Abstract
Background
Spontaneous calcium release evoking delayed after-depolarization is believed to cause CPVT, a lethal human arrhythmia provoked by exercise or emotional stress. Beta-adrenergic blockers are the drug of choice, but fail to achieve complete arrhythmia control in some patients. These individuals often require flecainide, device implantation and/or sympathetic denervation.
Objective
To optimize the arrhythmia therapy by pharmacological inhibition of the sympathetic nervous system in the CASQ2Δ/Δ mouse model of CPVT2.
Methods
A heart telemetry device was implanted for continuous ECG recording at rest and during provocation testing. Calcium transients and abnormal calcium release were studied in cardiomyocytes isolated from adult mice. Adrenergic receptor expression was determined by western blotting and confocal microscopy.
Results
Adult CASQ2Δ/Δ mice suffer from complex ventricular arrhythmia at rest and ventricular tachycardia during treadmill exercise and after epinephrine injection. Beta adrenergic blockers, propranolol and metoprolol attenuated arrhythmia at rest but not after stress. Reserpine had no efficacy in controlling arrhythmia. Agents with alpha blocking activity, phentolamine or labetalol, abolished both exercise and epinephrine-induced arrhythmia. To the contrary, injection of alpha adrenergic agonist phenylephrine reproducibly provoked VT. Isolated cardiomyocytes from CASQ2Δ/Δ mice had delayed calcium release waves upon exposure to sympathetic agonists which was abolished by phentolamine. Hearts of calsequestrin mutant mice expressed more alpha adreno-receptor1 compared to controls (p<0.05).
Conclusions
We identified a contribution of alpha adrenergic pathway to pathogenesis of catecholamine-induced arrhythmia. Alpha blockade emerges an effective therapy in the murine model of CPVT2 and should be tried in humans resistant to beta blockers.
Keywords: CPVT, calcium, calsequestrin, mouse model, arrhythmia, alpha adrenergic receptor, sympathetic blocker
Introduction
Catecholaminergic polymorphic ventricular tachycardia(CPVT) is a lethal human arrhythmia provoked by exercise or emotional stress1-3. CPVT is caused by mutations in genes that regulate intracellular Ca+2 homeostasis. Patients suffering from CPVT are at risk of ventricular fibrillation, seizures and sudden death triggered by exercise or emotional stress2,4,5.Two principal types of gene mutations cause the disease: heterozygous defects in the cardiac ryanodine receptor gene (RYR2) cause the autosomal dominant CPVT1, while homozygous mutations in the cardiac calsequestrin (CASQ2) cause the autosomal recessive CPVT23, 6. Calsequestrin is a sarcoplasmic reticulum (SR) protein which together with triadin and junction is essential for regulation of Ca+2 induced Ca+2 release through the ryanodine channel7. CASQ2 defects causing CPVT2 may be missense such as D307H6, which are assumed to impair the conformational changes occurring during Ca+2 binding and the ability to regulate Ca+2 transfer to the cytosol through the ryanodine channel or null-allele mutations8. We have previously described mouse models recapitulating the human phenotype for CPVT2. These gene-targeted mice are homozygous for either the D307H mutation (CASQ2D307H/D307H) or represent a CASQ2 knock-out (CASQ2Δ/Δ). Polymorphic and bidirectional VT may be provoked by physical or pharmacological stress9-11. The phenotype of CASQ2D307H/D307Hmice was attributed mainly to the degradation of the defective protein leading to a nearly complete protein deficiency, despite preserved mRNA levels10. We also observed some difference in arrhythmia severity between adult CASQ2D307H/D307H and CASQ2Δ/Δ mice and a decreased responsiveness to drug therapy in the latter9.
Beta-adrenergic blockers are the therapy of choice for human CPVT but they fail to achieve complete arrhythmia control in some patients9, 11.Such individuals often require additional drugs, device implantation and/or sympathetic denervation.
