Abstract
Neutrophil infiltration is a prominent feature in a number of pathologic conditions affecting horses including recurrent airway obstruction, ischemia-reperfusion injury, and laminitis. Cell signaling components involved in neutrophil migration represent targets for novel anti-inflammatory therapies. In order to migrate into tissue, neutrophils must respond to chemoattractant signals in their external environment through activation of adhesion receptors (i.e. integrins) and reorganization of the actin cytoskeleton. Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS), a highly conserved actin-binding protein, has a well demonstrated role in cytoskeletal dependent cellular functions (i.e. adhesion, spreading, and migration), but the details of MARCKS involvement in these processes remain vague. We hypothesized that MARCKS serves as a link between the actin cytoskeleton and integrin function in neutrophils. Using a MARCKS-specific inhibitor peptide known as MANS on equine neutrophils in vitro, we demonstrate that inhibition of MARCKS function significantly attenuates β2-integrin-dependent neutrophil functions including migration, adhesion, and immune complex-mediated respiratory burst. The MANS peptide did not, however, inhibit the β2-integrin-independent PMA mediated respiratory burst. These results attest to the essential role of MARCKS function in regulating neutrophil responses, and strongly implicate MARCKS as a potential regulator of β2-integrins in neutrophils.
Keywords: Neutrophil, Migration, Adhesion, Respiratory burst, Beta2-integrin, Inflammation
1. Introduction
Although they are essential for normal host defense, neutrophils feature prominently in the pathophysiology of a number of important equine diseases, including laminitis, ischemia-reperfusion injury and recurrent airway obstruction (Moore et al., 1995; Gerard et al., 1999; Little et al., 2005; Marinkovic et al., 2007; de la Rebiere de Pouyade and Serteyn, 2011). Mechanisms of neutrophil-mediated tissue injury include release of proteolytic enzymes and production of reactive oxygen species (Wong et al., 2012). Despite ample research efforts directed toward understanding neutrophil recruitment, activation, and mechanisms of injury, clinically applicable treatments for neutrophil-mediated diseases remain limited.
Neutrophils in systemic circulation are recruited to sites of tissue infection or inflammation by host- or bacterial-derived chemoattractants; first adhering to the luminal surface of post-capillary venules, then moving across the endothelium, and finally crawling through the extracellular matrix to their final destination (Colditz, 1985; Baggiolini, 1998). To accomplish this arduous journey, neutrophils must recognize, interpret, and physically migrate along a chemokine gradient through a process known as chemotaxis. Neutrophil chemotaxis is a complex process that requires coordinated participation of essential cell surface receptors, a hierarchy of secondary cell signaling molecules and dynamic restructuring of the actin cytoskeleton (Dillon et al., 1988; Foxman et al., 1997; Cicchetti et al., 2002). Thus, inhibition of cellular regulators of neutrophil chemotaxis could be utilized to prevent or minimize unwanted neutrophil accumulation in tissues, and are therefore potential targets for novel anti-inflammatory therapies.
In order to move from the vasculature to sites of tissue inflammation, neutrophils must adhere to, and crawl along, inflamed endothelium via an integrin-dependent process (Kurtel et al., 1992). Integrins are transmembrane receptors that consist of non-covalently bound heterodimers of α and β chains. While neutrophils express several integrin heterodimers from the β1, β2 and β3 families, intraluminal adhesion and migration are dependent on activation of β2-integrins specifically (Schmidt et al., 2013). There are three key steps to activation of β2-integrins in neutrophils. (1) Increased surface expression of β2-integrins is achieved when secretory vesicles, which contain high numbers of preformed β2-integrins on their membranes, fuse with the neutrophil plasma membrane during exocytosis. (2) Intermediate and high-affinity conformations of β2-integrins are induced by chemoattractant binding to G-protein coupled receptors (“inside-out” signaling) or by direct integrin-ligand binding (“outside-in” signaling). (3) Increased binding avidity occurs when integrins are released from their cytoskeletal constraints and are able to diffuse throughout the cell membrane, resulting in formation of clusters (Nishida et al., 2006; Schymeinsky et al., 2007).
While many of the signaling details regulating integrin affinity and avidity remain unclear, PKC-mediated release of cytoskeletal constraints is known to play a key role in β2-integrin activation (Springer, 1990; Hynes, 1992; Clark and Brugge, 1995; Rosales and Juliano, 1995; Zhou and Li, 2000; Larsson, 2006). As a prominent PKC substrate and actin-binding protein, the MARCKS protein (Myristoylated Alanine Rich C-Kinase Substrate) has been proposed as a key link between PKC, actin, and integrin molecules (Aderem, 1992; Hartwig et al., 1992a; Blackshear, 1993; Arbuzova et al., 2002). Indeed, previous research from our laboratory has demonstrated that inhibition of MARCKS function attenuates the β2-integrin-dependent processes of migration and adhesion in human neutrophils in vitro (Eckert et al., 2009). In the current study, our goal was to further investigate the potential link between β2-integrin-dependent neutrophil functions and MARCKS. To this end, we measured the β2-integrin-dependent neutrophil functions of migration, adhesion and respiratory burst in vitro, with and without MARCKS inhibition. These data were compared to results from similar experiments conducted with or without β2-integrin-specific inhibition. Equine neutrophils were utilized in order to gain comparative species data to complement our previous study, to expand on our previous results with a human-relevant animal model, and to conduct research relevant to veterinary species, as well as humans.
To block MARCKS function, we utilized the MARCKS-specific inhibitor peptide known as “MANS” (myristoylated n-terminal sequence) as previously described (Singer et al., 2004; Takashi et al., 2006; Eckert et al., 2009; Li et al., 2013, Ott et al., 2013). RNS (random n-terminal sequence), which is a scrambled version of the same 24 amino acids as MANS, was used as a control. To block β2-integrin function we inhibited the integrin β chain (CD18) with the F(ab1)2 portion of an anti-CD18 antibody (αCD18). Interestingly, these results show that inhibition of β2-integrin (using αCD18) or MARCKS (using 50 μM MANS) attenuates equine neutrophil migration, adhesion and respiratory burst to a similar degree. Our findings also demonstrate that MARCKS is essential for β2-integrin-dependent neutrophil functions, but is not essential for β2-integrin-independent functions (i.e. PMA-mediated respiratory burst) in equine neutrophils. Taken together, these results strongly suggest that MARCKS function is essential to β2-integrin-dependent processes in neutrophils. Studies are currently underway to determine which aspects of integrin activation and/or signaling are dependent on MARCKS function. Our findings support the assertion that inhibitors of MARCKS deserve further study as potential therapies for neutrophil mediated tissue injury.
