Abstract
Although the efficacy and effectiveness of lifestyle modifications and antihypertensive pharmaceutical treatment for the prevention and control of hypertension and concomitant cardiovascular disease have been demonstrated in randomized controlled trials, this scientific knowledge has not been fully applied in the general population, especially in low-income communities. This paper summarizes interventions to improve hypertension management and describes the rationale and study design for a cluster randomized trial testing whether a comprehensive intervention program within a national public primary care system will improve hypertension control among uninsured hypertensive men and women and their families. We will recruit 1,890 adults from 18 clinics within a public primary care network in Argentina. Clinic patients with uncontrolled hypertension, their spouses and hypertensive family members will be enrolled. The comprehensive intervention program targets the primary care system through health care provider education, a home-based intervention among patients and their families (home delivery of antihypertensive medication, self-monitoring of blood pressure, health education for medication adherence and lifestyle modification) conducted by community health workers, and a mobile health intervention. The primary outcome is net change in systolic blood pressure from baseline to month 18 between intervention and control groups among hypertensive study participants. The secondary outcomes are net change in diastolic blood pressure, blood pressure control, and cost-effectiveness of the intervention. This study will generate urgently needed data on effective, practical, and sustainable intervention programs aimed at controlling hypertension and concomitant cardiovascular disease in underserved populations in low- and middle-income countries.
Hypertension in Low- and Middle-Income Countries (LMICs)
Hypertension is a global public health challenge because of its high prevalence and concomitant increase in risk of cardiovascular disease (CVD).1,2 It was estimated that 26.4% of the world adult population in 2000 had hypertension and 29.2% were projected to have hypertension by 2025.1 Hypertension is a leading global risk factor for CVD and premature death.2 Approximately 80% of the attributable burden of hypertension is in LMICs.2 In these countries, the prevalence of hypertension continues to be high and is increasing while the proportion of hypertensive patients who are aware, treated, and controlled are unacceptably low.3 Hypertension detection, treatment and control in these countries are often burdened with strained healthcare systems, limited financial resources, and lack of prioritization of chronic disease care.3
Latin America has the highest estimated prevalence of hypertension in the world.1 The Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study reported the highest prevalence of hypertension (29.0%) in Buenos Aires, Argentina.4 In addition, 35.9% of Argentines with hypertension were unaware of their status, and only 18.0% were treated and controlled.4 Hypertension explained 37.0% of all coronary heart disease and stroke events in Argentina in 2005.5
The hypertension control rate is even lower in underserved populations in Argentina. For example, even though antihypertensive drugs are delivered free of charge at public primary care clinics under the Remediar+Redes program, only 57% of uninsured hypertensive patients were treated. In those treated, almost 75% of patients received medication for ≤4 months per year, and only 11.8% received it for ≥9 months per year.6
Lifestyle Modification and Antihypertensive Medications
Randomized clinical trials have demonstrated that lifestyle modification and antihypertensive medications lower blood pressure (BP) and risk of CVD.7,8 Proven, effective lifestyle interventions exist for the prevention and treatment of hypertension, including weight loss, decreased sodium intake, increased physical activity, reduced excessive alcohol intake, increased potassium intake, and consumption of a healthy diet rich in fruits, vegetables and low-fat dairy products with reduced saturated and total fat.7 Randomized controlled trials have demonstrated that treatment with any commonly-used antihypertensive medication regimen reduces the risk of major CVD events and total mortality.8 Although the efficacy and effectiveness of lifestyle modifications and antihypertensive medications on the prevention of hypertension and consequent CVD risk have been demonstrated in randomized controlled trials, this scientific knowledge has not been fully applied in the general population, especially in LMICs.9 Therefore, there is an urgent need to implement innovative strategies to improve hypertension treatment and control in LMICs.
Interventions to Improve Blood Pressure Control
Barriers to hypertension control have been identified at the health care system, health care provider, and patient levels. Lack of access to health care, medication costs, and poor insurance coverage are major health care system-level barriers to hypertension prevention and control.9 Additional barriers include multiple competing demands on physician time and lack of reimbursement for preventive counseling.10 Provider-level barriers include lack of adherence to guidelines, willingness to accept elevated BP, and failure to prioritize BP management among multiple chronic medical issues.9 Surveys have identified many reasons for provider failure to adhere to published guidelines including uncertainty that clinical BP levels are representative of patients’ usual BP, hypertension not being a priority for the visit, and patient non-adherence with medications already prescribed.11 Patient-level barriers to BP control are primarily related to therapy adherence, and include low perceived risks of high BP, low health literacy, lack of motivation, out-of-pocket medication costs, and adverse side effects.9,12 Adherence to antihypertensive medications is difficult because they are costly, prone to side effects, and no benefit is immediately observed.13 Because barriers to hypertension control exist at multiple levels (i.e., health care system, provider, and patient), a combination of effective interventions is needed to comprehensively address hypertension treatment and control.
