0.12 |
| Not married* | 267 | 57.4 | 71.9 | 28.1 | |
|
Change in Work/School after
Diagnosis |
|||||
| Yes* | 115 | 24.7 | 80.9 | 19.1 | 0.08 |
| No | 350 | 75.3 | 72.6 | 27.4 | |
| Ongoing Treatment ** | |||||
| Yes | 92 | 19.8 | 65.2 | 34.8 | 0.02 |
| No/Don’t know* | 373 | 80.2 | 76.9 | 23.1 | |
| Treatment Characteristics † | |||||
| Treatment Intensity | |||||
| Least Intense | 47 | 10.1 | 72.3 | 27.7 | 0.91 |
| Moderately Intense* | 290 | 62.4 | 75.2 | 24.8 | |
| Very Intense | 128 | 27.5 | 74.2 | 25.8 | |
| Health Characteristics † | |||||
| Stage at Diagnosis | |||||
| I/ II | 275 | 59.1 | 72.0 | 28.0 | 0.27 |
| III/ IV | 99 | 21.3 | 79.8 | 20.2 | |
| Unknown/ Unstaged | 91 | 19.6 | 76.9 | 23.1 | |
| Number of Symptoms | |||||
| 0-1 | 145 | 31.2 | 80.0 | 20.0 | 0.09 |
| 2-5* | 225 | 48.4 | 74.2 | 25.8 | |
| 6+ | 95 | 20.4 | 67.4 | 32.6 | |
| Number of Comorbidities | |||||
| 0 | 338 | 72.7 | 74.3 | 25.7 | 0.94 |
| 1 | 76 | 16.3 | 75.0 | 25.0 | |
| 2+ | 51 | 11.0 | 76.5 | 23.5 | |
| General Health | |||||
| Excellent/Very good | 211 | 45.4 | 78.7 | 21.3 | 0.02 |
| Good* | 174 | 37.4 | 75.3 | 24.7 | |
| Fair/Poor | 80 | 17.2 | 62.5 | 37.5 | |
Category includes the following number of missing values grouped together with the most common response category for analysis: Treatment Intensity=29; Education=1; Marital Status=1; Change in Work/School after Diagnosis=29; Ongoing Treatment=9; Number of Symptoms=13; General Health=4.
Refers to participants currently receiving treatment who were scheduled to receive future cancer treatment at follow-up.
Measured at Baseline, except age, sex, race, ongoing treatment (see **), and comorbidities, which were measured at diagnosis.
Treatment Characteristics
The medical record forms collected information on tumor characteristics, staging, and treatment.18 To account for differences in treatment, we used the Intensity of Treatment Rating Scale 2.0.19 Briefly, least intensive treatment included those who received surgery only, regardless of cancer type, and moderately intensive treatment included those with germ cell tumors who received chemotherapy or radiation, AJCC stage I-IIIa HL with non-bulky disease, NHL with AJCC I-III disease, and rhabdomyosarcoma with AJCC stage I-II disease. Finally, respondents with very intensive treatment included those with HL at AJCC stages IIIb-IV with bulky disease and/or a treated relapse, all Ewing or osteosarcomas, rhabdomyosarcomas with AJCC stages III-I,; NHL AJCC stage IV, and all ALL.
Statistical Approach
We used Chi-squared tests and multivariable logistic regression to examine associations between patient demographic and treatment characteristics and any period of insurance discontinuity up to 35 months after diagnosis. Logistic regression models include variables with an a priori hypothesis for inclusion, including age at diagnosis, sex, race/ethnicity, education, marital status, change in work/school after diagnosis, ongoing treatment, treatment intensity, symptoms, comorbidities, and general health. Missing item responses for all variables were grouped with the most common response to preserve our full sample size and provide more conservative estimates of effects than with respondents with missing data excluded from the analysis. To confirm that our results were not influenced by our categorization of missing data, we repeated our multivariable analyses excluding respondents with missing data. Additionally, because many of the factors influencing insurance discontinuity may be correlated, we used variance inflation factors (VIF) to ascertain whether any of the independent variables were correlated; the largest VIF was 1.65, indicating that multicollinearity was not a concern. Finally, as the effect of a college degree or marital status on insurance discontinuity may vary by age, we repeated our analyses removing those ≤25 as well as including interaction terms between age/education and marital status(p>0.10). In all sensitivity analyses described above, our primary conclusions remained unchanged (data not shown).
