Abstract
Background
Fibroblast growth factor-23 (FGF-23) is a hormone that promotes urinary phosphate excretion and regulates vitamin D metabolism. Circulating FGF-23 concentrations increase markedly in chronic kidney disease and are associated with increased risk of clinical cardiovascular events. FGF-23 may promote atrial fibrillation (AF) by inducing left ventricular hypertrophy and diastolic and left atrial dysfunction.
Methods and Results
We tested associations of circulating FGF-23 concentration with incident AF among 6,398 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) and 1,350 participants in the Cardiovascular Health Study (CHS), all free of clinical cardiovascular disease at baseline. Over 7.7 and 8.0 years median follow-up, we observed 291 and 229 incident AF events in MESA and CHS, respectively. In multivariable Cox proportional hazards models, each two-fold higher FGF-23 concentration was associated with a 41% higher risk of incident AF in MESA (HR 1.41 [95% CI 1.13-1.76], p=0.003) and a 30% higher risk of incident AF in CHS (HR 1.30 [95% CI 1.05-1.61], p=0.016), adjusting for potential confounding characteristics including kidney disease. Serum phosphate concentration was significantly associated with incident AF in MESA (HR 1.15 per 0.5 mg/dL [CI 1.02-1.31], p-value=0.023) but not CHS. In MESA, an association of low estimated glomerular filtration rate with incident AF was partially attenuated by adjusting for FGF-23.
Conclusions
Higher circulating FGF-23 concentration is associated with incident AF and may, in part, explain the link between chronic kidney disease and AF.
Keywords: atrial fibrillation, fibroblast growth factor, mineral, chronic kidney disease
Introduction
The global prevalence of atrial fibrillation (AF) and its associated costs are growing1-7. The identification of novel, modifiable risk factors may reveal new therapeutic targets for arrhythmia prevention. Alterations in mineral metabolism are particularly promising8-11.
Fibroblast growth factor-23 (FGF-23) is a bone-derived hormone that plays a central role in phosphate homeostasis. FGF-23 acts on the kidney to promote urinary phosphate excretion and inhibit the production of 1,25-dihydroxyvitamin D, thereby reducing gastrointestinal absorption of dietary phosphate. FGF-23 may directly induce left ventricular hypertrophy12. In human studies, higher circulating concentrations of FGF-23 have been associated with increased left ventricular mass13 as well as incident heart failure, myocardial infarction, and cardiovascular death14-17. Increased cardiac hypertrophy can lead to diastolic dysfunction and a rise in left-sided filling pressures resulting in left atrial dilation and fibrosis – an important structural precipitant for AF initiation18. Circulating FGF-23 concentrations rise substantially with chronic kidney disease (CKD) and FGF-23 excess may partially explain the known associations of CKD with incident AF19.
We tested the associations of FGF-23 with incident AF in longitudinal studies of the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). We secondarily examined whether associations of CKD with incident AF can be explained, in part, by elevated concentrations of FGF-23.
Methods
Study Population
MESA and CHS are community-based cohort studies designed to assess risk factors for cardiovascular disease20, 21.
Between 2000 and 2002, MESA enrolled 6,814 participants ages 45-84 years who were free of baseline cardiovascular disease, defined as physician-diagnosed myocardial infarction, angina or nitroglycerin use, stroke or transient ischemic attack, heart failure, current AF, or having undergone cardiovascular procedures (coronary artery bypass grafting, angioplasty, valve replacement, pacemaker or defibrillator implantation, any surgery on the heart or arteries). Participants were recruited by race/ethnicity (Caucasian, African-American, Hispanic, and Chinese descents) and were drawn from six U.S. regions. FGF-23 concentrations were measured for 6,552 MESA participants with frozen serum available at baseline. From this group, we excluded two participants with estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2 and three participants with implausibly high FGF-23 concentrations. Additionally, we eliminated another 149 additional participants with one or more of a self-reported history of transient AF prior to enrollment (N=55), AF noted at the baseline examination (N=1), or no follow-up for incident AF (N=98) leaving 6,398 participants in our analysis cohort.
CHS enrolled 5,201 patients ages ≥ 65 years between 1989 and 1990 from four U.S. communities. An additional 687 African American participants were enrolled between 1992 and 1993. FGF-23 was measured for 3,337 participants with frozen plasma available from the 1996-97 follow-up study visit (baseline for the current study). From this group, we excluded 1,987 participants with prevalent cardiovascular disease at the 1996-97 visit, defined as coronary artery disease, heart failure, stroke/transient ischemic attack, claudication, AF, and pacemaker implantation, leaving 1,350 participants in our analysis cohort.
The institutional review board at each site approved each respective study, and all participants provided written informed consent.
FGF-23
For MESA, FGF-23 was measured in previously unthawed serum using the Kainos immunoassay, which detects the full-length, biologically intact FGF-23 molecule via mid-molecule and distal epitopes. Assays were performed in 2011 with interassay coefficients of variation for singlicate high and low control samples of 6.7% and 12.4%, respectively22.
For CHS, FGF-23 was measured in previously unthawed plasma using the Immutopics C-terminal ELISA kit (Immutopics, San Clemente, California)14. Assays were performed in 2010, with intra-assay and interassay coefficients of variation of 7.4% and 10.6%, respectively14.
Atrial Fibrillation
Incident AF was identified using systematic reviews of hospital discharge diagnoses, inpatient and outpatient physician claims data, and study electrocardiograms. In MESA, these measures included review of hospitalization discharge diagnoses obtained during events data collection supplemented by study electrocardiograms as well as incident event dates identified using inpatient and outpatient physician claims data. In CHS, these measures included annual study electrocardiograms from 1996 to 1999, hospital discharge diagnoses during CHS events data collection, as well as inpatient, outpatient, and physician claims diagnoses of AF for participants enrolled in fee-for-service Medicare. Electrocardiograms in both studies were read at a centralized electrocardiographic reading center, EPICARE, at Wake Forest University. AF or atrial flutter that occurred during hospitalization for coronary bypass graft surgery or valve replacement surgery was excluded.
