Abstract
Sus1p is a common component of transcriptional co-activator, SAGA (Spt-Ada-Gcn5-Acetyltransferase), and mRNA export complex, TREX-2 (Transcription-export 2), and is involved in promoting transcription as well as mRNA export. However, it is not clearly understood how Sus1p promotes transcription. Here, we show that Sus1p is predominantly recruited to the upstream activating sequence of a SAGA-dependent gene, GAL1, under transcriptionally active conditions as a component of SAGA to promote the formation of pre-initiation complex (PIC) at the core promoter, and consequently, transcriptional initiation. Likewise, Sus1p promotes the PIC formation at other SAGA-dependent genes, and hence transcriptional initiation. Such function of Sus1p in promoting PIC formation and transcriptional initiation is not mediated via its role in regulation of SAGA’s histone H2B de-ubiquitylation activity. However, Sus1p’s function in regulation of histone H2B ubiquitylation is associated with transcriptional elongation, DNA repair and replication. Collectively, our results support that Sus1p promotes PIC formation (and hence transcriptional initiation) at the SAGA-regulated genes independently of histone H2B de-ubiquitylation, and further controls transcriptional elongation, DNA repair and replication via orchestration of histone H2B ubiquitylation, thus providing distinct functional insights of Sus1p in regulation of DNA transacting processes.
Keywords: Transcription, Sus1p, RNA polymerase II, DNA repair and replication
Introduction
Transcriptional initiation involves a coordinated assembly of various general transcription factors (GTFs) and RNA polymerase II at the core promoter to form the preinitiation complex (PIC). Such an assembly of transcription factors at the promoter is promoted by activator (1, 2). An activator binds to DNA upstream of TATA-box or core promoter via its DNA binding domain, and recruits a co-activator through its activation domain (1, 2). Co-activator then aids in the formation of PIC (1, 2). SAGA is a well-characterized co-activator (1, 3-6). It is a large multi-protein complex, and is involved in transcription of ~10% of genes in Saccharomyces cerevisiae (1). Although the SAGA complex was initially discovered in yeast, it is evolutionarily conserved from yeast to humans (1, 3, 4, 6). SAGA possesses two enzymatic activities: histone H3 acetyltransferase (HAT) and histone H2B ubiquitin protease or de-ubiquitylation (DUB) activity. These activities are crucial in regulating post-translational modifications of histones H3 and H2B, thereby causing an alteration of an active or inactive state of the chromatin. In yeast, SAGA is composed of at least 20 components which are organized into several modules such as DUB, HAT, TAF (TBP-associated factor) and SPT (Suppressor of Ty) (1, 3-9). The DUB module possesses histone H2B lysine 123 (K123) de-ubiquitylation or ubiquitin protease activity, and has four subunits, namely Ubp8p, Sgf11p, Sgf73p and Sus1p. The HAT module has histone H3 K9/14 HAT activity, and consists of Gcn5p, Ada2p, Ada3p and Sgf29p. A set of TAFs constitutes the TAF module that has been implicated in regulation of SAGA’s HAT activity and interaction with activator. The SPT module contains Tra1p, Spt3p, Spt7p, Spt8p, Ada1p and Spt20p, and contacts with activator and PIC to regulate transcriptional initiation in addition to its role in maintaining SAGA’s overall structural integrity. Thus, different modules of SAGA perform distinct functions in regulation of histone covalent modifications and transcriptional initiation.
Sus1p regulates SAGA’s histone H2B de-ubiquitylation activity by maintaining the association of Ubp8p (that has histone H2B de-ubiquitylation or ubiquitin protease activity) with SAGA (10-12). It is a small 11 kDa protein, and is evolutionarily conserved among eukaryotes. Its human homologue is ENY2 (13). Preliminary results suggest the links of ENY2 to different pathological states (10). In Drosophila, ENY2/E(Y)2 is involved in the barrier activity of Su(Hw)-dependent insulators (14). It also functions as a cofactor to regulate transcriptional activity of nuclear receptors (15). In yeast, transcription of ~9% genes is affected by Sus1p (12). Likewise, it is involved in gene expression in higher eukaryotes (13). Thus, Sus1p/ENY2 is an important regulator of gene expression for cellular functions.
Sus1p was initially identified by synthetic lethality screening with mRNA export adaptor Yra1 mutant allele (12). It also interacts physically with mRNA export factors, Thp1p and Sac3p, which are the integral components of the TREX-2 complex (10, 12). The TREX-2 complex binds to various nucleoporins of the nuclear pore complex, and mediates the export of mRNA from nucleus to cytoplasm (10-12). Thus, Sus1p is likely to affect mRNA export. Indeed, Sus1p has been shown to regulate mRNA export (10-12). Therefore, through its interaction with SAGA and TREX-2, Sus1p bridges transcription with mRNA export. However, it is not clearly understood how Sus1p regulates transcription. Here, we demonstrate that Sus1p stimulates the PIC formation (and hence transcriptional initiation) at the SAGA-regulated genes independently of its function in controlling SAGA’s histone H2B de-ubiquitylation activity. Further, Sus1p is involved in transcriptional elongation, DNA repair and replication via modulation of histone H2B deubiquitylation. These results provide significant mechanistic and functional insights of Sus1p as presented below.
Results
Sus1p is recruited to the UAS of a SAGA-dependent gene, GAL1, and promotes PIC formation at the core promoter for enhanced transcriptional initiation
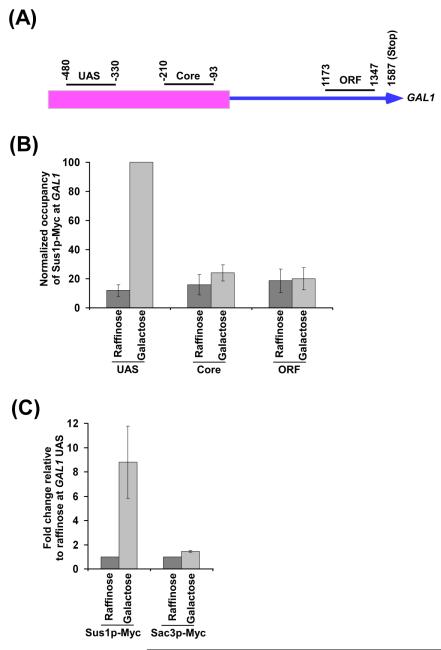
As mentioned above, Sus1p is a common component of SAGA and TREX-2, and thus, has been shown to be involved in transcription and mRNA export. Further, Sus1p has been implicated in translocation of GAL1 during active transcription (10, 11, 16, 17). Therefore, Sus1p functions at the interface of SAGA and TREX-2 in gene translocation, transcription and mRNA export. Hence, Sus1p is expected to be recruited to GAL1 during active transcription. In fact, we find that Sus1p is predominantly associated with the upstream activating sequence (UAS) of GAL1, but not with the core promoter or coding sequence in galactose-containing growth medium (Figures 1A and 1B). Sus1p is not found to be associated with GAL1 UAS in raffinose-containing growth medium (Figures 1A and 1B). Thus, Sus1p associates with the GAL1 UAS under transcriptionally active (galactose-containing growth medium), but not inactive (raffinose-containing growth medium), conditions. Like Sus1p, SAGA-associated Sgf73p and Ubp8p are recruited to the GAL1 UAS in a SAGA-dependent manner (18, 19). Similarly, the other components of SAGA are recruited to the GAL1 UAS under transcriptionally active conditions (18-22). However, Sac3p, a specific component of TREX-2, is not found to be associated with GAL1 UAS under transcriptionally active conditions (Figure 1C). These results support that Sus1p is recruited to the GAL1 UAS as a component of SAGA.
Figure 1.
Recruitment of Sus1p to the GAL1 UAS. (A) Schematic diagram showing the locations of different primer pairs at GAL1 for the ChIP analysis. The numbers are presented with respect to the position of the first nucleotide of the initiation codon (+1). (B) Recruitment of Sus1p to the UAS, but not core promoter or coding sequence, of the GAL1 gene. Yeast strain expressing Myc-epitope tagged Sus1p was grown at 30°C in yeast extract containing peptone plus 2% raffinose (YPR) up to an OD600 of 0.9 prior to formaldehyde-based in vivo crosslinking or induced for 90 min in YPG (yeast extract containing peptone plus 2% galactose) before crosslinking. Chromatin immunoprecipitation was performed as described in the Materials and Methods section. Immunoprecipitation was performed using a mouse monoclonal antibody against the c-Myc epitope-tag (9E10; Santa Cruz Biotechnology, Inc.). Primer pair located at the UAS, core promoter or coding sequence of the GAL1 gene (see Materials and Methods section) was used for PCR analysis of the immunoprecipitated DNA samples. The ratio of immunoprecipitate over the input in the autoradiogram was measured, and represented as ChIP signal. The maximum ChIP signal was set to 100. Other ChIP signals were normalized with respect to 100. The normalized ChIP signal (represented as normalized occupancy) is plotted in the form of a histogram. (C) Analysis of recruitment of Sus1p and Sac3p to the GAL1 UAS. Yeast strains expressing Myc-tagged Sus1p and Sac3p were grown, crosslinked and immunoprecipitated as in panel B. The ratio of immunoprecipitate over the input in the autoradiogram was measured in raffinose (YPR) and galactose (YPR). The fold change in galactose relative to raffinose is plotted in the form of a histogram.