Frequencies and percentages were then determined to evaluate 1) the level of doctor-recommended tests and treatments that were not covered by respondents’ insurance, 2) if they received the tests and treatments regardless of insurance coverage, and 3) whether insurance coverage had changed between the cancer diagnosis and the baseline survey. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). P-values were 2-sided, with p<0.05 considered significant.
Results
Approximately 70% of study participants were 25 years or older at diagnosis (Table 1). The majority of participants were male (61.7%), white (80.7%), were diagnosed with early stage disease (59.1% AJCC stage I/II), and did not change their work/school status after diagnosis (75.3%). Furthermore, more than 80% of survivors were not in ongoing treatment at baseline or follow-up. At baseline, most participants reported having at least two symptoms in the four weeks prior to completing the survey and 27% had at least one severe/chronic comorbidity.
Insurance Status
Over 25% (n=118) of AYA cancer survivors experienced some period of no coverage up to 35 months post-diagnosis (Table 1). Insurance rates were high in the initial year after diagnosis (6-14 months; 93.3%) but decreased substantially at follow-up (15-35 months; 85.2%) (Figure 1). Bivariate analysis demonstrated that individuals with lower levels of education (35.4%: high school or less vs. 17.9%: college graduate, p<0.01), those in ongoing cancer treatment at follow-up (34.8% in ongoing treatment vs. 23.1% not in treatment, p=0.02), and those with lower levels of general health at baseline were more likely to have at least some period of time without insurance (Table 1). After adjusting for other demographic and cancer characteristics, multivariable analysis indicated that compared to survivors ages 15-19, only older survivors ages 20-24 ( OR: 2.99, 95%CI:1.26-7.09) and ages 25-29 (OR: 3.35, 95%CI:1.49-7.55) and those with less education (high school or less vs. college graduate; OR: 2.80, 95%CI:1.55-5.06) were more likely to experience a lapse in coverage after diagnosis (Table 2).
Figure 1.
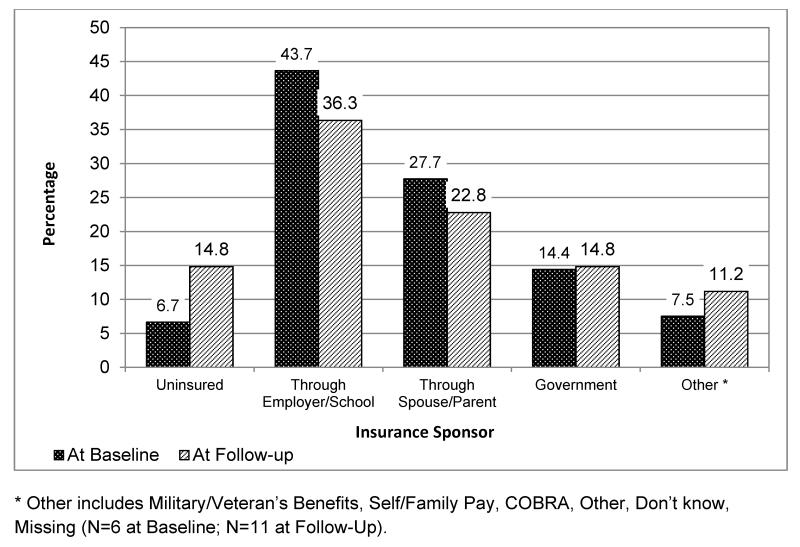
Health Insurance Sponsors among AYA Cancer Survivors, AYA HOPE Survey (N=465). The type of health insurance sponsor is reported at baseline (6-14 months post-diagnosis) and at follow-up (15-35 months post-diagnosis). Other includes Military/Veteran’s Benefits, Self/Family Pay, COBRA, Other, Don’t know, Missing (N=6 at Baseline; N=11 at Follow-Up).