Covariates
All covariates were ascertained at the time of FGF-23 measurement unless otherwise specified. Age, gender, race/ethnicity, highest attained education, smoking status, physical activity, and medication use were self-reported by questionnaire. Diabetes was defined as fasting glucose ≥126 mg/dl or use of hypoglycemic medications. Estimated glomerular filtration rate (eGFR) was calculated using serum concentrations of both creatinine and cystatin C equation to maximize precision23. Urine albumin excretion was quantified as the ratio of albumin to creatinine (ACR) in a single-voided urine sample. Serum calcium and phosphate were measured by indirect potentiometry on a DxC Synchron analyzer (Beckman Coulter Inc) and timed-rate colorimetric reaction method, respectively24. Serum 25-hydroxyvitamin D was measured by mass spectrometry25. Serum parathyroid hormone (PTH) was measured by Beckman-Coulter DxI automated two-site immunoassay (Beckman-Coulter, Inc., Brea, CA)26.
In CHS, left atrial diameter was measured in 1,256 participants (93%) and left ventricular end diastolic (LVED) mass and diameter were derived in 950 participants (70%) in 1994-1995 (two years prior to FGF-23 measurements) using M-Mode and two-dimensional echocardiography20. Left ventricular mass was determined using an autopsy-validated formula27. All study echocardiograms were interpreted at the CHS Echocardiography Reading Center at the University of Maryland20. In MESA, LVED mass and volume were measured in 5,003 participants by cardiac MRI using 1.5-Tesla magnets by blinded, central readers applying commercial software (MASS 4.2; Leiden, the Netherlands)28. In both cohorts, a congestive heart failure (CHF) event was defined as a physician diagnosis of heart failure, medical treatment for heart failure, and clinical signs or symptoms of heart failure14, 28.
Statistical Methods
MESA participants were considered at risk of incident AF from their baseline visit (2000-2002) until April 13, 2011. CHS participants were considered at risk from the 1996-1997 study visit (baseline for this study) until December 31, 2006. Unadjusted incidence rates of AF were calculated for each cohort and for each FGF-23 quartile.
Cox proportional hazards models were applied to test associations of FGF-23 with the hazard of developing AF during follow-up. Serial multivariable models were constructed. Initially, we adjusted for demographic data including age (continuous), gender, race/ethnicity, study site, and education (high school or less, high school to some college, college degree or more). Our second model additionally adjusted for potential confounders of the association between FGF-23 and AF, including physical characteristics (weight, height, height squared) as well as physiologic variables (physical activity, current smoking, diabetes [yes versus no], systolic blood pressure, LDL [continuous], use of lipid-lowering medications, use of hypertension medication, log urine ACR [continuous], and eGFR [continuous]). Our final model additionally adjusted for other serum markers of mineral metabolism: calcium, phosphate, 25-hydroxyvitamin D, and PTH. We used C-statistics and the net reclassification index to test whether FGF-23 improved prediction of AF when added to components of the Framingham AF risk score29-31.
To explore factors that may mediate the association of FGF-23 with AF, we performed additional analyses adjusted for LVED mass, volume, or diameter as well as interim CHF events, N-terminal pro-B-type natriuretic peptide (NT pro-BNP), C-reactive protein (CRP), left atrial diameter (CHS only), or interleukin 6 (IL-6) (MESA only).
Functional forms of the relationships between FGF-23 and incident AF were explored using penalized spline plots. We tested for heterogeneity by age, sex, race/ethnicity, eGFR, urine ACR, and chronic kidney disease (eGFR <60 mL/min/1.73m2, urine ACR ≥30 mg/g, or both)14 by including interaction terms of FGF-23 with each covariate, testing statistical significance using the Wald test. Additionally, to explore whether FGF-23 may mediate known associations of low eGFR with AF19, we assessed the association of eGFR < 60 mL/min/1.73m2 with the hazard of AF without and with adjustment for FGF-23.
Approximately 5% of subjects were missing covariate data on education, smoking, urine albumin, LDL cholesterol, or serum creatinine. In CHS, 58.4%, 58.3%, 60.4%, and 58.9% of participants were missing data on calcium, phosphate, 25-hydroxyvitamin D, and parathyroid hormone, respectively. These values were multiply imputed using chained equations on the basis of observed baseline covariates. Multiple analyses over the imputations were combined using Rubin's rules to account for variability in the imputation procedure32. Missing covariate data accounted for <1% of variation in coefficient estimates.
P-values less than 0.05 were considered statistically significant. All analyses were performed using R version 2.15 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant Characteristics
In MESA, mean age was 62.0 years, 53.5% of participants were women, and 38.9% were Caucasian (Table 1A). Participants with higher serum FGF-23 concentrations were more likely to be female and Caucasian, to have diabetes, to use lipid-lowering or anti-hypertensive medications, and to have lower eGFR and higher urine ACR. Participants with higher FGF-23 tended to have higher parathyroid hormone and 25-hydroxyvitamin D concentrations but no significant differences in serum phosphate or calcium concentrations were observed. Higher FGF-23 concentration was directly correlated with LVED mass.
