Abstract
CRM1 or XPO1 is the major nuclear export receptor in the cell, which controls the nuclear-cytoplasmic localization of many proteins and RNAs. CRM1 is also a promising cancer drug target as the transport receptor is overexpressed in many cancers and quite a few of its cargos are misregulated in the diseases and hence mislocalized to the cytoplasm. Atomic level understanding of CRM1 function has greatly facilitated recent drug discovery and development of CRM1 inhibitors to target a variety of malignancies. Numerous atomic resolution CRM1 structures are now available, explaining how the exporter recognizes nuclear export signals in its cargos, how RanGTP and cargo bind with positive cooperativity, how RanBP1 causes release of export cargos in the cytoplasm and how diverse inhibitors such as Leptomycin B and the new KPT-SINE compounds block nuclear export. This review summarizes the structure-function studies that explain CRM1-cargo recognition, release and inhibition.
Keywords: Nuclear export, NES, Karyopherin, Exportin, nuclear pore complex
1. Introduction
Nucleocytoplasmic transport is an essential process that governs the localization of numerous proteins and RNAs in eukaryotic cells. While small molecules can diffuse passively across through the nuclear pore complex (NPC), the majority of macromolecular transport across the nuclear envelope is mediated by nuclear transport receptors of the Karyopherin β (Kap) family. There are 19 known Kaps in human cells and they include import receptors called Importins, export receptors called Exportins and bidirectional Kaps that have both Importin and Exportin functions [1–9]. Kap proteins share low sequence identities (10–20%) but have similar physical properties such as molecular weights (90–150 kDa) and isoelectric points (pI = 4.0–5.0) [10]. Nuclear transport is achieved through interactions of cargos with Kap proteins, which in turn interact with nucleoporins that make up the NPC. Kap-cargo interactions are controlled by the GTPase Ran.
There are seven known Exportins in human cells. Of these, CRM1 (Chromosome region maintenance 1, also known as Exportin-1 or XPO1) is the most general and prevalently used export receptor. Other Exportins such as Cas, Exportin-t and Exportin-5 seem to be more specialized as they primarily export the import adaptor Importin-α, tRNAs and pre-miRNAs, respectively [11–13]. CRM1 was first identified through a mutation in its gene, which caused distortion in chromosome structure in Schizosaccharomyces pombe and was thus named Chromosomal Region Maintenance 1 [14]. CRM1 was later found to be an essential nuclear export receptor [15–21]. The discovery of CRM1’s nuclear export function was also accompanied by the finding that the natural product inhibitor Leptomycin B (LMB) is a very potent and specific inhibitor of CRM1 [17, 22, 23]. LMB facilitated the identification of numerous CRM1 cargos [24].
Like other Kaps, CRM1 also uses the Ran GTPase to load and unload cargos [25]. CRM1 binds cooperatively with cargos and RanGTP to form export complexes in the nucleus, which then translocate through the NPC via CRM1-nucleoporin interactions [21, 26–30]. CRM1 recognizes its export cargos through nuclear export signals or NESs in their polypeptide chains known as classical- or leucine-rich-NESs. These export signals are stretches of 8–15 amino acids, which contain patterns of hydrophobic residues [31–35]. Approximately 300 functionally diverse CRM1 cargos have been reported in the literature and information about these NES-containing proteins are archived in databases such as NESdb and ValidNESs [24, 36]. CRM1 cargos include many tumor suppressors and cell growth regulators such as p53, BRCA1/2, FOXO3, IκBα and Survivin [37–41]. Many of these cargo proteins are misregulated and then mislocalized to the cytoplasm in cancer cells [42]. CRM1 itself is also overexpressed in several malignancies and high levels of CRM1 protein is associated with lower survival rates in the patients [43–48]. CRM1 has recently been shown to be an effective drug target for various cancers as CRM1 inhibition restores nuclear localization and nuclear functions of tumor suppressors, leading to apoptosis of the cancer cells [43, 49–63]. Atomic level understanding of CRM1 function obtained from numerous structural studies was critical in the drug discovery endeavor to target this essential cellular process.
This review focuses on the atomic basis of CRM1-mediated nuclear export. There are now 27 crystal structures of CRM1 in the Protein Data Bank (PDB) (www.rcsb.org) [64]. Collectively, this large body of work explains various aspects of CRM1 function. Here we summarize the structure-function studies that explain CRM1-cargo recognition, release and inhibition.
2. CRM1 and the Ran cycle
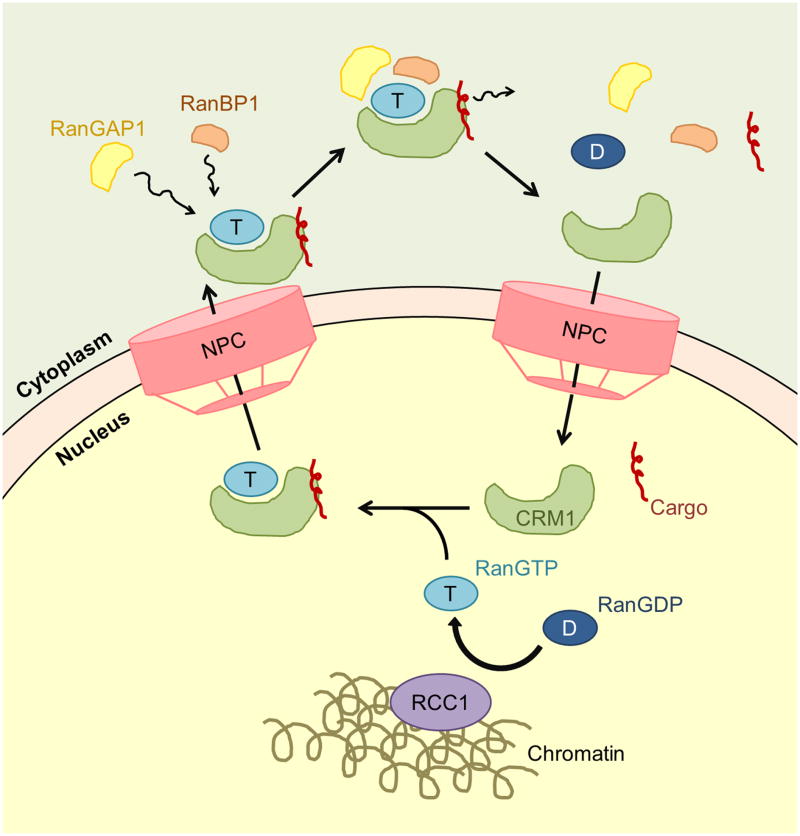
CRM1-mediated nuclear export requires the action of the small GTPase Ran. A RanGTP-RanGDP gradient is maintained across the nuclear envelope through compartmentalization of Ran regulators. Ran is predominantly in the GTP state in the nucleus because of efficient nucleotide exchange by its guanidine nucleotide exchange factor RCC1, which is tethered to chromatin through interactions with histones H2A and H2B (Fig. 1) [65–67]. In contrast, cytoplasmic Ran is predominantly in the GDP state because the GTPase-activating protein RanGAP1 that catalyzes hydrolysis of RanGTP to RanGDP is located in the cytoplasm or at the cytoplasmic fibrils of the NPC (Fig. 1) [68–70].
Figure 1. Schematic of the CRM1 nuclear export cycle.
In the nucleus, RanGTP is efficiently loaded with GTP by RCC1. RanGTP and cargo forms a complex with CRM1 and is exported through the nuclear pore complex to the cytoplasm. RanGAP1 and RanBP1 facilitate cargo release and RanGTP hydrolysis. CRM1 is then recycled back to the nucleus for another round of export.
Binary interactions of CRM1 with either RanGTP or export cargos are very weak, but CRM1 binds both ligands cooperatively to form the CRM1-cargo-RanGTP export complex (Fig. 1) [71, 72]. The loading process is further facilitated by the Ran binding protein RanBP3 through a still unknown mechanism [73, 74]. The CRM1-cargo-RanGTP export complex binds various nucleoporins in the NPC including Nup98 on the nucleoplasmic side, Nup214-Nup88 on the cytoplasmic side of the NPC and various FG repeat-containing nucleoporins [21, 26–30]. In the cytoplasm, the CRM1-cargo-RanGTP complex encounters RanBP1 and Nup358 (also known as RanBP2), which facilitate cargo release and interactions of RanGTP with RanGAP1 (Fig. 1) [75–79]. Finally, RanGAP1 catalyzes hydrolysis of RanGTP to RanGDP to end the nuclear export process and CRM1 is then recycled back to the nucleus for additional rounds of export (Fig. 1) [68].
3. A summary of CRM1 structures
Many crystal structures of CRM1 have been published in the last five years. 27 CRM1 structures are now available in the PDB [64]. CRM1 from several organisms (human, mouse, fungi C. thermophilum and S. cerevisiae) were used in these studies but CRM1 architecture appears conserved across these homologs. Structures of unliganded CRM1, a CRM1-cargo intermediate, CRM1-cargo-Ran export complexes, the post-release yeast CRM1-Ran-RanBP1 complex and several CRM1-inhibitor complexes, all provide snapshots of CRM1 in many steps of the nuclear export cycle and inform on various aspects of CRM1 function. We now have insights into the chemical basis of why unliganded CRM1 is inactive, how CRM1 recognizes NESs in the nucleus and releases them in the cytoplasm, and how various small molecule inhibitors block CRM1-mediated nuclear export.