Table 2.
Demographic, Treatment and Health Characteristics Associated with Insurance Discontinuity at Any Point Since Diagnosis(N=465)
| Adjusted Odds Ratio |
95%CI | |
|---|---|---|
| Demographic Characteristics † | ||
| Age at Diagnosis | ||
| 15-19(Reference) | 1.00 | |
| 20-24 | 2.99 | 1.26-7.09 |
| 25-39 | 3.35 | 1.49-7.55 |
| Sex | ||
| Male(Reference) | 1.00 | |
| Female | 0.71 | 0.43–1.16 |
| Race | ||
| white(Reference) | 1.00 | |
| Non-white | 0.91 | 0.52–1.60 |
| Education | ||
| ≤High School | 2.80 | 1.55–5.06 |
| Some College/Vocational School | 1.76 | 1.00–3.08 |
| ≥College Graduate*(Reference) | 1.00 | |
| Marital Status | ||
| Married/Living as married | 0.69 | 0.42–1.13 |
| Not married*(Reference) | 1.00 | |
|
Change in Work/School after
Diagnosis |
||
| Yes*(Reference) | 1.00 | |
| No | 0.70 | 0.40–1.23 |
| Ongoing Treatment** | ||
| Yes | 1.53 | 0.87–2.67 |
| No/Don’t know*(Reference) | 1.00 | |
| Treatment Characteristics † | ||
| Treatment Intensity | ||
| Least Intense | 1.32 | 0.62–2.79 |
| Moderately Intense*(Reference) | 1.00 | |
| Very Intense | 0.96 | 0.57–1.65 |
| Health Characteristics † | ||
| Number of Symptoms | ||
| 0-1 | 0.80 | 0.47–1.39 |
| 2-5*(Reference) | 1.00 | |
| 6+ | 1.09 | 0.59–1.37 |
| Number of Comorbidities | ||
| 0(Reference) | 1.00 | |
| 1 | 0.76 | 0.41–1.41 |
| 2+ | 0.65 | 0.30–1.37 |
| General Health | ||
| Excellent/Very good | 1.01 | 0.61–1.69 |
| Good*(Reference) | 1.00 | |
| Fair/Poor | 1.55 | 0.82–2.94 |
|
| ||
| C-statistic | 0.68 | |
Category includes the following number of missing values grouped together with the most common response category for analysis: Treatment Intensity=29; Education=1; Marital Status=1; Change in Work/School after Diagnosis=29; Ongoing Treatment=9; Number of Symptoms=13; General Health=4.
Refers to participants currently receiving treatment who were scheduled to receive future cancer treatment at follow-up.
Measured at Baseline, except age, sex, race, ongoing treatment (see **), and comorbidities, which were measured at diagnosis
CI=Confidence Interval.
Sponsors of and Changes in Insurance
The most common sponsors of insurance coverage at baseline and follow-up were employer/school-sponsored coverage, coverage through a spouse/parent, and Medicaid or other state-sponsored programs (43.7%, 27.7%, and 14.4% respectively at baseline) (Figure 1). Almost 30% of respondents stated that their insurance coverage had changed between diagnosis and the baseline survey. The most frequent reasons cited for this were: changing insurance companies (33.8%), becoming eligible for public insurance (29.5%), changing to a different type of coverage (23.0%), and losing coverage completely (15.1%) (Table 3).