Table 1. Baseline Characteristics of Participants in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS), by quartile of circulating FGF-23 concentration.
| A. MESA | |||||
|---|---|---|---|---|---|
| Fibroblast Growth Factor-23 (pg/mL) | |||||
| 1.4-30.5 | 30.6-37.7 | 37.8-46.5 | 46.6-590 | p-value | |
| Number of participants, N (%) | 1603 (25) | 1603 (25) | 1603 (25) | 1589 (25) | |
| Demographic Data | |||||
| Age, years | 60.7 (10.1) | 61.7 (10.0) | 62.2 (10.2) | 63.5 (10.2) | <0.001 |
| Male gender, N (%) | 664 (41.4) | 769 (48.0) | 766 (47.8) | 791 (49.8) | <0.001 |
| Race/Ethnicity, N (%) | <0.001 | ||||
| Black | 469 (29.3) | 433 (27.0) | 417 (26.0) | 401 (25.2) | |
| Chinese | 189 (11.8) | 210 (13.1) | 193 (12.0) | 192 (12.1) | |
| Hispanic | 426 (26.6) | 351 (21.9) | 348 (21.7) | 284 (17.9) | |
| White | 519 (32.4) | 609 (38.0) | 645 (40.2) | 712 (44.8) | |
| Education, N (%) | 0.87 | ||||
| College degree or more | 521 (32.6) | 602 (37.6) | 559 (35.0) | 586 (37.0) | |
| High school to some college | 458 (28.7) | 418 (26.1) | 456 (28.6) | 489 (30.9) | |
| High school or less | 617 (38.7) | 579 (36.2) | 581 (36.4) | 510 (32.2) | |
| Medical History | |||||
| Diabetes, N (%) | 196 (12.2) | 196 (12.2) | 170 (10.6) | 223 (14) | 0.31 |
| Current smoking, N (%) | 266 (16.7) | 212 (13.2) | 194 (12.2) | 141 (8.9) | <0.001 |
| Hypertension medication, N (%) | 499 (31.1) | 520 (32.4) | 565 (35.2) | 744 (46.9) | <0.001 |
| Lipid lowering medication, N (%) | 219 (13.7) | 239 (14.9) | 259 (16.2) | 312 (19.7) | <0.001 |
| Physical activity, N (%) | 0.003 | ||||
| Low | 506 (31.7) | 520 (32.5) | 518 (32.4) | 579 (36.5) | |
| Moderate | 512 (32.1) | 507 (31.7) | 552 (34.5) | 490 (30.9) | |
| High | 578 (36.2) | 572 (35.8) | 529 (33.1) | 517 (32.6) | |
| Physical Examination | |||||
| Height, cm | 164.9 (9.6) | 166.5 (9.9) | 166.7 (10.1) | 167.2 (10.2) | <0.001 |
| Weight, lb | 167 (37.5) | 170.8 (36.8) | 175 (38.4) | 178.7 (38.6) | <0.001 |
| BMI, kg/m2 | 27.8 (5.4) | 27.9 (5.2) | 28.5 (5.5) | 28.9 (5.6) | <0.001 |
| Systolic blood pressure, mmHg | 125 (22) | 125 (21) | 126 (21) | 129 (22) | <0.001 |
| Diastolic blood pressure, mmHg | 72 (11) | 72 (10) | 72 (10) | 72 (10) | 0.64 |
| Laboratory Measurements | |||||
| LDL-C, mg/dL | 117 (31) | 118 (32) | 118 (31) | 117 (32) | 0.85 |
| Interleukin 6, pg/mL* | 1.1 (0.7,1.8) | 1.1 (0.7,1.8) | 1.2 (0.8,1.9) | 1.3 (0.9,2.0) | <0.001 |
| C-reactive protein, mg/L* | 2.0 (0.8,4.5) | 1.7 (0.8,3.8) | 1.7 (0.8,4.0) | 2.1 (0.9,4.6) | <0.001 |
| NT pro-BNP, pg/mL* | 52.6 (24.2,106.5) | 54.2 (24.0,106.7) | 52.5 (23.1,109.8) | 57.6 (24.7,124.1) | 0.10 |
| eGFR, mL/min/1.73m2 | 91.4 (15.5) | 87.7 (15.9) | 84.5 (16.5) | 78 (19.3) | <0.001 |
| Urine albumin-creatinine ratio* | 5.4 (3.4,10.0) | 5.2 (3.3,10.0) | 5.0 (3.2,10.7) | 5.7 (3.4,13.8) | <0.001 |
| Serum calcium, mg/dL | 9.6 (0.4) | 9.6 (0.4) | 9.7 (0.4) | 9.7 (0.4) | <0.001 |
| Serum phosphorus, mg/dL | 3.6 (0.5) | 3.6 (0.5) | 3.7 (0.5) | 3.7 (0.6) | <0.001 |
| 25-hydroxyvitamin D, ng/mL | 23.9 (10.6) | 25.6 (10.4) | 25.9 (10.6) | 27.5 (12.0) | <0.001 |
| Parathyroid hormone, pg/mL* | 39.8 (30.7,51.7) | 40.1 (30.7,52.2) | 40.9 (31.5,53.5) | 41.6 (31.7,55.2) | 0.011 |
| FGF-23, pg/mL* | 26.0 (22.6,28.5) | 34.0 (32.4,35.8) | 41.7 (39.6,43.9) | 54.7 (50.0,62.9) | <0.001 |
| Structural Data | |||||
| LVED mass, g | 139.2 (37.7) | 144.5 (38.6) | 146.3 (39.1) | 149.5 (40.7) | <0.001 |
| B. CHS | |||||
|---|---|---|---|---|---|
| Fibroblast Growth Factor-23 (RU/mL) | |||||
| 0-52.7 | 52.8-67.9 | 68.0-93.7 | 93.8-14,200 | p-value | |
| Number of participants, N (%) | 356 (26) | 364 (27) | 344 (25) | 286 (21) | |
| Demographic Data | |||||
| Age, years | 76.6 (4.1) | 76.7 (3.8) | 76.9 (3.9) | 77.8 (4.3) | <0.001 |