2. Materials and methods
2.1. Donors and neutrophil isolation
Animal use protocols were reviewed and approved by the North Carolina State University IACUC review board. For all neutrophil experiments, 30–60 ml of whole blood was collected using heparinized syringes from the jugular vein of adult horses. As healthy members of the teaching animal unit herd at NCSU College of Veterinary Medicine, all donors were fed and housed under the same conditions and were receiving no medical treatment at the time of blood collection. Neutrophils were isolated from whole blood using Ficoll-Paque™ Plus (GE Healthcare, Sweden) density gradient centrifugation (Nauseef, 2007). Briefly, heparinized whole blood was aliquoted into 15 ml polypropylene conical tubes (Sarstedt) and allowed to settle at room temperature for 45–60 min. Up to 10 ml of leukocyte rich plasma was aspirated using a bulb syringe and layered on 5 ml of Ficoll in a separate 15 ml conical tube. Cells were then centrifuged at 1800 rpm for 20 min. The supernatant was discarded and remaining red blood cells within the cell pellet were removed by 60 s of hypotonic lysis. Isolated neutrophils (>96% by Wright’s Geimsa staining) were resuspended/washed in sterile HBSS (Cellgro, Inc.) without additives. Cell number and viability was quantified using trypan blue dye exclusion (1:1) and a manual hemocytometer count. Viability was routinely >99%. Final suspension of cells was in HBSS++ chemotaxis buffer [1× HBSS, 1 mM Ca2+, 1 mM Mg2+, 5% fetal bovine serum (Gemini bio-product)] at the indicated concentration for each experiment. With this isolation protocol, all experiments were completed within 4–6 h of blood collection. Neutrophils from individual donor horses were used for all time points and treatment conditions for each experiment (i.e. “n” represents a separate horse donor).
2.2. MARCKS inhibition using a cell permeant peptide
2.2.1. Peptide pretreatment
MANS and RNS peptides, as previously described, were synthesized by Genemed Synthesis, Inc. (San Francisco, CA, USA) (Singer et al., 2004). The sequence of MANS is identical to the first 24 amino acids of the human MARCKS protein: myristic acid-GAQFSKTAAKGEAAAERPGEAAVA. The RNS peptide is a randomly scrambled control: myristic acid-GTAPAAEGAGAEVKRASAEAKQAF. Where indicated, pretreatment of cell suspensions with indicated peptide concentrations occurred at 37 °C for 30 min. Neutrophils were confirmed viable after peptide treatment by trypan blue dye exclusion.
2.2.2. Effect of peptide without pretreatment
In addition to experiments in which neutrophils were pretreated with MANS and RNS, we also investigated the inhibitory effect of the MANS peptide without pretreatment. For these experiments, MANS and RNS (50 μM) were added to the cell suspension immediately prior to performing the assay.
2.3. Fluorescence labeling of neutrophils
For migration and adhesion experiments, isolated neutrophils (1 × 107/ml in HBSS) were incubated with the fluorescent dye calcein am (Anaspec, Fremont, CA) at 2 μg/ml for 30 min at room temperature. Cells were then centrifuged at 1000 rpm for 8 min and resuspended in HBSS++ (chemotaxis buffer) to the appropriate final experimental concentration.
2.4. β2-integrin inhibition
As a positive control for β2-integrin inhibition in both adhesion and respiratory burst assays, isolated equine neutrophils were pretreated with indicated concentrations of α-human CD18 F(ab1)2 (Ancell Corp, Bayport, MN) at 37 °C for 30 min. Anti-human IgG1 F(ab1)2 (Ancell Corp, Bayport, MN) was used as an isotype control. The F(ab1)2 fragment, as opposed to whole antibody, was utilized to avoid unintended neutrophil Fcγ receptor crosslinking and activation.
2.5. Western blotting
PMA (ACROS organics, Belgium), platelet-activating factor (PAF) (Cayman Chemical, Ann Arbor, MI) and leukotriene B4 (LTB4) (Sigma, St. Louis, MO) stimulated equine neutrophils (2.5 × 107 cells/ml) were lysed with RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 5 mM sodium pyrophosphate and 50 mM sodium fluoride) containing EDTA-free protease and phosphatase inhibitor tablets (Roche, Germany). Lysates were incubated on ice with agitation for 20 min and then cleared by centrifugation for 10 min at 9000 × g at 4 °C. Protein concentrations were determined by BCA assay (Pierce, Rockford, IL). Samples were then diluted in 5× Sample Buffer containing 2-ME and boiled for 5 min prior to storing at −20 °C and subsequent analysis by 10% SDS-PAGE (Invitrogen). Resolved samples were transferred to Immobilon-P PVDF membrane (Millipore), blocked in 5% non-fat dry milk (Labscientific, Livingston, NJ) in sterile Dulbecco’s powdered phosphate-buffered saline (PBS) (GIBCO, Gland Island, NY) for 1 h prior to overnight incubation with phospho-MARCKS or total MARCKS primary antibodies (Santa Cruz) at 4 °C. Following 2 h incubation with the appropriate HRP-conjugated secondary antibody (Santa Cruz) the PVDF membrane was developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and radiograph film.