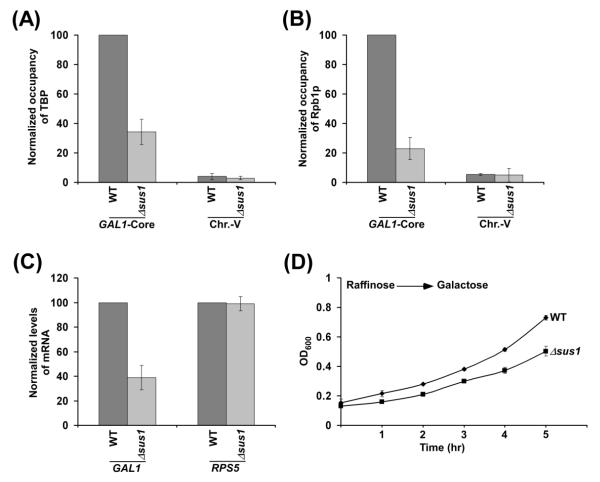
Next, we analyzed the function of Sus1p in formation of the PIC at the core promoter of GAL1. In this direction, we determined the recruitment of TBP and RNA polymerase II to the GAL1 core promoter in the presence and absence of Sus1p. TBP nucleates the assembly of GTFs to form the PIC. Thus, the formation of PIC and hence RNA polymerase II recruitment to the GAL1 core promoter would be impaired in the absence of TBP. We find that the recruitment of TBP to the GAL1 core is significantly decreased in the absence of Sus1p (Figure 2A). Likewise, the recruitment of the largest subunit (Rpb1p) of RNA polymerase II to the GAL1 core promoter is reduced in the Δsus1 strain (Figure 2B). Rpb1p maintains the structural integrity of RNA polymerase II (23). Thus, reduced recruitment of Rpb1p (Figure 2B) implies decreased association of RNA polymerase II with the GAL1 core promoter in the absence of Sus1p. As a non-specific DNA control, we analyzed the ChIP signal at the transcriptionally inactive region of chromosome V as done previously (24). As expected, a background level of ChIP signal was observed at the inactive region in chromosome V (Figures 2A and 2B). Together, these results support that Sus1p promotes the formation of PIC at the GAL1 core promoter.
Figure 2.
Requirement of Sus1p for recruitment of TBP and RNA polymerase II to the GAL1 core promoter. (A and B) Sus1p is required for recruitment of TBP and RNA polymerase II to the GAL1 core promoter. Yeast strains were grown and crosslinked as in Figure 1B. Immunoprecipitation was carried using an anti-TBP antibody (obtained from Michael R. Green; University of Massachusetts Medical School) against TBP or 8WG16 antibody (Covance, Inc.) against the carboxy terminal domain of Rpb1p. Immunoprecipitated DNA was amplified by PCR using primer pair targeted to the GAL1 core promoter or an inactive region within chromosome V. The maximum ChIP signal was set to 100, and other ChIP signals were normalized with respect to 100. The normalized ChIP signal (represented as normalized occupancy) is plotted in the form of a histogram. (C) Transcription. Total cellular RNA was prepared from the wild type and Δsus1 strains, and mRNA levels from the GAL1 and RPS5 genes were quantitated by primer extension analysis. Yeast cells were grown as in Figure 1B. The mRNA level of wild type strain was set to 100, and mRNA level of the Δsus1 strain was normalized with respect to 100. Normalized levels of mRNA are plotted in the form of a histogram. (D) Growth analysis. Both the wild type and Δsus1 strains were initially grown in YPR up to an OD600 of 0.1 and then switched to YPG at 30 °C. Subsequently, OD600 was measured for next 5 hr, and plotted as a function of time.
As mentioned above, transcriptional initiation involves the PIC formation at the core promoter. Since Sus1p facilitates the PIC formation at the GAL1 core promoter, transcription of GAL1 would likely to be decreased in the absence of Sus1p. To test this, we carried out primer extension analysis in the Δsus1 and wild type strains. Our primer extension analysis reveals that transcription of GAL1 is impaired in the Δsus1 strain (Figure 2C; Supplementary Figure S1). As a control, we also analyzed transcription of a SAGA-independent gene, RPS5, in the presence and absence of Sus1p. Since RPS5 is a SAGA-independent gene (20), we would not observe reduced RPS5 transcription in the Δsus1 strain. Indeed, we do not find an alteration of RPS5 transcription in the Δsus1 strain in comparison to the wild type equivalent (Figure 2C). Therefore, our results support that Sus1p promotes transcription of the SAGA-dependent GAL1 gene, but not SAGA-independent RPS5 gene. Such transcriptional stimulatory role of Sus1p is mediated via facilitated formation of the PIC at the GAL1 core promoter (Figures 2A and 2B). Consistently, the Δsus1 strain grows slowly in comparison to the wild type equivalent in galactose-containing growth medium that requires expression of GAL genes including GAL1 (Figure 2D).
Sus1p facilitates the PIC formation by enhancing recruitment of Sgf73p to the GAL1 UAS
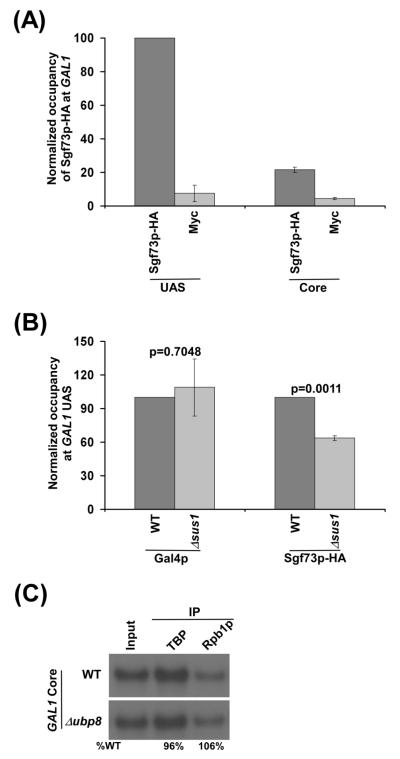
Next, we analyzed how Sus1p promotes the PIC formation and hence transcriptional initiation. Sus1p has been biochemically shown to reside within DUB module of SAGA that contains Sgf73p, Upb8p, Sus1p and Sgf11p (7-9, 25, 26). Sgf73p plays a crucial function in maintaining SAGA’s global structural integrity, and hence PIC formation and transcriptional initiation (19, 27). Further, Sgf73p links DUB module with the rest of SAGA (5, 7-9, 25). Sus1p may regulate the association of Sgf73p with SAGA, and control the PIC formation via Sgf73p. To test this, we analyzed the association of Sgf73p with the GAL1 UAS in the presence and absence of Sus1p. In this direction, we tagged Sgf73p by HA epitope at its chromosomal locus in the wild type and Δsus1 strains, and then performed the ChIP assay. We find that Sgf73p is predominantly associated with the GAL1 UAS (Figure 3A). An anti-Myc was used as a non-specific antibody, and generated background signal (Figure 3A). Interestingly, we find that the association of Sgf73p with SAGA is decreased in the absence of Sus1p in comparison to its wild type equivalent (Figure 3B). However, the association of activator Gal4p with the GAL1 UAS is not altered in the Δsus1 strain (Figure 3B). Thus, Sus1p facilitates the recruitment of Sgf73p to the GAL1 UAS. Such facilitated recruitment of Sgf73p to the GAL1 UAS in the presence of Sus1p is likely to enhance the PIC formation at the core promoter and hence transcriptional initiation, as our previous studies (19) demonstrated the stimulatory role of Sgf73p in PIC formation and transcriptional initiation.
Figure 3.
