Abstract
Purpose
Placebo and randomization are important concepts that must be understood before youth can safely participate in HIV vaccine studies or other biomedical trials for HIV prevention. These concepts are central to the phenomenon of preventive misconception which may be associated with an increase in risk behavior among study participants related to mistaken beliefs. Persuasive messaging, traditionally used in the field of marketing, could enhance educational efforts associated with randomized clinical trials.
Methods
Two educational brochures were designed to increase knowledge about HIV vaccine clinical trials via 1 and 2-sided persuasive messaging. Through the Adolescent Medicine Trials Network, 120 youth were enrolled, administered a mock HIV vaccine trial consent, and then randomized to receive either no supplemental information or one of the two brochures.
Results
The 2-sided brochure group in which common clinical trial misconceptions were acknowledgedand then refuted had significantly higher scores on knowledge of randomization and interpretation of side effects than the consent-only control group, and willingness to participate in an HIV vaccine trial was not decreased with the use of this brochure.
Conclusion
Two sided persuasive messaging improves understanding of the concepts of randomization and placebo among youth who would consider participating in an HIV vaccine trial. Further evaluation of this approach should be considered for at-risk youth participating in an actual trial of a biomedical intervention for HIV prevention.
Keywords: adolescent clinical trials, HIV vaccine trials, preventive misconception, adolescents, HIV
Background and Introduction
Given that a large proportion of HIV infections globally occur among young persons aged 15-24,1 youth will be a target population for an approved HIV vaccine or other biomedical prevention modality. Therefore, once a candidate biomedical prevention approach, including HIV vaccination, demonstrates promising results in phase 3 trials among adults, adolescents will need to be enrolled in clinical trials in order to obtain an indication for adolescent administration. Protection of adolescents enrolled in clinical trials is particularly important given their developing cognitive and emotional capacities.2-4 Thus, appropriate provisions must be made to ensure that youth will not only understand what is involved with participation in an HIV vaccine trial, but also be adequately protected from risks that may be associated with trial participation.5;6
One specific issue repeatedly raised in discussions around adolescent participation in HIV vaccine trials is behavioral disinhibition, or the concern that adolescents who participate will practice riskier sexual behaviors.2 This concern is based on Risk Compensation Theory, which suggests that persons have an inherent set-point that determines their willingness to take risks.7;8 According to this theory, any modification in the environment that reduces the external probability of risk will lead an individual to increase their risk-related behaviors (i.e., disinhibit), thereby neutralizing the benefits of risk-reduction strategies.
For behavioral disinhibition to be attributed to participation in a preventive clinical trial, the phenomenon of preventive misconception must be present.2 Preventive misconception is the tendency of participants in preventative clinical trials to make two cognitive errors: 1) to overestimate the probability that they have been assigned to the experimental versus the control condition; and 2) to assume that an unproven experimental intervention is effective at preventing infection.2;9 If trial participants practice riskier sexual behaviors because they believe they are receiving a protective vaccine, trial participation could be harmful. Although most studies have not found increased risk behavior in the context of HIV vaccine clinical trials,10;11 the desire for, and expectation of, protection has been identified as a motivation for vaccine trial participation,12;13 suggesting that every effort should be made to minimize preventive misconception. It is standard to monitor for any increased risk behavior in an HIV vaccine trial. Still, ensuring protection is particularly important for adolescents, as they are a vulnerable subject population.
Prospective research trial participants may not fully understand the information they receive during the informed consent process. To date, efforts to better inform clinical trial participants have largely focused on modification of consent forms, but several difficulties have been encountered.14 Any HIV vaccine trial will almost certainly involve multiple research sites, each with its own requirements for construction of a consent form. Furthermore, by necessity, such consent forms typically include legalistic and medical language that may be difficult to simplify. To circumvent these issues, we focused on development and assessment of supplemental material that specifically addresses the issue of preventive misconception for use with adolescents.