The sympathetic nervous system plays a crucial role in the regulation of cardiac function. Norepinephrine (NE) released from sympathetic neurons innervating the heart affects the heart rate, cardiac contractility, blood flow, substrate utilization and mediates cardiomyocytes hypertrophy. The effects of NE are mediated by nine different receptors (α1A-, α1B-, α1D-, α2A-, α2B-, α2C, β1-, β2- and β3). These receptors are part of a larger superfamily of G-protein coupled receptors that mediate the effects of hormones and neurotransmitters12, 13.
In this study we attempted to optimize CPVT therapy by targeting several components of the autonomic nervous system. We hereby report that alpha adrenergic receptor blockers protect against arrhythmia and abolish abnormal calcium release in cardiomyocytes fromCASQ2 knockout mice.
Methods
Animals
The study was approved by the Institutional Committee for Animal Care and Use and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Experiments were carried out on gene targeted C57BL/6 mice modeling CPVT210, homozygous for off-frame exon 9 deletion (CASQ2Δ/Δ) or D307H mutation (CASQ2D307H/D307H) in comparison to age-matched wild type (WT) mice. Mice were maintained and bred in a pathogen-free facility on regular rodent chow with free access to water and 12-hour light and dark cycles.
Unless otherwise stated, experiments were performed in adult (6-8 month old) male mice.
In vivo Pharmacological Testing
CASQ2Δ/Δ mice were implanted with a telemetry device (DSI St. Paul MM, device weight 3.8g) as previously described14. Provocation testing was performed at baseline and 15 min after IP drug injection of each drug (Table 1). A stock solution of Reserpine (MP Biomedicals) was prepared by diluting 4mg/ml in DMSO(Sigma). Mice were injected 0.5 μg/g of the primary stock. Provocation testing was repeated 24 hours after reserpine looking for the delayed effect of this drug. Phentolamine (Novartis) and labetalol (Trandate, Perrigo Israel Pharmaceuticals Ltd.) were used in doses previously tested in mice15.
Tables Table 1 Pharmacological testing in adult CASQ2△/△mice
| Agent and Dose | Mechanism of Action | No. mice | Sinus Rate eats/min | Rest | Exercise | Epinephrine | mice with VT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VPC s | NSV T | SV T | VPC s | NSV T | SV T | VPC s | NSV T | SVT | |||||
| Saline 0.1 ml | Control | 6 | 657±22 | 6 | 6 | 0 | 6 | 6 | 4 | 6 | 6 | 5 | 6 |
| Reserpine acute 0.5mg/g | Neurotransmitter depletion | 3 | 648±10 | 3 | 2 | 0 | 3 | 3 | 1 | 3 | 3 | 3 | 3 |
| Reserpine – 0.5mg/g | Neurotransmitter depletion | 3 | 637± 6 | 3 | 2 | 0 | 3 | 3 | 2 | 2 | 3 | ||
| Propranolol 10mg/g | β1+pβ2 Blocker | 6 | 456±31¶ | 3 | 1 | 0 | 6 | 3 | 0 | 6 | 5 | 0 | 5 |
| Metoprolol 10mg/g | β1 Blocker | 6 | 449±11¶ | 2 | 0 | 0 | 6 | 5 | 0 | 0 | 0 | 0 | 5 |
| Phentolamine 50 mg/g | α Blocker | 6 | 510±45 * | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0† |
| Labetalol 20 mg/g | β1+β2+α1 Blocker | 7 | 409± 6¶ | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0† |
| Labetalol 10 mg/g | β1+β2+α1 Blocker | 6 | 460±42¶ | 2 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | 2* |
Abbreviation: VPCs, ventricular premature contractions; NSVT, non-sutained ventricular tachycardia; SVT, sustained ventricular tachycardia; Sinus rate (beats/min); mice with VT, a criterion for disease expression defined as ventricular tachycardia under any condition;
P<0.05
P<0.01
P<0.005. The sinus rate was determined at rest.