3.1 CRM1 architecture
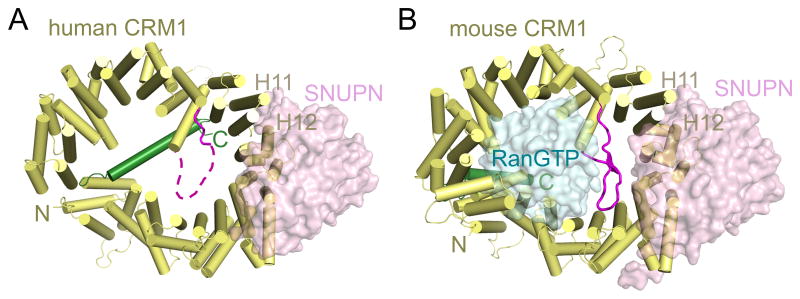
CRM1 is a 120 kDa protein that is composed of 21 HEAT repeats. HEAT repeats are helix-coil-helix tandem repeats, named after the proteins Huntingtin, Elongation factor 3 (EF3), regulatory unit A of protein phosphatase 2A (PP2A), and TOR1 that were first identified to contain these repeats [80]. HEAT repeats in Kaps are composed of antiparallel α-helices A and B, which stack and align such that the A helices form the outer convex surface and the B helices form the inner concave surface of the solenoid, ring- or spiral-shaped protein [81–83]. The structure of a small fragment of CRM1 was reported in 2004 but the first full-length CRM1 structures were reported in 2009 [72, 84, 85]. The latter are structures of CRM1-cargo and CRM1-cargo-RanGTP complexes [84, 85]. Full length CRM1 proteins adopt ring shapes with their N-terminal HEAT repeats in close proximity with their C-terminal HEAT repeats (Fig. 2A, B). The outer and inner diameters of the CRM1 rings are approximately 85 Å and 40 Å, and the ternary complex is more compact than the binary complex. Several key structural features of CRM1 deserve mention here. First, the helices of HEAT repeats 11 and 12 (abbreviated H11 and H12) form a hydrophobic groove on the outer surface of CRM1 that binds NES peptides (Fig. 2A, B) [84]. Second, the last CRM1 helix or the B helix of H21 (H21B) can adopt drastically different conformations. In the ternary CRM1-cargo-RanGTP complex, helix H21B packs next to H21A and aligns with the CRM1 ring (Fig. 2B) [85]. In the binary CRM1-cargo intermediate, H21B no longer packs against H21A but crosses the CRM1 ring with the C-terminal tail that follows H21B interacting with the inner concave surfaces of repeats H9 and H10 (Fig. 2A) [84]. Finally, the long conserved loop that connects H9A and H9B also adopts different conformations. This loop, known as the H9 loop, adopts a beta-hairpin structure in the CRM1-cargo-RanGTP complex (Fig. 2B) but is disordered in the CRM1-cargo intermediate (Fig. 2A). Conformational changes involving these key structural elements are crucial for CRM1 function and will be discussed more extensively below.
Figure 2. Architecture of full-length CRM1.
A) Crystal structures of the human CRM1-SNUPN complex (PDB ID: 3GB8) and B) the mouse CRM1-RanGTP-SNUPN complex (3GJX). The 21 HEAT repeats of CRM1 are in yellow with individual helices drawn as cylinders. The two key switch features of CRM1, the H9 Loop and the C-terminal extension (helix H21B and C-terminal tail), are in magenta and green respectively. H11 and H12 that make up the NES binding groove are labelled. SNUPN (cyan) and RanGTP (pink) are shown as surface representations.
3.2 Structures of CRM1-cargo complexes: Cargo recognition
CRM1 recognizes NES sequences in its cargos. NESs were first discovered in the cAMP-dependent protein kinase inhibitor (PKI) and the human immunodeficiency virus type 1 (HIV-1) Rev protein where short stretches of leucine-rich sequences are transplantable and sufficient to direct nuclear exit [34, 35, 86]. Yeast two-hybrid screening of randomized Rev NES established the first NES consensus of L-X(2,3)-[LIVFM]-X(2,3)-L-X-[LI] [87]. Analysis of the first 75-entry NES database named NESbase resulted in an expanded and widely used NES consensus sequence of Φ1-X(2,3)-Φ2-X(2,3)-Φ3-X-Φ4, (Φn is Leu, Ile, Val, Phe or Met, and X can be any other amino acid) [33, 88]. Based on results of a random peptide library screen, Kosugi et al. further expanded the NES consensus to accommodate additional spacings between hydrophobic NES residues thus generating multiple classes of the NES (class 1a–d, 2, 3; Table 1) [32]. To date, over 200 NES-containing cargos have been identified mostly through the use of the LMB inhibitor and information on these cargos and their putative NESs has been compiled into two similar databases, each named NESdb and ValidNES [24, 33, 36].
Table 1.
Six classes of classical NESs and their consensus sequences. Φn represents Leu, Ile, Val, Phe or Met, and X can be any other amino acid.
| NES Class | Consensus sequence |
|---|---|
| 1a | Φ1XXXΦ2XX–Φ3X–Φ4 |
| 1b | Φ1XX–Φ2XX–Φ3X–Φ4 |
| 1c | Φ1XXXΦ2XXXΦ3X–Φ4 |
| 1d | Φ1XX–Φ2XXXΦ3X–Φ4 |
| 2 | Φ1X– –Φ2XX–Φ3X–Φ4 |
| 3 | Φ1XX–Φ2XXXΦ3XXΦ4 |
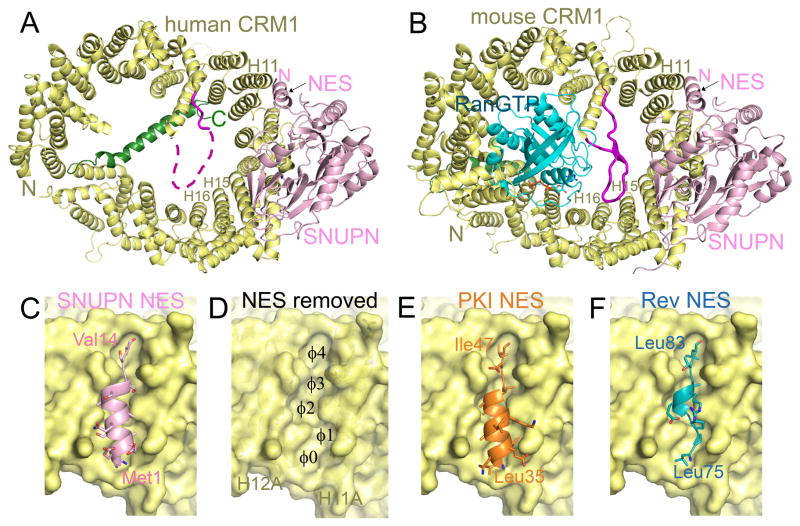
The first structures of cargo-bound CRM1 are of human CRM1 bound to its cargo Snurportin-1 (SNUPN) and mouse CRM1 bound to SNUPN and RanGTP (Fig. 3A, B) [84, 85]. SNUPN is a U snRNP-specific nuclear import adaptor, which has an N-terminal Importin-β binding (IBB) domain and a nucleotide binding domain (NBD) that recognizes the trimethylguanosine (m3G) cap structure of snRNP [71, 89]. SNUPN binds to the outer convex surface of CRM1 that span H11-16 (Fig 3A, B). The N-terminal helix of SNUPN binds in a hydrophobic groove that is formed by CRM1 repeats H11 and H12, the NBD of SNUPN makes extensive polar interactions with the outer surfaces of H12-14 and the C-terminal tail of SNUPN interacts with the outer surfaces of H14-H16. RanGTP binds inside the CRM1 ring, making many contacts with the concave surface of CRM1 (Fig. 3B).
Figure 3. Cargo-bound CRM1 complexes.
A) Structures of the same human CRM1-SNUPN and B) mouse CRM1-RanGTP-SNUPN complexes in Fig 2, but shown here with more details to explain CRM1-cargo recognition. Surface representations of the NES binding groove of the mouse CRM1-RanGTP-SNUPN complex, C) with the SNUPN NES bound, D) the NES peptide removed to view the underlying Φ0-4 pockets in the groove, E) the PKI NES bound (3NBY) and F) the HIV-1 Rev-NES bound (3NBZ). NES peptides are shown in cartoon with their side chains in sticks. The three peptides have diverse amino acid sequences and adopt different secondary structures to accommodate their hydrophobic side chains into the same Φ0-4 hydrophobic pockets.