The primary objective of this study was to evaluate supplemental educational brochures designed to increase knowledge about HIV vaccine clinical trials via persuasive messaging, with a particular focus on topics central to preventive misconception. Persuasive communication theory suggests that, when seeking to persuade or inform, one can employ either a 1-sided or a 2-sided message.14;15 In the context of the present study, the goal of which was to inform rather than persuade, a 1-sided message involved a straight forward presentation of pertinent facts associated with participation in a clinical trial (e.g., “You will have an equal chance of getting the vaccine or the placebo”). In contrast, a 2-sided message presents common misconceptions, but then refutes them with factual information (e.g., “Some people think they have a better than equal chance of being in the vaccine group. This is not true. You will have an equal chance of getting the vaccine or the placebo”). Two-sided messages are hypothesized to be more effective at adequately conveying complex information.15;16
A secondary objective of this study was to investigate the extent to which numeracy, health literacy, and impulsive decision-making were associated with knowledge about specific aspects of an HIV vaccine clinical trial, such as randomization, interpretation of side effects, and recognition that the vaccine is experimental.
We hypothesized that a 2-sided brochure would result in greater knowledge about HIV vaccine clinical trials than a 1-sided brochure or consent-only conditions and that there would be no difference across the three groups with respect to willingness-to-participate in a clinical trial. We also hypothesized that poorer numeracy, poorer health literacy, and higher impulsive decision-making would be associated with lower knowledge about HIV vaccine clinical trials.
METHODS
Brochure Development
Prototype brochures were created by Drs. Zimet and Lally in conjunction with Dr. Richard Goldsworthy of The Academic Edge, Inc., a company with expertise in development of health education materials. The brochures delivered 1- or 2-sided messages around the topic areas of randomization and unknown efficacy of a candidate HIV vaccine. The brochures were revised based on feedback by adolescent members of Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) community advisory boards about appearance and content. The brochure with 1-sided messages gave accurate information about vaccine trial randomization, interpretation of common side effects, and unproven efficacy of vaccine. The brochure with 2-sided messages acknowledged some beliefs that are at odds with the information content and presented counter-arguments (See Figure 1 for images of the brochures showing the messages).
Figure 1.
Displayed are the inner contents of the brochures using 1-sided (Three Imortant Facts...) and 2-sided (Three Commmon Misunderstandings...) messages.
Participants and Procedures
To evaluate the brochures, one hundred and twenty youth were enrolled from four ATN sites in the United States, located in New Orleans, New York City, Baltimore, and San Francisco. Study randomization was stratified by age (under 18 vs. 18 or older) and gender; enrollment was offered to 16-19 year old women and men who were sexually active with men, and who indicated that they would be willing to consider participating in an actual HIV vaccine trial.
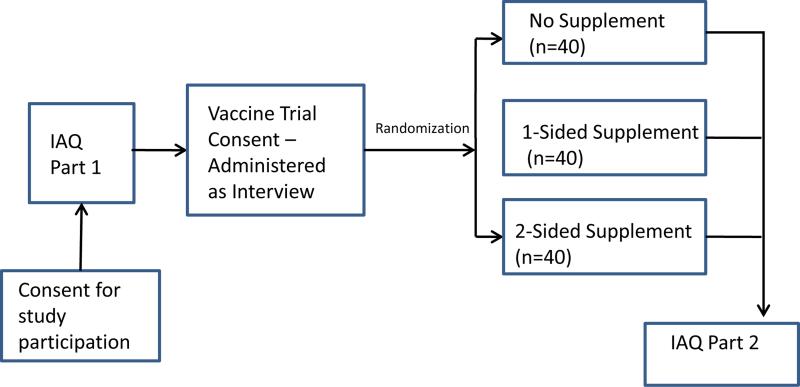
Institutional review board approval and waivers of parental informed consent were granted at each of the four sites. Waivers of parental consent were used in this study as participation included only minimal risk. All subjects provided written informed consent. The sequence of study events is shown in Figure 2. After adolescents consented to study participation they completed an Interviewer Administered Questionnaire (IAQ Part 1). After completion of IAQ Part 1, participants were administered a mock HIV vaccine trial consent form. This consent form was based on a standard HIV Vaccine Trial Consent template provided to us by the HIV Vaccine Trials Network (HVTN). Participants were then randomized into one of three conditions: 1) No supplemental information control group; 2) Supplemental information with 1-sided messages; or 3) Supplemental information with 2-sided messages. Randomization was done at each site and within each age/gender stratum using a randomized block method with fixed block size k = 3. Participants completed IAQ Part 2 after reading through the consent and brochure (if applicable to their assigned group) with the research assistant. The research assistant was able to answer and clarify any questions or concerns the participant might have had while reading through the consent and brochure. A subset of adolescents (n=33) were recruited after IAQ Part 2 to participate in qualitative debriefing interviews. The findings from these interviews are beyond the scope of this paper and are reported elsewhere.17
Figure 2.