2.6. Migration & adhesion assays
2.6.1. Neutrophil migration
The 3 micron pore size Neuro Probe ChemoTx® system consisting of a 96-well microtiter plate was used to assess neutrophil migration (Frevert et al., 1998). Each upper well was loaded with 4 × 104 calcein-labeled neutrophils in 20 μl of the appropriate media. Treatment groups were tested in triplicate. HBSS++ was used for chemotaxis; while HBSS++ with chemoattractant (concentration equal to the bottom wells) was used to measure chemokinesis. Cells were allowed to migrate for 1 h at 37 °C toward lower wells containing HBSS++, HBSS++ with chemoattractant (10 nM LTB4 or 10 nM PAF), or HBSS++ with VC (EtOH). Following the hour migration, non-migrated cells were removed from the top of the membrane with a cell scraper. Following centrifugation at 1000 rpm for 1 min, fluorescence of the bottom wells was measured (485 nm excitation, 530 nm emission) using an fMax fluorescence plate reader (Molecular Devices). Percent migration was calculated by dividing the fluorescence of the experimental bottom wells by the fluorescence of bottom wells containing 4 × 104 cells.
2.6.2. Neutrophil adhesion
To assess PMA and LTB4 mediated adhesion, 1 × 105 calcein-labeled neutrophils (100 μl) from the various treatment groups were allowed to settle for 10 min in individual wells of Immulon2HB flat bottom 96-well plates (Thermo Fischer Scientific) coated with 5% FCS. The addition of 10 ng/ml PMA per well (final concentration) or vehicle control (DMSO), or 10 nM LTB4 per well (final concentration) or vehicle control (EtOH) was followed by indicated experimental incubation times at 37°C. Treatment groups were tested in triplicate.
For insoluble immune complex (IIC)-mediated adhesion, experimental and blank wells of Immulon2HB plates were each coated with 200 μl of 100 μg/ml BSA (Sigma) and incubated overnight at 4 °C (Jones et al., 1998). The next day, wells were emptied and washed 3 times with sterile PBS, followed by the addition of either 5 μg/well (low density) or 20 μg/well (high density) rabbit-anti-BSA antibody (Sigma) in 200 μl sterile PBS. Plates were incubated at 37 °C for 2 h; then immediately prior to the assay the wells were emptied and washed three times with sterile PBS. 1 × 105 calcein-labeled neutrophils (100 μl) from the various treatment groups were then added to individual wells and incubated at 37 °C for 30 min.
Following indicated incubation times, neutrophil adhesion was assessed using an fMax fluorescence plate reader (Molecular Devices). After an initial fluorescence reading (485 nm excitation, 530 nm emission) the plates were gently dumped and washed with 150 μl sterile PBS and a second fluorescence reading was obtained. This procedure was repeated for a total of three to four washes to remove non-adhered cells. Fluorescence after each washing was divided by the initial fluorescence to calculate percent adhesion. The first wash that demonstrated less than 10% adhesion of non-stimulated cells (third wash on average) was considered the final result.
2.7. Neutrophil ROS generation
To conduct respiratory burst experiments, isolated neutrophils were resuspended in HBSS++ with 2% FCS to a final concentration of 3.0 × 106/ml. Cells were then incubated with indicated treatments at 37 °C for 30 min prior to each experiment. 3.0 × 105 cells (100 μl) from various treatment groups were placed in individual wells of 5% FBS or IIC coated Immulon HB2 plates. For PMA-stimulated respiratory burst, cells were allowed to settle for 10 min prior to the addition of dihydrorhodamine-123 (DHR-123) (Sigma) (10 μM final concentration) and PMA (100 ng/ml final concentration) (Hurley et al., 2006). In the case of IIC-mediated respiratory burst, DHR-123 was added immediately following addition of cells to the well. An fMax fluorescence plate reader (Molecular Devices) was used to measure initial fluorescence (485 nm excitation, 530 nm emission) followed by a fluorescence reading every 15 min for 120 min. Results are reported as nm fluorescence.
2.8. Statistical analysis
Data are reported as mean ± SEM. Data were analyzed by one way repeated measures ANOVA (Holm–Sidak multiple comparison testing) assuming equal variance, with p < 0.05 considered statistically significant.
3. Results
3.1. MARCKS amino-terminus is highly conserved
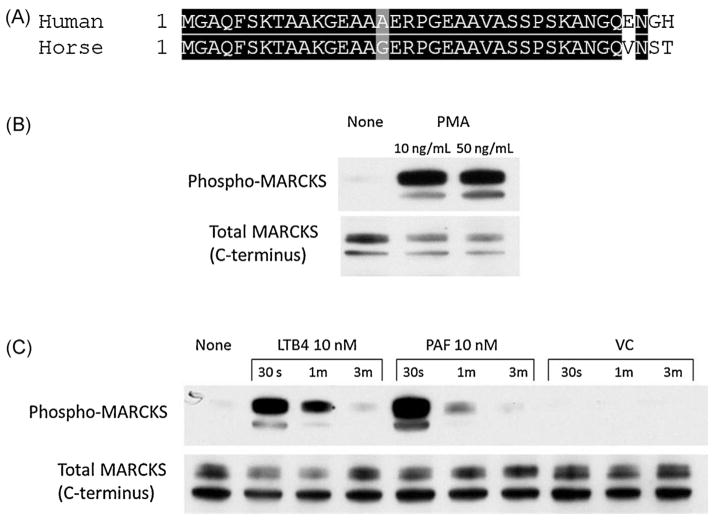
Myristoylated Alanine-Rich C Kinase Substrate (MAR-CKS) is both highly conserved and ubiquitously expressed in mammalian species (Li et al., 2013). A specific inhibitor of MARCKS, the MANS peptide, is identical to the first 24 amino acids of human MARCKS (NCBI RefSeq: NP 002347.5). In order to establish the suitability of MANS as an inhibitor of equine MARCKS, we investigated available equine reference sequences. Because there was no identifiable protein or nucleotide sequence for equine MARCKS present in GenBank, we employed the human MARCKS mRNA sequence (NCBI RefSeq: NM 002356.5) as a query to search (BLASTn) the equine genome database. This search identified a highly homologous region on chromosome 10 (EquCab2.0 scaffold 12, whole genome shotgun sequence, RefSeq: NW 001867364.1) with an E value of 0.0. Although the entire sequence homology was >80%, a significant portion of the coding region was missing from the reference genome; therefore a full length protein sequence could not be predicted. However an alignment between the N-terminus of the two sequences shows that the first 34 amino acids of the equine MARCKS protein are nearly identical to human MARCKS (Fig. 1A).