Arrhythmia was studied with heart rhythm telemetry at rest, during treadmill exercise (Exer-6M, Columbus Instruments, OH, USA) and after IP injection of epinephrine 0.1mg/kg (Teva, Israel). In brief, mice were forced to exercise on a rodent treadmill, gradually increasing the speed up to a maximum of 15 m/min. Then, after 3 minutes of rest, mice were injected with epinephrine, followed by additional 5 minutes of telemetric recording. Ventricular tachycardia (VT) was defined as four or more consecutive ventricular beats. All other ventricular arrhythmias, i.e. premature beats, ventricular bigeminy, couplets and triplets were all defined as premature ventricular contractions (PVCs). Animal expressing CPVT was defined by developing VT (either sustained or non-sustained) at rest, during exercise or pharmacological stress 9
Experiments with phenylephrine
Phenylephrine 1mg/g(West-Ward Pharmaceutical, Eatontown, NJ, USA) or saline was injected IP to 3-month old mice following 10 minutes of recording at rest. These young animals do not have arrhythmia under resting conditions. An ECG recording was initiated 5 min after the injection and continued for another 10 minutes. On the next day mice were pretreated by IP verapamil (2.5 mg/g, Abbott, IL, USA) prior to phenylephrine.
Cardiomyocyte isolation from adult mice and intracellular Ca+2 measurements
Adult ventricular cardiomyocytes were isolated from CASQ2Δ/Δ mice using a Langendorff isolated heart perfusion and enzymatic digestion as previously described16. Cells were incubated at 37°C in 2% CO2 until used. [Ca2+] i from individual adult cardiomyocytes was measured as above. Measurements were performed in electrically paced cells at 1 Hz via platinum electrodes, under baseline conditions, with pharmaceutical agents isoproterenol 10 μM (Monico Spa, Venice), norepinephrine 10μM (Hospira, Lake Forest, IL) and phentolamine 10μM, or their combination.
Intracellular free calcium [Ca+2] i from individual adult cardiomyocytes was measured using the indicator indo-1-AM under a Zeiss epi-fluorescent inverted microscope (Zeiss, Germany). All the measurements were made on the day of heart isolation. Adult cardiomyocytes were incubated with 5μM indo-1-AM and 3μM pluronic acid for 30 min in glucose-enriched Tyrode's solution at 25°C. After incubation, the cells were rinsed with glucose-enriched Tyrode's and transferred to a chamber (RC-47FSLP Warner Instruments) on the microscope. Calcium measurements were made in cells in suspension. Indo-1 loaded cells were exited at 340 nm and the emitted light then split by a dichroic mirror into two photomultipliers (Hamamatsu, Japan), with input filters at 410 and 490 nm for indo-1. The fluorescence ratio (R) of 410/490 nm, which is proportional to [Ca+2]i, was input to the SAMPLE program written by Dr. D. Kaplan, the Biological Institute, Ness-Ziona, Israel. Analysis was performed at 25°C.
Protein extraction and Western blot analysis
Proteins were extracted from frozen hearts and separated as previously described14. Electro-blotting to Hybond-C Extra membranes (Habersham, USA) was followed by incubation with rabbit anti-alpha 1 adrenergic receptor antibodies (1:400), (Acris Antibodies, Germany). For secondary detection, we used DyLight800 goat anti-rabbit (Pierce Biotechnology, Thermo Fisher Scientific, town, USA) (1:10000). Reactive bands were detected and quantified by the Odyssey® Infrared Imaging System (Li-Cor Biotechnology, town, USA).