Coverage of Doctor-Recommended Tests and Treatments
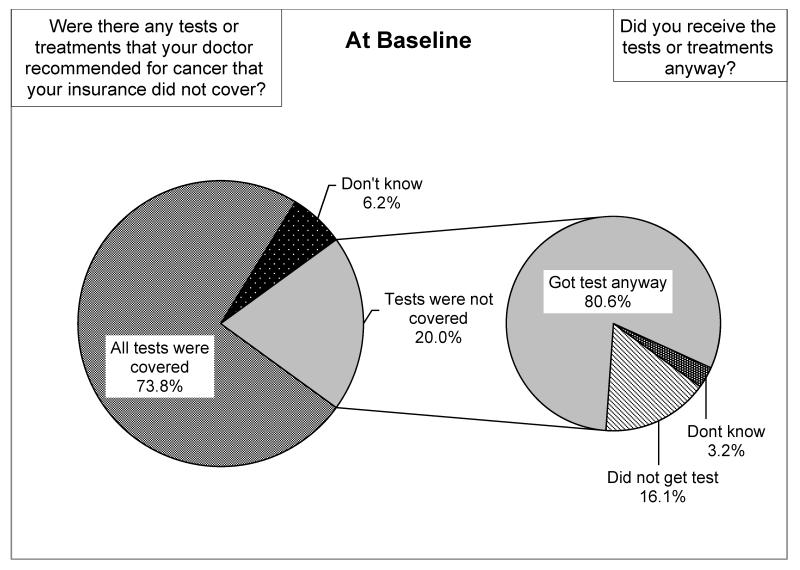
One-fifth of cancer survivors indicated there were doctor-recommended tests/treatments not covered by their insurance at the time of the baseline survey. However, 80.6% of the individuals with non-covered tests/treatments stated that they chose to receive the tests/treatments regardless of insurance coverage (Figure 2). Furthermore, out of those 20% of cancer survivors who reported non-coverage of recommended test/treatments by the time of the baseline survey, 34% also experienced some gap in insurance between diagnosis and follow-up (data not shown).
Figure 2.
Coverage of Doctor Recommended Cancer Tests/Treatments (n=465). The percentage of participants who indicated that there were doctor-recommended tests or treatments not covered by their insurance in the baseline survey as well as whether they received the test/treatments regardless of insurance coverage.
Discussion
In our study of recently diagnosed AYA cancer survivors recruited from population-based cancer registries, we found that insurance rates substantially decreased with time since diagnosis. Insurance rates were high in the initial year after diagnosis (6-14 months; 93.3%), but decreased substantially at follow-up (15-35 months; 85.2%), with those older at diagnosis and with lower levels of education most likely to lack insurance. Importantly, 20% of survivors indicated that their doctors recommended tests/treatments not covered by their insurance. Despite certain limitations, such as the lower response rates of males and minorities in this study, this is one of the first studies to examine changes in insurance coverage among survivors of AYA cancer, a population already at high risk of lacking insurance or being underinsured.
Overall, our insurance rates at the time of the follow-up survey are similar to rates found in the Childhood Cancer Survivor Study (CCSS), a study that included >5-year cancer survivors diagnosed before the age of 21 years and ranging in age up to 48 years at time of study.3 Specifically, Park et al. found adult survivors in the CCSS to have lower rates of insurance in their 1994-1998 baseline survey (83.9% insured) than at 6-year follow-up in 2000-2002 (88%).3 However, at both time-points, adult survivors had significantly lower rates of health insurance compared to their sibling controls. Additionally, in a single-site study of long-term (>10 years post-diagnosis) childhood cancer survivors, Crom et al. found insurance rates of 82.7% among those treated at St. Jude’s Research Hospital from1962-1992.20 Our findings may differ slightly from those of the CCSS as well as the St. Jude study as our study included recently diagnosed AYAs from a population-based sample of respondents, which may be more representative of all cancer patients compared to studies including survivors treated at only one institution or non-population based studies. While our insurance rates are higher than the 2008 national average for 15-39 year-olds (76.0%), the concerning trend of substantially decreasing rates of insurance after diagnosis may hamper access to necessary lifelong survivorship care.1
Our finding that almost 30% of respondents stated that their insurance had changed between diagnosis and the baseline survey 6-14 months after diagnosis suggests that many AYAs and their families experience significant occupational, socioeconomic, and other life transitions after diagnosis. The most frequently cited reasons for changing included becoming eligible for public insurance, switching to a different coverage sponsor or losing coverage completely. Changes in insurance coverage may be accompanied by changes in networks of covered providers, eligible benefits, and out-of-pocket service costs. While this increase in the percentage of AYAs who lack insurance may not be permanent and may improve farther from diagnosis, it will be critical to develop interventions to help young adults stay insured as they transition out of treatment and back into their everyday lives of completing education and entering/re-entering the workforce.