Interventions to address the previously described barriers have been developed and tested in clinical trials conducted primarily in high-income countries (HICs). These interventions include physician education, family-based education, self-monitoring of blood pressure, use of community health workers as part of the healthcare delivery team, and mobile health interventions14–28. Interactive physician educational programs have resulted in improvements in professional practice and BP control. After implementation of a large-scale hypertension program consisting of physician education on the use of an evidence-based clinical practice guideline, the hypertension control rate in a large healthcare delivery system almost doubled from 43.6% in 2001 to 80.4% in 2009 (p for trend <0.001).14 In a meta-analysis, physician education interventions specific to BP control have resulted in a median reduction in systolic BP of 3.3 mmHg and diastolic BP of 0.6 mmHg.15
BP lowering trials with a patient education component in multi-factorial interventions have been successful in lowering BP and controlling hypertension. A systematic review of hypertension management interventions found a median reduction of 8.1 mmHg for systolic and 3.8 mmHg for diastolic BP, as well as median 19.2% and 17.0% increases in systolic and diastolic BP control, respectively, in studies with a patient education component.15 Patient reminder strategies (encouraging patients to keep appointments or adhere to treatment) have been effective for improving BP control in hypertension.15,16 In comparison to individual education, family-based education has the added advantages of providing social support and accountability, and targets shared lifestyle changes, such as food preparation and leisure time activities. Social support has a positive impact on many chronic disease outcomes, including hypertension.17 In addition, spouses of patients with hypertension are more likely to have high BP than spouses of normotensives,18 so targeting interventions at the family could also lower BP of high risk individuals who are not the primary patient.
Home BP monitoring is an effective tool in the management of hypertension. Compared to measurements in a physician’s office or clinic, home BP monitoring minimizes the “white coat” effect and allows for frequent and multiple readings.19 Since some physicians are hesitant to treat hypertension based on clinic measurements alone, home BP monitoring can provide additional BP measurements leading to better treatment decisions. Home BP monitoring using electronic cuffs has been shown to be more effective in reducing BP and reaching target BP goals than BP monitoring in a clinical setting alone.20 A recent trial comparing home BP monitoring used for antihypertensive therapy adjustment to usual care found a 9.7 mm Hg greater reduction in systolic BP (p<0.001), a 5.1 greater reduction in diastolic BP (p<0.001), and an 18.2% greater control rate (p=0.005) in the intervention group compared to usual care after one year.21
Community health workers (CHW) can increase the capacity of an already overburdened health care system by using health care resources effectively and increasing the quality of care.22 The addition of CHW to the clinical team is an example of organizational or team change, which addresses systems-level barriers to hypertension prevention and control by simplifying the health care provider’s tasks and transferring some responsibility for patient care to another team member (task shifting). Task shifting from physicians to other team members was an important component of a large-scale hypertension program in an integrated healthcare delivery system that was able to almost double hypertension control rates in eight years, because it addressed patient barriers by reducing appointment times, providing increased scheduling flexibility, and decreasing healthcare costs.14 Team change strategies have resulted in median reductions in systolic BP of 9.7 mmHg and in diastolic BP of 4.2 mmHg in a meta-analysis.15 In addition, CHW may remove barriers to BP control and medication adherence due to cultural, educational, and language differences between community members and the health care system.23 A systematic review of randomized trials using CHW to implement BP control programs found significant improvement in 7 of 8 studies, primarily in poor, urban, minority communities.24 Trials in LMICs have also shown reductions in BP using CHW-delivered programs.25
Mobile health (mHealth), which uses mobile telecommunication and multimedia technologies for health-related purposes, has great potential for supporting behavior change. For example, in a trial of mobile phone text messages coupled with BP telemonitoring in diabetic patients with uncontrolled systolic hypertension, daytime ambulatory systolic BP decreased by 9.1 mm Hg in the intervention group vs. 1.5 mm Hg in the control group (p<0.005).26 A review of text messaging or email interventions identified 16 randomized trials of which 10 reported significant improvement in outcomes and 6 reported differences suggesting positive trends.27 In addition, mHealth trials in LMICs have demonstrated some improvements in chronic disease management.28
Recent randomized trials have shown that a comprehensive intervention strategy is more effective in hypertension control than individual components. For example, in the Hypertension Improvement Project, the greatest BP control was seen in the group with physician and patient interventions.29 Similarly, reviews examining the translation of guidelines into practice have demonstrated that isolated strategies are largely ineffective, whereas integration of multiple intervention strategies (even those that are ineffective in isolation) in the appropriate context and setting results in improved outcomes.30 Given the complex nature of barriers to hypertension prevention and control, a comprehensive, sustainable approach to interventions focusing on multiple domains is not only advantageous but necessary.7,12
Hypertension Control Program in Argentina (HCPIA)
Objectives
The overall objective of the HCPIA study is to test whether a comprehensive intervention program within a national public primary health care system in a LMIC will improve hypertension control among uninsured hypertensive patients and their families in Argentina. The specific aims of this cluster randomized trial are:
To test whether a comprehensive intervention program will lower BP among uncontrolled hypertensive patients over an 18-month period, compared to usual care;
To evaluate whether a comprehensive intervention program will improve hypertension control among hypertensive patients over an 18-month period, compared to usual care; and
To estimate the cost-effectiveness of this comprehensive intervention program compared to usual care.