Dong et al. observed that residues 4LSQALASSFSV14 of the N-terminal helix of SNUPN match the class 1c NES consensus (consensus Φ1- Φ4 hydrophobic residues are in bold; Table 1) [84]. They showed that this NES-like sequence of SNUPN was sufficient and essential for CRM1 binding and nuclear export, indicating that the segment is indeed a functional NES. Most importantly, mutations of the binding site on CRM1 (hydrophobic residues in H11 and H12) abolished binding to different NES peptides, suggesting that the CRM1 hydrophobic groove that SNUPN NES occupies is indeed the general NES binding site [84]. The NES helix of SNUPN extends one more turn N-terminal of Leu4 (Φ1 position) but breaks at Phe12 (Φ3) and then continues for another 3 residues in an extended conformation (Fig. 3C). The entire SNUPN NES adopts a helix-loop conformation. Met1 of the SNUPN NES was thus designated as Φ0, the first hydrophobic residue of this NES which has five hydrophobic residues pointing right into the binding groove. The NES binding groove on CRM1 is wide at the bottom where the N-terminus of the NES binds but its width tapers towards the middle of the groove where the bound NES helix transitions to a loop and then remains narrow the rest of the way (Fig. 3D). The structure of the groove suggests that it would not accommodate an entirely helical NES, consistent with the finding that experimentally identified NESs are more likely to adopt helix-loop secondary structure when compared to computationally generated sequences that match the NES consensus but are not functional NESs [31].
NESs can have very diverse sequences (Table 1). In order to understand how other NES sequences bind CRM1, Güttler et al. replaced the SNUPN NES with NESs from cargos PKI and HIV-1 Rev and solved structures of these chimeric NES-SNUPN bound to CRM1 and RanGTP (Fig. 3E, F) [90]. The PKI NES, with sequence 35LNELALKLAGLDI47, matches the class 1a NES consensus (Table 1) and binds in a similar helix-loop conformation as SNUPN NES (Fig. 3E). PKI Leu35 is Φ0 and its Φ1-4 residues bind to the same hydrophobic pockets within the groove as SNUPN NES. The Rev NES sequence (73LQLPPLERLTL83) has long posed a conundrum as its proline-rich content is a significant departure from the expected helical NESs. The spacings between its leucine residues are consistent with a class 1b NES consensus (Table 1) but its structure bound to CRM1 revealed different consensus hydrophobic residues and a different NES consensus. The Rev NES binds in the CRM1 NES groove in an almost entirely loop-like conformation (Fig. 3F). Leu75, Pro76, Leu78, Leu81 and Leu83 are the Φ0-4 residues that bind to the same five hydrophobic pockets that bind the Φ0-4 residues of the PKI and SNUPN NESs. An important conclusion of the Güttler et al. study is that the CRM1 groove conformation did not vary despite sequence and structural variations of the three bound NESs. However, since structures of only three different NESs (each of NES classes 1a, 1b and 1c) bound to CRM1 are available, it remains uncertain how other NESs, especially those in classes 1d, 2 and 3 bind CRM1 and the extent of conformations that NESs adopt when bound to CRM1.
The CRM1-SNUPN-RanGTP structure also provided important insights into how CRM1 binds RanGTP [85, 90]. CRM1 wraps around Ran, which interacts with the concave surfaces of HEAT repeats H1-H9, H17 and H19 (Fig. 3B). Important CRM1-Ran interactions also involve the 23-residue H9 loop of CRM1, which adopts a beta hairpin structure that contacts RanGTP and binds near repeats H15 and H16 of CRM1 (Fig. 3B). Molecular dynamics simulations suggested that H9 loop interactions are important for maintaining rigidity of the CRM1 ring [91].
Structures of CRM1-SNUPN also suggested how cytoplasmic ligands of SNUPN, the m3G-cap of snRNAs and Importin-β1, help release SNUPN from CRM1. The m3G cap binds the same site on the SNUPN NBD as CRM1, explaining mutually exclusive binding of the two SNUPN ligands [85, 92, 93]. m3G-cap binding is also expected to disrupt intramolecular contacts between the SNUPN IBB and NBD and thus free the former to bind Importin-β1, which imports SNUPN back into the nucleus [93, 94]. Although this exact mechanism is likely specific to SNUPN, cytoplasmic ligands of multi-domain cargos may contribute in general to cargo release from CRM1 in the cytoplasm.
3.3 Unliganded CRM1: the inactive state and positive cooperativity of Ran and cargo
Structures of the CRM1-SNUPN and CRM1-SNUPN-RanGTP complexes showed how the karyopherin binds NESs and RanGTP, but mechanistic understanding of positive cooperativity between CRM1, RanGTP and cargo to form the ternary export complex remained unclear until structures of unliganded CRM1 were solved. Three different crystal structures of unliganded CRM1 (human, yeast and C. thermophilum) were reported in 2013, finally showing pictures of the inactive state of CRM1 [95–97].
In general, unliganded CRM1 is less compact than cargo-bound CRM1 and accordingly, the termini of unliganded CRM1 proteins are farther apart. Most importantly, the NES binding grooves of the unliganded proteins are closed – helices H11A and H12A have moved closer together, rendering the NES groove inaccessible to NES peptides (compare Fig. 4D to the open groove in Fig. 3D) [91]. Two other structural features are clearly different in the unliganded state. First, the H9 loop of CRM1 interacts with the back of the NES binding groove (compare Fig. 4A–C with Fig. 3B). The loop interacts with helices H11B and H12B, which rotated to bring helices H11A and H12A closer to achieve NES groove closure [95, 96]. Results from molecular dynamics simulations and mutagenesis of the H9 loop support the notion that H9 loop-NES groove interactions are key in controlling the opening and closing of the groove [91, 98]. Second, the C-terminal helix H21B (also known as the C-extension) of unliganded CRM1 crosses the CRM1 ring in a conformation similar to that seen in the binary CRM1-SNUPN complex (Fig. 3A, B, 4A–C). In the cross-ring conformation, the last 11–15 residues or the C-terminal tail of CRM1 contacts both the back of the NES groove and the H9 loop. The C-terminal tail stabilizes the inhibitory configuration of the H9 loop and thus the closed or inactive configuration of the NES binding groove, as shown by mutagenesis of tail residues which activates CRM1 [95, 99]. Two major structural rearrangements appear to be important for full activation of CRM1 - the C-terminal tail and H9 loop of CRM1 has to be displaced from the back of the NES binding groove (H11-H12) to its active position at H15-H16, and the C-terminal H21B helix has to rearrange into the toroid of the CRM1 ring. Two models involving these rearrangements have been proposed to explain the cooperativity of RanGTP and cargo binding to CRM1.
Figure 4. Unliganded CRM1.
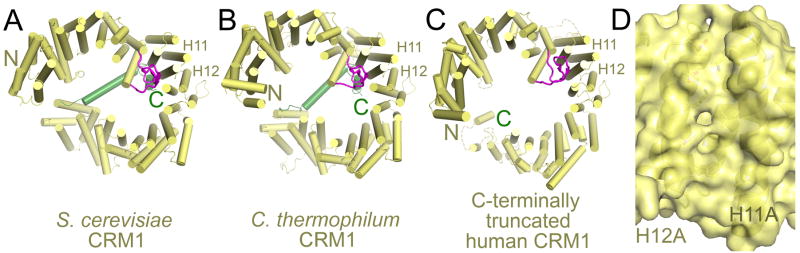
A) Unliganded S. cerevisiae CRM1 (3VYC), B) unliganded C. thermophilum CRM1 (4FGV) and C) the unliganded C-terminally truncated mutant of human CRM1 (4BSM). All three unliganded CRM1 proteins have similar conformations with their H9 loops associating with H11-H12 at the back of the NES groove. H11 and H12 that make up the NES binding groove are labelled. The H9 loops and C-terminal extensions are in magenta and green, respectively. D) The closed NES binding groove in unliganded S. cerevisiae CRM1 is shown as surface representation with underlying helices and side chains shown as cartoons and sticks.
Saito et al. proposed that assembly of the CRM1-RanGTP-cargo complex occurs in an induced fit mechanism [95]. Superposition of unliganded and cargo-bound CRM1 structures showed that Ran can bind to N-terminus of unliganded CRM1. This observation led them to suggest that Ran binding is the first step in export complex formation. Ran binding would displace the ring-crossing C-terminal H21B helix and its subsequent rearrangement into the ring toroid would facilitate CRM1 ring closure, conformational transition of H9 loop and hence the NES groove opening. It was suggested that energy required for this drastic conformational change is greater than that gained from the CRM1-Ran binding, and that NES binding provides the additional boost to stabilize the active conformation of CRM1, hence explaining the positive cooperativity between RanGTP and cargo binding to CRM1 [95].
