Abstract
There is a significant need for novel treatments that will improve traumatic brain injury (TBI) outcomes. One potential neuroprotective mechanism is to increase oxygen binding proteins such as neuroglobin. Neuroglobin has a high affinity for oxygen, is an effective free radical scavenger, and is neuroprotective within the brain following hypoxia and ischemia. The purpose of this study was to determine whether neuroglobin overexpression improves sensorimotor outcomes following TBI in transgenic neuroglobin overexpressing (NGB) mice. Additional study aims were to determine if and when an endogenous neuroglobin response occurred following TBI in wild-type (WT) mice, and in what brain regions and cell types the response occurred. Controlled cortical impact (CCI) was performed in adult (5 month) C57/BL6 WT mice, and NGB mice constitutively overexpressing neuroglobin via the chicken beta actin promoter coupled with the cytomegalovirus distal enhancer. The gridwalk task was used for sensorimotor testing of both WT and NGB mice, prior to injury, and at 2, 3, and 7 days post-TBI. NGB mice displayed significant reductions in the average number of foot faults per minute walking at 2, 3, and 7 days post-TBI when compared to WT mice at each time point. Neuroglobin mRNA expression was assessed in the injured cortex of WT mice prior to injury, and at 1, 3, 7, and 14 days post-TBI using quantitative real time polymerase chain reaction (qRT-PCR). Neuroglobin mRNA was significantly increased at 7 days post-TBI. Immunostaining showed neuroglobin primarily localized to neurons and glial cells in the injured cortex and ipsilateral hippocampus of WT mice, while neuroglobin was present in all brain regions of NGB mice at 7 days post-TBI. These results showed that overexpression of neuroglobin reduced sensorimotor deficits following TBI, and that an endogenous increase in neuroglobin expression occurs during the subacute period. Increasing neuroglobin expression through novel therapeutic interventions during the acute period after TBI may improve recovery.
Keywords: Neuroglobin, Traumatic Brain Injury, Transgenic, Overexpression, Neuroprotective, Sensorimotor
Introduction
Traumatic brain injury (TBI) is a major public health concern with severe consequences that include long-term loss of function, profound disability, and death. Accordingly, there is a significant need for novel treatments that will improve TBI outcomes. Improved TBI outcomes may be achieved through treatments that target globin genes (e.g., hemoglobin, myoglobin, cytoglobin, and neuroglobin). The globin gene family translates proteins that are vital for binding oxygen within various body tissues. Following upregulation, globin proteins also play a neuroprotective role in the brain [12, 26]. The work presented here focuses on neuroglobin, one of the most recently characterized members of the globin gene family. Neuroglobin was first described in 2000 as a protein with a high affinity for binding oxygen that is present in the brain [4]. In addition to its ability to bind oxygen, neuroglobin also functions as an antioxidant and free radical scavenger [18]. Neuroglobin is expressed throughout the central and peripheral nervous systems of vertebrates [4, 14, 23, 28]. The primary sites of neuroglobin expression are the limbic system and cerebral cortex [14]. Neuron survival is enhanced via an increased endogenous production of neuroglobin following hypoxia and ischemia [13]. Therefore, neuroglobin has been suggested to be neuroprotective in experimental models of stroke [17, 27] and in TBI [22, 28].
Previous research has demonstrated that transgenic neuroglobin overexpressing (NGB) mice display significantly reduced cortical lesion volumes in comparison to wild-type (WT) mice 21 days after TBI [28]. Furthermore, overexpression of neuroglobin in Wistar rats significantly reduced neuron necrosis and apoptosis after TBI in comparison to controls [22]. In humans, genetic polymorphisms in neuroglobin have been shown to positively influence recovery in TBI patients [6]. The present study was designed to determine whether NGB mice displayed improved sensorimotor recovery after TBI in comparison to WT mice. In addition, related study objectives were to determine if and when an endogenous neuroglobin response occurred following TBI, and in what brain regions and cell types the response occurred. It was hypothesized that overexpression of neuroglobin would reduce sensorimotor deficits following TBI in NGB mice when compared to WT mice.