Recruitment of Sgf73p to the GAL1 UAS. (A) Recruitment of Sgf73p to the UAS, but not core promoter, of the GAL1 gene. The wild type strain expressing HA-epitope tagged Sgf73p was grown and crosslinked as in Figure 1B. Immunoprecipitation was performed using a mouse monoclonal antibody (F-7; Santa Cruz Biotechnology, Inc.) against the HA epitope-tag. An anti-Myc was used as a non-specific antibody. The ratio of immunoprecipitate over the input in the autoradiogram was measured, and represented as ChIP signal. The maximum ChIP signal was set to 100, and other ChIP signals were normalized with respect to 100. The normalized ChIP signal (represented as normalized occupancy) is plotted in the form of a histogram. (B) Sus1p facilitates the recruitment of Sgf73p to the GAL1 UAS. Wild type and Δsus1 strains expressing HA-epitope tagged Sgf73p were grown, crosslinked and immunoprecipitated as in Figure 1B. The ChIP signal of the wild type strain was set to 100, and the ChIP signal of the Δsus1 strain was normalized with respect to 100. The normalized ChIP signal (represented as normalized occupancy) is plotted in the form of a histogram. The Student’s t test of Microsoft Excel 2003 (with tail = 2 and types = 3) was used to determine the p values for statistical significance of the change in the ChIP signals from three biologically independent experiments. The changes were considered to be statistically significant at p < 0.05. (C) Ubp8p does not regulate the recruitment of TBP and RNA polymerase II (Rpb1p) to the GAL1 core promoter. Yeast cells were grown and crosslinked as in Figure 1B. Immunoprecipitation was carried out as in Figures 2A and 2B.
The stimulatory function of Sus1p in PIC formation at GAL1 is not mediated via histone H2B de-ubiquitylation
We find that Sus1p promotes Sgf73p recruitment and hence PIC formation at GAL1. In addition, Sus1p may play an additional function in the PIC formation via an impaired recruitment of Ubp8p (or concurrent reduction of histone H2B deubiquitylation), as previous studies demonstrated the role of Sus1p in recruitment of Ubp8p and hence histone H2B de-ubiquitylation (25, 28, 29). Intriguingly, we find that the loss of Ubp8p does not alter the recruitment of TBP and the the largest subunit (Rpb1p) of RNA polymerase II to the GAL1 core promoter following 90 min transcriptional induction (Figure 3C). Likewise, previous studies (30) demonstrated the dispensability of Ubp8p for recruitment of the Rpb3p component of RNA polymerase II to GAL1. Thus, the PIC formation at GAL1 is not regulated by histone H2B deubiquitylation. Therefore, Sus1p does not regulate the PIC formation at GAL1 via Ubp8p (or histone H2B de-ubiquitylation).
Sus1p facilitates the PIC formation (and hence transcriptional initiation) at the SAGA-dependent ADH1 and PHO84 genes independently of histone H2B de-ubiquitylation
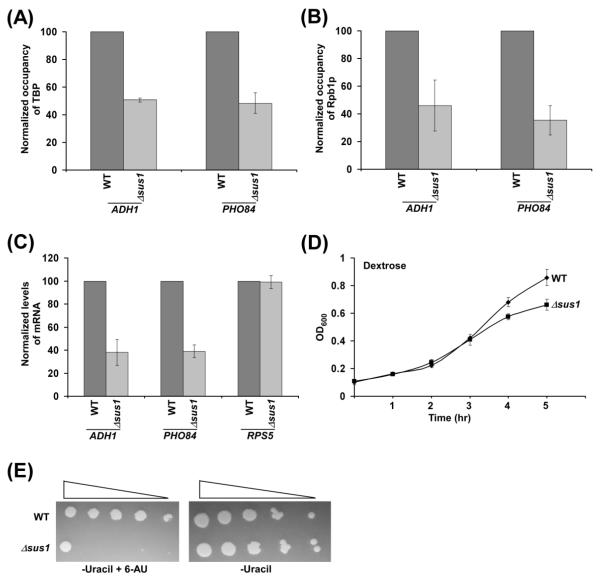
Our results demonstrate that Sus1p promotes the PIC formation (and hence transcriptional initiation) at a SAGA-dependent gene, GAL1. To determine whether Sus1p plays similar functions at other SAGA-dependent genes, we analyzed the formation of PIC at the core promoters of two other SAGA-dependent genes such as ADH1 and PHO84 in the presence and absence of Sus1p. We find that the recruitment of TBP and RNA polymerase II to the core promoters of these genes is significantly impaired in the absence of Sus1p (Figures 4A and 4B). Thus, like the results at GAL1, Sus1p facilitates the formation of PIC at the core promoters of the SAGA-dependent ADH1 and PHO84 genes. Therefore, transcription of these genes is likely to be impaired in the absence of Sus1p. Indeed, we find that transcription of these genes is reduced in the Δsus1 strain in comparison to the wild type equivalent (Figure 4C; Supplementary Figure S1). As a control, we show that transcription of a SAGA-independent gene, RPS5, is not altered in the absence of Sus1p (Figure 4C). The stimulatory role of Sus1p in transcriptional initiation of ADH1 and PHO84 is not mediated via histone H2B deubiquitylation, as our previous studies (18) demonstrated that PIC formation (and transcriptional initiation) at the promoters of these genes is not altered in the absence of Ubp8p. Thus, similar to the results at GAL1, Sus1p promotes the PIC formation (and hence transcriptional initiation) independently of histone H2B de-ubiquitylation at other SAGA-regulated genes that are constitutively active in dextrose-containing growth medium. Consistently, the growth of the Δsus1 strain is decreased under similar dextrose-containing growth medium (Figure 4D).
Figure 4.
Sus1p promotes the PIC formation at the ADH1 and PHO84 core promoters. (A and B) Sus1p is required for recruitment of TBP and RNA polymerase II to the ADH1 and PHO84 core promoters. Yeast strains were grown in YPD (yeast extract containing peptone plus 2% dextrose) up to an OD600 of 1.0 prior to crosslinking. Immunoprecipitation was carried as in Figures 2A and 2B. The ChIP signal of the wild type strain was set to 100, and the ChIP signal of the Δsus1 strain was normalized with respect to 100. The normalized ChIP signal (represented as normalized occupancy) is plotted in the form of a histogram. (C) Transcription. Total cellular RNA was prepared from the wild type and Δsus1 strains, and mRNA levels from the ADH1, PHO84 and RPS5 genes were quantitated by primer extension analysis. Yeast cells were grown as in panels A and B. (D) Growth analysis. Both wild type and Δsus1 strains were initially grown in YPD up to an OD600 of 0.1 at 30 °C. Subsequently, OD600 was measured for next 5 hr in YPD, and plotted as a function of time. (E) Growth analysis of the Δsus1 and wild type strains in the solid SC-uracil medium containing 2% dextrose with or without 6-AU (100 μg/ml) at 30 °C.
Sus1p promotes transcriptional elongation, DNA repair and replication
SAGA, Ubp8p or Sus1p has been previously shown to be associated with the coding sequence of certain genes, and regulates histone H2B ubiquitylation (30-32). The loss of histone H2B ubiquitylation in the absence of histone H2B ubiquitylation enzymes (or in histone H2B K123R point mutant) impairs transcriptional elongation (33-39). Thus, an enhanced level of histone H2B ubiquitylation at the coding sequence in the absence of Sus1p or Ubp8p would affect transcriptional elongation. Indeed, it has been demonstrated previously that the loss of Ubp8p or increased histone H2B ubiquitylation lowers the association of Ctk1p (Serine-2 kinase for carboxy terminal domain of the largest subunit of RNA polymerase II), Set2p (histone H3 K36 methyltransferase) and serine-2-phosphorylated RNA polymerase II with the active coding sequence (30). Consequently, the null mutation of Ubp8p (or enhanced histone H2B ubiquitylation; 18, 26, 30, 40) impairs the cellular growth in solid SC-uracil medium containing 6-AU (6-Azauracil) (30). 6-AU decreases nucleotide pools for transcriptional elongation by RNA polymerase II, and thus, results in slow (or impaired) growth phenotype upon deletion or mutation of the factors involved in transcriptional elongation (30). Thus, impaired growth of the Δubp8 strain in the presence of 6-AU (30) implicates the role of Ubp8p or histone H2B de-ubiquitylation in transcriptional elongation. Since the loss of Sus1p enhances histone H2B ubiquitylation (25, 28, 29) similar to the Δubp8 strain, transcriptional elongation is thus likely to be impaired in the Δsus1 strain. Indeed, the growth of the Δsus1 strain is impaired in SC-uracil medium containing 6-AU (Figure 4E), indicating the role of Sus1p in facilitation of transcriptional elongation. Thus, the loss as well as gain (or excess) of histone H2B ubiquitylation impairs transcriptional elongation, implicating an appropriate balance of histone H2B ubiquitylation and de-ubiquitylation in the maintenance of normal transcriptional elongation.