Monecke et al. proposed that RanGTP and NES bind CRM1 through a conformational selection rather than an induced-fit mechanism [96]. Their molecular dynamics simulations suggested that CRM1 ring compaction, which also corresponds with an open NES groove, is the main factor that determines CRM1 activity. EM and SAXS analysis of full length CRM1 revealed intrinsic flexibility of the CRM1 ring, which samples both extended and compact conformations [91, 96]. Normal mode analysis of C-terminally truncated human CRM1 showed increased CRM1 flexibility with the NES groove fluctuating to various widths, suggesting that the C-terminal helix is important in controlling conformation flexibility of CRM1 [96, 97]. Monecke et al. suggested that binary interactions with either RanGTP or cargo stabilize the compact and active state of CRM1, making it energetically favorable for binding of the second ligand to form the ternary CRM1-RanGTP-cargo complex. Despite their differences, both the Saito et al. and the Monecke et al. models point to the same key features that control CRM1 activation - the H9 loop, the C-terminal H21B helix rearrangement, and CRM1 ring compaction.
3.4 Structure of the yeast CRM1-Ran-RanBP1 complex: Cargo release
RanBP1 is a member of the Ran binding protein family, and has one Ran binding domain (RBD). Nup358 or RanBP2 is another member of the family and has 4 RBDs. RanBP1 and Nup358 were previously shown to facilitate disassembly of the CRM1-RanGTP-cargo complex and RanGAP-catalyzed RanGTP hydrolysis in the cytoplasm [75, 76, 79, 100, 101]. Nup358 is part of the cytoplasmic fibrils of the NPC while RanBP1 is a shuttling protein that is mostly located in the cytoplasm [102–104]. Human RanBP1 contains an NES at its C-terminus but yeast RanBP1 has no obvious NES in its sequence as further supported by its LMB-insensitive actions [102, 104, 105]. The absence of NES in yeast RanBP1 suggested that cargo dissociation from the yeast CRM1 export complex does not occur through direct competition with a RanBP1 NES. The structure of the yeast CRM1-RanGTP-RanBP1 complex and accompanying kinetics analysis of cargo dissociation by Koyama et al. explained how RanBP1 causes export cargo release in the cytoplasm [98].
Individual RBDs of RanBP1 and RanBP2 accelerate cargo release from the CRM1-Ran-NES complex by 2–3 orders of magnitude and mutations of hydrophobic residues in the CRM1 H9 loop decrease the acceleration effect significantly [98]. These biochemical findings suggested an allosteric mechanism for RBD-mediated cargo release that involves the H9 loop of CRM1, and the structure of the yeast CRM1-RanGTP-RanBP1 complex showed how the allosteric mechanism was achieved (Fig. 5A). The C-terminal tail of RanGTP is disordered in other Karyopherin-Ran complexes but becomes ordered and is wrapped around RanBP1 in the Karyopherin-RanGTP-RanBP1 complexes like the CRM1-Ran-RanBP1 complex [82, 98]. Mutagenesis showed that the C-terminal tail of Ran is critical for cargo release, likely because it displaced the CRM1 H9 loop from its active state seatbelt configuration at H15-H16 (compare Fig. 5B and Fig. 3B) [98, 106]. The H9 loop in the CRM1-Ran-RanBP1 complex interacts with the back of the NES binding groove (H11-H12) instead, in a configuration essentially identical to the H9 loop in inactive unliganded CRM1 (compare Fig. 5B and Fig. 4A–C). Residues that maintain this behind-the-groove, inactive state H9 loop conformation was found to be critical for RanBP1-induced cargo release, consistent with the view that the inactive H9 loop configuration induces NES groove closure and NES release [91, 95, 98].
Figure 5. The CRM1-RanGTP-RanBP1 complex.
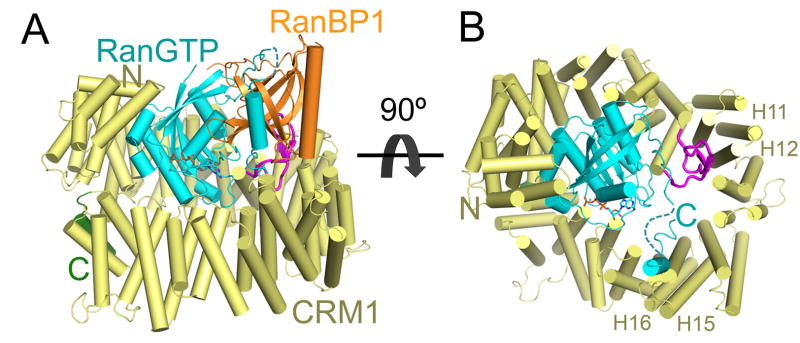
Structure of the CRM1-RanGTP-RanBP1 complex (3M1I). A) CRM1 is yellow with its H9 loop in magenta and C-terminal extension in green. RanGTP is in cyan with GTP in sticks, and RanBP1 is orange. B) Same as A, but RanBP1 is removed to better view the C-terminal tail of RanGTP and the H9 loop of CRM1. Interactions of RanBP1 with the C-terminal tail of RanGTP and positioning of the latter results in steric clash of the Ran tail with the CRM1 H9 loop of CRM1 in its active configuration at H15-H16 (as seen in Fig. 3B) and stabilization of the inactive conformation of the H9 loop at H11-H12. Relevant HEAT repeats 11, 12, 15 and 16 are labelled.
3.5 CRM1 inhibition by Leptomycin-B
Leptomycin B (LMB) was first discovered as an anti-fungal antibiotic produced by Streptomyces bacterial strains [107]. It was shown to possess anti-tumor activity and was tested for anti-cancer properties in pre-clinical studies [108–110]. A phase I clinical trial, however, showed large dose-dependent toxicity deeming LMB unsuitable for clinical use [111]. In attempts to reduce potential off-target effects of LMB, several modified versions of LMB were studied and reported to have improved pharmacological properties [112, 113].
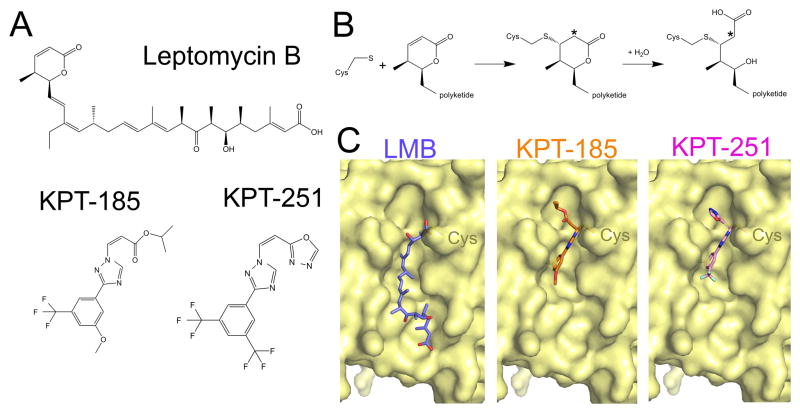
LMB is a 540 Da polyketide with an α,β-unsaturated δ-lactone ring (Fig. 6A), which is covalently conjugated through Michael addition to a reactive cysteine residue in the CRM1 NES groove (Cys528 in human CRM1) (Fig. 6B) [114]. Recently solved structures of LMB (and related lactone polyketide compounds) bound to CRM1 showed how LMB binds in the CRM1 groove and also revealed an unexpected mechanism of action for this widely used inhibitor (Fig. 6C) [115]. CRM1-LMB structures were solved using a mutant S. cerevisiae CRM1 (wild type is LMB-insensitive with a threonine residue in place of the reactive NES groove cysteine) with a threonine to cysteine mutation to confer LMB-sensitivity. The long LMB lactone polyketide occupies the NES groove lengthwise, almost filling up the groove (Fig. 6C), which adopts an inhibitor-bound conformation that is intermediate between that of open NES-bound groove and the closed unliganded CRM1 groove. The polyketide of LMB makes hydrophobic interactions with the same CRM1 groove residues that contact NESs.
Figure 6. CRM1 bound to small molecule inhibitors.
A) Chemical structures of Leptomycin B (LMB), KPT-185 and KPT-251. B) Chemical reactions showing conjugation of LMB to the groove cysteine residue of CRM1 and the subsequent hydrolysis. Positions of α-protons of the Michael reaction sites are denoted by asterisks. The CRM1 NES groove bound to C) LMB (4HAT), D) KPT-185 (4GMX) E) KPT-251 (4GPT). The reactive cysteine residue in the CRM1 NES groove which these inhibitors conjugate to is labelled.
As expected, the alkene in the LMB lactone forms a covalent bond with the thiol of the cysteine residue in the NES groove, but the CRM1-bound lactone had undergone an unexpected chemical reaction where the lactone ring was hydrolyzed to a hydroxy acid (Fig. 6B, C). Using a combination of chemical analysis, NMR spectroscopy, mutagenesis and structure determination of CRM1 mutants, Sun et al. determined that the mechanism of inhibition by LMB involved an initial covalent bond formation through Michael addition between its lactone and the reactive CRM1 cysteine, followed by an unexpected hydrolysis of the bound lactone [115]. The latter reaction is driven by a bound water as the nucleophile and an oxyanion hole composed of basic residues in the CRM1 groove. The resulting hydroxyl and carboxylate groups of the hydrolyzed lactone and inhibitor’s terminal carboxylate group all make extensive electrostatic/polar interactions with residues at the top and bottom of the NES groove, probably important for LMB’s extensive and stable coverage of the NES groove to prevent NES binding.