Methods
Adult (5 month) male C57/BL6 WT, and transgenic (B6.Cg-Tg(CAG-Ngb,-EGFP)1Dgrn/J; The Jackson Laboratory, ME) NGB mice were used for this study. NGB mice overexpressed neuroglobin via the chicken beta actin promoter coupled with the cytomegalovirus distal enhancer. Animal care and use procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee and conducted according to the Institute of Laboratory Animal Research guidelines.
A controlled cortical impact (CCI) was used to induce a moderate TBI as previously described [19]. The CCI injury model targeted the primary and secondary motor and primary somatosensory cortical regions. CCI reproduces many of the features of brain injuries including motor deficits, memory loss, and neuron loss [7]. Responses following controlled cortical impact include glial activation, increased cytokine expression, blood brain barrier disruption, and neuron loss [20, 21]. The CCI procedure was performed while mice were anaesthetized with 2.5% isoflurane in 30% O2 and air. Briefly, a midline incision was made to expose the skull. A Wild operating microscope was used to view the skull at 60X magnification, and a 3.5 mm diameter craniotomy was performed with a dental drill on the right side of the mid-sagittal suture, with the following coordinates: Anterior-Posterior (AP) coordinates centered at bregma, 2.5 mm lateral to the midline. Drilling was performed in a careful manner to leave the dura intact, and avoid blood vessels traveling through the superior sagittal sinus. Once the brain was exposed, the cortex was impacted (3 mm diameter injury tip centered at 2.5 mm lateral to bregma) with a custom made, electronically controlled, CCI injury device (P01-23×80, LinMot Inc., Zurich, Switzerland). CCI impact parameters were as follows: impact velocity (1.5 m/s), impact depth (1.0 mm), and contact time (85 ms). These impact parameters have resulted in a low (5%) mortality rate. Following impact, the skin was closed with a sterile suture. The duration of each CCI procedure was approximately 30 minutes.
Sensorimotor deficits (i.e., foot faults) were evaluated using the gridwalk task as previously described [19]. 6 WT and 7 NGB mice were tested on the gridwalk at four time points (1 day pre-TBI, 2 days post-TBI, 3 days post-TBI, and 7 days post-TBI). Mice were given one trial per day, at the same time of day, and scored by an observer who was unaware of the groups. Mice were placed on a grid area measuring 32 cm×20 cm×50 cm with 11×11 mm diameter openings. Mice were allowed to walk on the grid for five minutes, during which their total walking time was measured, and the number of foot faults for each foot counted. Any step passing through a grid hole was considered a foot fault. Foot fault data was normalized by dividing the total counted foot faults by the total time spent walking to obtain a measure of foot faults per minute of walking. Data were expressed as the mean number of foot faults per minute ± SEM. Two-way repeated measures ANOVA with a Fisher's LSD post hoc for multiple comparisons was used for analyzing significant differences in mean number of foot faults per minute at each time-point.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR) was utilized for our gene expression time course study as previously described [1]. 6 WT mice were sacrificed via intraperitoneal injection of beuthanasia prior to TBI, and at 1 day, 3 days, 7 days, and 14 days post-TBI. Animals were decapitated at each time point, and whole brains were removed and stored in RNA later (Ambion, Austin, TX) for 24 hours at 4°C to preserve the RNA. After the 24 hour incubation period, whole brains were dissected in order to collect the injured cortex (right hemisphere). Samples were homogenized and total RNA was extracted, purified, and quantified using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). Complementary DNA (cDNA) was synthesized by using 1 μg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA). The cDNA and gene specific primers (see Supplementary Materials for primer sequences) were subjected to PCR amplification using an Applied Biosystems 7300 Real Time PCR System Machine (Applied Biosystems, Foster City, CA). PCR reactions were conducted in duplicate, and GAPDH was used as the housekeeping gene. Sequence Detection Software 2.0 (Applied Biosystems, Foster City, CA) was used to collect and analyze data. Neuroglobin gene expression was calculated relative to GAPDH by the ΔΔCt method, and reported in accordance with The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [5]. A two-way ANOVA was used for analyzing significant differences in mRNA expression between time points. Fisher's LSD was used for post hoc comparisons.