Immunostaining of isolated cardiomyocytes
Fresh isolated cardiomyocytes were added to 10μl laminin (Sigma, town, Germany) on a cover glass and were fixed with 100% methanol (Bio-Labs, town Israel) incubation for 10 min. Cells were washed with PBS (Sigma, Germany) and the membrane was perforated with PBS containing triton X-100 (Sigma, Germany).Blocking was performed with PBS containing 5% BSA (Sigma, Germany) for 1 hour and samples were incubated overnight with primary antibodies, either beta 1 adreno-receptor (rabbit polyclonal antibody 1:100, SC-568, CA USA) or alpha 1 adrenergic receptor (rabbit polyclonal 1:1000, Acris Town, Germany). Afterwards cells were incubated for 2 hours at room temperaturewith1:1500 secondary antibody, goat anti-rabbit polyclonal IgG- H&L (FITC,ABCAM, Cambridge, UK) and with Hoechst (Invitrogen, Paisley, UK) for 10 min. The cells were visualized and photographed using a Leica TCS SP5 Confocal Imaging System (Leica Microsystems, Wetzlar, Germany) and x63 oil objectives.
Statistical analyses were performed when appropriate using Student's t or Fisher's exact test. Data are presented as mean± SD and statistical significance was accepted at p<0.05.
Results
In vivo studies
Adult CASQ2Δ/Δ mice, implanted with the telemetry device, underwent provocation testing at baseline and after drug injection to evoke CPVT (Table 1).
All mutant mice studied in this experiment suffered from severe arrhythmia including VT at baseline. Βeta adrenergic blockers were insufficiently effective against stress-induced VT irrespective of 1 selectivity. They prevented sustained ventricular tachycardia but not VPCs or non sustained VT.
Since patients that do not respond to β-blockers are treated by sympathetic denervation17, we tried to induce sympathectomy by targeting sympathetic nerve endings with reserpine17. Unfortunately, CASQ2Δ/Δ mice did not respond to this treatment and all had VT during the provocation testing immediately or 24 hours after drug administration.
The non-selective α adrenergic blocker phentolamine had a pronounced antiarrhythmic efficacy. Because any pharmacological therapy for human CPVT should include beta blockade, we proceeded to experiment with the combined +1blocker, labetalol (having a 'β to α1' antagonism ratio of 3:1). This drug prevented VT in a dose-dependent fashion (Table 1, Fig 1).
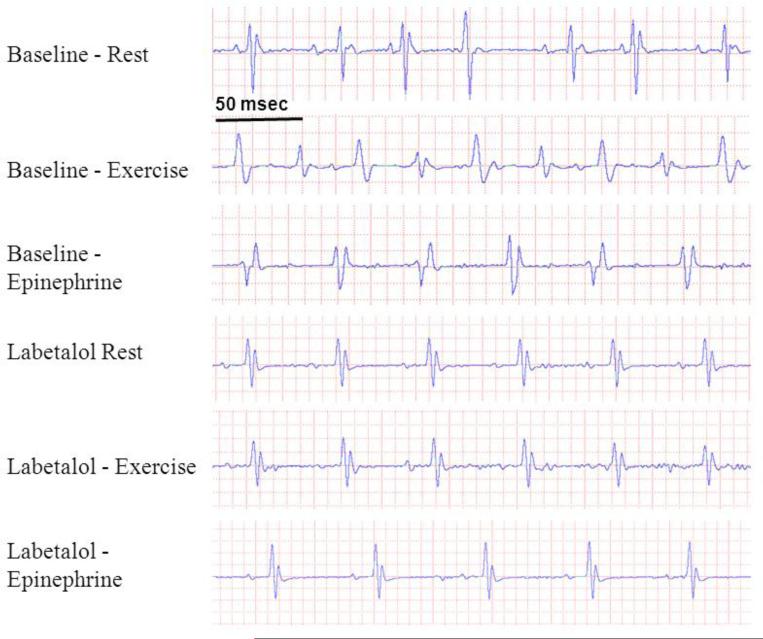
Figure 1.
Representative ECG traces from an 8 month old CASQ2Δ/Δ mouse undergoing provocation testing at baseline and after injection of labetalol. Sinus rhythm with multiple ventricular premature beats was recorded at rest, bidirectional VT after exercise and polymorphic VT after adrenaline. Labetalol evoked sinus bradycardia and eliminated the arrhythmia.