| Male gender | 128 (36.0) | 119 (32.7) | 86 (25.0) | 54 (18.9) | <0.001 |
| Black race | 65 (18.3) | 47 (12.9) | 39 (11.3) | 22 (7.7) | <0.001 |
| Education | 0.10 | ||||
| College degree or more | 93 (26.2) | 81 (22.4) | 83 (24.2) | 58 (20.3) | |
| High school to some college | 100 (28.2) | 86 (23.8) | 83 (24.2) | 79 (27.6) | |
| High school or less | 162 (45.6) | 195 (53.9) | 177 (51.6) | 149 (52.1) | |
| Medical History | |||||
| Diabetes, N (%) | 35 (9.8) | 32 (8.8) | 38 (11.0) | 38 (13.3) | 0.11 |
| Smoking Status | 0.008 | ||||
| Never | 197 (56.3) | 197 (55.2) | 174 (50.9) | 151 (53.9) | |
| Former | 140 (40.0) | 144 (40.3) | 135 (39.5) | 96 (34.3) | |
| Current | 13 (3.7) | 16 (4.5) | 33 (9.6) | 33 (11.8) | |
| Antihypertensive Medications | 133 (37.4) | 159 (43.7) | 174 (50.6) | 155 (54.2) | <0.001 |
| Lipid Lowering Medications | 21 (5.9) | 32 (8.8) | 29 (8.4) | 28 (9.8) | 0.10 |
| Walks for exercise | 217 (61.0) | 202 (55.5) | 185 (53.8) | 138 (48.3) | 0.001 |
| Physical Examination | |||||
| BMI, kg/m2 | 26.2 (4.5) | 26.4 (4.1) | 27.4 (4.3) | 27.9 (5.2) | <0.001 |
| Systolic Blood Pressure, mmHg | 136 (19) | 138 (19) | 137 (19) | 137 (20) | 1.00 |
| Laboratory Measurements | |||||
| LDL-C, mg/dl | 127 (33) | 132 (33) | 134 (34) | 130 (34) | 0.19 |
| C-reactive protein, mg/L* | 1.8 (0.9,4.3) | 2.0 (1.0,4.4) | 2.6 (1.1,5.5) | 2.4 (1.3,5.4) | 0.001 |
| NT pro-BNP, pg/dL* | 93.8 (51.7,171.2) | 97.3 (51.0,165.2) | 104.2 (53.8,179.6) | 101.4 (60.0,198.6) | 0.12 |
| eGFR, mL/min/1.73m2 | 72.9 (13.5) | 67.9 (12.8) | 64.7 (13.2) | 57.1 (17) | <0.001 |
| Urine albumin - creatinine ratio* | 7.9 (4.7,14.5) | 7.7 (4.4,14.1) | 7.8 (4.9,16.4) | 10.0 (5.3,21.5) | 0.004 |
| Serum calcium, mg/dL | 9.6 (0.5) | 9.8 (0.6) | 9.8 (0.5) | 9.9 (0.6) | 0.006 |
| Serum phosphorus, mg/dL | 3.7 (0.5) | 3.8 (0.5) | 3.8 (0.6) | 4.0 (0.6) | 0.89 |
| 25-hydroxyvitamin D, ng/mL | 28.4 (9.9) | 30.1 (11.3) | 29.5 (12.1) | 28.8 (11.1) | 0.95 |
| Parathyroid hormone, pg/mL* | 49.0 (37.0,61.5) | 46.0 (36.0,62.0) | 49.0 (38.0,62.0) | 55.5 (42.0,76.0) | <0.001 |
| FGF-23, RU/mL* | 44.7 (39.1,48.7) | 60.0 (54.1,63.8) | 77.5 (72.0,84.6) | 119.7 (101.5,150.0) | <0.001 |
| Structural Data | |||||
| LVED mass, g | 139.3 (42.8) | 137.3 (39.5) | 140.9 (42.6) | 144.8 (42.6) | 0.12 |
| LVED diameter, cm | 4.7 (0.5) | 4.6 (0.5) | 4.7 (0.5) | 4.6 (0.6) | 0.46 |
| Left atrial diameter, cm | 3.8 (0.5) | 3.9 (0.6) | 3.9 (0.6) | 3.9 (0.6) | 0.01 |
Entries are N (%) for categorical variables and mean (± standard deviation) for continuous variables except where indicated by *, which denotes median (interquartile range).
Abbreviations: BMI, body mass index; LDL-C, low density lipoprotein cholesterol; HDL, high density lipoprotein; NT pro-BNP, N-terminal pro-B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; FGF-23, fibroblast growth factor-23; LVED, left ventricular end-diastolic
In CHS, mean age was 77 years, 71.3% of participants were women, and 87.2% were Caucasian (Table 1B). In addition, compared with MESA, mean eGFR was lower and geometric mean urine ACR was higher. Similar to MESA, participants with higher FGF-23 were more likely to use anti-hypertensive medications and tended to have lower eGFR and higher urine ACR. Higher FGF-23 was directly correlated with left atrial diameter but not LVED mass. In adjusted analyses, higher plasma FGF-23 concentration was associated with greater left atrial diameter, adjusting for demographic variables (Supplemental Table 1A). This association was attenuated and no longer statistically significant with adjustment for potential confounders. Higher FGF-23 concentration was associated with LVED Mass and unlike left atrial diameter, this relationship remained strong and significant despite attenuation when fully adjusted (Supplemental Table 1B).