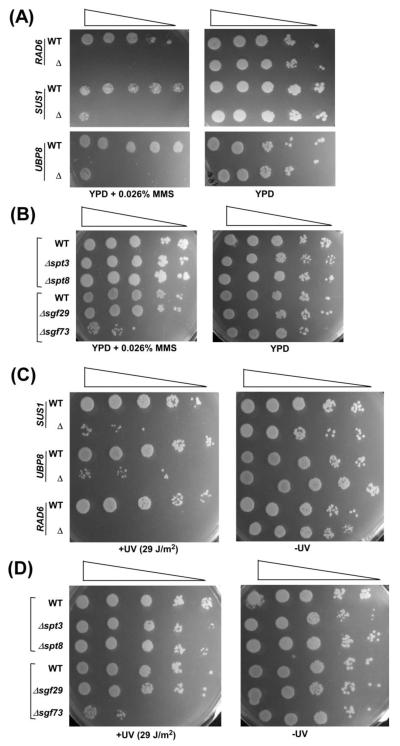
In addition to its stimulatory function in transcriptional elongation, histone H2B ubiquitylation also promotes DNA repair (34, 41, 42). Since Sus1p is involved in modulation of histone H2B ubiquitylation via maintaining the association of Ubp8p with SAGA (25, 28, 29), it is likely to be involved in DNA repair. To test this, we analyzed the growth of the wild type and Δsus1 strains in solid YPD growth medium with or without 0.026% MMS (Methyl methanosulfonate; DNA damaging agent). We find that the Δsus1 strain does not grow on solid YPD medium containing 0.026% MMS (Figure 5A), while it grows normally in the absence of MMS (Figure 5A). These results indicate that Sus1p promotes DNA repair. Such stimulatory function of Sus1p in DNA repair may be mediated via its role in maintaining SAGA’s histone H2B de-ubiquitylation activity. If yes, Ubp8p would enhance DNA repair. To test this, we analyzed the growth of the wild type and Δubp8 strains in solid growth medium with or without 0.026% MMS. We find that, similar to the Δsus1 strain, the Δubp8 strain does not grow on solid YPD medium with 0.026% MMS, but rather grows fine in the absence of MMS (Figure 5A). Similarly, the deletion of SGF73 (that impairs recruitment of Ubp8p, and hence histone H2B deubiquitylation; 43) shows MMS-sensitivity (Figure 5B). However, the deletion of SPT3, SPT8 and SGF29 (that do not alter SAGA’s global structural integrity, and hence histone H2B de-ubiquitylation; 5, 20, 22, 44-48) do not affect the growth of yeast cells in the presence of 0.026% MMS (Figure 5B). Thus, the loss of Sus1p, Ubp8p, Sgf73p or enhanced histone H2B ubiquitylation impairs DNA repair. Likewise, the Δrad6 strain does not grow on solid YPD medium containing 0.026% MMS (Figure 5A), consistent with previous studies (34, 41) that demonstrated the role of Rad6p (histone H2B ubiquitin conjugase) or histone H2B ubiquitylation in facilitation of DNA repair. Thus, the loss as well as gain (or excess) of histone H2B ubiquitylation have adverse effects on DNA repair. Similar results are also found following UV-induced DNA damage (Figures 5C and 5D).
Figure 5.
Sus1p facilitates DNA repair. (A and B) Growth analysis of the Δsus1, Δrad6, Δubp8, Δspt3, Δspt8, Δspt29, Δsgf73 and wild type strains in the solid YPD medium with or without 0.026% MMS at 30 °C. (C and D) Growth analysis of the Δsus1, Δrad6, Δubp8, Δspt3, Δspt8, Δspt29, Δsgf73 and wild type strains in the solid YPD medium with or without UV (254 nm) exposure (29 J/m2).
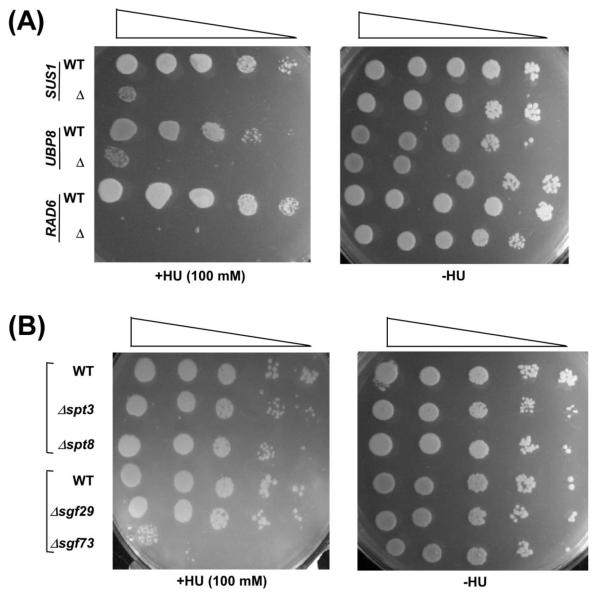
A recent study (49) has implicated the role of histone H2B ubiquitylation in DNA replication. Consistently, the absence of Rad6 impairs cellular growth in response to replication stress by HU (Hydroxyurea) (Figure 6A). However, it remains unknown whether an excess of histone H2B ubiquitylation or impairment of histone H2B deubiquitylation in the absence of Ubp8p or Sus1p has any effect on DNA replication. To test this, we analyzed the growth of the Δubp8 and Δsus1 strains in solid YPD growth medium containing HU. We find that the growth of the Δubp8 cells is dramatically impaired in the presence of HU, similar to the growth defect of the Δrad6 strain in HU (Figure 6A). However, the growth of the Δspt3, Δspt8 and Δsgf29 strains (that do not regulate histone H2B de-ubiquitylation) is not altered in solid growth medium containing HU (Figure 6B). These results support that Ubp8p is essential for DNA replication. Similar results are also found in the absence of Sus1 (Figure 6A) that is essential to maintain DUB activity of Ubp8 (25, 28, 29). Likewise, the growth of the Δsgf73 strain (that impairs histone H2B de-ubiquitylation; 43) is decreased in the presence of HU (Figure 6B). Thus, histone H2B de-ubiquitylation affects DNA replication.
Figure 6.
Sus1p facilitates DNA replication. (A and B) Growth analysis of the Δsus1, Δrad6, Δubp8, Δspt3, Δspt8, Δspt29, Δsgf73 and wild type strains in the solid YPD medium with or without 100 mM HU.
Discussion
Although previous studies (12) implicated Sus1p in transcription, it was not clear how Sus1p affects transcription. Here, we show that Sus1p is recruited to the UAS of a SAGA-dependent gene, GAL1, as a component of SAGA. Subsequently, it promotes the formation of PIC at the core promoter. Enhanced formation of PIC by Sus1p consequently increases transcriptional initiation of GAL1. Likewise, Sus1p promotes the formation of PIC (and hence transcriptional initiation) at other SAGA-dependent genes such as ADH1 and PHO84. These results show how Sus1p controls transcriptional initiation.
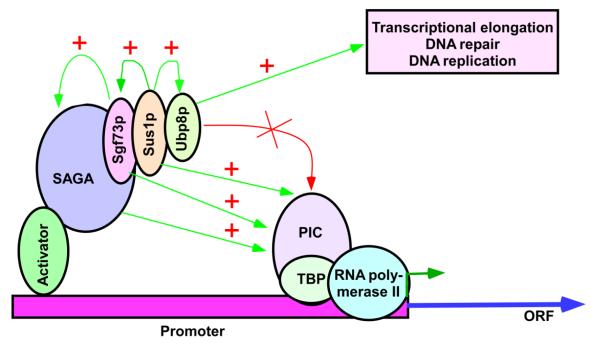
The absence of Sus1p impairs the recruitment of Ubp8p (25), and hence histone H2B de-ubiquitylation (25, 28, 29). However, an impaired histone H2B de-ubiquitylation in the absence of Ubp8p does not alter PIC formation (Figure 3C; 18, 30). Thus, Sus1p promotes the PIC formation independently of histone H2B de-ubiquitylation (Figure 7). Further, histone H2B ubiquitylation facilitates histone H3 K4 methylation via histon trans-tail cross-talk (50, 51, 52). However, similar to histone H2B de-ubiquitylation, histone H3 K4 methylation is dispensable for PIC formation (18, 33). Therefore, Sus1p facilitates PIC formation at the SAGA-regulated genes independently of histone H2B deubiquitylation or histone H3 K4 methylation.
Figure 7.
Schematic diagram showing the roles of Sus1p in different DNA transacting processes. Sus1p is essential for association of Ubp8p with SAGA (25). Ubp8p is dispensable for PIC formation (Figure 3C; 18), but favors transcriptional elongation (Figure 4E; 30, 31), DNA repair (Figure 5), and DNA replication (Figure 6). Sus1p also facilitates the association of Sgf73p with SAGA (Figure 3B). Sgf73p is essential for overall structural integrity of SAGA and hence PIC formation (19, 27). Thus, Sus1p appears to promote the PIC formation via Sgf73p. Sus1p and Sgf73p may also have additional direct roles in stimulation of PIC as described in the discussion section. “+”, stimulatory effect; PIC, pre-initiation complex; and ORF, open reading frame.