Immunohistochemistry was conducted to assess the location of specific cell types expressing neuroglobin as previously described [20]. 4 WT and 4 NGB mice were sacrificed by transcardial perfusion under anesthesia (isoflurane) at 7 days post-TBI. Brains were fixed by perfusion with 4% buffered formaldehyde, dissected, postfixed overnight, cryoprotected in 30% sucrose in 0.1 M phosphate buffer (pH 7.2), and sectioned through the coronal plane at 50 μm on a microtome. Sections were rinsed in PBS with 0.4 mg/ml glycine for 5 min, permeabilized with 0.2% Triton X-100, and quenched for peroxidase with 3% H2O2. Sections were incubated overnight at 4°C with the primary antibody (neuroglobin rabbit anti-mouse 1:200, Sigma AB-N7162). After overnight incubation, all sections were rinsed with PBS and incubated with a biotinylated secondary antibody (1:500 goat anti-rabbit; Vector Labs BA-1000) for 2 hours at room temperature. An ABC Elite kit (Vector Labs, Burlingame, CA) was used to produce the immunoperoxidase reaction. Color was visualized using diaminobenzidine (DAB) solution (Vector Labs, Burlingame, CA). A Nikon inverted stage microscope was used to visualize all sections, and digital images were captured with a SPOT microscope camera (Diagnostic Instruments, Sterling Heights, MI).
Results
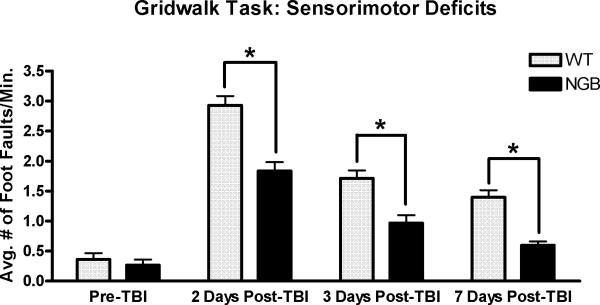
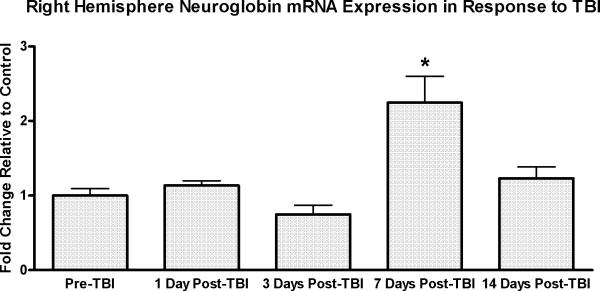
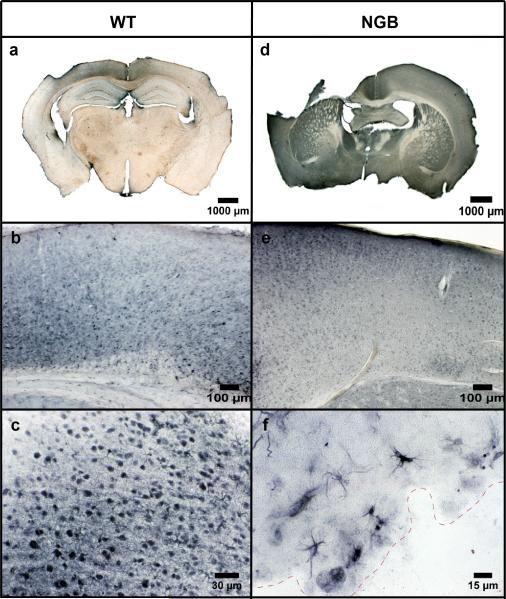
The gridwalk task revealed unilateral sensorimotor forelimb and hindlimb deficits (i.e., foot faults) in both WT and NGB mice at 2 days, 3 days, and 7 days post-TBI (Figure 1). However, NGB mice displayed a significant (p=.001) reduction in the average number of foot faults per minute walking in comparison to WT mice at all post-TBI time points (2, 3, and 7 days). Furthermore, at 7 days post-TBI, NGB mice demonstrated a reduction in the average number of foot faults per minute of walking that was similar to pre-TBI averages. qRT-PCR analysis (Figure 2) showed a significant (p≤.005) increase in neuroglobin mRNA expression in the right hemisphere of WT mice 7 days post-TBI when compared to mRNA levels at all other time points. CCI induced a lesion cavity within the right hemisphere that was seen 7 days post-TBI in both WT and NGB mice (Figure 3 a, d). Immunohistochemistry revealed neuroglobin protein localization within neurons and glial cells in the injured cortex near the injury site, and ipsilateral hippocampus of WT mice 7 days post-TBI (Figure 3 a, b, c). In addition, neuroglobin protein was observed in neurons and glial cells throughout the brain of NGB mice 7 days post-TBI (Figure 3 d, e, f).
Figure 1.