Study design
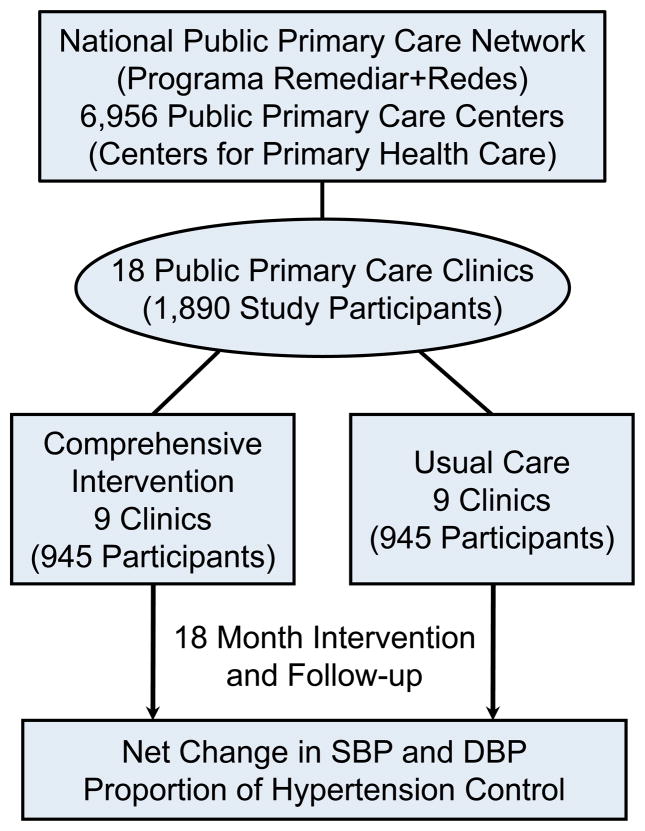
The HCPIA is a cluster randomized trial in which 18 primary care clinics (Centers for Primary Health Care, CPHC) within a national public primary care network are assigned to the intervention or control groups (Figure 1). The trial will recruit 1,890 study participants from the CPHCs: 9 clinics with approximately 945 participants in the intervention group and 9 clinics with similar participants in the control group. Men and women with uncontrolled hypertension from the participating primary care clinics and their spouses as well as adult hypertensive family members will be enrolled. The comprehensive intervention program, including health care provider education, home-based intervention among patients and their families (home delivery of antihypertensive medication, home BP monitoring, health education for medication adherence and lifestyle modification) conducted by CHWs, and a mobile health intervention, will last for 18 months. If proven effective, the study findings will be disseminated and the program will be scaled-up to the entire national public primary care network in Argentina.
Figure 1.
Study Design of the Hypertension Control Program in Argentina (HCPIA)
Study population
Eighteen CPHCs from the provinces of Buenos Aires (6 clinics), Misiones (4 clinics), Tucuman (4 clinics), Corrientes (2 clinics), and Entre Rios (2 clinics) have been selected for this trial from a national public primary care network (Remediar+Redes Program). These CPHCs provide health care to underserviced populations without health insurance and are funded by the Argentina Ministry of Health. The eligibility criteria for CPHCs are presented in Table 1. The 18 selected CPHCs, which fulfill the eligibility criteria, were matched by district and randomized to either the intervention or the control group.