FGF-23 and Atrial Fibrillation
In MESA, 291 incident AF events were observed, with an incidence rate of 6.2 per 1000 person-years. In CHS, 229 incident AF events were observed, with an incidence rate of 25.6 per 1000 person-years. In both MESA and CHS, higher FGF-23 concentrations were associated with higher unadjusted incidence rates of AF (Table 2 and top panels of Figure 1).
Table 2. Associations of circulating FGF-23 concentrations with incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS).
| A. MESA | |||||
|---|---|---|---|---|---|
| FGF-23 (pg/mL) | Events | Incidence Rate | Model 1 | Model 2 | Model 3 |
| 1.4-30.5 | 39 | 3.3 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 30.6-37.7 | 62 | 5.2 | 1.39 (0.93 to 2.07) | 1.38 (0.92 to 2.06) | 1.37 (0.91 to 2.05) |
| 37.8-46.5 | 91 | 7.7 | 1.93 (1.32 to 2.82) | 1.72 (1.17 to 2.53) | 1.72 (1.17 to 2.53) |
| 46.6-590 | 99 | 8.4 | 1.90 (1.31 to 2.76) | 1.40 (0.95 to 2.05) | 1.38 (0.94 to 2.04) |
| per doubling | 1.79 (1.45 to 2.21) | 1.41 (1.13 to 1.76) | 1.41 (1.13 to 1.76) | ||
| p-value | <0.001 | 0.003 | 0.003 | ||
| B. CHS | |||||
|---|---|---|---|---|---|
| FGF-23 (RU/mL) | Events | Incidence Rate | Model 1 | Model 2 | Model 3 |
| 0-52.7 | 53 | 21.0 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 52.8-67.9 | 63 | 24.2 | 1.16 (0.81 to 1.67) | 1.20 (0.82 to 1.74) | 1.20 (0.83 to 1.74) |
| 68.0-93.7 | 60 | 25.3 | 1.24 (0.85 to 1.79) | 1.23 (0.83 to 1.83) | 1.23 (0.83 to 1.82) |
| 93.8-14,200 | 53 | 31.8 | 1.54 (1.03 to 2.28) | 1.51 (0.99 to 2.30) | 1.52 (1.00 to 2.32) |
| per doubling | 1.31 (1.07 to 1.60) | 1.29 (1.05 to 1.60) | 1.30 (1.05 to 1.61) | ||
| p-value | 0.010 | 0.018 | 0.016 | ||
Results from Cox proportional hazards regression models for association of circulating FGF-23 with incident atrial fibrillation in MESA(A) and CHS(B). Model 1 is adjusted for age, gender, race/ethnicity, study site, and attained education. Model 2 is additionally adjusted for low density cholesterol, use of lipid-lowering medications, current smoking, diabetes, physical activity, height, height squared, weight, urine albumin-creatinine-ratio, estimated glomerular filtration rate, systolic blood pressure, and use of hypertension medication. Model 3 is adjusted as per Model 2 with the addition of the serum concentrations of calcium, phosphate, 25-hydroxyvitamin D, and parathyroid hormone. Incidence rates are per 1000 person-years. P-values are derived from the models assessing FGF-23 as a continuous variable. Abbreviations: FGF-23, fibroblast growth factor-23
Figure 1.
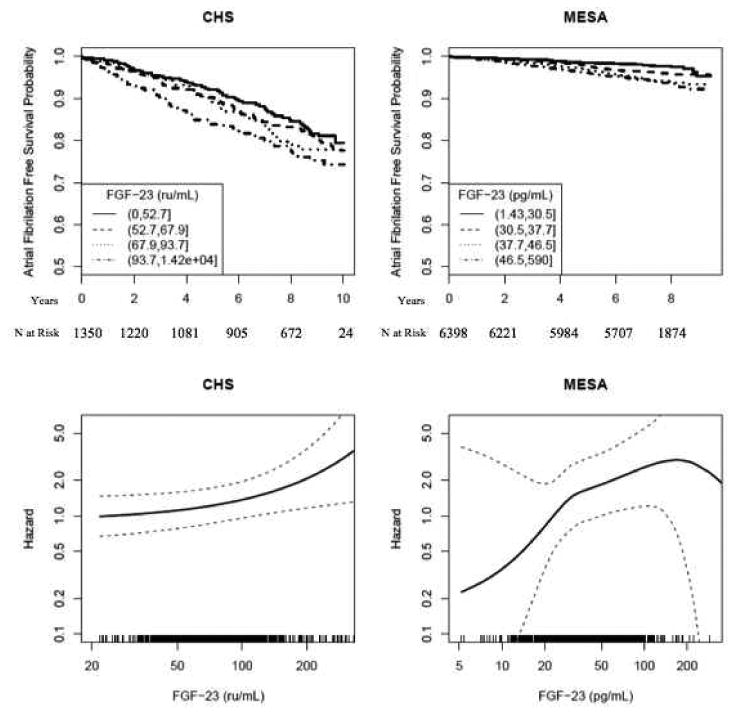
Associations of circulating FGF-23 concentration with atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Unadjusted Kaplan-Meier Curves are shown in the upper panels and restricted cubic spline plots, adjusted for demographic characteristics and potential confounding variables (Model 2 as in Table 2) are shown in the lower panels.
In adjusted analyses, risk of incident AF increased monotonically with FGF-23 concentration in each cohort (bottom panels of Figure 1). Adjusting for demographics and potential confounding characteristics, each two-fold higher FGF-23 concentration was associated with a 41% higher risk of AF in MESA (HR 1.41 [95% confidence interval 1.13-1.76], p=0.003) and a 29% higher risk of AF in CHS (HR 1.29 [95% confidence interval 1.05-1.60], p=0.018). Assessing FGF-23 concentration in quartiles, risk increased in a graded fashion in CHS, while the third quartile demonstrated the highest risk in MESA (Table 2). Further adjustment for other biomarkers of mineral metabolism did not substantially attenuate the association of FGF-23 with AF (Table 2, model 3). Addition of FGF-23 to components of the Framingham AF risk score did not improve AF prediction in MESA or CHS (Supplemental Tables 2-4).