Study Flow
Measures
IAQ Part 1 was used to obtain demographic and risk behavior information as well as assess the predictors of interest, subjective numeracy, impulsive decision making, and health literacy. The Subjective Numeracy Scale is a brief, validated measure of numeracy.18 It consists of eight items addressing self-assessed mathematical ability and preference for display of numeric information. The Impulsive Decision-Making Scale includes 12 questions that have been validated as a brief assessment of impulsivity.19 Health Literacy was assessed with the Rapid Estimate of Adult Literacy in Medicine-Short Form (REALM-SF), a validated 7-item measure.20
IAQ Part 2 was composed of the outcome measures: knowledge about HIV vaccine trials and willingness to participate in an HIV vaccine trial. With respect to knowledge, two items measured understanding of randomization (e.g., “... whether I would be in the vaccine group or the placebo group, it would be like the flip of a coin”). Two items measured interpretation of side-effects (e.g., “If... my arm felt sore from the shots that would tell me that I got the vaccine, not the placebo”). Two items measured unproven efficacy of the experimental vaccine (e.g., “If I took part in an HIV vaccine study and got the vaccine, that would mean that I would be protected from HIV infection”). Knowledge about topic areas covered by the vaccine trial consent, but not covered by the brochures was assessed with four items (e.g., “If I took part in an HIV vaccine study, I could leave the study at any time I wanted to”). Respondents were asked to respond to all knowledge items using a 5-point Likert-type response scale ranging from “Strongly Disagree” to “Strongly Agree”. Scales for each knowledge area (i.e., randomization, interpretation of side-effects, unproven efficacy, and non-brochure topics) were created by calculating the mean value across the items.
Willingness-to-participate (WTP) was evaluated with three items, each of which began with the question stem, “If offered the chance, how likely would you be to participate in a clinical trial for a preventive HIV vaccine that required 3 shots over a 6 month period...?”. Respondents were asked to respond to these three items using a 5-point Likert-type scale ranging from “Definitely Not Participate” to “Definitely Participate”. A WTP Scale was created by calculating the mean value across the three items. The internal reliability for the scale was very good (Cronbach’s coefficient alpha = .8).
Statistical Methods
All statistical tests were performed using SAS™ 9.2 software [SAS 9.2, 2009, SAS Institute, Cary, NC]. Means and proportions were generated to describe the study population. One-way analysis of variance (ANOVA) was used to assess the association between the 3 Intervention Groups (Consent Alone, Consent and 1-Sided, Consent and 2-Sided) and study outcome variables, with Tukey post-hoc tests used to identify pairwise differences. Outcome variables included knowledge scales and the WTP scale. Finally, Pearson product-moment correlation coefficients were used to measure the association of health literacy, subjective numeracy, and impulsive decision-making with the outcome variables.
RESULTS
Study Subject Characteristics
Table 1 presents the characteristics of the study population. The mean age of participants was 17.7 (SD = 1.1) and 50% were male. With respect to race/ethnicity, 64% identified as non-Hispanic Black/African-American, 14% as non-Hispanic White, and 21% as Hispanic. Study participants in the three experimental groups did not differ significantly by any of the demographic characteristics or by the key predictors, subjective numeracy, impulsive decision-making, and health literacy, indicating that randomization was successful.
Table 1.