Table 1.
Eligibility Criteria for Study Clinics and Participants
|
Eligibility Criteria for Study Clinics (Centers for Primary Health Care)
|
|
|
Eligibility Criteria for Study Participants
|
Inclusion Criteria:
|
Exclusion Criteria:
|
CPHC = Center for Primary Health Care
Study participants are recruited from the participating CPHCs with minimum eligibility criteria so that the intervention can be tested in “real world” clinical settings (Table 1). Each CPHC will recruit 45–50 indicated hypertensive men and women and their spouses, as well as adult family members with uncontrolled hypertension. CPHC research nurses review the clinic appointment schedules daily to identify patients with hypertension and meet with patients after their physician visit for a screening to assess their eligibility. Eligible patients are invited, along with their family, to participate in the study.
Interventions
This study tests a comprehensive intervention program addressing system, provider, and patient barriers to hypertension prevention and control by integrating individual strategies previously proven effective (Table 2). CPHCs randomized to the intervention group receive a three component intervention lasting for 18 months: physician education, home intervention delivered by CHWs, and an mHealth intervention.
Table 2.
Strategies to Overcome Barriers to Hypertension Prevention and Control
| Barrier | General approach | Specific strategy to overcome barrier |
|---|---|---|
| Systems Level | ||
|
| ||
| Insufficient time of physician/provider | Team change |
|
| Lack of reimbursement for TLC counselling | Team change |
|
| Lack of continuity of care | Team change |
|
| Limited free medications | Policy change |
|
|
| ||
| Provider Level | ||
|
| ||
| Lack of adherence to treatment guidelines, “clinical inertia” | Physician education |
|
| Uncertainty that office BP represents usual BP | Home BP monitoring |
|
|
| ||
| Patient Level | ||
|
| ||
| Poor adherence to medications | Reminders, Family-support, Patient education, Home BP monitoring |
|
| Hypertension knowledge/ risk perception | Patient education |
|
| Poor memory | Reminders, Family support, Patient education |
|
| Low health literacy | Patient education |
|
| Poor motivation | Reminders, Family-support, Patient education, Home BP monitoring |
|
| Medication costs | Policy change, Physician education, Patient education |
|
| Adverse effects | Physician education, Patient education |
|
TLC = therapeutic lifestyle change; CHW = community health worker
The physician education program consists of an online continuing education course on hypertension management, an on-site intensive training and certification, and annual distance learning modules for re-certification. The program focuses on standard treatment algorithms for stepped-care management based on hypertension guidelines,31,32 including both lifestyle modifications (weight loss, sodium reduction, physical activity, moderation of alcohol intake, and adoption of a potassium rich, DASH diet) and pharmacologic treatment (assessment of CVD risk factors and absolute risk, use of a treatment algorithm for initial drug choices, a stepped-care approach to titrating medications, and follow-up visits), and emphasizing the importance of and improvement in medication adherence.
Community health workers (CHW) serve as a source of education, motivation, and social support and as facilitators of healthcare utilization for patients and their families. CHWs are trained in facilitating behavioral change through BP monitoring, medication management, and lifestyle modifications during a 2-day interactive training session followed by an onsite field testing and certification. CHWs visit participants’ homes monthly for the first six months of the intervention and every other month thereafter. CHWs educate and train participants and their families about medication adherence, home BP monitoring, and lifestyle modification. All study participants are given a pill box and a home BP monitor. CHWs also deliver antihypertensive medications to patients’ homes and help patients to schedule appointments with primary care physicians when necessary. During follow-up home visits, CHW will provide tailored counselling to address barriers to hypertension self-management, including medication adherence, and effective behavior change.
Individualized text messages to promote lifestyle changes and reminders to reinforce medication adherence are sent out weekly. Messages are based on hypertension status and perceived barriers to behavioral change identified during CHW motivational counselling sessions and consist of motivational statements and behavior-change techniques to reinforce in-person education interventions.
CPHCs randomized to the control group continue with usual care without study intervention.
Outcomes
The primary outcome is net change in systolic BP, defined as the difference between the intervention and control groups in BP changes from baseline to termination of the intervention, in hypertensive participants. Secondary outcomes include net change in diastolic BP, the proportion of hypertensive patients with controlled hypertension (BP <140/90 mmHg), self-reported antihypertensive medication adherence among treated patients, cost-effectiveness of the intervention, and net changes in BP, body weight, and waist circumference among all participants.