Gridwalk task for evaluation of sensorimotor deficits. Data represents the average number of foot faults per minute of locomotion prior to TBI, and at 2, 3, and 7 day's post-TBI. NGB mice demonstrated significant reductions in the average number of foot faults per minute of walking at all post-TBI time points when compared to WT controls (*p=.001). Sensorimotor recovery was nearly complete at 7 days post-TBI for NGB mice. n=6 (WT) n=7 (NGB); mean ± SEM.
Figure 2.
qRT-PCR study of injured cortex (right hemisphere) neuroglobin gene expression in WT mice. Y-axis is the average fold change relative to GAPDH. Data represents neuroglobin expression prior to TBI, and at 1, 3, 7, and 14 day's post-TBI. A significant (*p≤ .005) late upregulation in neuroglobin expression occurred at 7 days post-TBI when compared to mRNA levels at all other time points. n=6 at each time point; mean ± SEM.
Figure 3.
Coronal immunostained sections (50 μm) showing localization of neuroglobin protein in WT (a, b, c) and NGB mice (d, e, f) at 7 days post-TBI. Neuroglobin was present in the injured cortex and ipsilateral hippocampus of WT mice (a). NGB mice displayed neuroglobin throughout the cortical and subcortical tissues (d). The injured cortex of both WT and NGB mice demonstrated neuroglobin localization in neurons (b, e). Neuroglobin was observed in both neurons and glial cells within the injured cortex of WT mice (c), and the subcortical white matter inferior to the injury site (dashed red line) in NGB mice (f). Magnification at 1X (a, d), 10X (b, e), 40X (c), and 60X (f).
Discussion
The gridwalk task data demonstrated that NGB mice displayed a significant reduction in the number of foot faults (i.e., sensorimotor deficits) per minute walking at all post-TBI time points when compared to WT mice. This result showed a link between neuroglobin overexpression and significantly improved sensorimotor outcomes after TBI. Interestingly, this finding is in contrast to the results of a recent study conducted by Zhao et al., which showed no significant differences in recovery of sensorimotor function between NGB mice and WT mice at multiple post-TBI time points [28]. A disparity in the results may exist because Zhao et al used a 10-point neurological severity score (NNS) for evaluating post-TBI neurological dysfunction while the present study used the gridwalk task. One limitation of the NNS is that the test cannot evaluate sensorimotor deficits that occur while walking because the NNS only measures the ability of mice to walk on beams of various widths, but it does not evaluate accuracy (i.e., foot faults) of locomotion [24]. The gridwalk task is a well validated test used to assess sensorimotor function following TBI [3, 19], and since the NNS does not measure foot faults, it is less sensitive than the gridwalk task at assessing sensorimotor deficits that occur while walking [24]. In addition, differences in post-TBI sensorimotor function and recovery may have existed because our CCI injury site and impact parameters were different than those of Zhao et al.