Characteristics of study sample
| Overall n = 120 | Intervention Groups |
p-value* | |||
|---|---|---|---|---|---|
| Consent Alone n = 42 | Consent 1-Sided n = 39 | Consent 2-Sided n = 39 | |||
| Race, n (%) | |||||
| Black | 76(64%) | 25(59%) | 25(64%) | 26(67%) | 0.55 |
| White | 17(14%) | 7(17%) | 7(18%) | 3(8%) | |
| Other | 26(22%) | 10(24%) | 6(16%) | 10(26%) | |
| Hispanic (Spanish) or Latino origin, n (%) | |||||
| Hispanic | 25(21%) | 9(21%) | 7(18%) | 9(23%) | 0.89 |
| Non_Hispanic | 95(79%) | 33(79%) | 32(82%) | 30(77) | |
| Birth Gender, n (%) | |||||
| Male | 60(50%) | 20(48%) | 20(51%) | 20(51%) | 0.95 |
| Female | 60(50%) | 22(52%) | 19(49%) | 19(49%) | |
| Age | |||||
| Mean (SD) | 17.69 (1.07) | 17.67 (0.93) | 17.79 (1.13) | 17.62 (1.16) | 0.75 |
| Subjective Numeracy | |||||
| Mean (SD) | 3.93 (0.94) | 3.96 (0.99) | 3.77 (0.78) | 4.04 (1.01) | 0.42 |
| Impulsive Decision-Making | |||||
| Mean (SD) | 2.90 (0.66) | 2.99 (0.61) | 2.90 (0.67) | 2.82 (0.69) | 0.49 |
| Health Literacy | |||||
| Mean (SD) | 5.48 (1.51) | 5.40 (1.73) | 5.54 (1.27) | 5.51 (1.50) | 0.92 |
P-value is from ANOVA for continuous variables and from Fisher's Exact test or Chi-Square statistics for categorical variables
Effect of Brochures on Knowledge Outcomes
For the knowledge outcomes (see Table 2), ANOVA tests revealed significant intervention effects on randomization (overall p < .01) and interpretation of side effects (overall p < .01). Pair-wise comparisons based on Tukey post-hoc tests indicated that the 2-sided brochure group had significantly higher scores on knowledge of randomization and interpretation of side effects than the consent-only control group. The 1-sided brochure group did not differ on any knowledge measure from either the consent-only or the 2-sided brochure group. Although neither brochure group had significantly higher knowledge related to unproven efficacy, the non-significant trend was in the same direction as the other brochure-related knowledge scales (p < .12). Non-brochure knowledge did not differ across intervention groups.
Table 2.
Effect of intervention on outcome variables
| Outcome Variables | Intervention Groups |
||
|---|---|---|---|
| Consent Alone | Consent & 1-Sided | Consent & 2- Sided | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Knowledge Scales | |||
| Randomization* | 3.61 (0.48)a | 3.88 (0.54)a,b | 3.95 (0.56)b |
| Interpretation of Side-Effects* | 3.48 (0.96)a | 3.87 (0.77)a,b | 4.03 (0.77)b |
| Unproven Efficacy | 3.73 (0.91) | 4.04 (0.75) | 4.05 (0.70) |
| Non-Brochure Topics | 4.13 (0.55) | 4.13 (0.48) | 4.26 (0.54) |
| Willingness-to-Participate* | 3.50 (0.91)a | 2.92 (0.97)b | 3.46 (0.90)a |
p<.01 for overall ANOVA.
Superscripts that differ indicate significant pair-wise differences.
With respect to willingness-to-participate, the overall ANOVA was significant (p < .01), with post-hoc tests indicating that the 1-sided brochure group had significantly lower scores on this scale compared to either the consent-only or the 2-sided brochure group, which, in turn, did not differ from each other (see Table 2).
Factors Associated with Knowledge
Table 3 shows that health literacy and subjective numeracy were significantly correlated with most of the knowledge variables. Health literacy showed significantly positive correlations with randomization (r = 0.26, p < .01), unproven efficacy(r = 0.35, p <0.01) and non-brochure topics (r = 0.35, p <0.01). Subjective numeracy had only a borderline significant correlation with unproven efficacy (r = 0.17, p < 0.06), but showed significantly positive correlations with randomization (r = 0.28, p < 0.01) and non-brochure topics (r = 0.26, p < 0.01) in addition to interpretation of side effects (r = 0.23, p < 0.01).
Table 3.