Data collection
Study nurses collect all study data at baseline, 6, 12, and 18 months of follow-up in participants’ homes using standard questionnaires and measurement methods. Two visits between 1 and 14 days apart at baseline and at 18 months termination visits are conducted so that repeated BP measurements can be obtained.
The study nurses administer validated questionnaires obtaining medical history, lifestyle risk factors (e.g., smoking, alcohol drinking, diet, and physical activity), adherence to antihypertensive medications, health facility utilization, and costs.33–35 The International Physical Activity Questionnaire is used for physical activity,34 and the 8-item Morisky Medication Adherence (MMAS) questionnaire is used for medication adherence.35
Three BP measurements are obtained at each data collection visit, and the mean of the three measurements will be used. BP is measured according to a standard protocol recommended by the American Heart Association.36 BP is measured with the participant in a seated position after 5 minutes of quiet rest. In addition, participants are advised to avoid alcohol, cigarettes, coffee/tea and exercise for at least 30 minutes before their BP measurement. An auto-BP cuff (Intellisense Digital Blood Pressure Monitor; model: OMRON HEM-907 XL) is used and one of four cuff sizes (pediatric, regular adult, large or thigh) is chosen on the basis of each participant’s arm circumference.
Study nurses take anthropometric measurements on individuals in light clothing without shoes using a standard protocol. Two measurements are obtained at each visit for weight, height, and waist circumference and the arithmetic means will be used for analyses.
Data analysis and statistical power
We will test the primary research hypothesis that there is a greater reduction in mean systolic BP in the intervention group than in the control group using mixed-effects regression analysis with participants and clinics included as random effects and intervention group, time, and the group-by-time interaction as estimable fixed effects. Results from the primary analyses among individuals who completed the entire study will be compared to results from a conservative strategy where all missing values are replaced by the baseline values, with a resulting net change of 0 mm Hg in BP. Logistic regression analyses will be used for categorical secondary outcomes. Since we are measuring subjects at multiple time points, we will use GEE for these analyses.
The proposed trial is designed to provide 80% statistical power to detect a ≥3.86 mmHg reduction in systolic BP at a significance level of 0.05 using a 2-tailed test. An 85% follow-up rate, an inter-cluster correlation of 0.06 and a standard deviation of 10.0 mm Hg are assumed, and the cluster design is taken into consideration in the power calculation.
Approval has been obtained from the Institutional Review Boards of both Tulane University and the Institute of Clinical Effectiveness and Health Policy. The proposed study is conducted following strict guidelines for the protection of the rights of human volunteers. Informed consent is signed by all participants during screening.
Conclusion
The HCPIA trial is designed with an implementation focus and has several unique aspects. We have taken advantage of intervention strategies previously proven effective to develop a comprehensive intervention program, which will be tested and implemented in a national public primary care network in Argentina. The home-based intervention will target the family members of hypertensive patients who are at high risk for hypertension. Family-based interventions are especially effective for lifestyle change and provide social support for adherence to intervention programs. This study will use a mobile health intervention for supporting behaviour change. This study will employ CHWs to implement the intervention program, which is a practical and sustainable approach for large-scale and long-term interventions. This study targets the uninsured population and may, thereby, reduce health disparities in hypertension prevention and control. The results from the proposed study, if effective, will have an immediate impact in the real world through the dissemination and scale-up of the intervention program to the entire national public primary care network in Argentina.
This study will generate urgently needed data on effective, practical, and sustainable intervention programs aimed at control of hypertension and concomitant CVD. The results from this trial may be directly used by other primary care settings and healthcare systems in LMICs for the prevention and control of hypertension.
Acknowledgments
The Hypertension Control Program in Argentina is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (U01HL114197) under the Global Alliance for Chronic Diseases programme.