Fig. 1.
MARCKS protein is expressed in equine neutrophils. (A) Box-shade demonstrating homology of amino-terminus of human MARCKS sequence (NCBI RefSeq: NP 002347.5) with predicted amino-terminus of equine MARCKS. The equine MARCKS sequence was derived from alignment of human MARCKS mRNA (NCBI RefSeq: NW 001867364.1) with EquCab2.0 scaffold 12 whole genome shotgun sequence. Western blots of MARCKS phosphorylation in equine neutrophils stimulated with PMA (B) or cytokines LTB4 and PAF (C). Human mAbs for total MARCKS and phospho-MARCKS consistently detected a single protein dimer at a molecular weight of 50 kDa.
As a prominent protein kinase C (PKC) substrate, MARCKS is rapidly phosphorylated following neutrophil stimulation (Thelen et al., 1991). Treatment of isolated equine neutrophils with PMA resulted in rapid MARCKS phosphorylation, as detected by western blot (Fig. 1B). Equine neutrophils stimulated with known chemoattractants, leukotriene B4 (LTB4) and platelet activating factor (PAF) showed maximal MARCKS phosphorylation at 30 s with a return to baseline by 3 min (Fig. 1C). This pattern of transient phosphorylation followed by de-phosphorylation is a key feature of MARCKS’ reversible, phosphorylation-dependent association with the plasma membrane, and is thought to be an essential mechanism for MARCKS regulation of the actin cytoskeleton (Disatnik et al., 2004). Based on identifiable nucleotide sequence homology, cross-reactivity with human MARCKS antibody and a pattern of phosphorylation consistent with reports in other species, we concluded that the MARCKS protein present in equine neutrophils is structurally and functionally homologous to human MARCKS, and therefore that the MANS peptide should be an effective MARCKS inhibitor in equine neutrophils.
3.2. MANS peptide pretreatment inhibits equine neutrophil migration
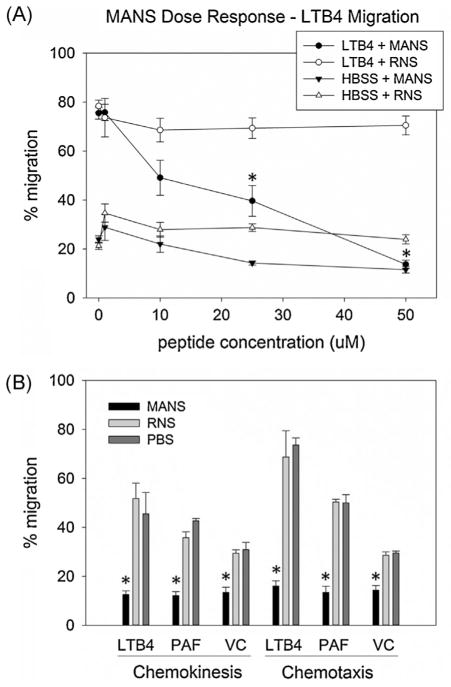
Cell permeant peptides MANS (myristoylated N-terminal sequence) and RNS (random N-terminal sequence) were used to investigate the requirement for MARCKS function in equine neutrophil migration in vitro. In isolated equine neutrophils pretreated with varying concentrations of MANS (1–50 μM), a dose dependent decrease in chemotaxis toward LTB4, was observed, starting at 10 μM. Directed migration of equine neutrophils pretreated with 50 μM MANS peptide was markedly attenuated, with only 14% migration toward 10 nM LTB4 compared to the maximal migration of 76% seen in neutrophils pretreated with PBS (“0” μM MANS) (Fig. 2A). Pretreatment of cells with up to 50 μM RNS had no effect on LTB4 mediated neutrophil chemotaxis. The IC50 of MANS, 29.3 μM, was determined by plotting the % reduction in migration of MANS vs. vehicle control treated cells, against MANS concentration (data not shown).
Fig. 2.
MANS peptide inhibits equine neutrophil migration toward multiple chemoattractants. MANS peptide demonstrated a dose dependent inhibition (A) of LTB4 stimulated equine neutrophil migration in vitro. (B) Compared to RNS 50 μM treatment, MANS 50 μM significantly inhibited both chemoattractant stimulated (gradient and non-gradient) and non-stimulated (VC) migration. Data presented as mean ± SEM, *p < 0.05 MANS vs. RNS, n = 6.
We next investigated MANS effect on both random (chemokinesis) and directed (chemotaxis) neutrophil migration using uniform and polarized gradients of LTB4 and PAF. PBS treated neutrophils responded to both cytokines (LTB4 and PAF) with significant chemotaxis (74% and 50%, respectively) and chemokinesis (45% and 42%, respectively) compared to “background migration” of cells not exposed to chemoattractant (VC). Consistent with the effects seen on chemotaxis, only 16% and 13% of neutrophils pretreated with 50 μM MANS migrated to the lower well in polarized gradients of LTB4 and PAF, respectively. Pretreatment with 50 μM MANS also inhibited equine neutrophil chemokinesis, as only 13% of LTB4-stimulated cells and 12% of PAF-stimulated migrated across the membrane (Fig. 2B). Pretreatment of equine neutrophils with 50 μM RNS had no significant effect on chemotaxis or chemokinesis compared to PBS treated control. Because MANS peptide inhibited both directed (chemotaxis) and non-directed (chemokinesis) migration, we were able to establish that MARCKS function is not limited to pathways involving the neutrophil’s ability to “sense” direction. The next aspect of neutrophil migration that we chose to investigate was adhesion.