More importantly, Sun et al. found that CRM1-driven LMB hydrolysis has important chemical and thus biological consequences. The α-proton of the hydrolyzed inhibitor is less acidic than that of the unhydrolyzed lactone (positions of α-protons are denoted by asterisks in Fig. 6B). Consequently, the reverse Michael addition (to break the CRM1-LMB covalent bond) is expected to be much less facile for the conjugated hydroxy acid than the conjugated unhydrolyzed lactone ring [116, 117]. Consistent with the chemistry, Sun et al. showed that LMB inhibits CRM1 with non-detectable reversibility but LMB inhibition of a CRM1 mutant that cannot hydrolyze LMB was instead slowly reversible, with 20–30% of the bound LMB deconjugated and released after 24 hours. Therefore, hydrolysis of LMB by CRM1 is critical in stabilizing the covalent bond between inhibitor and CRM1 [115].
3.6 CRM1 inhibition by the KPT-SINE inhibitors
The toxicity of LMB in the clinic has led to searches of other small molecule inhibitors of CRM1. A few novel compounds have been identified and they all inhibit CRM1 covalently like LMB [113, 118]. Recently, Karyopharm Therapeutics Inc. has used a method called Consensus Induced Fit Docking (cFID) to computationally dock small molecule compounds into the CRM1 NES groove, and successfully identified a number of new active compounds [119]. Docking small molecules into a rigid binding groove is usually problematic because proteins are flexible molecules that often sample multiple conformations. Various approaches using ensembles of protein structure generated from crystal structures or from simulations have been shown to be successful but require tremendous computational power [120–123]. The cFID approach was developed to account for protein flexibility while keeping computational costs low. The method uses docking results of various ligands to a receptor surface and the different docked poses are combined to generate a hybrid ligand, which is then used to optimize the receptor surface to accommodate for various ligand-binding modes. Success of the cFID docking method has led to the development of selective inhibitors of nuclear export (SINEs) that also bind CRM1 covalently [50, 52].
Two KPT-SINE inhibitors (KPT-330 or Selinexor and KPT-335 or Verdinexor) are now in clinical trials for solid tumors and blood cancers, and numerous studies on anti-tumor effects of these inhibitors in several different malignancies have been published in the last two years [43, 49–62]. CRM1 inhibition by KPT-SINE compounds caused nuclear retention of tumor suppressors and pro-apoptotic signals such as IκB, Survivin, p53, NPMc mutant, p27 and FOXO, leading to cell-cycle arrest and apoptosis of cancerous cells [49–52, 54, 58–60, 63]. The administration of KPT compounds in animals was much less toxic than LMB.
Structures of KPT-SINE inhibitors bound to CRM1 an accompanying biochemical studies may have revealed some insights to explain why these inhibitors are less toxic than LMB [50, 52]. KPT-SINE compounds such as KPT-185 and KPT-251 are small compounds (~350 Da compared to the 540 Da LMB) that share a phenyl triazole scaffold with different Michael addition acceptor side chains (Fig. 6A) [50]. The KPT inhibitors conjugate to the same reactive cysteine in the CRM1 groove as LMB (Fig. 6C) but there are two key differences in their mechanisms of action compared to LMB [50, 52, 115]. First, the smaller KPT inhibitors occupy only the top third of the NES groove leaving ~60% of the hydrophobic groove open and unoccupied (Fig. 6C). CRM1-KPT interactions are exclusively hydrophobic in nature with the trifluoromethyl groups of the inhibitors buried deep in the NES binding groove. The second difference between KPTs and LMB involves their Michael acceptor moieties or reactive enones. Although the reactive enone of KPT-185 is chemically similar to that of LMB, it was not hydrolyzed by CRM1 after conjugation. The lack of hydrolysis for KPT-185 could be explained by the positioning of its enone and attached side chain deep in the NES groove, away from potential nucleophiles and the oxyanion hole that facilitate hydrolysis of LMB. As mentioned above, 20–30% of non-hydrolyzed LMB was released from CRM1 after 24 hours. KPT-185’s conjugation and inhibition activity is also 40–60% reversed after 24 hour, likely due to the lack of hydrolysis of its enone [115]. Therefore, although KPT-SINEs bind covalently to CRM1, they are not irreversible inhibitors but in fact are slowly-reversible inhibitors. The slow reversibility of CRM1-KPT interactions may contribute to their improved tolerance in animals since withdrawal of the drug may allow sufficient deconjugation of the inhibitor from CRM1 and allow essential nuclear export to resume in normal cells. The KPT-SINEs may bind long enough to kill cancer cells but due to the reversible nature of their interactions with CRM1, they may be released in time to spare normal cells.
4. Conclusion and Perspectives
The first crystal structure of a small fragment of CRM1 was solved in 2004, the first structures of full length CRM were reported in 2009 and structural knowledge of CRM1-mediated nuclear export has grown tremendously since then [72]. It is now clear that CRM1 is a ring-shaped karyopherin, which uses its outer convex surface to bind protein cargos. CRM1-NES recognition is achieved through anchoring of key hydrophobic residues of the NES into the hydrophobic NES binding groove, and variation in NES sequences is accommodated through variation of secondary structures of the bound NESs. The CRM1 protein adopts multiple conformations, including an inactive unliganded state with a closed NES groove and an active state with the groove open and NES-bound. These two conformations are stabilized by two different conformations of the CRM1 H9 loop, which are in turn controlled by different conformations of the CRM1 C-terminal extension (the H21B helix and C-terminal tail). Similarly, RanBP1, which accelerates cargo release when bound to RanGTP and CRM1, also stabilizes the inactive groove-closed state of CRM1 through stabilization of the inactive configuration of the CRM1 H9 loop. Finally, thus far, inhibition of CRM1 nuclear export by small molecule compounds has been achieved through direct conjugation of the inhibitors to the reactive cysteine residue in the NES groove of CRM1. However, the mechanisms of inhibition are not the same for all CRM1 inhibitors. LMB is first covalently conjugated to CRM1 and then hydrolyzed by the protein. LMB hydrolysis stabilizes the covalent CRM1-LMB interaction causing the inhibitor to be irreversibly bound to CRM1. The persistent shutdown of CRM1-mediated nuclear export is expected to be highly deleterious. KPT-SINEs, on the other hand, are not hydrolyzed by CRM1 and conjugate to CRM1 in a slowly reversible manner, which is potentially favorable for tolerance of the drugs.
The large body of structural knowledge that is currently available for CRM1 has contributed to atomic level and mechanistic understanding of many steps in the CRM1 nuclear export cycle. However, structural analysis could still inform on several outstanding questions and guide future discoveries pertaining to CRM1-mediated nuclear export. The most glaring gap in current structural knowledge of this transport pathway concerns the lack of CRM1-nucleoporin structures. Are there different classes of CRM1-nucleoporin interactions, such as ones for docking onto the NPC and others for translocation? The mechanism of RanBP3 facilitated cargo loading in the nucleus is also unclear at this time, and structural work of complexes containing CRM1 and RanBP3 should shed light on this process. In addition to its hundreds of protein cargos, CRM1 also exports a subset of mRNAs in a Ran-dependent and LMB-sensitive fashion [20, 124–131]. mRNA export by CRM1 likely requires NES-containing adaptors but it is currently not known if there is a general mRNA adaptor for CRM1 or specific adaptors for different mRNAs [132]. It is also not known if CRM1 shares binding interfaces with mRNA or other nucleic acid cargos. Structural analysis of CRM1-nucleic acid complexes may be possible and useful once identities of their adaptors are known. Finally, recent reports suggest that CRM1 is a relevant target for cancer and potentially inflammatory diseases, and efforts to design and develop CRM1 inhibitors for therapeutics has and should continue benefiting from the large body of structural data on the pathway [133–137]. Atomic understanding of the different steps of the CRM1 cycle and the different conformational states of CRM1 should guide discovery of reversible inhibitors for the NES binding groove and perhaps allosteric inhibitors that control CRM1 activity through binding at sites far from the NES groove.
Acknowledgments
This work is funded by the National Institutes of Health R01-GM069909 (YMC), Welch Foundation (I-1532; YMC), Leukemia and Lymphoma Society Scholar award (YMC), CPRIT (RP120352; YMC) and the UT Southwestern Endowed Scholars Program (YMC). We thank Q. Sun and S. Fu for critical comments.
Footnotes
Conflict of Interest
YMC, consultancy, Karyopharm Therapeutics Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuh Min Chook, Email: YuhMin.Chook@UTSouthwestern.edu.
Ho Yee Joyce Fung, Email: HoYeeJoyce.Fung@UTSouthwestern.edu.