Pearson correlation coefficients between participant characteristics and outcome measures
| Health Literacy | Subjective Numeracy | Impulsive Decision-Making | |
|---|---|---|---|
| Knowledge Scales | |||
| Randomization | .26* | .28* | −.18 |
| Interpretation of Side Effects | .13 | .23* | −.11 |
| Unproven Efficacy | .35* | .17 | −.16 |
| Non-Brochure | .35* | .26* | −.10 |
| Willingness-to-Participate | −.11 | .16 | .06 |
p<01
DISCUSSION
This study demonstrates that use of a supplemental brochure that delivers 2-sided messaging among youth considering participation in an HIV vaccine trial improves targeted knowledge around the concepts related to preventive misconception as compared to the use of an informed consent document alone, and does not compromise willingness to participate. In partial confirmation of our hypothesis, the 2-sided brochure resulted in higher knowledge scores for placebo and randomization than the consent-only control group. Understanding these concepts is critical in order to guard against the phenomenon of preventive misconception. Among youth, willingness-to-participate in an HIV vaccine trial was not compromised with the use of this 2-sided brochure. In contrast, the 1-sided brochure decreased willingness to participate in an HIV vaccine clinical trial, a result that we did not find for the 2-sided brochure.
It is interesting to note that the utilization of 2-sided messaging has a potential benefit of providing prospective protection against subsequent attitude slippage (i.e., changing attitudes in the face of introduction of new information).21 This effect may be particularly important in HIV vaccine clinical trials, when participants may sometimes incorrectly interpret side-effects (e.g., local tenderness, mild fever) as an indication of assignment to the experimental vaccine group.22 The finding that the 2-sided messages, in contrast to the 1-sided messages, did not compromise willingness-to-participate also was an encouraging result, which may be explained by Attribution Theory.23 This theory would predict that the acknowledgement of contrary beliefs inherent in 2-sided messages would increase the perceived trustworthiness and credibility of the message source, an effect that would not be expected with 1-sided messages.15;24;25
Persuasive communication techniques have been evaluated and used for years in marketing research, with a primary focus on consumer decision-making and purchasing behaviors. More recently, researchers have applied some of these marketing approaches to health behavior and health decision-making.26-29 To our knowledge, these kinds of techniques have not previously been applied to efforts to maximize potential clinical trial participants’ understanding of research and to minimize the occurrence of preventive misconception. However, as indicated by our results, persuasive message communication may be well-suited for the task of educating individuals recruited to participate in HIV prevention clinical trials.
This study also demonstrates that youth participants with higher baseline scores on health literacy and subjective numeracy scales demonstrated superior knowledge of important study characteristics regardless of the intervention group to which they were assigned. Although this finding is not surprising, to our knowledge the importance of health literacy and numeracy have not previously been demonstrated among adolescents. Some work does support the importance of both health literacy and numeracy for clinical trial participation among adults.30,31 This set of results suggests that screening for health literacy and numeracy among potential trial participants could be important. Individuals identified as low in either of these domains may require additional time with study staff to allow for additional education around key elements of clinical trials. We did not find that impulsive decision-making was associated with knowledge about clinical trials. However, impulsivity may still be a factor in terms of behavioral disinhibition in the context of clinical trial participation and deserves further study.