3.3. MANS peptide pretreatment inhibits PMA- and IIC-induced adhesion
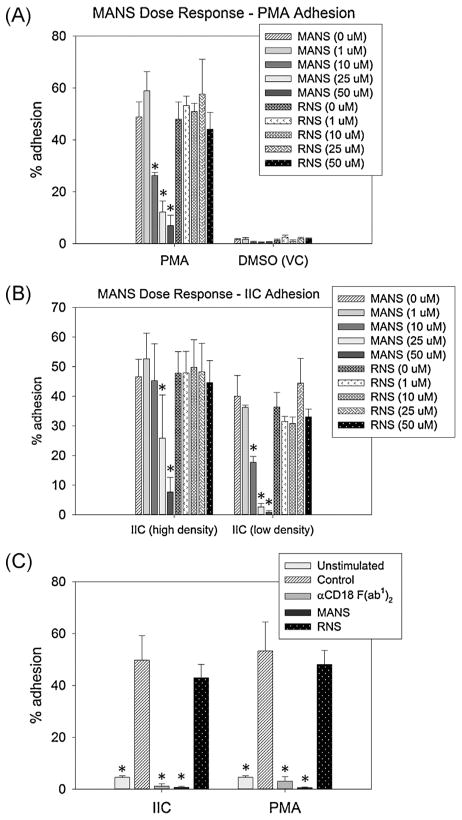
In order to migrate along activated endothelium, neutrophils must first adhere to the endothelial surface through β2-integrin (CD11a/CD18 and CD11b/CD18) dependent ligand interactions. Therefore, we next investigated whether MANS was interfering with neutrophil migration via inhibition of β2-integrin dependent adhesion. Stable neutrophil adhesion was induced with two different neutrophil activators, phorbol 12-myristate 13-acetate (PMA) or insoluble immune complexes (IIC). PMA is a synthetic mimic of diacylglycerol and a direct activator of novel and conventional PKCs, whereas IIC ligates and activates Fcγ receptors. High- and low-densities of IIC were used to investigate varying degrees of IIC-mediated neutrophil activation. Both methods induce stable neutrophil adhesion through β2-integrin activation. Treatment with PMA stimulated approximately 50% adhesion of neutrophils that received pretreatment with PBS only (MANS and RNS “0 μM”), while unstimulated cells (VC) showed less than 3% “background” adhesion. Neutrophil groups that were pretreated with 10, 25 and 50 μM MANS had 26%, 12% and 7% adherent cells following 30 min PMA treatment, respectively; which was significantly less than cells treated with VC (DMSO) (Fig. 3A). Similar results were observed with IIC-mediated adhesion. Of the PBS treated (MANS and RNS “0 μM”) neutrophils added to wells coated with either high (20 μg/well) or low (5 μg/well) density IIC, approximately 47% and 38% adhered, respectively. On high density IIC coated wells, only 8% of 50 μM MANS pretreated neutrophils adhered, a significant reduction compared to PBS treated cells. On low density IIC coated wells, 25 and 50 μM MANS treatment significantly inhibited neutrophil adhesion (3% and 1%, respectively) compared to PBS treated cells (Fig. 3B).
Fig. 3.
MANS peptide inhibits β2-integrin dependent equine neutrophil adhesion. MANS peptide, but not RNS, demonstrated a dose dependent inhibition of PMA- (A) and IC-mediated (B) equine neutrophil adhesion in vitro. (C) Both MANS 50 μM and αCD18 F(ab1)2 (30 μg/ml shown) significantly inhibited PMA- and IIC-mediated equine neutrophil adhesion. Data presented as mean ± SEM, *p < 0.05 vs. PBS (“MANS 0 μM”), n = 6.
We next examined the effect of 50 μM MANS pretreatment, along with specific inhibition of β2-integrins, on PMA- and IIC-mediated equine neutrophil adhesion. As shown in Fig. 3C, less than 1% of neutrophils treated with 50 μM MANS remained adherent following PMA or IIC-activation, significantly less than the nearly 50% adhesion observed with PBS (MANS “0 μM”) treated cells. A similar attenuation of adhesion was seen with specific β2-integrin inhibition using either 10 μg/ml (data not shown) or 30 μg/ml αCD18 F(ab1)2 pretreatment (Fig. 3C).
3.4. MANS inhibits neutrophil adhesion and migration without pretreatment
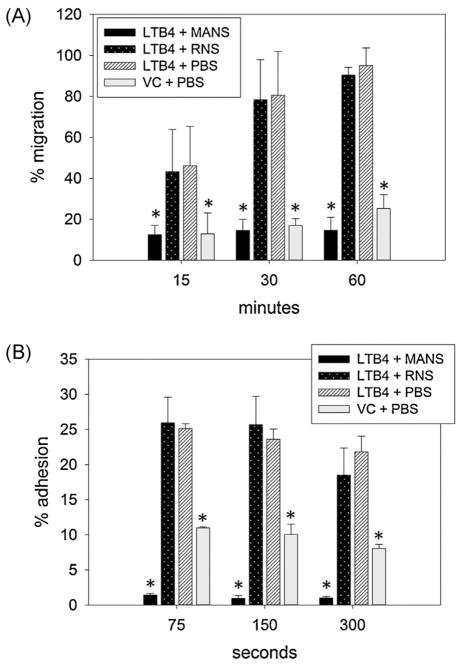
The clinical benefit of minimizing neutrophil mediated tissue injury has been demonstrated in animal models of sepsis, ischemia-reperfusion injury, acute and chronic airway disease and arthritis (Kurtel et al., 1992; Fujishima and Aikawa, 1995; Moore et al., 1995; Kraan et al., 2000; Cazzola et al., 2012; Uriarte et al., 2013; Berger et al., 2014). However, most patients that would benefit from therapies targeting neutrophil migration would not realistically have an opportunity for “pretreatment” prior to neutrophil activation. With this in mind, the next set of experiments investigated MANS effect on neutrophil migration and adhesion without pretreatment. Using 3 micron pore sized chemotaxis plates we measured directed migration of calcein-labeled equine neutrophils toward LTB4 at 15, 30 and 60 min. At these respective time points, 46%, 80% and 95% of PBS treated neutrophils migrated to the lower wells. These percentages were not significantly different in RNS treated cells. However, 50 μM MANS peptide, even without pretreatment, significantly inhibited LTB4 induced migration at all three time points when compared to treatment with RNS (Fig. 4A). We next investigated whether MANS peptide, without pretreatment, would inhibit the rapid and transient adhesion induced by chemoattractant activation. The percentage of PBS treated equine neutrophils adherent at 75, 150 and 300 s following LTB4 stimulation was 25%, 24% and 22%, respectively. Even without pretreatment, MANS inhibition proved to be extremely rapid, with a significant inhibition of LTB4-induced adhesion seen at all three time points. There was no difference in percent adhesion with RNS treatment. Interestingly, the percent adhesion of MANS treated cells was also significantly less than the average unstimulated adhesion of approximately 10% seen in unstimulated (VC) neutrophils (Fig. 4B).