References
- 1.O’Reilly AJ, Dacks JB, Field MC. Evolution of the karyopherin-beta family of nucleocytoplasmic transport factors; ancient origins and continued specialization. PloS one. 2011;6:e19308. doi: 10.1371/journal.pone.0019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran EJ, Bolger TA, Wente SR. SnapShot: nuclear transport. Cell. 2007;131:420. doi: 10.1016/j.cell.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Chook YM, Suel KE. Nuclear import by karyopherin-betas: recognition and inhibition. Biochimica et biophysica acta. 2011;1813:1593–606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti E, Izaurralde E. Nucleocytoplasmic transport enters the atomic age. Current opinion in cell biology. 2001;13:310–9. doi: 10.1016/s0955-0674(00)00213-1. [DOI] [PubMed] [Google Scholar]
- 5.Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–51. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 6.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annual review of cell and developmental biology. 1999;15:607–60. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 7.Ullman KS, Powers MA, Forbes DJ. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–70. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 8.Weis K. Importins and exportins: how to get in and out of the nucleus. Trends in biochemical sciences. 1998;23:185–9. doi: 10.1016/s0968-0004(98)01204-3. [DOI] [PubMed] [Google Scholar]
- 9.Chook YM, Blobel G. Karyopherins and nuclear import. Current opinion in structural biology. 2001;11:703–15. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Farmer A, Chook YM. Recognition of nuclear targeting signals by Karyopherin-beta proteins. Current opinion in structural biology. 2010;20:782–90. doi: 10.1016/j.sbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leisegang MS, Martin R, Ramirez AS, Bohnsack MT. Exportin t and Exportin 5: tRNA and miRNA biogenesis - and beyond. Biological chemistry. 2012;393:599–604. doi: 10.1515/hsz-2012-0146. [DOI] [PubMed] [Google Scholar]
- 12.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes & development. 2003;17:3011–6. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW. The role of exportin-t in selective nuclear export of mature tRNAs. The EMBO journal. 1998;17:7430–41. doi: 10.1093/emboj/17.24.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adachi Y, Yanagida M. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. The Journal of cell biology. 1989;108:1195–207. doi: 10.1083/jcb.108.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–60. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–11. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 17.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–4. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 18.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, et al. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. The Journal of biological chemistry. 1997;272:29742–51. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 19.Neville M, Stutz F, Lee L, Davis LI, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Current biology: CB. 1997;7:767–75. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 20.Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–50. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 21.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, et al. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. The EMBO journal. 1997;16:807–16. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. The Journal of biological chemistry. 1994;269:6320–4. [PubMed] [Google Scholar]
- 23.Wolff B, Sanglier JJ, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chemistry & biology. 1997;4:139–47. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 24.Xu D, Grishin NV, Chook YM. NESdb: a database of NES-containing CRM1 cargoes. Molecular biology of the cell. 2012;23:3673–6. doi: 10.1091/mbc.E12-01-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macara IG. Transport into and out of the nucleus. Microbiology and molecular biology reviews: MMBR. 2001;65:570–94. doi: 10.1128/MMBR.65.4.570-594.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutten S, Kehlenbach RH. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Molecular and cellular biology. 2006;26:6772–85. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C. The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFkappaB activation in Drosophila. Journal of cell science. 2006;119:4409–19. doi: 10.1242/jcs.03201. [DOI] [PubMed] [Google Scholar]
- 28.Bernad R, Engelsma D, Sanderson H, Pickersgill H, Fornerod M. Nup214-Nup88 nucleoporin subcomplex is required for CRM1-mediated 60 S preribosomal nuclear export. The Journal of biological chemistry. 2006;281:19378–86. doi: 10.1074/jbc.M512585200. [DOI] [PubMed] [Google Scholar]
- 29.Marg A, Shan Y, Meyer T, Meissner T, Brandenburg M, Vinkemeier U. Nucleocytoplasmic shuttling by nucleoporins Nup153 and Nup214 and CRM1-dependent nuclear export control the subcellular distribution of latent Stat1. The Journal of cell biology. 2004;165:823–33. doi: 10.1083/jcb.200403057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka M, Asally M, Yasuda Y, Ogawa Y, Tachibana T, Yoneda Y. The mobile FG nucleoporin Nup98 is a cofactor for Crm1-dependent protein export. Molecular biology of the cell. 2010;21:1885–96. doi: 10.1091/mbc.E09-12-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D, Farmer A, Collett G, Grishin NV, Chook YM. Sequence and structural analyses of nuclear export signals in the NESdb database. Molecular biology of the cell. 2012;23:3677–93. doi: 10.1091/mbc.E12-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Nuclear Export Signal Consensus Sequences Defined Using a Localization-Based Yeast Selection System. Traffic. 2008;9:2053–62. doi: 10.1111/j.1600-0854.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- 33.la Cour T, Kiemer L, Molgaard A, Gupta R, Skriver K, Brunak S. Analysis and prediction of leucine-rich nuclear export signals. Protein engineering, design & selection: PEDS. 2004;17:527–36. doi: 10.1093/protein/gzh062. [DOI] [PubMed] [Google Scholar]
- 34.Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–73. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 35.Fischer U, Huber J, Boelens WC, Mattaj IW, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–83. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 36.Fu SC, Huang HC, Horton P, Juan HF. ValidNESs: a database of validated leucine-rich nuclear export signals. Nucleic acids research. 2013;41:D338–43. doi: 10.1093/nar/gks936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. The EMBO journal. 1999;18:1660–72. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X, Saito H, Miki Y, Nakanishi A. A CRM1-mediated nuclear export signal governs cytoplasmic localization of BRCA2 and is essential for centrosomal localization of BRCA2. Oncogene. 2008;27:2969–77. doi: 10.1038/sj.onc.1210968. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez JA, Henderson BR. Identification of a functional nuclear export sequence in BRCA1. The Journal of biological chemistry. 2000;275:38589–96. doi: 10.1074/jbc.M003851200. [DOI] [PubMed] [Google Scholar]
- 40.Knauer SK, Bier C, Habtemichael N, Stauber RH. The Survivin-Crm1 interaction is essential for chromosomal passenger complex localization and function. EMBO reports. 2006;7:1259–65. doi: 10.1038/sj.embor.7400824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson C, Van Antwerp D, Hope TJ. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IkappaBalpha. The EMBO journal. 1999;18:6682–93. doi: 10.1093/emboj/18.23.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochemical pharmacology. 2012;83:1021–32. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima K, Kornblau SM, Ruvolo V, Dilip A, Duvvuri S, Davis RE, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121:4166–74. doi: 10.1182/blood-2012-08-447581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou F, Qiu W, Yao R, Xiang J, Sun X, Liu S, et al. CRM1 is a novel independent prognostic factor for the poor prognosis of gastric carcinomas. Medical oncology. 2013;30:726. doi: 10.1007/s12032-013-0726-1. [DOI] [PubMed] [Google Scholar]
- 45.Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, et al. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncology reports. 2009;21:229–35. [PubMed] [Google Scholar]
- 46.Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65:153–9. doi: 10.1227/01.NEU.0000348550.47441.4B. discussion 9–60. [DOI] [PubMed] [Google Scholar]
- 47.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clinical and investigative medicine Medecine clinique et experimentale. 2009;32:E315. [PubMed] [Google Scholar]
- 48.Noske A, Weichert W, Niesporek S, Roske A, Buckendahl AC, Koch I, et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer. 2008;112:1733–43. doi: 10.1002/cncr.23354. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y, Holloway MP, Nguyen K, McCauley D, Landesman Y, Kauffman MG, et al. XPO1 (CRM1) inhibition represses STAT3 activation to drive a survivin-dependent oncogenic switch in triple negative breast cancer. Mol Cancer Ther. 2014 doi: 10.1158/1535-7163.MCT-13-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–34. doi: 10.1182/blood-2012-05-429506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–65. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etchin J, Sun Q, Kentsis A, Farmer A, Zhang ZC, Sanda T, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia. 2013;27:66–74. doi: 10.1038/leu.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salas Fragomeni RA, Chung HW, Landesman Y, Senapedis W, Saint-Martin JR, Tsao H, et al. CRM1 and BRAF inhibition synergize and induce tumor regression in BRAF-mutant melanoma. Molecular cancer therapeutics. 2013;12:1171–9. doi: 10.1158/1535-7163.MCT-12-1171. [DOI] [PubMed] [Google Scholar]