Additionally our study found that specific groups of the AYA survivor population were more likely to be uninsured, including those older at diagnosis and those with lower levels of education. In previous studies, long-term survivors of childhood cancer have had more difficulty obtaining and keeping health insurance compared to their siblings who did not have cancer.2,3 These findings highlight the potential benefit for AYA cancer survivors as the measures from the ACA of 2010 are fully implemented.10,21 The goal of the ACA is to increase access to healthcare among vulnerable populations, particularly young adults, as it allows individuals to stay on their parents’ insurance until the age of 26, eliminates insurance coverage limits, does not exclude preexisting conditions (such as cancer) and expressly prohibits termination of coverage, each of which may play a critical role in obtaining and retaining insurance coverage and utilizing recommended healthcare for this entire AYA population.22-24 With the difficulties of obtaining health insurance coverage in the years following diagnosis, the ACA presents important opportunities to extend insurance options and coverage to a population that will require long-term medical care and surveillance to address the physical and psychological challenges of survivorship.7 Our findings are timely as AYA cancer survivors should soon have access to a wider range of health insurance options with the state and federal run insurance exchanges opening for enrollment as of October 2013.21,22 However, future studies should explore the potentially burdensome out-of-pocket costs required by these new plans and the ACA’s impact on AYA cancer survivors.25 Ensuring that this population has access to information about health insurance choices and best practices for maintaining health insurance will be critical first steps to promote access to recommended treatment and surveillance care in AYAs.
Finally, we found that 20% of AYA indicated that there were doctor-recommended tests/treatments not covered by insurance, pointing toward potential underinsurance in this population (i.e., individuals have insurance but benefits may not extend to all recommended services). As a result, individuals may be left to pay out-of-pocket for recommended services not covered by insurance. Alternatively, hospital-based cancer treatment centers may provide charity care or non-profit organizations may provide financial assistance for patients in active treatment to cover these treatments. Regardless, cost has consistently been cited as a reason for forgoing medical care in AYA cancer survivors,7 who are more likely to be financially unstable due to the transition in education and employment after diagnosis. A qualitative CCSS study showed that out-of-pocket costs were particularly burdensome for young adult cancer survivors.26 Copayments, high deductibles, other out-of-pocket costs, as well as transportation, childcare, and lost wages, can be burdensome to young adults with chronic diseases, especially when they are faced with education or early career debt.27 A recent study using Behavioral Risk Factor Surveillance System (BRFSS) data found that AYAs with cancer were 55%-67% more likely than those without chronic disease to report forgoing medical care due to out-of-pocket costs, which can lead to financial hardship, despite similar levels of health insurance.7,28 Additionally, previous studies have highlighted that survivors face coverage exclusions/restrictions more often than their siblings.3 The ACA has the potential to address these critical issues of insurance coverage and high out-of-pocket costs of care. However, with such a large proportion of AYAs indicating recommended services are not covered by insurance, future research must examine continued barriers to appropriate insurance coverage and concerns with costs of care, to inform development of evidence-based interventions and education for this population, their families, medical staff, and care providers.