Fig. 4.
MANS peptide inhibits migration and adhesion without pretreatment. Addition of peptide concurrent with LTB4 stimulation demonstrated that MANS 50 μM rapidly inhibits equine neutrophil adhesion (A) and migration (B). Data presented as mean ± SEM, *p < 0.05 vs. LTB4 + PBS, n = 6.
3.5. MANS inhibits β2-integrin dependent respiratory burst
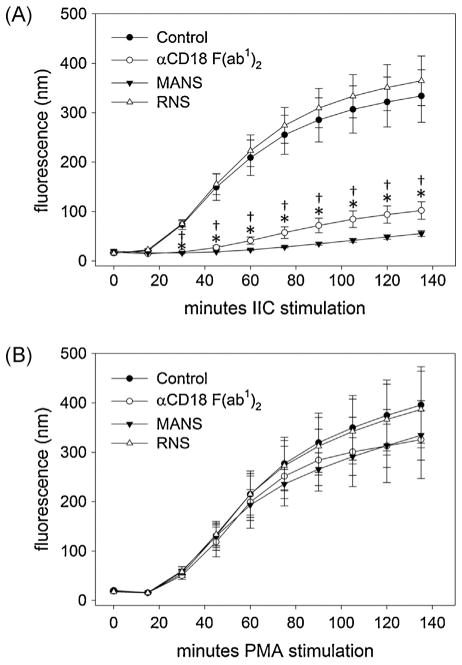
Our findings to this point clearly demonstrate similar effects of MARCKS inhibition and β2-integrin inhibition on neutrophil migration and adhesion. Therefore, we chose to further investigate the apparent link between MARCKS and β2-integrins by determining the effect of MANS on generation of reactive oxygen species (i.e. H2O2) stimulated in a β2-integrin dependent (IIC) or independent (PMA) manner. With both PMA and IIC stimulation, DHR-123 detected a robust and sustained production of intracellular H2O2 in equine neutrophils for over 2 h (Fig. 5A and B). Inhibition of β2-integrin with αCD18 F(ab1)2 caused an average 70% reduction in the IIC-mediated respiratory burst compared to untreated cells (Fig. 5A), while PMA-mediated respiratory burst was unaffected (Fig. 5B). Similar to αCD18 F(ab1)2, pretreatment of equine neutrophils with MANS peptide significantly attenuated IIC-mediated respiratory burst (approximately 80% inhibition compared to UTC), but had no effect on PMA-mediated respiratory burst. This result demonstrates that MANS treatment does not interfere with the assembly of the NADPH complex or generation of ROS, but with activation and/or signaling upstream of PKC activation in a manner consistent with inhibition of β2-integrins.
Fig. 5.
MANS peptide inhibits β2-integrin dependent respiratory burst. MANS peptide (50 μM) significantly inhibited IC-stimulated respiratory burst (A), but had no significant effect on respiratory burst stimulated by PMA (B). As demonstrated by inhibition of β2-integrin with αCD18 F(ab1)2 (30 μg/ml), IC-stimulated respiratory burst is β2-integrin dependent, while PMA-stimulated respiratory burst is β2-integrin independent. Respiratory burst was measured by addition of DHR-123. Data presented as mean ± SEM, *p < 0.05 MANS vs. control, †p < 0.05 αCD18 F(ab1)2 vs. control, n = 6.
4. Discussion
The current study was conducted to investigate the potential link between β2-integrins and the actin binding protein MARCKS, in equine neutrophils. Consistent with our previous findings in human neutrophils (Eckert et al., 2009), these results confirm that inhibition of MARCKS with the MANS peptide attenuates β2-integrin dependent migration (Fig. 2) and adhesion (Fig. 3) of equine neutrophils in vitro. Using LTB4 to stimulate migration and adhesion, we further demonstrate that MANS is an effective inhibitor of these neutrophil functions in vitro, even without pretreatment. Finally, we report that MARCKS function is necessary for IIC-mediated respiratory burst, but is not required for the β2-integrin independent process of PMA-mediated respiratory burst. This novel finding suggests that MANS mediated MARCKS inhibition disrupts β2-integrin signaling upstream of PKC activation. This significant discovery opens the door to exciting hypotheses regarding the role of MARCKS in integrin-dependent pathways.
Our ultimate goal with MARCKS inhibition is the development of therapeutics that will prevent or minimize neutrophil mediated tissue injury in acute and/or chronic inflammatory diseases. Most patients that would benefit from this type of treatment already have increased levels of endogenous inflammatory cytokines causing various degrees of neutrophil activation. In these clinical situations, a drug requiring pretreatment would have very limited applications. For this reason, we investigated chemotaxis and adhesion of equine neutrophils exposed simultaneously to MANS peptide and chemoattractant. Our results demonstrate that even without pretreatment, the MANS peptide rapidly inhibits equine neutrophil migration and adhesion in response to LTB4 (Fig. 4A and B). To more closely simulate conditions in vivo, future in vitro studies will determine if functions of previously activated neutrophils (i.e. stable adhesion, chemotaxis) can be disrupted by MANS treatment.