Neuroglobin is an oxygen-binding protein that supplies oxygen to hypoxic tissue. Hypoxia commonly occurs as a secondary injury response to TBI, and many neuroprotective hypoxia-inducible genes (hypoxia-inducible factor-1 alpha, vascular endothelial growth factor, heme oxygenase-1, and erythropoietin) are upregulated after TBI to assist in promoting cell survival [1]. In conjunction with these hypoxia-inducible genes, neuroglobin expression is also known to increase in neurons responding to hypoxia [17]. This increase in neuroglobin expression protects neurons from cell death and reactive oxygen species damage [17, 18]. The qRT-PCR results indicated that there is a late, but significant, increase in neuroglobin mRNA expression 7 days post-TBI in WT mice. The subacute (i.e., 7 days post-TBI) increase in neuroglobin expression occurred later in comparison to the response of other hypoxia inducible genes that are known to be upregulated at 3 days post-TBI [1]. This delayed increase may reduce the neuroprotective potential of the endogenous neuroglobin response because significant neuron cell death occurs in adult mice at 3 days post-TBI [20]. The delayed increase in neuroglobin gene expression observed in our study at the 7 day post-TBI time point is in contrast to the findings of Di Pietro et al., which showed an acute increase in neuroglobin expression [9]. However, it is important to note that in opposition to our study design Di Pietro et al. employed rats instead of mice, and used a diffuse head injury model that induced different injury severities (i.e. mild and severe TBI). Furthermore, neuroglobin expression was quantified at different time points in comparison to our study. Differences in study design and methods make direct comparisons between the results of these studies difficult. The immunostaining performed 7 days post-TBI confirmed a greater accumulation of neuroglobin protein near the injury site and ipsilateral hippocampus when compared to the uninjured contralateral cortex and hippocampus in WT mice. The presence of neuroglobin protein within the injured cortex of WT mice coincided with the significantly increased right hemisphere expression of neuroglobin mRNA observed 7 days post-TBI. NGB mice demonstrated neuroglobin protein throughout the brain, and neuroglobin was localized in neurons and glial cells of both WT and NGB mice. Our immunostaining results are in agreement with the findings of DellaValle, et al., who showed similar in vivo neuroglobin localization in mouse models of TBI, cerebral malaria, and autoimmune encephalitis [8].
Neuroglobin has multiple neuroprotective effects that operate by different mechanisms, and several studies suggest that neuroglobin may positively affect TBI outcomes. Neuroglobin inhibits the intrinsic apoptosis pathway by maintaining cytochrome c in a non-apoptotic oxidation state [11], protects neurons from nitric oxide toxicity [16], and prevents mitochondrial aggregation in hypoxic neurons [17]. In cell culture models, elevating neuronal neuroglobin reduced oxidative stress and increased intracellular adenosine tri-phosphate (ATP) by activating mitochondrial ATP sensitive potassium channels [2]. Furthermore, neuroglobin is known to positively affect metal homeostasis in neurons during hypoxic conditions. Hypoxic neurons display increased intracellular concentrations of calcium, iron, copper, and zinc [10]. Increased accumulation of these metals promotes inflammation, mitochondrial dysfunction, uncontrolled reactive oxygen species production, altered neurotransmitter release, neurotoxicity, and cell death [10]. Neuroglobin inhibits calcium influx, reduces cellular uptake of iron, copper, and zinc, and inhibits both necrosis and apoptosis [10]. The mechanisms underlying modulation of metal homeostasis by neuroglobin in response to hypoxia have not been clearly defined.