In MESA, adjustment for left ventricular mass and volume, NT pro-BNP, CHF events, or inflammatory markers did not attenuate the magnitude of association of FGF-23 with AF (Supplemental Table 5). Similarly, in CHS, adjustment for left atrial diameter, CHF events, NT pro-BNP, or CRP, did not attenuate the magnitude of association of FGF-23 with AF (Supplemental Table 5). However, in a subset of 950 participants with measures of LVED mass and diameter accounting for 155 incident AF events, further adjustment for left ventricular mass or diameter modestly attenuated this association, which subsequently did not reach statistical significance.
No significant heterogeneity in the association of FGF-23 concentration with AF was observed among subgroups defined by age, gender, or race/ethnicity (Figure 2, all p-values for interaction >0.1). The risk of AF associated with higher FGF-23 appeared somewhat greater in participants with low eGFR, elevated urine ACR, or CKD (Figure 2); this heterogeneity was nominally significant only evaluating CKD and only in MESA (p-value for interaction=0.04).
Figure 2.
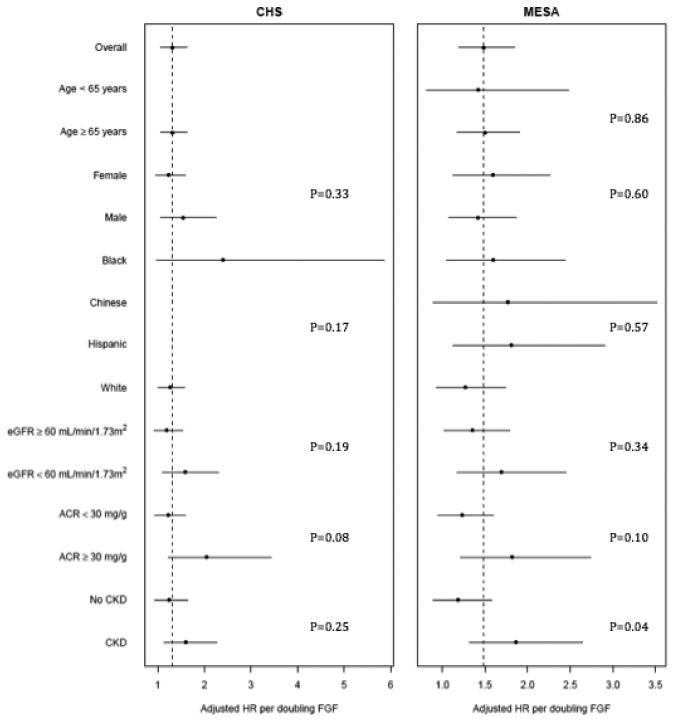
Associations of circulating FGF-23 concentration with atrial fibrillation by subgroup in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). P-values are for the interaction between FGF-23 and the respective covariate. Abbreviations: eGFR, estimated glomerular filtration rate; ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease (defined as an eGFR <60 mL/min/1.73m2 or ACR ≥ 30 mg/g); FGF, fibroblast growth factor.
Other Mineral Metabolism Markers
In analyses of MESA adjusted for demographics and potential confounding characteristics (as per Table 2, model 2), each 0.5 mg/dL higher serum phosphate concentration was associated with a 15% higher risk of incident AF (HR 1.15 [95% confidence interval 1.02-1.31], p-value=0.023) (Supplemental Table 6). In CHS, serum phosphate concentration was not significantly associated with incident AF (HR 1.02 per 0.5 mg/dL, [95% confidence interval 0.78-1.34], p-value=0.88). Serum concentrations of calcium, 25-hydroxyvitamin D, and PTH were not significantly associated with incident AF in either cohort (Supplemental Table 6).
Low eGFR and Atrial Fibrillation
In MESA, eGFR <60 mL/min/1.73m2 was associated with a 62% higher risk of incident AF (HR 1.62, [95% confidence interval 1.19-2.20]), adjusting for age, gender, race/ethnicity, study site, education, LDL cholesterol, use of lipid-lowering and antihypertensive medications, smoking, diabetes, physical activity, height, weight, systolic blood pressure, and urine ACR. With further adjustment for FGF-23, eGFR <60 mL/min/1.73m2 was associated with a 43% higher risk of incident AF (HR 1.43, [95% confidence interval 1.05-1.95]). In CHS, eGFR <60 mL/min/1.73m2 was not significantly associated with incident AF with or without adjustment for FGF-23.
Discussion
In two large community-based cohorts free of baseline cardiovascular disease, we demonstrated consistent associations of higher circulating FGF-23 concentration with increased risk of incident AF. These associations remained significant after accounting for other biomarkers of mineral metabolism, eGFR, urine ACR, and heart failure events. Adjusting for FGF-23 attenuated the association of low eGFR with incident AF in MESA, suggesting that FGF-23 may mediate, in part, the known association of CKD with AF.
Seiler and colleagues previously examined correlations of plasma FGF-23 concentration with left ventricular function and prevalent AF in patients admitted for elective coronary angiography11. Higher FGF-23 values were associated with poor left ventricular function and an increased prevalence of AF (OR 3.13 [1.82-5.40], p<0.001) 11. Unlike our study, Seiler et al. studied participants in a specific clinical context with a high proportion of pre-existing cardiovascular disease, raising the possibility of residual confounding. Moreover, they studied cross-sectional correlations with AF. We were able to assess the development of new-onset AF over long-term follow-up in well-characterized, community-based populations without prevalent cardiovascular disease. This approach supports a temporal relationship between FGF-23 and AF while reducing the likelihood of confounding.