The current report adds significant information to our previous understanding of MARCKS function in neutrophils. In this study we observed that MANS peptide treatment of equine neutrophils significantly inhibits IIC-mediated respiratory burst, but fails to inhibit PMA-mediated respiratory burst. As shown by pretreatment of neutrophils with an αCD18 antibody F(ab1)2 (Fig. 5), the result seen with MANS treatment is consistent with those of β2-integrin inhibition. Insoluble IC activation of oxidative burst is triggered by FcγRIII-receptors, but this process also requires the cytoskeletal dependent activation of β2-integrins (Jones et al., 1998, 2001; Williams and Solomkin, 1999). PMA, which activates respiratory burst at the level of novel and/or conventional PKCs, is independent of β2-integrin and the actin cytoskeleton (Lehmeyer et al., 1979). Inhibition of both IIC- and PMA-mediated respiratory burst by the pan-PKC inhibitor staurosporine confirmed that both methods of stimulation rely on a PKC dependent pathway (data not shown). Because our findings demonstrate that MANS treated neutrophils are able to undergo respiratory burst when stimulated by PMA, but not by IIC, we conclude that at least one aspect of MARCKS function is essential in β2-integrin dependent processes in neutrophils between the level of β2-integrin receptors and PKC activation.
Beta2-integrins, composed of two non-covalently bound α-(CD11a, CD11b and CD11c) and β-(CD18) subunits, are trans-membrane adhesion proteins essential to neutrophil functions including adhesion, migration, degranulation, respiratory burst and phagocytosis. Prior to neutrophil activation, β2-integrins are expressed in an inactive or “bent” conformation in relatively small numbers on the cell surface, and movement within the membrane is restricted by cytoskeletal constraints. Upon neutrophil stimulation, β2-integrins undergo several changes: (1) their surface expression is increased by exocytosis of secretory vesicles, as well as tertiary and specific granules; (2) their conformation changes from low- to intermediate- or high-affinity in order to engage specific ligands and matrix proteins; and (3) release from the actin cytoskeleton leads to increased lateral mobilization and cluster formation, termed “avidity”. Neutrophil β2-integrins are unique in terms of receptors because they can originate (“outside-in”) or be the target of (“inside-out”) activation signals. During “inside-out” signaling integrins are the downstream target of intracellular signaling initiated by cell surface receptors that recognize mediators of neutrophil activation (i.e. LTB4, PAF). During “outside-in” signaling, β2-integrins engage specific ligands and initiate signaling that regulates processes from cytoskeletal rearrangement and degranulation, to cytokine production and apoptosis (Nishida et al., 2006; Ley et al., 2007; Luo et al., 2007; Schymeinsky et al., 2007). However, neither of these processes occurs independently, and the complexity of this network is why so many questions regarding the regulation and function of neutrophil β2-integrins remain unanswered. The results reported here indicate that MARCKS function is essential to β2-integrin dependent equine neutrophil functions. What remains unclear is whether that function is relevant to “outside-in” or “inside-out” β2-integrin signaling/activation, or both.
Previous reports linking MARCKS to β1-integrin dependent processes in myoblasts and oligodendrocytes clearly demonstrate that MARCKS is an essential regulator of the actin cytoskeleton downstream of integrin-mediated PKC activation (Disatnik et al., 2004; Siskova et al., 2006). While our results show that inhibition of MARCKS function with the MANS peptide disrupts β2-integrin dependent functions of adhesion, migration and respiratory burst in neutrophils, the mechanism of MANS inhibition has yet to be determined. Eckert et al., (2009) previously showed that the MANS peptide does not affect spreading or polarization of human neutrophils following PMA or fMLF stimulation, respectively, and had minimal effect on the fMLF induced rise in F-actin. Additionally, these authors showed no effect of MANS on up-regulation of CD18 following either PMA or fMLF stimulation of human neutrophils in vitro (Eckert et al., 2009). We are currently conducting studies to investigate both MARCKS role in, and the effect of MANS on, additional aspects of β2-integrin activation and signaling in neutrophils.
5. Conclusions
Viable therapeutic options are needed to treat neutrophil mediated tissue damage in human and veterinary species alike. In horses, neutrophils play a key role in the pathophysiology of significant diseases including laminitis, recurrent airway obstruction and ischemia-reperfusion injury. Research identifying key cellular regulators of neutrophil functions will assist in the development of novel anti-inflammatory therapies. Based on the promising results of the current study, as well as key in vivo findings by our colleagues (Singer et al., 2004; Takashi et al., 2006; Li et al., 2013, Ott et al., 2013), we hope to move forward with investigation of MANS or other MARCKS inhibitors as potential anti-inflammatory therapy for recurrent airway obstruction in the horse.
Acknowledgments
This study was funded by the Morris Animal Foundation Established Investigator Award (D12EQ-017) (SLJ) and by NIH R37 HL36982 (KBA). MKS received funding from the Ruth L. Kirschstein National Research Service Award T32 RR024394 and is currently supported by Office of the Director National Institutes of Health under award number 5K01OD015136. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank Dr. Jeffery Yoder for his assistance with interpretation of equine MARCKS nucleotide sequence data.
Footnotes
Conflict of interest statement
KBA holds 150,000 founders shares of a start-up biotech company, BioMarck, and serves as a scientific consultant and member of the scientific advisory board without monetary compensation. KBA receives over $100,000 yearly in research grants from National Institutes of Health (NIH) and U.S. Environmental Protection Agency. KBA is Editor-in-Chief of the American Journal of Respiratory Cell and Molecular Biology and receives a stipend of <$100,000 per year from the American Thoracic Society for this. The remaining authors have declared that no competing interests exist.