Sus1p facilitates the recruitment of Sgf73p (Figure 3B) that is essential for SAGA’s overall structural integrity, PIC formation and transcriptional initiation (19, 27). Thus, Sus1p appears to facilitate the PIC formation via Sgf73p (Figure 7). Further, a compromised overall structural integrity of SAGA in the absence of Sus1p (via decreased recruitment of Sgf73p) would reduce SAGA’s HAT activity. Hence, decreased PIC formation at GAL1 in the Δsus1 strain might be mediated via impaired histone H3 K9/14 acetylation. However, we rule out this possibility, as our previous studies (19, 20) demonstrated that SAGA’s HAT activity or Gcn5p (that has HAT activity) is dispensable for the PIC formation at GAL1. Thus, Sus1p’s function in promoting the PIC formation is not mediated via SAGA’s HAT activity.
Like Sus1p, Sgf73p promotes the PIC formation independently of histone H2B de-ubiquitylation, but rather via the maintenance of overall structural integrity of SAGA (Figure 7). Sgf73p may also play an additional direct role in regulation of the PIC formation and transcriptional initiation. In support of this possibility, a very recent study (53) has implicated the interaction of Sgf73p with proteasome in regulation of transcription. Further, Sgf73p has been implicated to interact with Mediator (54) that promotes transcription (21). Thus, Sgf73p is likely to play a direct role in PIC formation in addition to its role in maintaining SAGA’s overall structural integrity. Likewise, Sus1p may play a direct function in addition to its role in stimulation of PIC formation via Sgf73p. In support of this possibility, Sus1p is found to be a peripheral component of SAGA (5), and has been implicated to interact with Mediator (5) that facilitates GAL1 transcription (21).
Although both the Sgf73p and Sus1p components of SAGA’s DUB module are involved in the PIC formation independently of histone H2B de-ubiquitylation, it is not clear whether Sgf73p functions through Sus1p (as the loss of Sgf73p impairs recruitment of Sus1p; 31), Sus1p functions through Sgf73p or independently. We find that the loss of Sus1p impairs the recruitment of Sgf73p and PIC formation (Figures 2A, 2B and 3B). Similarly, the loss of Sgf73p impairs the recruitment of Sus1p (31), and reduces the PIC formation (19). Thus, it is likely that the functions of Sus1p and Sgf73p are mutually dependent in stimulating the formation of PIC, and subsequently transcriptional initiation. Consistently, Sus1p and Sgf73p are found to interact with each other (5, 12, 31, 43).
In addition to promoting transcriptional initiation, Sus1p also facilitates transcriptional elongation (Figure 4E). Such function appears to be mediated via its role in regulation of histone H2B de-ubiquitylation (Figure 7). Recent studies (30) have implicated that enhanced histone H2B ubiquitylation in the absence of Ubp8p decreases transcription elongation. An excess histone H2B ubiquitylation decreases the recruitment of Ctk1p (that phosphorylates RNA polymerase II for recruitment of Set2p and mRNA 3′-end processing factors to control transcriptional elongation and mRNA processing; 55-59) to the coding sequence, thus lowering transcriptional elongation (30). On the other hand, the loss of histone H2B ubiquitylation also impairs transcriptional elongation (33-39). Therefore, an appropriate balance of histone H2B ubiquitylation and deubiquitylation is essential for optimal transcriptional elongation. Ctk1p is not involved in transcriptional initiation. Thus, while histone H2B de-ubiquitylation is dispensable for PIC formation (18), it plays an important role in maintaining proper level of histone H2B ubiquitylation for optimal transcriptional elongation.
Further, we show here that Sus1p or histone H2B de-ubiquitylation is essential for DNA repair and replication (Figures 5, 6 and 7). We find that an excess of histone H2B ubiquitylation in the absence of Sus1p or Ubp8p (25, 28, 29) impairs DNA repair and replication (Figures 5A, 5C and 6A). On the other hand, the loss of histone H2B ubiquitylation in the absence of Rad6p decreases DNA repair and replication (Figures 5A, 5C and 6A). Thus, the loss or gain of histone H2B ubiquitylation reduces DNA repair and replication. An appropriate balance of this modification is essential for optimal DNA repair and replication. Sus1p or Ubp8p play important roles in maintaining such balance. Likewise, Sgf73p and Sgf11p (that are required for recruitment of Ubp8p, and hence histone H2B de-ubiquitylation; 26, 40, 43) would also regulate the balance of histone H2B ubiquitylation/de-ubiquitylation, and thus DNA repair and replication. Indeed, we show here that, like Sus1p and Ubp8p, Sgf73p is required for DNA repair and replication (Figures 5B, 5D and 6B). However, the Spt3p, Spt8p and Sgf29p components of SAGA (that are dispensable for SAGA’s overall structural integrity, and hence histone H2B de ubiquitylation activity; 5, 20, 22, 44-48) are not required for DNA repair and replication (Figures 5B, 5D and 6B). Thus, histone H2B de-ubiquitylation activity of SAGA regulates DNA repair and replication.
In summary, Sus1p promotes the PIC formation independently of histone H2B deubiquitylation, while it favors transcriptional elongation, DNA repair and replication via modulation of histone H2B de-ubiquitylation. These results provide significant functional insights of Sus1p in regulation of DNA transacting processes. Since Sus1p is conserved among eukaryotes, similar regulatory mechanisms/functions are likely to exist in humans.
Materials and Methods
Plasmids
The plasmids, pFA6a-13Myc-KanMX6 and pFA6a-3HA-His3MX6 (60), were used for genomic tagging of the proteins of interest by Myc and HA epitopes, respectively. Plasmids, pRS416 and pRS413, were used for PCR-based knock-out of the genes of interest such as SGF29, SGF73 and UBP8.
Yeast strains
The yeast (S. cerevisiae) strain bearing SUS1 knockout (Δsus1) and its isogenic wild-type equivalent were obtained from the Rodríguez-Navarro laboratory (Centro de Investigación Príncipe Felipe, Spain; 12). The yeast strain harboring the null mutation of RAD6 (STY2; Δrad6 in FM392) and the wild-type equivalent (STY1; FM392) were obtained from the Shilatifard laboratory (Ali Shilatifard, Stowers Institute for Medical Research; purchased from Research Genetics). The wild type yeast strain, YKH045, was obtained from the Osley laboratory (Mary Ann Osley, University of New Mexico Health Science Center) (61). Yeast strains harboring null mutation in SPT3 (FY294) and SPT8 (FY462), and their isogenic wild-type equivalent, FY631, were obtained from the Winston laboratory (Fred Winston, Harvard Medical School, Boston, MA) (44). Multiple HA epitope tags were added at the original chromosomal locus of SGF73 in the Δsus1 and wild type strains to generate GDY76 (sus1Δ, Sgf73p-HA) and GDY77 (Sgf73p-HA), respectively. Likewise, multiple Myc epitope tags were added at the chromosomal loci of SUS1 and SAC3 in W303a to generate GDY24 (Sus1p-Myc) and GDY27 (Sac3p-Myc), respectively. The endogenous UBP8 gene of YKH045 was disrupted, using a PCR-based gene knockout method to generate the ASY19 (Δubp8) strain (18). Likewise, the SGF29 and SGF73 genes of W303a strain were deleted to generate ASY8 (Δsgf29) and ASY9 (Δsgf73), respectively (19, 22).
Growth media
Yeast strains were grown in YPD (yeast extract, peptone plus 2% dextrose) up to an OD600 of 1.0 at 30 °C for the studies at the ADH1, PHO84 and RPS5 genes. For studies at GAL1, yeast cells were grown in YPR (yeast extract, peptone plus 2% raffinose) up to an OD600 of 0.9 at 30 °C, and then switched to YPG (yeast extract, peptone plus 2% galactose) for 90 min. For growth curve analysis in YPG, yeast cells were grown in YPR at 30 °C up to an OD600 of 0.1, and then switched to YPG. Subsequently, OD600 was measured at different time points. The growth analysis was also performed for yeast cells continuously grown in YPD.