Based upon our observations and prior studies, we postulate that increasing neuroglobin expression prior to 7 days post-TBI would maximize its potential for neuroprotection, thereby improving sensorimotor outcomes. Since neuroglobin is an intracellular protein that is not capable of crossing cell membranes, direct administration of neuroglobin is not a practical treatment intervention [15]. However, previous research has demonstrated that endogenous neuroglobin production can be upregulated pharmacologically by deferoxamine, cinnamic acid, and valproic acid [15]. Deferoxamine is an iron chelator known to increase hemin (ferric protoporphyrin IX), an oxidation product of heme [15]. Hemin initiates transcription and translation of neuroglobin in neurons via the soluble guanylate cyclase-protein kinase G (sGC-PKG) signal transduction pathway [29]. In addition, Deferoxamine induces neuroglobin expression by increasing levels of hypoxia-inducible factors (HIF-1α and HIF-2α) in cortical neurons [15, 25, 29]. Cinnamic acid and valproic acid induce neuroglobin protein expression in cultured neurons in vitro [15], but the mechanisms by which these small molecules enhance neuroglobin production have not been determined. Currently, no in vivo TBI studies exist investigating whether administration of cinnamic acid or valproic acid induces neuroglobin in the brain. Increasing neuroglobin via pharmacological treatments during the acute period after TBI may improve sensorimotor outcomes. More research is clearly warranted to determine whether pharmacological treatments are effective, and to define the optimal therapeutic window for neuroprotection after TBI.
Supplementary Material
Highlights.
Sensorimotor outcomes were improved post-TBI in transgenic neuroglobin mice
Neuroglobin gene expression increased 7 days post-TBI in wild-type mice
Neuroglobin protein is present throughout the brain of transgenic neuroglobin mice
Increasing neuroglobin early after TBI may improve outcomes
Acknowledgments
This project was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103418. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. This work was also supported by an award from the University of Kansas Alzheimer's Disease Center (P30 AG035982) and the Landon Center on Aging.
References
- 1.Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009;26:1557–1566. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antao ST, Duong TT, Aran R, Witting PK. Neuroglobin overexpression in cultured human neuronal cells protects against hydrogen peroxide insult via activating phosphoinositide-3 kinase and opening the mitochondrial K(ATP) channel. Antioxidants & redox signaling. 2010;13:769–781. doi: 10.1089/ars.2009.2977. [DOI] [PubMed] [Google Scholar]
- 3.Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 4.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 5.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 6.Chuang PY, Conley YP, Poloyac SM, Okonkwo DO, Ren D, Sherwood PR, Hravnak M, Alexander SA. Neuroglobin genetic polymorphisms and their relationship to functional outcomes after traumatic brain injury. J Neurotrauma. 2010;27:999–1006. doi: 10.1089/neu.2009.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colicos MA, Dixon CE, Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- 8.DellaValle B, Hempel C, Kurtzhals JA, Penkowa M. In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis. Glia. 2010;58:1220–1227. doi: 10.1002/glia.21002. [DOI] [PubMed] [Google Scholar]
- 9.Di Pietro V, Lazzarino G, Amorini AM, Tavazzi B, D'Urso S, Longo S, Vagnozzi R, Signoretti S, Clementi E, Giardina B, Lazzarino G, Belli A. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic Biol Med. 2014;69C:258–264. doi: 10.1016/j.freeradbiomed.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Duong TT, Witting PK, Antao ST, Parry SN, Kennerson M, Lai B, Vogt S, Lay PA, Harris HH. Multiple protective activities of neuroglobin in cultured neuronal cells exposed to hypoxia re-oxygenation injury. J Neurochem. 2009;108:1143–1154. doi: 10.1111/j.1471-4159.2008.05846.x. [DOI] [PubMed] [Google Scholar]