To test if isolated α adrenergic stimulation would induce arrhythmia in CASQ2Δ/Δ mice we injected young animals (which do not have arrhythmia at rest) with a α1 agonist, phenylephrine. Mice (n=3) developed VT after being treated with phenylephrine but not with saline vehicle (Fig 2). This arrhythmia could be abrogated by pretreatment with verapamil which was previously shown by us to prevent CPVT in CASQ2 mutant mice9.
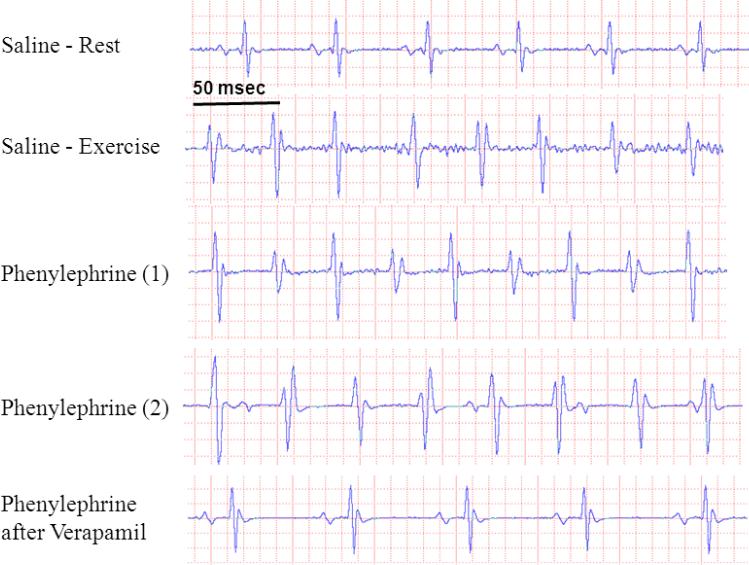
Figure 2.
Representative ECG traces from a 16 week old CASQ2 Δ/Δ mouse showing normal sinus rhythm at baseline but polymorphic VT could be evoked by exercise. Ventricular arrhythmia appeared after injection of phenylephrine: trace (1) shows an example of bidirectional VT and (2) shows a polymorphic VT with a prominent atrio-ventricular dissociation. There was no arrhythmia when phenylephrine was administered after pretreatment with verapamil. n=3.
Studies in isolated cardiomyocytes
Calcium transients were compared between WT and CASQ2Δ/Δ cardiomyocytes on baseline, after exposure to norepinephrine and to phentolamine (Table 2). WT transients had a higher amplitude and ascent rate at baseline. The descent rate followed a similar trend but did not reach statistical significance. An adrenergic agonist noradrenalin significantly augmented the transient in the WT. The area under curve representing the total systolic calcium release was significantly higher in WT cells compared to CASQ2Δ/Δ at baseline (24 23 vs. 16 ±10 unit, p=0.0012) and after noradrenaline (40 ±39 vs. 15 ±8, p=0.014). In CASQ2Δ/Δcells noradrenaline did not affect the parameters of initial calcium release but often triggered a second wave of delayed calcium release which was never seen in the WT (Figure 3). The blocker phentolamine, on its own, had no significant effect on transient parameters. However it did attenuate the physiological and the pathological effects of norepinephrine on WT and CASQ2Δ/Δcells, respectively.
Table 2.