ChIP assay
The ChIP assay was performed as described previously (18, 20, 21, 45, 62, 63). Briefly, yeast cells were treated with 1% formaldehyde, collected and resuspended in lysis buffer. Following sonication, cell lysate (400 μl lysate from 50 ml of yeast culture) was precleared by centrifugation, and then 100 μl lysate was used for each immunoprecipitation. Immunoprecipitated protein–DNA complexes were treated with proteinase K, the cross-links were reversed, and DNA was purified. Immunoprecipitated DNA was dissolved in 20 μl TE 8.0 (10 mM Tris HCl pH 8.0 and 1 mM EDTA), and 1 μl of immunoprecipitated DNA was analyzed by PCR. The PCR reactions (a total of 23 cycles) contained [α-32P]dATP (2.5 μCi for each 25-μl reaction), and the PCR products were detected by autoradiography after separation on a 6% polyacrylamide gel. As a control, “input” DNA was isolated from 5 μl of lysate without going through the immunoprecipitation step and suspended in 100 μl of TE 8.0. To compare the PCR signal arising from the IP DNA with that from the input DNA, 1 μl of input DNA was used for PCR analysis. Serial dilutions of input and IP DNA samples were used to assess the linear range of PCR amplification as described previously (45, 63; Supplementary Figure S2). The PCR data presented here are within the linear range of PCR analysis.
For analysis of Sus1p, Sac3p and Sgf73p recruitment, the above ChIP protocol was modified as described previously (18, 19, 22, 24, 64, 65). Briefly, a total of 800 μl lysate was prepared from 100 ml of yeast culture. Following sonication, 400 μl lysate was used for each immunoprecipitation (using 10 μl of anti-HA or anti-Myc antibody and 100 μl of protein A/G plus agarose beads from Santa Cruz Biotechnology, Inc.), and immunoprecipitated DNA sample was dissolved in 10 μl TE 8.0 of which 1 μl was used for PCR analysis (a total of 23 cycles). In parallel, PCR analysis for input DNA was performed using 1 μl DNA that was prepared by dissolving purified DNA from 5 μl lysate in 100 μl TE 8.0. The primer pairs used for PCR analysis were as follows:
| ADH1 (Core): | 5′-GGTATACGGCCTTCCTTCCAGTTAC-3′ 5′-GAACGAGAACAATGACGAGGAAACAAAAG-3′ |
| PHO84 (Core): | 5′-GATCCACTTACTATTGTGGCTCGT-3′ 5′-GTTTGTTGTGTGCCCTGGTGATCT-3′ |
| RPS5 (Core): | 5′-GGCCAACTT CTACGCTCACGTTAG-3′ 5′-CGGTGTCAGACATCTT TGGAATGGTC-3′ |
| GAL1 (UAS): | 5′-CGCTTAACTGCTCATTGCTATATTG-3′ 5′-TTGTTCGGAGCAGTGCGGCGC-3′ |
| GAL1 (Core): | 5′-ATAGGATGATAATGCGATTAGTTTTTTAGCCTT-3′ 5′-GAAAATGTTGAAAGTATTAGTTAAAGTGGTTATGCA-3′ |
| GAL1 (ORF): | 5′-CAGAGGGCTAAGCATGTGTATTCT-3′ 5′-GTCAATCTCTGGACAAGAACATTC-3′ |
| Chromosome-V: | 5′-GGCTGTCAGAATATGGGGCCGTAGTA-3′ 5′-CACCCCGAAGCTGCTTTCACAATAC-3′ |
Autoradiograms were scanned and quantitated by the National Institutes of Health image 1.62 program. Immunoprecipitated DNAs were quantitated as the ratio of immunoprecipitate to input, and represented as ChIP signal. The maximum ChIP signal was set to 100, and other ChIP signals were normalized with respect to 100. The normalized ChIP signal (represented as normalized occupancy) is plotted in the form of a histogram with standard deviation (S.D.; Microsoft Excel 2003). ORF, open reading frame; UAS, upstream activating sequence; and Core, core promoter.
Total RNA preparation
The total RNA was prepared from yeast cell culture following the standard protocol. Briefly, 10 ml yeast culture of a total OD600 of 1.0 in YPD (or 90 min induced yeast culture in YPG) was harvested, and then suspended in 100 μl RNA preparation buffer (500 mM NaCl, 200 mM Tris–HCl, 100 mM Na2EDTA, and 1% SDS) along with 100 μl phenol/chloroform/isoamyl alcohol and 100 μl volume equivalent of glass beads (acid washed; Sigma). Subsequently, yeast cell suspension was vortexed with a maximum speed (10 in a VWR mini-vortexer; cat. no. 58816-121) five times (30 s each). The cell suspension was placed in ice for 30 s between pulses. After vortexing, 150 μl RNA preparation buffer and 150 μl phenol/chloroform/isoamyl alcohol were added to the yeast cell suspension followed by vortexing for 15 s with maximum speed on a VWR mini-vortexer. The aqueous phase was collected following 5 min of centrifugation at maximum speed in a microcentrifuge machine. The total RNA was isolated from the aqueous phase by precipitation with ethanol.
Primer extension analysis
Primer extension analysis was performed as described previously (18-21). Briefly, total RNA was prepared from 10 ml of yeast culture as described above. Fifteen micrograms of total RNA was used in the primer extension analysis using 32P-labeled primer and AMV reverse transcriptase (Promega). The average primer extension signal of the biologically independent experiments is reported with S.D. (Microsoft Excel 2003). The primers used for analysis of PHO84, ADH1, RPS5, and GAL1 mRNAs were as follows:
| ADH1: | 5′-TATCCTTGTGTTCCAATTTACCGTGG-3′ |
| RPS5: | 5′-GACTGGGGTGAATTCTTCAACAACTTC-3′ |
| PHO84: | 5′-GAAGACTTCTTTCAGCAACATG-3′ |
| GAL1: | 5′-CCTTGACGTTAAAGTATAGAGG-3′ |
Analysis of transcriptional elongation on solid growth medium
The growth of the Δsus1 and wild type cells was analyzed on plates containing solid SC-uracil (plus 2% dextrose) with or without 100 μg/ml 6-AU. Both wild type and Δsus1 strains were transformed with a low copy number plasmid expressing the URA3 gene, inoculated in liquid SC-uracil medium (with 2% dextrose), and grown up to an OD600 of 0.2 at 30°C. Subsequently, yeast cells were suspended in fresh liquid SC-uracil medium (with 2% dextrose), and grown up to an OD600 of 0.4 prior to spotting (3 μl) on solid SC-uracil medium (plus 2% dextrose) with or without 100 μg/ml 6-AU. Yeast cells were spotted with serial dilutions. Yeast cells were grown at 30 °C, and photographed after 2 or 3 days. Growth analysis was carried out in biological triplicates, and consistent results were obtained. One representative set is included in Figure 4E.
Analysis of DNA repair on solid growth medium
The growth of the Δrad6, Δsus1, Δubp8, Δspt3, Δspt8, Δspt29, Δsgf73 and wild type cells was analyzed on plates containing solid YPD with or without 0.026% MMS (129925–5G, Sigma). Yeast cells were inoculated in YPD, and grown up to an OD600 of 0.2 at 30 °C. Subsequently, yeast cells were suspended in fresh YPD medium, and grown up to an OD600 of 0.4 at 30 °C prior to spotting (3 μl) on solid medium with serial dilutions. Yeast cells were grown at 30 °C, and photographed after 2, 3 or 4 days. Similar experiments were also performed for UV-induced DNA damage. Briefly, yeast cells were spotted on solid YPD, and then exposed to UV (254 nm) light (29 J/m2). Following exposure to UV light, YPD plates were wrapped by aluminum foil to avoid light, and incubated at 30 °C. Cells were photographed after 2, 3 or 4 days. Growth analysis was carried out in biological duplicates or triplicates, and consistent results were obtained. One representative set is included in Figure 5.
Analysis of DNA replication on solid growth medium
The growth of the Δrad6, Δsus1, Δubp8, Δspt3, Δspt8, Δspt29, Δsgf73 and wild type cells was analyzed on plates containing solid YPD with or without 100 mM HU. Yeast cells were inoculated in YPD, and grown up to an OD600 of 0.2 at 30 °C. Subsequently, yeast cells were suspended in fresh YPD medium, and grown up to an OD600 of 0.4 at 30 °C prior to spotting (3 μl) on solid medium with serial dilutions. Yeast cells were grown at 30 °C, and photographed after 2, 3 or 4 days. Growth analysis was carried out in biological duplicates, and consistent results were obtained. One representative set is included in Figure 6.
Supplementary Material
Highlights.
 Sus1p enhances Sgf73p recruitiment, PIC formation and transcription initiation.
Sus1p enhances Sgf73p recruitiment, PIC formation and transcription initiation. Sus1p promotes PIC formation independently of histone H2B de-ubiquitylation.
Sus1p promotes PIC formation independently of histone H2B de-ubiquitylation. Sus1p also facilitates transcriptional elongation, DNA repair and replication.