References
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71:713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MAR-CKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Berger C, Rossaint J, Van Aken H, Westphal M, Hahnenkamp K, Zarbock A. Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients. J Immunol. 2014;192:367–376. doi: 10.4049/jimmunol.1301363. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993;268:1501–1504. [PubMed] [Google Scholar]
- Cazzola M, Page CP, Calzetta L, Matera MG. Emerging anti-inflammatory strategies for COPD. Eur Respir J. 2012;40:724–741. doi: 10.1183/09031936.00213711. [DOI] [PubMed] [Google Scholar]
- Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002;13:220–228. doi: 10.1177/154411130201300302. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Colditz IG. Margination and emigration of leucocytes. Surv Synth Pathol Res. 1985;4:44–68. doi: 10.1159/000156964. [DOI] [PubMed] [Google Scholar]
- de la Rebiere de Pouyade G, Serteyn D. The role of activated neutrophils in the early stage of equine laminitis. Vet J. 2011;189:27–33. doi: 10.1016/j.tvjl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Dillon SB, Verghese MW, Snyderman R. Signal transduction in cells following binding of chemoattractants to membrane receptors. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55:65–80. doi: 10.1007/BF02896561. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Boutet SC, Pacio W, Chan AY, Ross LB, Lee CH, Rando TA. The bi-directional translocation of MARCKS between membrane and cytosol regulates integrin-mediated muscle cell spreading. J Cell Sci. 2004;117:4469–4479. doi: 10.1242/jcs.01309. [DOI] [PubMed] [Google Scholar]
- Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol. 2009;42:586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods. 1998;213:41–52. doi: 10.1016/s0022-1759(98)00016-7. [DOI] [PubMed] [Google Scholar]
- Fujishima S, Aikawa N. Neutrophil-mediated tissue injury and its modulation. Intensive Care Med. 1995;21:277–285. doi: 10.1007/BF01701489. [DOI] [PubMed] [Google Scholar]
- Gerard MP, Blikslager AT, Roberts MC, Tate LP, Jr, Argenzio RA. The characteristics of intestinal injury peripheral to strangulating obstruction lesions in the equine small intestine. Equine Vet J. 1999;31:331–335. doi: 10.1111/j.2042-3306.1999.tb03826.x. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992a;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Hurley DJ, Parks RJ, Reber AJ, Donovan DC, Okinaga T, Vandenplas ML, Peroni JF, Moore JN. Dynamic changes in circulating leukocytes during the induction of equine laminitis with black walnut extract. Vet Immunol Immunopathol. 2006;110:195–206. doi: 10.1016/j.vetimm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones SL, Knaus UG, Bokoch GM, Brown EJ. Two signaling mechanisms for activation of alphaM beta2 avidity in polymorphonuclear neutrophils. J Biol Chem. 1998;273:10556–10566. doi: 10.1074/jbc.273.17.10556. [DOI] [PubMed] [Google Scholar]
- Jones SL, Sharief Y, Chilcoat CD. Signaling mechanism for equine neutrophil activation by immune complexes. Vet Immunol Immunopathol. 2001;82:87–100. doi: 10.1016/s0165-2427(01)00350-6. [DOI] [PubMed] [Google Scholar]
- Kraan MC, de Koster BM, Elferink JG, Post WJ, Breedveld FC, Tak PP. Inhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double-blind clinical trial in fifteen patients. Arthritis Rheum. 2000;43:1488–1495. doi: 10.1002/1529-0131(200007)43:7<1488::AID-ANR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kurtel H, Tso P, Granger DN. Granulocyte accumulation in postischemic intestine: role of leukocyte adhesion glycoprotein CD11/CD18. Am J Physiol. 1992;262:G878–G882. doi: 10.1152/ajpgi.1992.262.5.G878. [DOI] [PubMed] [Google Scholar]
- Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lehmeyer JE, Snyderman R, Johnston RB., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979;54:35–45. [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li J, D’Annibale-Tolhurst MA, Adler KB, Fang S, Yin Q, Birkenheuer AJ, Levy MG, Jones SL, Sung EJ, Hawkins EC, Yoder JA, Nordone SK. A myristoylated alanine-rich C kinase substrate-related peptide suppresses cytokine mRNA and protein expression in LPS-activated canine neutrophils. Am J Respir Cell Mol Biol. 2013;48:314–321. doi: 10.1165/rcmb.2012-0278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D, Tomlinson JE, Blikslager AT. Post operative neutrophilic inflammation in equine small intestine after manipulation and ischaemia. Equine Vet J. 2005;37:329–335. doi: 10.2746/0425164054529472. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic D, Aleksic-Kovacevic S, Plamenac P. Cellular basis of chronic obstructive pulmonary disease in horses. Int Rev Cytol. 2007;257:213–247. doi: 10.1016/S0074-7696(07)57006-3. [DOI] [PubMed] [Google Scholar]
- Moore RM, Muir WW, Granger DN. Mechanisms of gastrointestinal ischemia-reperfusion injury and potential therapeutic interventions: a review and its implications in the horse. J Vet Intern Med. 1995;9:115–132. doi: 10.1111/j.1939-1676.1995.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–594. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Ott LE, Sung EJ, Melvin AT, Sheats MK, Haugh JM, Adler KB, Jones SL. Fibroblast migration is regulated by myristoylated alanine-rich C-kinase substrate (MARCKS) protein. PLoS ONE. 2013;8:e66512. doi: 10.1371/journal.pone.0066512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C, Juliano RL. Signal transduction by cell adhesion receptors in leukocytes. J Leukoc Biol. 1995;57:189–198. doi: 10.1002/jlb.57.2.189. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Schymeinsky J, Mocsai A, Walzog B. Neutrophil activation via beta2 integrins (CD11/CD18): molecular mechanisms and clinical implications. Thromb Haemost. 2007;98:262–273. [PubMed] [Google Scholar]
- Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- Siskova Z, Baron W, de Vries H, Hoekstra D. Fibronectin impedes myelin sheet-directed flow in oligodendrocytes: a role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking. Mol Cell Neurosci. 2006;33:150–159. doi: 10.1016/j.mcn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Takashi S, Park J, Fang S, Koyama S, Parikh I, Adler KB. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol. 2006;34:647–652. doi: 10.1165/rcmb.2006-0030RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Uriarte SM, Rane MJ, Merchant ML, Jin S, Lentsch AB, Ward RA, McLeish KR. Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock. 2013;39:286–292. doi: 10.1097/SHK.0b013e318282c9a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Solomkin JS. Integrin-mediated signaling in human neutrophil functioning. J Leukoc Biol. 1999;65:725–736. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]
- Wong DM, Moore RM, Brockus CW. Mechanisms of oxidative injury in equine disease. Compend Contin Educ Vet. 2012;34:E6. [PubMed] [Google Scholar]
- Zhou X, Li J. Macrophage-enriched myristoylated alanine-rich C kinase substrate and its phosphorylation is required for the phorbol ester-stimulated diffusion of beta 2 integrin molecules. J Biol Chem. 2000;275:20217–20222. doi: 10.1074/jbc.M909129199. [DOI] [PubMed] [Google Scholar]