This study has several limitations. First, this was not an actual HIV vaccine clinical trial. Youth who actually participate in a trial may be different from those willing to enroll in a study that essentially assesses understanding of trial concepts. However, a key aim of the ATN is to provide a research infrastructure and access to high risk HIV infected and uninfected youth for biomedical HIV treatment and prevention trials. Participants were specifically recruited through ATN sites so that results would be applicable to the adolescents most likely to be enrolled in future biomedical prevention trials. Next, the youth under age 18 were able to enroll in this study without parental consent. As an actual HIV vaccine trial would pose greater than minimal risk to participants, those under 18 years of age would require parental involvement in the consenting process. Finally, although this study assessed understanding of trial concepts related to prevention misconception, true efficacy of the supplemental brochures will need to be assessed in an actual HIV vaccine study, with behavioral as well as knowledge outcomes. Adolescents at risk for HIV infection, who would qualify as candidates for a biomedical prevention trial, represent a vulnerable group. Ethical implementation of this kind of research must include assurances that risk of harm will be minimized, including risks related to preventive misconception and behavioral disinhibition.17 It is essential that effective behavioral sexual risk-reduction interventions are provided for all adolescent participants in biomedical HIV prevention clinical trials research.2
This study demonstrates that youth, even those as young as 16, can understand key concepts required for participation in an HIV vaccine trial. The use of a supplemental brochure that employs 2-sided messaging can help to convey the concepts of randomization and placebo that are critical to protect against harms that might be associated with trial participation. Youth will need to participate in trials that investigate the effectiveness of biomedical approaches to HIV prevention, and future research should investigate the use of a supplemental 2-sided brochure in actual pre-exposure prophylaxis (PrEP), microbicide, and/or HIV vaccine trials in order to determine the feasibility and utility of such a tool.32
Acknowledgements
ATN 076 was supported by The Adolescent Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health [U01 HD 040533] through the National Institute of Child Health and Human Development, with supplemental funding from the National Institutes on Drug Abuse and Mental Health. All authors who have contributed significantly to the work are listed here. Results from Qualitative Interviews conducted during this project have been published in Journal of Medical Ethics, 2013 Jan 25 [Epub ahead of print]. Preliminary data from the project was presented at Society for Adolescent Health and Medicine Annual Meeting in Toronto, April 2010, and Seattle, March 2011, and the AIDS Vaccine meeting 2010 in Atlanta. Further analysis of qualitative interviews will be presented at the Annual Conference for the Society of Social Work and Research, San Antonio Texas, January 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest Dr. Lally is a recipient of funding from Merck for ongoing trials of HPV vaccines. Dr. Zimet is a past recipient of grants from Merck related to HPV vaccination and an unrestricted program development grant from GlaxoSmithKline related to cervical cancer prevention.
Implications and Contribution
It is critical that participants in HIV vaccine trials understand the concepts of placebo and randomization. Mistaken beliefs could be associated with an increase in risk behavior. Through the Adolescent Trials Network (ATN), we have designed, tested, and demonstrated the effectiveness of using a simple brochure to help explain these concepts.
References
- 1.UNAIDS UNAIDS Report on the Global AIDS Epidemic 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf. 2012. 5-19-2013.
- 2.Hosek SG, Zimet GD. Behavioral considerations for engaging youth in HIV clinical research. J Acquir Immune Defic Syndr. 2010;54:S25–S30. doi: 10.1097/QAI.0b013e3181e15c22. [DOI] [PubMed] [Google Scholar]
- 3.Kafaar Z, Swartz L, Kagee A, et al. Adolescent participation in HIV vaccine trials: Cognitive developmental considerations. S Afr J Psychol. 2007;37:576–94. [Google Scholar]
- 4.Blake DR, Lemay CA, Kearney MH, et al. Adolescents' understanding of research concepts: A focus group study. Arch Pediatr Adolesc Med. 2011;165(6):533–39. doi: 10.1001/archpediatrics.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindegger G, Milford C, Ranchod C, et al. Potential behavioural and psychological contributions to ethical HIV vaccine trials in South Africa. S Afr J Psychol. 2006;36:715–33. [Google Scholar]
- 6.Slack C, Strode A, Fleischer T, et al. Enrolling adolescents in HIV vaccine trials: Reflections on legal complexities from South Africa. BMC Med Ethics. 2007;8:5. doi: 10.1186/1472-6939-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilde GJS. Risk homeostasis theory: An overview. Inj Prev. 1998;4:89–91. doi: 10.1136/ip.4.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilde GJS. The theory of risk homeostasis: Implications for safety and health. Risk Anal. 1982;2:209–25. [Google Scholar]