Sus1p also facilitates transcriptional elongation, DNA repair and replication. Such functions of Sus1p are mediated via modulation of H2B de-ubiquitylation
Such functions of Sus1p are mediated via modulation of H2B de-ubiquitylation Sus1p differentially regulates DNA transacting processes via H2B deubiquitylation
Sus1p differentially regulates DNA transacting processes via H2B deubiquitylation
Acknowledgements
We thank Michael R. Green for TBP antibody; and Susana Rodríguez-Navarro, Ali Shilatifard, Mary Ann Osley and Fred Winston for yeast strains. The work in the Bhaumik laboratory was supported by a National Institutes of Health grants (1R15GM088798-01 and 2R15GM088798-02), Scientist development grant (0635008N) from American Heart Association (National Affiliate), a grant-in-aid (10GRNT4300059) from American Heart Association (Greater Midwest Affiliate), a grant (06-52) from American Cancer Society, a Mallinckrodt Foundation grant, and Excellence in Academic Medicine (EAM) awards of SIU-School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhaumik SR. Distinct regulatory mechanisms of eukaryotic transcriptional activation by SAGA and TFIID. Biochim. Biophys. Acta. 2011;1809:97–108. doi: 10.1016/j.bbagrm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaumik SR, Malik S. Diverse regulatory mechanisms of eukaryotic transcriptional activation by the proteasome complex. Crit Rev Biochem Mol Biol. 2008;43:419–433. doi: 10.1080/10409230802605914. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Navarro S. Insights into SAGA function during gene expression. EMBO Rep. 2009;10:843–850. doi: 10.1038/embor.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spedale G, Timmers HT, Pijnappel WW. ATAC-king the complexity of SAGA during evolution. Genes Dev. 2012;26:527–41. doi: 10.1101/gad.184705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KK, Sardiu ME, Swanson SK, Gilmore JM, Torok M, Grant PA, Florens L, Workman JL, Washburn MP. Combinatorial depletion analysis to assemble the network architecture of the SAGA and ADA chromatin remodeling complexes. Mol Syst Biol. 2011;7:503. doi: 10.1038/msb.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker SP, Grant PA. The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene. 2007;26:5329–40. doi: 10.1038/sj.onc.1210603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köhler A, Zimmerman E, Schneider M, Hurt E, Zheng N. Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module. Cell. 2010;141:606–617. doi: 10.1016/j.cell.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C. Structural insights into the assembly and function of the SAGA deubiquitinating module. Science. 2010;328:1025–1029. doi: 10.1126/science.1190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samara NL, Ringel AE, Wolberger C. A role for intersubunit interactions in maintaining SAGA deubiquitinating module structure and activity. Structure. 2012;20:1414–24. doi: 10.1016/j.str.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galán A, Rodríguez-Navarro S. Sus1/ENY2: a multitasking protein in eukaryotic gene expression. Crit Rev Biochem Mol Biol. 2012;47:556–68. doi: 10.3109/10409238.2012.730498. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Oliver E, Garcia-Molinero V, Rodriguez-Navarro S. mRNA export and gene expression: the SAGA-TREX-2 connection. Biochim. Biophys. Acta. 2012;1819:555–565. doi: 10.1016/j.bbagrm.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Navarro S, Fischer T, Luo MJ, Antunez O, Brettschneider S, Lechner J, Perez Ortin JE, Reed R, Hurt E. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell. 2004;116:75–86. doi: 10.1016/s0092-8674(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 13.Kopytova DV, Krasnov AN, Orlova AV, Gurskiy DY, Nabirochkina EN, Georgieva SG, Shidlovskii YV. ENY2: couple, triple…more? Cell Cycle. 2010;9:479–81. doi: 10.4161/cc.9.3.10610. [DOI] [PubMed] [Google Scholar]
- 14.Kurshakova M, Maksimenko O, Golovnin A, Pulina M, Georgieva S, Georgiev P, Krasnov A. Evolutionarily conserved E(y)2/Sus1 protein is essential for the barrier activity of Su(Hw)-dependent insulators in Drosophila. Mol. Cell. 2007;27:332–338. doi: 10.1016/j.molcel.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Lang G, Ito S, Bonnet J, Metzger E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG, Krasnov A, et al. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol. Cell. 2008;29:92–101. doi: 10.1016/j.molcel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin J-C, Hurt EC, Nehrbass U. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–773. doi: 10.1038/nature04752. [DOI] [PubMed] [Google Scholar]
- 17.Luthra R, Kerr SC, Harreman MT, Apponi LH, Fasken MB, Ramineni S, Chaurasia S, Valentini SR, Corbett AH. Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J Biol Chem. 2007;282:3042–3049. doi: 10.1074/jbc.M608741200. [DOI] [PubMed] [Google Scholar]
- 18.Shukla A, Stanojevic N, Duan Z, Sen P, Bhaumik SR. Ubp8p, a histone deubiquitinase whose association with SAGA is mediated by Sgf11p, differentially regulates lysine 4 methylation of histone H3 in vivo. Mol. Cell. Biol. 2006;26:3339–3352. doi: 10.1128/MCB.26.9.3339-3352.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shukla A, Bajwa P, Bhaumik SR. SAGA-associated Sgf73p facilitates formation of the preinitiation complex assembly at the promoters either in a HAT-dependent or independent manner in vivo. Nucleic Acids Res. 2006;34:6225–6232. doi: 10.1093/nar/gkl844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhaumik SR, Green MR. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes & Dev. 2001;15:1935–1945. doi: 10.1101/gad.911401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaumik SR, Raha T, Aiello DP, Green MR. In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes Dev. 2004;18:333–343. doi: 10.1101/gad.1148404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla A, Lahudkar S, Durairaj G, Bhaumik SR. Sgf29p facilitates the recruitment of TATA box binding protein but does not alter SAGA’s global structural integrity in vivo. Biochemistry. 2012;51:706–14. doi: 10.1021/bi201708z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik S, Bagla S, Chaurasia P, Duan Z, Bhaumik SR. Elongating RNA polymerase II is disassembled through specific degradation of its largest but not other subunits in response to DNA damage in vivo. J. Biol. Chem. 2008;283:6897–6905. doi: 10.1074/jbc.M707649200. [DOI] [PubMed] [Google Scholar]
- 24.Uprety B, Lahudkar S, Malik S, Bhaumik SR. The 19S proteasome subcomplex promotes the targeting of NuA4 HAT to the promoters of ribosomal protein genes to facilitate the recruitment of TFIID for transcriptional initiation in vivo. Nucleic Acids Res. 2012;40:1969–1983. doi: 10.1093/nar/gkr977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler A, Pascual-Garcia P, Llopis A, Zapater M, Posas F, Hurt E, Rodríguez-Navarro S. The mRNA export factor Sus1 is involved in Spt/Ada/Gcn5 acetyltransferasemediated H2B deubiquitinylation through its interaction with Ubp8 and Sgf11. Mol. Biol. Cell. 2006;17:4228–4236. doi: 10.1091/mbc.E06-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingvarsdottir K, Krogan NJ, Emre NC, Wyce A, Thompson NJ, Emili A, Hughes TR, Greenblatt JF, Berger SL. H2B ubiquitin protease Ubp8 and Sgf11 constitute a discrete functional module within the Saccharomyces cerevisiae SAGA complex. Mol Cell Biol. 2005;25:1162–72. doi: 10.1128/MCB.25.3.1162-1172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon SJ, Pray-Grant MG, Schieltz D, Yates JR, 3rd, Grant PA. Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity. Proc Natl Acad Sci U S A. 2005;102:8478–82. doi: 10.1073/pnas.0503493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hossain MA, Claggett JM, Nguyen T, Johnson TL. The cap binding complex influences H2B ubiquitination by facilitating splicing of the SUS1 pre-mRNA. RNA. 2009;15:1515–27. doi: 10.1261/rna.1540409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klöckner C, Schneider M, Lutz S, Jani D, Kressler D, Stewart M, Hurt E, Köhler A. Mutational uncoupling of the role of Sus1 in nuclear pore complex targeting of an mRNA export complex and histone H2B deubiquitination. J. Biol. Chem. 2009;284:12049–56. doi: 10.1074/jbc.M900502200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyce A, Xiao T, Whelan KA, Kosman C, Walter W, Eick D, Hughes TR, Krogan NJ, Strahl BD, Berger SL. H2B ubiquitylation acts as a barrier to Ctk1 nucleosomal recruitment prior to removal by Ubp8 within a SAGArelated complex. Mol. Cell. 2007;27:275–288. doi: 10.1016/j.molcel.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Pascual-García P, Govind CK, Queralt E, Cuenca-Bono B, Llopis A, Chavez S, Hinnebusch AG, Rodríguez-Navarro S. Sus1 is recruited to coding regions and functions during transcription elongation in association with SAGA and TREX2. Genes Dev. 2008;22:2811–2822. doi: 10.1101/gad.483308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govind CK, Zhang F, Qiu H, Hofmeyer K, Hinnebusch AG. Gcn5 promotes acetylation, eviction, and methylation of nucleosomes in transcribed coding regions. Mol. Cell. 2007;25:31–42. doi: 10.1016/j.molcel.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Shukla A, Bhaumik SR. H2B-K123 ubiquitination stimulates RNAPII elongation independent of H3-K4 methylation. Biochem Biophys Res Commun. 2007;359:214–220. doi: 10.1016/j.bbrc.2007.05.105. [DOI] [PubMed] [Google Scholar]