As one of the largest population-based cohorts of AYA cancer survivors with a broad range of cancer diagnoses, our study provides important data on changes in insurance coverage over time among AYAs. Additional strengths of this study were the availability of patient-reported education level, health status, ongoing therapy, and tests and treatments not covered by insurance, as well as the ability to track longer-term changes in self-reported outcomes. While our study provides important data on changes in insurance status among AYAs, we acknowledge several limitations. First, our study relied on self-reported outcomes to understand changes in and sponsors of insurance. While other data sources may provide additional information on out-of-pocket costs and healthcare access, our study identifies a broad range of problems leading to lack of insurance that AYA cancer patients experience after diagnosis. Second, due to our sample size, we were unable to examine factors associated with being uninsured by age-group or with losing coverage. Third, our study did not examine the quality of employer-sponsored insurance, which may have important implications for medical care coverage among survivors. Fourth, our study cohort may not be generalizable to all AYA cancer survivors, as our cohort was predominately white, had to read and write English to be eligible, and did not include survivors diagnosed with all types of cancer. Thus, our findings may overestimate insurance rates in the larger AYA cancer survivor population, as lack of insurance has been traditionally higher among minority groups and males. As a result, future studies should evaluate how factors associated with lack of insurance identified in our study apply to larger, more diverse AYA populations.
Overall, the findings from our study provide insight into important factors related to lack of insurance in the vulnerable population of AYA cancer survivors. The Patient Protection and ACA of 2010 has the potential to influence both access to insurance and utilization of necessary healthcare for young adults.23,24 Therefore, ensuring that this population has access to information about health insurance choices and best practices for maintaining health insurance will be critical first steps to promote access to recommended treatment and surveillance in AYAs, particularly older survivors (25-39) and those with less education. With a growing U.S. population of more than 500,000 AYA cancer survivors, the majority of whom will require consistent, long-term medical care to address their survivorship needs, ongoing monitoring of new policies under the ACA to extend access to and uptake of insurance coverage beyond initial treatment will be required.
Acknowledgments
Funding: NCI Contracts: HHSN261201000024C; HSN261201000025C; HHSN261201000032C; HHSN261201000027C; HHSN261201000026C; HHSN261201000140C; HHSN261201000037C; HHSN261201000033C; HHSN261201000034C; HHSN261201000035C; HHSN261201000029C; HHSN261201000031C; HHSN261201000028C; HHSN261201000030C. Dr. Parsons is supported by K07CA175063 and Dr. Keegan is supported by the Stanford Cancer Institute.
References
- 1.Health insurance coverage of young adults aged 19 to 25: 2008, 2009, and 2011 . United States Census Bureau; [Accessed March 20, 2013]. 2012. at http://www.census.gov/prod/2012pubs/acsbr11-11.pdf. [Google Scholar]
- 2.Casillas J, Castellino SM, Hudson MM, et al. Impact of insurance type on survivor-focused and general preventive health care utilization in adult survivors of childhood cancer: the Childhood Cancer Survivor Study (CCSS) Cancer. 2011;117:1966–75. doi: 10.1002/cncr.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park ER, Li FP, Liu Y, et al. Health insurance coverage in survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2005;23:9187–97. doi: 10.1200/JCO.2005.01.7418. [DOI] [PubMed] [Google Scholar]
- 4.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401–9. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhoff AC, Lyles CR, Fluchel M, Wright J, Leisenring W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118:5964–72. doi: 10.1002/cncr.27537. [DOI] [PubMed] [Google Scholar]
- 8.Adams SH, Newacheck PW, Park MJ, Brindis CD, Irwin CE., Jr Health insurance across vulnerable ages: patterns and disparities from adolescence to the early 30s. Pediatrics. 2007;119:e1033–9. doi: 10.1542/peds.2006-1730. [DOI] [PubMed] [Google Scholar]
- 9.Adolescent and Young Adult Oncology Progress Review Group . Closing the gap: research and care imperatives for adolescents and young adults with cancer. National Institutes of Health; Bethesda, MD: 2006. [Google Scholar]
- 10.Bleyer A, Ulrich C, Martin S. Young adults, cancer, health insurance, socioeconomic status, and the Patient Protection and Affordable Care Act. Cancer. 2012;118:6018–21. doi: 10.1002/cncr.27685. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Survivorship Research: Estimated U.S. Cancer Prevalence [Accessed October 14, 2013];2013 at http://cancercontrol.cancer.gov/ocs/prevalence/prevalence.html#age.