FGF-23 may be linked with AF through direct effects on the myocardium12. Higher circulating FGF-23 concentration has been associated with adverse cardiac remodeling, including greater left ventricular mass, an increased prevalence of left ventricular hypertrophy, and lower ejection fraction12, 13. Similarly, among CHS participants in our study, higher concentrations of FGF-23 were strongly associated with greater left ventricular mass, as previously observed in a broader group of CHS participants that included those with baseline cardiovascular disease33.
Interestingly, adjusting for left ventricular mass and size modestly attenuated the association between FGF-23 and AF in CHS but not MESA. These mediation analyses are limited by measurement of left ventricular dimensions at a single point in time, and as a result, the degree to which the association of FGF-23 with AF can be explained by left ventricular hypertrophy is not entirely clear.
Changes in left ventricular function may lead to increased left atrial size, a known risk factor for atrial dysrhythmias. In CHS, FGF-23 was correlated with left atrial size but this association was attenuated and non-significant with adjustment for potential confounders. These results may reflect misclassification of left atrial size34. In the initial CHS echocardiographic reading, only left atrial anteroposterior diameter was measured35. Since the left atrium is an asymmetric, three-dimensional structure which does not dilate uniformly, one-dimensional left atrial measures ultimately fail to accurately capture the true extent of left atrial enlargement36.
Since structural changes in the left ventricle and left atrium do not clearly explain the observed associations of FGF-23 with AF, other mechanisms should also be considered. FGF-23 has been associated with endothelial dysfunction and vascular calcification37-40 – both of which could enhance automaticity and trigger episodes of AF. FGF-23 has also been associated with systemic inflammation41. AF is well known to be associated with an inflammatory milieu42, 43 and elevated CRP levels are a known risk factor for incident AF44. However, adjustment for single measures of CRP and IL-6 did not attenuate the association of FGF-23 with AF making the role of these mechanisms unclear. Finally, the association of FGF-23 with AF could be mediated by inhibiting the production of 1,25-dihydroxyvitamin D (1,25(OH)2D, the active vitamin D hormone). The PRIMO trial examined the effects of a 1,25(OH)2D analogue on cardiac structure over 48 weeks45. Although there were no differences between treatment and placebo groups with respect to the primary outcome of left ventricular mass index, there were significant differences in left atrial volume index, brain natriuretic peptide levels, and congestive heart failure hospitalizations between groups45, 46. In light of these findings, FGF-23 and 1,25(OH)2D may affect cardiac structure and function without an overt effect on the left ventricle.
Impaired GFR is a strong risk factor for AF19, and circulating FGF-23 concentrations rise markedly with progressive stages of chronic kidney disease47-49 Therefore, kidney disease is an important potential confounder of the FGF-23-AF relationship. Nevertheless, we saw strong associations of FGF-23 with AF after adjusting for eGFR as well as urine ACR, a complementary marker of kidney disease. Interestingly, the association of eGFR <60 mL/min/1.73m2 with incident AF observed in MESA was substantially attenuated by adjustment for FGF-23. One potential explanation for this observation is that FGF-23 mediates, in part, the known association of eGFR with AF. Low eGFR was not associated with incident AF in CHS, with or without adjustment for FGF-23, perhaps because of the strong competing risk of death in this older population50.
These observations implicate a renal-mineral metabolism axis that may play an important role in cardiovascular disease, including arrhythmogenesis. This relationship may be bidirectional. Recent studies suggest that successful arrhythmia suppression may be dependent on changes in renal function in patients with mild to moderate kidney disease as well as resistant hypertension51, 52. Alternatively, Bansal and colleagues have shown that incident AF in a large population of patients with CKD was associated with a 67% increase in the rate of ESRD53. FGF-23 may be an important catalyst in this bidirectional axis with both renal and cardiovascular implications.
Higher serum phosphate concentration was associated with increased risk of AF in MESA, but not CHS. In the Atherosclerosis Risk in Communities Study (ARIC), another community-based cohort study, each 1 mg/dl higher serum phosphate was associated with a 13% greater risk of incident AF9. In comparison, participants in MESA were free of cardiovascular disease at baseline, and the association of serum phosphate with incident AF was stronger. Reasons for heterogeneity across cohorts are not clear. Importantly, a single serum phosphate concentration may not be a robust marker of abnormal phosphate homeostasis. FGF-23 may be a more durable indicator of altered phosphate metabolism, potentially explaining the strong positive associations with AF observed in both MESA and CHS.
Our study has limitations. First, AF events were not adjudicated and were not classified by duration (paroxysmal versus persistent versus permanent). Second, FGF-23 did not improve AF risk prediction. However, we evaluated the contribution of FGF-23 to risk prediction in isolation, and a comprehensive evaluation of the renal-mineral metabolism axis may further improve risk prediction. Importantly, a lack of clear difference in risk prediction does not eliminate an important role for FGF-23 in AF pathogenesis. Third, limited intermediate outcomes were available to evaluate potential mechanisms. Most importantly, the study is observational in nature and observed associations may not be causal. In particular, there is a complex interplay between hormones regulating mineral metabolism, including 1, 25-dihydroxyvitamin D, PTH, and klotho in addition to FGF-23, and this endocrine axis is strongly influenced by kidney disease. We adjusted for several markers of mineral metabolism as well as eGFR and urine ACR, but residual confounding of the association of FGF-23 with AF may persist.
Strengths of our study include the community-based cohorts with broad ranges of age and racial/ethnic diversity, the longitudinal design, the ability to reduce potential confounding by excluding participants with clinical cardiovascular disease and adjusting for well-ascertained potential confounding characteristics, the long duration of follow-up with large numbers of events, and the replication of results in two independent cohorts.