- 34.Sen R, Lahudkar S, Durairaj G, Bhaumik SR. Functional analysis of Bre1p, an E3 ligase for histone H2B ubiquitylation, in regulation of RNA polymerase II association with active genes and transcription in vivo. J Biol Chem. 2013;288:9619–9633. doi: 10.1074/jbc.M113.450403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao T, Kao CF, Krogan NJ, Sun ZW, Greenblatt JF, Osley MA, Strahl BD. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol. Cell Biol. 2005;25:637–651. doi: 10.1128/MCB.25.2.637-651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell. 2008;31:57–66. doi: 10.1016/j.molcel.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Tanny JC, Erdjument-Bromage H, Tempst P, Allis CD. Ubiquitylation of histone H2B controls RNA polymerase II transcription elongation independently of histone H3 methylation. Genes Dev. 2007;21:835–847. doi: 10.1101/gad.1516207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen R, Bhaumik SR. Transcriptional stimulatory and repressive functions of histone H2B ubiquitin ligase. Transcription. 2013 Oct 3;4(5) doi: 10.4161/trns.26623. [DOI] [PubMed] [Google Scholar]
- 39.Batta K, Zhang Z, Yen K, Goffman DB, Pugh BF. Genome-wide function of H2B ubiquitylation in promoter and genic regions. Genes Dev. 2011;25:2254–65. doi: 10.1101/gad.177238.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee KK, Florens L, Swanson SK, Washburn MP, Workman JL. The deubiquitylation activity of Ubp8 is dependent upon Sgf11 and its association with the SAGA complex. Mol Cell Biol. 2005;25:1173–82. doi: 10.1128/MCB.25.3.1173-1182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood A, Krogan NJ, Dover J, Schneider J, Heidt J, Boateng MA, Dean K, Golshani A, Zhang Y, Greenblatt JF, Johnston M, Shilatifard A. Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol. Cell. 2003;11:267–274. doi: 10.1016/s1097-2765(02)00802-x. [DOI] [PubMed] [Google Scholar]
- 42.Chernikova SB, Razorenova OV, Higgins JP, Sishc BJ, Nicolau M, Dorth JA, Chernikova DA, Kwok S, Brooks JD, Bailey SM, Game JC, Brown JM. Deficiency in mammalian histone H2B ubiquitin ligase Bre1 (Rnf20/Rnf40) leads to replication stress and chromosomal instability. Cancer Res. 2012;72:2111–2119. doi: 10.1158/0008-5472.CAN-11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohler A, Schneider M, Cabal GG, Nehrbass U, Hurt E. Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export. Nat. Cell Biol. 2008;10:707–715. doi: 10.1038/ncb1733. [DOI] [PubMed] [Google Scholar]
- 44.Sterner DE, Grant PA, Roberts SM, Duggan LJ, Belotserkovkaya R, Pacella LA, Winston F, Workman JL, Berger SL. Functional organization of the yeast SAGA complex: Distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhaumik SR, Green MR. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol Cell Biol. 2002;22:7365–7371. doi: 10.1128/MCB.22.21.7365-7371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sermwittayawong D, Tan S. SAGA binds TBP via its Spt8 subunit in competition with DNA: implications for TBP recruitment. EMBO J. 2006;25:3791–800. doi: 10.1038/sj.emboj.7601265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, Eriksson P, Bhoite LT, Stillman DJ. Regulation of TATA-binding protein binding by the SAGA complex and the Nhp6 high-mobility group protein. Mol Cell Biol. 2003;23:1910–21. doi: 10.1128/MCB.23.6.1910-1921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warfield L, Ranish JA, Hahn S. Positive and negative functions of the SAGA complex mediated through interaction of Spt8 with TBP and the N-terminal domain of TFIIA. Genes Dev. 2004;18:1022–34. doi: 10.1101/gad.1192204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trujillo KM, Osley MA. A role for H2B ubiquitylation in DNA replication. Mol Cell. 2012;48:734–46. doi: 10.1016/j.molcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shukla A, Chaurasia P, Bhaumik SR. Histone methylation and ubiquitination with their cross-talk and roles in gene expression and stability. Cell. Mol. Life Sci. 2009;66:1419–1433. doi: 10.1007/s00018-008-8605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JS, Shukla A, Schneider J, Swanson SK, Washburn MP, Florens L, Bhaumik SR, Shilatifard A. Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell. 2007;131:1084–1096. doi: 10.1016/j.cell.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 52.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–16. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 53.Lim S, Kwak J, Kim M, Lee D. Separation of a functional deubiquitylating module from the SAGA complex by the proteasome regulatory particle. Nat Commun. 2013;4:2641. doi: 10.1038/ncomms3641. [DOI] [PubMed] [Google Scholar]
- 54.Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Duempelfeld B, Edelmann A, Heurtier MA, Hoffman V, Hoefert C, Klein K, Hudak M, Michon AM, Schelder M, Schirle M, Remor M, Rudi T, Hooper S, Bauer A, Bouwmeester T, Casari G, Drewes G, Neubauer G, Rick J. M,, Kuster, B., Bork P, Russell RB, Superti-Furga G. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- 55.Ahn SH, Keogh MC, Buratowski S. Ctk1 promotes dissociation of basal transcription factors from elongating RNA polymerase II. EMBO J. 2009;28:205–212. doi: 10.1038/emboj.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol Cell. 2004;13:67–76. doi: 10.1016/s1097-2765(03)00492-1. [DOI] [PubMed] [Google Scholar]
- 57.Krogan NJ, Kim M, Tong A, Golshani A, Cagney G, Canadien V, Richards DP, Beattie BK, Emili A, Boone C, Shilatifard A, Buratowski S, Greenblatt J. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol. 2003;23:4207–4218. doi: 10.1128/MCB.23.12.4207-4218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood A, Shukla A, Schneider J, Lee JS, Stanton JD, Dzuiba T, Swanson SK, Florens L, Washburn MP, Wyrick J, Bhaumik SR, Shilatifard A. Ctk complex-mediated regulation of histone methylation by COMPASS. Mol Cell Biol. 2007;27:709–720. doi: 10.1128/MCB.01627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao T, Shibata Y, Rao B, Laribee RN, O’Rourke R, Buck MJ, Greenblatt JF, Krogan NJ, Lieb JD, Strahl BD. The RNA polymerase II kinase Ctk1 regulates positioning of a 5′ histone methylation boundary along genes. Mol Cell Biol. 2007;27:721–731. doi: 10.1128/MCB.01628-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 61.Henry KW, Wyce A, Lo WS, Duggan LJ, Emre NC, Kao CF, Pillus L, Shilatifard A, Osley MA, Berger SL. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes & Dev. 2003;17:2648–2663. doi: 10.1101/gad.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhaumik SR, Green MR. Interaction of Gal4p with components of transcription machinery in vivo. Methods Enzymol. 2003;370:445–454. doi: 10.1016/S0076-6879(03)70038-X. [DOI] [PubMed] [Google Scholar]
- 63.Shukla A, Stanojevic N, Duan Z, Shadle T, Bhaumik SR. Functional analysis of H2B-Lys-123 ubiquitination in regulation of H3-Lys-4 methylation and recruitment of RNA polymerase II at the coding sequences of several active genes in vivo. J Biol Chem. 2006;281:19045–54. doi: 10.1074/jbc.M513533200. [DOI] [PubMed] [Google Scholar]
- 64.Malik S, Shukla A, Sen P, Bhaumik SR. The 19S proteasome subcomplex establishes a specific protein interaction network at the promoter for stimulated transcriptional initiation in vivo. J. Biol. Chem. 2009;284:35714–35724. doi: 10.1074/jbc.M109.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malik S, Chaurasia P, Lahudkar S, Durairaj G, Shukla A, Bhaumik SR. Rad26p, a transcription-coupled repair factor, is recruited to the site of DNA lesion in an elongating RNA polymerase II-dependent manner in vivo. Nucleic Acids Res. 2010;38:1461–1477. doi: 10.1093/nar/gkp1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.