- 54.Walker CJ, Oaks JJ, Santhanam R, Neviani P, Harb JG, Ferenchak G, et al. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood. 2013 doi: 10.1182/blood-2013-04-495374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013 doi: 10.1038/leu.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2013 doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. British journal of haematology. 2013;161:117–27. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, et al. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. The Journal of urology. 2013;189:2317–26. doi: 10.1016/j.juro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang K, Wang M, Tamayo AT, Shacham S, Kauffman M, Lee J, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Experimental hematology. 2013;41:67–78. e4. doi: 10.1016/j.exphem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Azmi AS, Aboukameel A, Bao B, Sarkar FH, Philip PA, Kauffman M, et al. Selective inhibitors of nuclear export block pancreatic cancer cell proliferation and reduce tumor growth in mice. Gastroenterology. 2013;144:447–56. doi: 10.1053/j.gastro.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner JG, Dawson J, Emmons MF, Cubitt CL, Kauffman M, Shacham S, et al. CRM1 Inhibition Sensitizes Drug Resistant Human Myeloma Cells to Topoisomerase II and Proteasome Inhibitors both In Vitro and Ex Vivo. Journal of Cancer. 2013;4:614–25. doi: 10.7150/jca.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.London CA, Bernabe LF, Barnard S, Kisseberth WC, Borgatti A, Henson M, et al. Preclinical Evaluation of the Novel, Orally Bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in Spontaneous Canine Cancer: Results of a Phase I Study. PloS one. 2014;9:e87585. doi: 10.1371/journal.pone.0087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120:1765–73. doi: 10.1182/blood-2012-04-423160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic acids research. 2000;28:235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makde RD, England JR, Yennawar HP, Tan S. Structure of RCC1 chromatin factor bound to the nucleosome core particle. Nature. 2010;467:562–6. doi: 10.1038/nature09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG. Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B. Science. 2001;292:1540–3. doi: 10.1126/science.292.5521.1540. [DOI] [PubMed] [Google Scholar]
- 67.Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991;354:80–2. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- 68.Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2587–91. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hopper AK, Traglia HM, Dunst RW. The yeast RNA1 gene product necessary for RNA processing is located in the cytosol and apparently excluded from the nucleus. The Journal of cell biology. 1990;111:309–21. doi: 10.1083/jcb.111.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. The Journal of cell biology. 1996;135:1457–70. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paraskeva E, Izaurralde E, Bischoff FR, Huber J, Kutay U, Hartmann E, et al. CRM1-mediated recycling of snurportin 1 to the cytoplasm. The Journal of cell biology. 1999;145:255–64. doi: 10.1083/jcb.145.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petosa C, Schoehn G, Askjaer P, Bauer U, Moulin M, Steuerwald U, et al. Architecture of CRM1/Exportin1 suggests how cooperativity is achieved during formation of a nuclear export complex. Molecular cell. 2004;16:761–75. doi: 10.1016/j.molcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 73.Englmeier L, Fornerod M, Bischoff FR, Petosa C, Mattaj IW, Kutay U. RanBP3 influences interactions between CRM1 and its nuclear protein export substrates. EMBO reports. 2001;2:926–32. doi: 10.1093/embo-reports/kve200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nemergut ME, Lindsay ME, Brownawell AM, Macara IG. Ran-binding protein 3 links Crm1 to the Ran guanine nucleotide exchange factor. The Journal of biological chemistry. 2002;277:17385–8. doi: 10.1074/jbc.C100620200. [DOI] [PubMed] [Google Scholar]
- 75.Kehlenbach RH, Dickmanns A, Kehlenbach A, Guan T, Gerace L. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. The Journal of cell biology. 1999;145:645–57. doi: 10.1083/jcb.145.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bischoff FR, Gorlich D. RanBP1 is crucial for the release of RanGTP from importin beta-related nuclear transport factors. FEBS letters. 1997;419:249–54. doi: 10.1016/s0014-5793(97)01467-1. [DOI] [PubMed] [Google Scholar]
- 77.Bischoff FR, Krebber H, Smirnova E, Dong W, Ponstingl H. Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. The EMBO journal. 1995;14:705–15. doi: 10.1002/j.1460-2075.1995.tb07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seewald MJ, Korner C, Wittinghofer A, Vetter IR. RanGAP mediates GTP hydrolysis without an arginine finger. Nature. 2002;415:662–6. doi: 10.1038/415662a. [DOI] [PubMed] [Google Scholar]
- 79.Bernad R, van der Velde H, Fornerod M, Pickersgill H. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Molecular and cellular biology. 2004;24:2373–84. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrade MA, Bork P. HEAT repeats in the Huntington’s disease protein. Nature genetics. 1995;11:115–6. doi: 10.1038/ng1095-115. [DOI] [PubMed] [Google Scholar]
- 81.Cingolani G, Petosa C, Weis K, Muller CW. Structure of importin-beta bound to the IBB domain of importin-alpha. Nature. 1999;399:221–9. doi: 10.1038/20367. [DOI] [PubMed] [Google Scholar]
- 82.Chook YM, Blobel G. Structure of the nuclear transport complex karyopherin-beta2-Ran x GppNHp. Nature. 1999;399:230–7. doi: 10.1038/20375. [DOI] [PubMed] [Google Scholar]
- 83.Okada C, Yamashita E, Lee SJ, Shibata S, Katahira J, Nakagawa A, et al. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–9. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- 84.Dong X, Biswas A, Suel KE, Jackson LK, Martinez R, Gu H, et al. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458:1136–41. doi: 10.1038/nature07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monecke T, Guttler T, Neumann P, Dickmanns A, Gorlich D, Ficner R. Crystal structure of the nuclear export receptor CRM1 in complex with Snurportin1 and RanGTP. Science. 2009;324:1087–91. doi: 10.1126/science.1173388. [DOI] [PubMed] [Google Scholar]
- 86.Wen W, Harootunian AT, Adams SR, Feramisco J, Tsien RY, Meinkoth JL, et al. Heat-stable inhibitors of cAMP-dependent protein kinase carry a nuclear export signal. The Journal of biological chemistry. 1994;269:32214–20. [PubMed] [Google Scholar]
- 87.Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Molecular and cellular biology. 1996;16:4207–14. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.la Cour T, Gupta R, Rapacki K, Skriver K, Poulsen FM, Brunak S. NESbase version 1.0: a database of nuclear export signals. Nucleic acids research. 2003;31:393–6. doi: 10.1093/nar/gkg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huber J, Cronshagen U, Kadokura M, Marshallsay C, Wada T, Sekine M, et al. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. The EMBO journal. 1998;17:4114–26. doi: 10.1093/emboj/17.14.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guttler T, Madl T, Neumann P, Deichsel D, Corsini L, Monecke T, et al. NES consensus redefined by structures of PKI-type and Rev-type nuclear export signals bound to CRM1. Nature structural & molecular biology. 2010;17:1367–76. doi: 10.1038/nsmb.1931. [DOI] [PubMed] [Google Scholar]
- 91.Dolker N, Blanchet CE, Voss B, Haselbach D, Kappel C, Monecke T, et al. Structural determinants and mechanism of mammalian CRM1 allostery. Structure. 2013;21:1350–60. doi: 10.1016/j.str.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 92.Strasser A, Dickmanns A, Luhrmann R, Ficner R. Structural basis for m3G-cap-mediated nuclear import of spliceosomal UsnRNPs by snurportin1. The EMBO journal. 2005;24:2235–43. doi: 10.1038/sj.emboj.7600701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong X, Biswas A, Chook YM. Structural basis for assembly and disassembly of the CRM1 nuclear export complex. Nat Struct Mol Biol. 2009;16:558–60. doi: 10.1038/nsmb.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitrousis G, Olia AS, Walker-Kopp N, Cingolani G. Molecular basis for the recognition of snurportin 1 by importin beta. The Journal of biological chemistry. 2008;283:7877–84. doi: 10.1074/jbc.M709093200. [DOI] [PubMed] [Google Scholar]