Footnotes
Disclaimers: None
References
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet. 2012;380:611–9. doi: 10.1016/S0140-6736(12)60861-7. [DOI] [PubMed] [Google Scholar]
- 4.Schargrodsky H, Hernandez-Hernandez R, Champagne BM, et al. CARMELA: Assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121:58–65. doi: 10.1016/j.amjmed.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein A, Colantonio L, Bardach A, et al. Estimation of the burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of preventative interventions to reduce this burden in Argentina. BMC Public Health. 2010;10:627. doi: 10.1186/1471-2458-10-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernztein RDI. Uso de medicamentos en hipertensión arterial en el primer nivel de atención pública argentina: La experiencia del programa remediar. Revista Argentina de Cardiología. 2009;77:187–195. [Google Scholar]
- 7.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: Clinical and public health advisory from the national high blood pressure education program. JAMA. 2002;288:1882–8. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PKHJ. Blood pressure reduction. In: Buring JE, Manson JE, Ridker PM, editors. Clinical trials in cardiovascular disease. 2. Philadelphia: W.B. Saunders; 2004. [Google Scholar]
- 9.Walsh JM, Sundaram V, McDonald K, et al. Implementing effective hypertension quality improvement strategies: Barriers and potential solutions. J Clin Hypertens (Greenwich) 2008;10:311–6. doi: 10.1111/j.1751-7176.2008.07425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: A model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166–71. [PubMed] [Google Scholar]
- 11.Lin ND, Martins SB, Chan AS, et al. Identifying barriers to hypertension guideline adherence using clinician feedback at the point of care. AMIA Annu Symp Proc. 2006:494–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens (Greenwich) 2008;10:644–6. doi: 10.1111/j.1751-7176.2008.08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borzecki AM, Oliveria SA, Berlowitz DR. Barriers to hypertension control. Am Heart J. 2005;149:785–94. doi: 10.1016/j.ahj.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe MG, Lee GA, Young JD, et al. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: A systematic review. Med Care. 2006;44:646–57. doi: 10.1097/01.mlr.0000220260.30768.32. [DOI] [PubMed] [Google Scholar]
- 16.Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: A systematic review. Br J Gen Pract. 2010;60:e476–88. doi: 10.3399/bjgp10X544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker B, Szalai JP, Paquette M, et al. Marital support, spousal contact and the course of mild hypertension. J Psychosom Res. 2003;55:229–33. doi: 10.1016/s0022-3999(02)00551-2. [DOI] [PubMed] [Google Scholar]
- 18.Pyke SD, Wood DA, Kinmonth AL, et al. Change in coronary risk and coronary risk factor levels in couples following lifestyle intervention. The British Family Heart Study. Arch Fam Med. 1997;6:354–60. doi: 10.1001/archfami.6.4.354. [DOI] [PubMed] [Google Scholar]
- 19.Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: A systematic review. Am J Hypertens. 2011;24:123–34. doi: 10.1038/ajh.2010.194. [DOI] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Kerry SM, Forbes L, et al. Blood pressure control by home monitoring: Meta-analysis of randomised trials. BMJ. 2004;329:145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized trial. JAMA. 2013;310:46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witmer A, Seifer SD, Finocchio L, et al. Community health workers: Integral members of the health care work force. Am J Public Health. 1995;85:1055–8. doi: 10.2105/ajph.85.8_pt_1.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownstein JN, Bone LR, Dennison CR, et al. Community health workers as interventionists in the prevention and control of heart disease and stroke. Am J Prev Med. 2005;29:128–33. doi: 10.1016/j.amepre.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435–47. doi: 10.1016/j.amepre.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Cappuccio FP, Kerry SM, Micah FB, et al. A community programme to reduce salt intake and blood pressure in ghana [ISRCTN88789643. BMC Public Health. 2006;6:13. doi: 10.1186/1471-2458-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan AG, Irvine MJ, McIsaac WJ, et al. Effect of home blood pressure monitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60:51–57. doi: 10.1161/HYPERTENSIONAHA.111.188409. [DOI] [PubMed] [Google Scholar]
- 27.Wei J, Hollin I, Kachnowski S. A review of the use of mobile phone text messaging in clinical and healthy behaviour interventions. J Telemed Telecare. 2011;17:41–8. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- 28.Beratarrechea A, Lee AG, Wilner JM, et al. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemed J E Health. 2014;20:75–82. doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svetkey LP, Pollak KI, Yancy WS, Jr, et al. Hypertension improvement project: Randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009;54:1226–33. doi: 10.1161/HYPERTENSIONAHA.109.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325:925. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. Latin American Expert Group. J Hypertens. 2009;27:905–22. doi: 10.1097/HJH.0b013e32832aa6d2. [DOI] [PubMed] [Google Scholar]
- 33.Perman G, Rossi E, Waisman GD, et al. Cost-effectiveness of a hypertension management programme in an elderly population: a Markov model. Cost Eff Resour Alloc. 2011;9:4. doi: 10.1186/1478-7547-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 35.Morisky DE, Ang A, Krousel-Wood MA, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: An AHA scientific statement from the council on high blood pressure research professional and public education subcommittee. J Clin Hypertens (Greenwich) 2005;7:102–9. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]