- 9.Simon AE, Wu AW, Lavori PW, et al. Preventive misconception: Its nature, presence, and ethical implications for research. Am J Prev Med. 2007;32:370–374. doi: 10.1016/j.amepre.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 10.van Griensven F, Keawkungwal J, Tappero JW, et al. Lack of increased HIV risk behavior among injection drug users participating in the AIDSVAX B/E HIV vaccine trial in Bangkok, Thailand. AIDS. 2004;18:295–301. doi: 10.1097/00002030-200401230-00020. [DOI] [PubMed] [Google Scholar]
- 11.Lampinen JM, Chan K, Remis RS, et al. Sexual risk behaviour of Canadian participants in the first efficacy trial of a preventive HIV-1 vaccine. Can Med Assoc J. 2005;172:479–83. doi: 10.1503/cmaj.1031785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macqueen KM, Buchbinder S, Douglas JM, et al. The decision to enroll in HIV vaccine efficacy trials: Concerns elicited from gay men at increased risk for HIV infection. AIDS Res Human Retroviruses. 1994;10:S261–S264. [PubMed] [Google Scholar]
- 13.Colfax G, Buchbinder S, Vamshidar G, et al. Motivations for participating in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005;39:359–64. doi: 10.1097/01.qai.0000152039.88422.ec. [DOI] [PubMed] [Google Scholar]
- 14.Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: A systematic review. JAMA. 2004;292:1593–601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 15.Crowley A, Hoyer W. An integrative framework for understanding two-side persuasion. J Consum Res. 1994;20:561–74. [Google Scholar]
- 16.Eisend M. Understanding two-sided persuasion: An empirical assessment of theoretical approaches. Psychol Marketing. 2007;24:615–40. [Google Scholar]
- 17.Ott MA, Alexander AB, Lally M, et al. Preventive misconception and adolescents' knowledge about HIV vaccine trials. J Med Ethics. doi: 10.1136/medethics-2012-100821. Published Online first 1/25/2013. doi: 10.1136/medethics-2012-100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, et al. Measuring numeracy without a math test: Development of the Subjective Numeracy Scale. Med Dec Making. 2007;27:672–80. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 19.Donohew L, Zimmerman R, Cupp PS, et al. Sensation seeking, impulsive decision-making, and risky sex: Implications for risk-taking and design of interventions. Pers Indiv Differ. 2000;28:1079–91. [Google Scholar]
- 20.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45:1026–33. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 21.Burgoon M, Pfau M, Birk TS. An inoculation theory explanation for the effects of corporate issue/advocacy advertising campaigns. Commun Res. 1995;22:485–505. [Google Scholar]
- 22.Johnson MO. Belief of vaccine receipt in HIV vaccine trials: Further cautions. J Acquir Immune Defic Syndr. 1999;21:413–16. [PubMed] [Google Scholar]
- 23.Eagly A, Chaiken S. The psychology of attitudes. Harcourt Brace College Publishers; Fort Worth: 1993. [Google Scholar]
- 24.Settle RB, Golden LL. Attribution theory and advertiser credibility. J Marketing Res. 1974;11(May):181–85. [Google Scholar]
- 25.Kamins MA, Brand MJ, Heoke SA, et al. Two-sided versus one-sided celebrity endorsements: The impact on advertising effectiveness and credibility. J Advertising. 1989;18(2):4–10. [Google Scholar]
- 26.Cox AD, Cox D, Zimet GD. Understanding consumer responses to product risk information. J Marketing. 2006;70:79–91. [Google Scholar]
- 27.Cox AD, Cox D, Cyrier R, et al. Can self-prediction overcome barriers to hepatitis B vaccination? A randomized controlled trial. Health Psychol. 2012;31:97–105. doi: 10.1037/a0025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox DS, Cox AD, Sturm L, et al. Behavioral interventions to increase HPV vaccination acceptability among mothers of young girls. Health Psychol. 2010;29:29–39. doi: 10.1037/a0016942. [DOI] [PubMed] [Google Scholar]
- 29.Nan X. Communicating to young adults about HPV vaccination: Considerations of message framing, motivation, and gender. Health Commun. 2012;27:10–18. doi: 10.1080/10410236.2011.567447. [DOI] [PubMed] [Google Scholar]
- 30.Evans KR, Lewis MJ, Hudson SV. The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J Cancer Educ. 2012;27:299–305. doi: 10.1007/s13187-011-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinfurt KP, Depuy V, Castel LD, et al. Understanding of an aggregare probability statement by patients who are offered participation in Phase I clinical trials. Cancer. 2005;103:140–147. doi: 10.1002/cncr.20730. [DOI] [PubMed] [Google Scholar]
- 32.Rudy BJ, Kapogiannis BG, Lally MA, et al. Youth-specific considerations in the development of PrEP, microbicide, and vaccine research trials. J Acquir Immune Defic Syndr. 2010;54(Suppl 1):S31–S42. doi: 10.1097/QAI.0b013e3181e3a922. [DOI] [PMC free article] [PubMed] [Google Scholar]