- 95.Saito N, Matsuura Y. A 2.1-A-resolution crystal structure of unliganded CRM1 reveals the mechanism of autoinhibition. Journal of molecular biology. 2013;425:350–64. doi: 10.1016/j.jmb.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 96.Monecke T, Haselbach D, Voss B, Russek A, Neumann P, Thomson E, et al. Structural basis for cooperativity of CRM1 export complex formation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:960–5. doi: 10.1073/pnas.1215214110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dian C, Bernaudat F, Langer K, Oliva MF, Fornerod M, Schoehn G, et al. Structure of a truncation mutant of the nuclear export factor CRM1 provides insights into the auto-inhibitory role of its C-terminal helix. Structure. 2013;21:1338–49. doi: 10.1016/j.str.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Koyama M, Matsuura Y. An allosteric mechanism to displace nuclear export cargo from CRM1 and RanGTP by RanBP1. The EMBO journal. 2010;29:2002–13. doi: 10.1038/emboj.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fox AM, Ciziene D, McLaughlin SH, Stewart M. Electrostatic interactions involving the extreme C terminus of nuclear export factor CRM1 modulate its affinity for cargo. The Journal of biological chemistry. 2011;286:29325–35. doi: 10.1074/jbc.M111.245092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Askjaer P, Bachi A, Wilm M, Bischoff FR, Weeks DL, Ogniewski V, et al. RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase. Molecular and cellular biology. 1999;19:6276–85. doi: 10.1128/mcb.19.9.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maurer P, Redd M, Solsbacher J, Bischoff FR, Greiner M, Podtelejnikov AV, et al. The nuclear export receptor Xpo1p forms distinct complexes with NES transport substrates and the yeast Ran binding protein 1 (Yrb1p) Molecular biology of the cell. 2001;12:539–49. doi: 10.1091/mbc.12.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kunzler M, Gerstberger T, Stutz F, Bischoff FR, Hurt E. Yeast Ran-binding protein 1 (Yrb1) shuttles between the nucleus and cytoplasm and is exported from the nucleus via a CRM1 (XPO1)-dependent pathway. Molecular and cellular biology. 2000;20:4295–308. doi: 10.1128/mcb.20.12.4295-4308.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilken N, Senecal JL, Scheer U, Dabauvalle MC. Localization of the Ran-GTP binding protein RanBP2 at the cytoplasmic side of the nuclear pore complex. European journal of cell biology. 1995;68:211–9. [PubMed] [Google Scholar]
- 104.Plafker K, Macara IG. Facilitated nucleocytoplasmic shuttling of the Ran binding protein RanBP1. Molecular and cellular biology. 2000;20:3510–21. doi: 10.1128/mcb.20.10.3510-3521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richards SA, Lounsbury KM, Carey KL, Macara IG. A nuclear export signal is essential for the cytosolic localization of the Ran binding protein, RanBP1. The Journal of cell biology. 1996;134:1157–68. doi: 10.1083/jcb.134.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vetter IR, Nowak C, Nishimoto T, Kuhlmann J, Wittinghofer A. Structure of a Ran-binding domain complexed with Ran bound to a GTP analogue: implications for nuclear transport. Nature. 1999;398:39–46. doi: 10.1038/17969. [DOI] [PubMed] [Google Scholar]
- 107.Hamamoto T, Uozumi T, Beppu T. Leptomycins A and B, new antifungal antibiotics. III. Mode of action of leptomycin B on Schizosaccharomyces pombe. The Journal of antibiotics. 1985;38:1573–80. doi: 10.7164/antibiotics.38.1573. [DOI] [PubMed] [Google Scholar]
- 108.Komiyama K, Okada K, Tomisaka S, Umezawa I, Hamamoto T, Beppu T. Antitumor activity of leptomycin B. The Journal of antibiotics. 1985;38:427–9. doi: 10.7164/antibiotics.38.427. [DOI] [PubMed] [Google Scholar]
- 109.Shao C, Lu C, Chen L, Koty PP, Cobos E, Gao W. p53-Dependent anticancer effects of leptomycin B on lung adenocarcinoma. Cancer chemotherapy and pharmacology. 2011;67:1369–80. doi: 10.1007/s00280-010-1434-6. [DOI] [PubMed] [Google Scholar]
- 110.Roberts BJ, Hamelehle KL, Sebolt JS, Leopold WR. In vivo and in vitro anticancer activity of the structurally novel and highly potent antibiotic CI-940 and its hydroxy analog (PD 114,721) Cancer chemotherapy and pharmacology. 1986;16:95–101. doi: 10.1007/BF00256156. [DOI] [PubMed] [Google Scholar]
- 111.Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. British journal of cancer. 1996;74:648–9. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mutka SC, Yang WQ, Dong SD, Ward SL, Craig DA, Timmermans PB, et al. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer research. 2009;69:510–7. doi: 10.1158/0008-5472.CAN-08-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Monovich L, Koch KA, Burgis R, Osimboni E, Mann T, Wall D, et al. Suppression of HDAC nuclear export and cardiomyocyte hypertrophy by novel irreversible inhibitors of CRM1. Biochimica et biophysica acta. 2009;1789:422–31. doi: 10.1016/j.bbagrm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 114.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9112–7. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun Q, Carrasco YP, Hu Y, Guo X, Mirzaei H, Macmillan J, et al. Nuclear export inhibition through covalent conjugation and hydrolysis of Leptomycin B by CRM1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1303–8. doi: 10.1073/pnas.1217203110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arnett EM, Harrelson JA. Ion pairing and reactivity of enolate anions. 7. A spectacular example of the importance of rotational barriers: the ionization of Meldrum’s acid. Journal of the American Chemical Society. 1987;109:809–12. [Google Scholar]
- 117.Taylor EA, Palmer DR, Gerlt JA. The lesser “burden borne” by o-succinylbenzoate synthase: an “easy” reaction involving a carboxylate carbon acid. J Am Chem Soc. 2001;123:5824–5. doi: 10.1021/ja010882h. [DOI] [PubMed] [Google Scholar]
- 118.Sakakibara K, Saito N, Sato T, Suzuki A, Hasegawa Y, Friedman JM, et al. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood. 2011;118:3922–31. doi: 10.1182/blood-2011-01-333138. [DOI] [PubMed] [Google Scholar]
- 119.Kalid O, Toledo Warshaviak D, Shechter S, Sherman W, Shacham S. Consensus Induced Fit Docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. Journal of computer-aided molecular design. 2012;26:1217–28. doi: 10.1007/s10822-012-9611-9. [DOI] [PubMed] [Google Scholar]
- 120.Osguthorpe DJ, Sherman W, Hagler AT. Exploring protein flexibility: incorporating structural ensembles from crystal structures and simulation into virtual screening protocols. The journal of physical chemistry B. 2012;116:6952–9. doi: 10.1021/jp3003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang SY, Zou X. Ensemble docking of multiple protein structures: considering protein structural variations in molecular docking. Proteins. 2007;66:399–421. doi: 10.1002/prot.21214. [DOI] [PubMed] [Google Scholar]
- 122.Sherman W, Beard HS, Farid R. Use of an induced fit receptor structure in virtual screening. Chemical biology & drug design. 2006;67:83–4. doi: 10.1111/j.1747-0285.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 123.Verkhivker GM, Bouzida D, Gehlhaar DK, Rejto PA, Freer ST, Rose PW. Computational detection of the binding-site hot spot at the remodeled human growth hormone-receptor interface. Proteins. 2003;53:201–19. doi: 10.1002/prot.10456. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe M, Fukuda M, Yoshida M, Yanagida M, Nishida E. Involvement of CRM1, a nuclear export receptor, in mRNA export in mammalian cells and fission yeast. Genes to cells: devoted to molecular & cellular mechanisms. 1999;4:291–7. doi: 10.1046/j.1365-2443.1999.00259.x. [DOI] [PubMed] [Google Scholar]
- 125.Cuevas IC, Frasch AC, D’Orso I. Insights into a CRM1-mediated RNA-nuclear export pathway in Trypanosoma cruzi. Molecular and biochemical parasitology. 2005;139:15–24. doi: 10.1016/j.molbiopara.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Kimura T, Hashimoto I, Nagase T, Fujisawa J. CRM1-dependent, but not ARE-mediated, nuclear export of IFN-alpha1 mRNA. Journal of cell science. 2004;117:2259–70. doi: 10.1242/jcs.01076. [DOI] [PubMed] [Google Scholar]
- 127.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. The Journal of cell biology. 2006;175:415–26. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schutz S, Chemnitz J, Spillner C, Frohme M, Hauber J, Kehlenbach RH. Stimulated expression of mRNAs in activated T cells depends on a functional CRM1 nuclear export pathway. Journal of molecular biology. 2006;358:997–1009. doi: 10.1016/j.jmb.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 129.Prechtel AT, Chemnitz J, Schirmer S, Ehlers C, Langbein-Detsch I, Stulke J, et al. Expression of CD83 is regulated by HuR via a novel cis-active coding region RNA element. The Journal of biological chemistry. 2006;281:10912–25. doi: 10.1074/jbc.M510306200. [DOI] [PubMed] [Google Scholar]
- 130.Brennan CM, Gallouzi IE, Steitz JA. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. The Journal of cell biology. 2000;151:1–14. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murphy R, Wente SR. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–60. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- 132.Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends in cell biology. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 133.Vancurova I, Vancura A. Regulation and function of nuclear IkappaBalpha in inflammation and cancer. American journal of clinical and experimental immunology. 2012;1:56–66. [PMC free article] [PubMed] [Google Scholar]
- 134.Zerfaoui M, Errami Y, Naura AS, Suzuki Y, Kim H, Ju J, et al. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. Journal of immunology. 2010;185:1894–902. doi: 10.4049/jimmunol.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bouayad D, Pederzoli-Ribeil M, Mocek J, Candalh C, Arlet JB, Hermine O, et al. Nuclear-to-cytoplasmic relocalization of the proliferating cell nuclear antigen (PCNA) during differentiation involves a chromosome region maintenance 1 (CRM1)-dependent export and is a prerequisite for PCNA antiapoptotic activity in mature neutrophils. The Journal of biological chemistry. 2012;287:33812–25. doi: 10.1074/jbc.M112.367839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Garcia-Yague AJ, Rada P, Rojo AI, Lastres-Becker I, Cuadrado A. Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. The Journal of biological chemistry. 2013;288:5506–17. doi: 10.1074/jbc.M112.439190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muller PA, van de Sluis B, Groot AJ, Verbeek D, Vonk WI, Maine GN, et al. Nuclear-cytosolic transport of COMMD1 regulates NF-kappaB and HIF-1 activity. Traffic. 2009;10:514–27. doi: 10.1111/j.1600-0854.2009.00892.x. [DOI] [PubMed] [Google Scholar]