- 12.Harlan LC, Lynch CF, Keegan THM, et al. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE study. J Cancer Surviv. 2011;5:305–14. doi: 10.1007/s11764-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surveillance Epidemiology and End Results Program 2013 Accessed at http://seer.cancer.gov/
- 14.Behavioral Risk Factor Surveillance System 2013 Accessed at http://aspe.hhs.gov/hsp/06/catalog-ai-an-na/brfss.htm.
- 15.Parsons HM, Harlan LC, Lynch CF, et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012;30:2393–400. doi: 10.1200/JCO.2011.39.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keegan TH, Lichtensztajn DY, Kato I, et al. Unmet adolescent and young adult cancer survivors information and service needs: a population-based cancer registry study. J Cancer Surviv. 2012;6:239–50. doi: 10.1007/s11764-012-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: the predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. Journal of clinical epidemiology. 1997;50:517–28. doi: 10.1016/s0895-4356(97)00045-0. [DOI] [PubMed] [Google Scholar]
- 18.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29:4045–53. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werba BE, Hobbie W, Kazak AE, et al. Classifying the intensity of pediatric cancer treatment protocols: the intensity of treatment rating scale 2.0. Pediatr Blood Cancer. 2007;48:673–7. doi: 10.1002/pbc.21184. [DOI] [PubMed] [Google Scholar]
- 20.Crom DB, Lensing SY, Rai SN, Snider MA, Cash DK, Hudson MM. Marriage, employment, and health insurance in adult survivors of childhood cancer. J Cancer Surviv. 2007;1:237–45. doi: 10.1007/s11764-007-0026-x. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson J, Ruccione K, Reaman GH. Health care reform 2010: expected favorable impact on childhood cancer patients and survivors. Cancer J. 2010;16:554–62. doi: 10.1097/PPO.0b013e3181feee83. [DOI] [PubMed] [Google Scholar]
- 22.Moy B, Polite BN, Halpern MT, et al. American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011;29:3816–24. doi: 10.1200/JCO.2011.35.8903. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser Family Foundation; [Accessed March 20, 2013]. 2011. Summary of the New Health Reform Law. The Henry J. at http://www.kff.org/healthreform/upload/8061.pdf. [Google Scholar]
- 24.Sommers BD, Buchmueller T, Decker SL, Carey C, Kronick R. The Affordable Care Act has led to significant gains in health insurance and access to care for young adults. Health affairs. 2013;32:165–74. doi: 10.1377/hlthaff.2012.0552. [DOI] [PubMed] [Google Scholar]
- 25.Gruber J, Perry I. Will the Affordable Care Act make health insurance affordable? Issue Brief (Commonw Fund) 2011;2:1–15. [PubMed] [Google Scholar]
- 26.Park ER, Kirchhoff AC, Zallen JP, et al. Childhood Cancer Survivor Study participants’ perceptions and knowledge of health insurance coverage: implications for the Affordable Care Act. J Cancer Surviv. 2012;6:251–9. doi: 10.1007/s11764-012-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson JL, Collins SR. Young, uninsured, and seeking change: health coverage of young adults and their views on health reform. Findings from the Commonwealth fund Survey of Young Adults (2009) Issue Brief (Commonw Fund) 2009;73:1–22. [PubMed] [Google Scholar]
- 28.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–7. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]