Conclusion
Higher circulating FGF-23 concentrations were associated with an increased risk of developing AF. Elevated FGF-23 may explain, in part, the excess risk of AF observed in CKD.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA and CHS studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Funding Sources: This research was supported by grants R01HL096875, R01HL102214, and R01HL080295 as well as contracts N01HC95159 through N01HC95169 and contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 from the National Heart, Lung, and Blood Institute (NHLBI). Additional support was provided by grant AG023629 from the National Institute on Aging (NIA) and by grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources (NCRR).
Dr. de Boer receives research funding from Abbott Laboratories.
Footnotes
Conflict of Interest Disclosures: Dr. Ix has received an honorarium from Shire Pharmaceuticals.
References
- 1.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–672. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: The rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the united states. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in scotland, 1986--1996. Eur Heart J. 2001;22:693–701. doi: 10.1053/euhj.2000.2511. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: An economic analysis of atrial fibrillation in the uk. Heart. 2004;90:286–292. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the united states, 1985 through 1999: Implications for primary prevention. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 8.Khan AM, Lubitz SA, Sullivan LM, Sun JX, Levy D, Vasan RS, Magnani JW, Ellinor PT, Benjamin EJ, Wang TJ. Low serum magnesium and the development of atrial fibrillation in the community: The framingham heart study. Circulation. 2013;127:33–38. doi: 10.1161/CIRCULATIONAHA.111.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez FL, Agarwal SK, Grams ME, Loehr LR, Soliman EZ, Lutsey PL, Chen LY, Huxley RR, Alonso A. Relation of serum phosphorus levels to the incidence of atrial fibrillation (from the atherosclerosis risk in communities [aric] study) Am J Cardiol. 2013;111:857–862. doi: 10.1016/j.amjcard.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rienstra M, Lubitz SA, Zhang ML, Cooper RR, Ellinor PT. Elevation of parathyroid hormone levels in atrial fibrillation. J Am Coll Cardiol. 2011;57:2542–2543. doi: 10.1016/j.jacc.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, Steimle C, Ege P, Fehrenz M, Rogacev KS, Scheller B, Bohm M, Fliser D, Heine GH. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–2696. doi: 10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 12.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. Fgf23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: Chs (cardiovascular health study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, Investigators H. Fgf-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix gla protein with mortality in coronary artery disease: The heart and soul study. Ann Inter Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH. Fgf-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983–3989. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: A mechanistic appraisal. Circulation. 2012;126:2353–2362. doi: 10.1161/CIRCULATIONAHA.112.113233. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: The atherosclerosis risk in communities (aric) study. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J, Rock D, de Boer IH. Fibroblast growth factor-23 and cardiovascular disease in the general population: The multi-ethnic study of atherosclerosis. Circ Heart Fail. 2014 Mar 25; doi: 10.1161/CIRCHEARTFAILURE.113.000952. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, Investigators CE. Estimating glomerular filtration rate from serum creatinine and cystatin c. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linefsky JP, O'Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, Siscovick DS, Kestenbaum B. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: The cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, Kestenbaum B, de Boer IH. Estimating mean annual 25-hydroxyvitamin d concentrations from single measurements: The multi-ethnic study of atherosclerosis. Am J Clin Nutr. 2013;97:1243–1251. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosworth C, Sachs MC, Duprez D, Hoofnagle AN, Ix JH, Jacobs DR, Jr, Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf) 2013;79:429–436. doi: 10.1111/cen.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 28.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: The mesa (multi-ethnic study of atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paynter NP, Cook NR. A bias-corrected net reclassification improvement for clinical subgroups. Med Decis Making. 2013;33:154–162. doi: 10.1177/0272989X12461856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 31.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (framingham heart study): A community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 33.Jovanovich A, Ix JH, Gottdiener J, McFann K, Katz R, Kestenbaum B, de Boer IH, Sarnak M, Shlipak MG, Mukamal KJ, Siscovick D, Chonchol M. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231:114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E Recommendations for chamber quantification: A report from the american society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc of Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: The cardiovascular health study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 36.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: Physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 37.Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA, European Uremic Toxin Work G Fgf23 is independently associated with vascular calcification but not bone mineral density in patients at various ckd stages. Osteoporos Int. 2012;23:2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 38.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Srivaths PR, Goldstein SL, Silverstein DM, Krishnamurthy R, Brewer ED. Elevated fgf 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C. Fgf-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78:679–685. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 41.Manghat P, Fraser WD, Wierzbicki AS, Fogelman I, Goldsmith DJ, Hampson G. Fibroblast growth factor-23 is associated with c-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int. 2010;21:1853–1861. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- 42.Boos CJ, Anderson RA, Lip GY. Is fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 43.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: A systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 44.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 45.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin d therapy and cardiac structure and function in patients with chronic kidney disease: The primo randomized controlled trial. JAMA. 2012;307:674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 46.Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, Pritchett Y, Chang Y, Agarwal R, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Singh B, Zehnder D, Pachika A, Manning WJ, Shah A, Solomon SD, Thadhani R. Vitamin d reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164:902–909. e902. doi: 10.1016/j.ahj.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 48.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of fgf-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 49.Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, Fukagawa M. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 50.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin c and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 51.Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi Y, Takahashi A, Kuwahara T, Okubo K, Fujino T, Takagi K, Nakashima E, Kamiishi T, Hikita H, Hirao K, Isobe M. Renal function after catheter ablation of atrial fibrillation. Circulation. 2011;124:2380–2387. doi: 10.1161/CIRCULATIONAHA.111.047266. [DOI] [PubMed] [Google Scholar]
- 53.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