- 11.Fago A, Mathews AJ, Brittain T. A role for neuroglobin: resetting the trigger level for apoptosis in neuronal and retinal cells. IUBMB life. 2008;60:398–401. doi: 10.1002/iub.35. [DOI] [PubMed] [Google Scholar]
- 12.Fiocchetti M, De Marinis E, Ascenzi P, Marino M. Neuroglobin and neuronal cell survival. Biochim Biophys Acta. 2013;1834:1744–1749. doi: 10.1016/j.bbapap.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg DA, Jin K, Khan AA. Neuroglobin: an endogenous neuroprotectant. Curr Opin Pharmacol. 2008;8:20–24. doi: 10.1016/j.coph.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hundahl CA, Allen GC, Hannibal J, Kjaer K, Rehfeld JF, Dewilde S, Nyengaard JR, Kelsen J, Hay-Schmidt A. Anatomical characterization of cytoglobin and neuroglobin mRNA and protein expression in the mouse brain. Brain Res. 2010;1331:58–73. doi: 10.1016/j.brainres.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Jin K, Mao XO, Xie L, John V, Greenberg DA. Pharmacological induction of neuroglobin expression. Pharmacology. 2011;87:81–84. doi: 10.1159/000322998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin K, Mao XO, Xie L, Khan AA, Greenberg DA. Neuroglobin protects against nitric oxide toxicity. Neurosci Lett. 2008;430:135–137. doi: 10.1016/j.neulet.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan AA, Mao XO, Banwait S, DerMardirossian CM, Bokoch GM, Jin K, Greenberg DA. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008;22:1737–1747. doi: 10.1096/fj.07-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Wu Y, Ren C, Lu Y, Gao Y, Zheng X, Zhang C. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins. 2011;79:115–125. doi: 10.1002/prot.22863. [DOI] [PubMed] [Google Scholar]
- 19.Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 21.Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang A, Feng X, Wang H, Wang J, Hang X, Yang Y, Wang Z, Zhou D. Neuroglobin upregulation offers neuroprotection in traumatic brain injury. Neurol Res. 2012;34:588–594. doi: 10.1179/1743132812Y.0000000052. [DOI] [PubMed] [Google Scholar]
- 23.Shang A, Liu K, Wang H, Wang J, Hang X, Yang Y, Wang Z, Zhang C, Zhou D. Neuroprotective effects of neuroglobin after mechanical injury. Neurol Sci. 2012;33:551–558. doi: 10.1007/s10072-011-0772-4. [DOI] [PubMed] [Google Scholar]
- 24.Shelton SB, Pettigrew DB, Hermann AD, Zhou W, Sullivan PM, Crutcher KA, Strauss KI. A simple, efficient tool for assessment of mice after unilateral cortex injury. J Neurosci Methods. 2008;168:431–442. doi: 10.1016/j.jneumeth.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian SF, Yang HH, Xiao DP, Huang YJ, He GY, Ma HR, Xia F, Shi XC. Mechanisms of neuroprotection from hypoxia-ischemia (HI) brain injury by up-regulation of cytoglobin (CYGB) in a neonatal rat model. J Biol Chem. 2013;288:15988–16003. doi: 10.1074/jbc.M112.428789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Z, Liu N, Liu J, Yang K, Wang X. Neuroglobin, a Novel Target for Endogenous Neuroprotection against Stroke and Neurodegenerative Disorders. International journal of molecular sciences. 2012;13:6995–7014. doi: 10.3390/ijms13066995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao S, Yu Z, Zhao G, Xing C, Hayakawa K, Whalen MJ, Lok JM, Lo EH, Wang X. Neuroglobin-overexpression reduces traumatic brain lesion size in mice. BMC Neurosci. 2012;13:67. doi: 10.1186/1471-2202-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Sun Y, Jin K, Greenberg DA. Hemin induces neuroglobin expression in neural cells. Blood. 2002;100:2494–2498. doi: 10.1182/blood-2002-01-0280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.