Ca2+ transients in isolated cardiomyocytes from adult WT and CASQ2△/△ mice
| Paramete r | Baseline | P value vs. WT | NE | P value vs. Base | PHENTOL | P value vs. Base | NE + PHENTOL | P value vs. Base | |
|---|---|---|---|---|---|---|---|---|---|
| Wildtype | Amplitu de (a.u.) | 0.081±0.0 65 | NA | 0.187 ±0.141 | 0.004 | 0.105±0.074 | NS | 0.140±0.120 | NS |
| Rise rate (a.u.\sec) | 1.45±1.54 | NA | 3.48±3.96 | 0.028 | 1.78±1.15 | NS | 3.95±5.16 | NS | |
| Decay rate (a.u.\sec ) | 0.45±0.48 | NA | 1.29±1.17 | 0.004 | 0.51±0.44 | NS | 1.35±1.48 | NS | |
| AUC (a.u./100) | 24±23 | NA | 40±39 | 0.13 | 29±24 | NS | 31±18 | NS | |
| CASQ 2 knockout | Amplitu de (a.u.) | 0.056±0.034 | 0.005 | 0.059±0.026 | NS | 0.062±0.014 | NS | 0.055±0/016 | NS |
| Rise rate (a.u.\sec ) | 0.84±0.70 | 0.004 | 1.20±0.98 | NS | 0.96±0.43 | NS | 1.18±0.76 | NS | |
| Decay rate (a.u.\sec) | 0.30±0.36 | NS | 0.38±0.37 | NS | 0.36±0.10 | NS | 0.29±0.12 | NS | |
| AUC (a.u./100 ) | 16±10 | 0.0012 | 15±8 | NS | 13±4 | NS | 14±5 | NS |
Abbreviations: a.u., arbitrary units; AUC, area under curve; NE, nor-epinephrine; PHENTOL, phentolamine; NA, non-available; NS, non-significant; all values representmean±SD from ≥12 cells obtained from ≥3 animals.
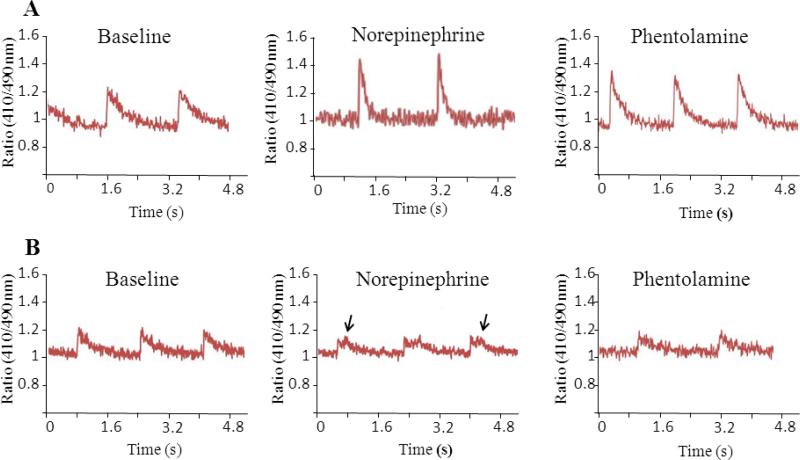
Figure 3.
Calcium transients in cardiomyocytes isolated from wild type (A) and CASQ2Δ/Δ (B) mice. Representative traces are shown on baseline, after exposure to norepinephrine and then after adding phentolamine. Note the repetitive abnormal calcium release waves in CASQ2Δ/Δ cells exposed to norepinephrine (arrows) which were seen in 4/12 cells studied and disappeared after adding phentolamine (mean±SD, n=3-5 mice/group).
After documenting the protection of blockade against abnormal calcium release on combined (+) adrenergic stimulation with norepinephrine we examined if blockade could protect from calcium irregularities induced by a selective adrenergic agonist. As illustrated in Figure 4, Isoproterenol induced repetitive delayed calcium release, severely prolonging the transient in CASQ2Δ/Δcardiomyocytes. Phentolamine reproducibly abolished isoproterenol-induced abnormal calcium release in all cells tested.
Figure 4.
Calcium transient measurement in isolated CASQ2Δ/Δ cardiomyocytes. agonist isoproterenol induces abnormal calcium release waves in 6/6 cells (n=2 mice), which were abolished by adding an blocker phentolamine (B).
Adrenergic alpha 1 (ADRA1) receptor expression in the heart of CASQ2 mutant mice
ADRA1proteinlevels were assessed in hearts of 14-16-week-old WT or either CASQ2Δ/Δ or CASQ2D307H/D307H mutant mice. The postsynaptic 1adreno-receptor was significantly elevated in total heart homogenates from either CASQ2 mutant mouse compared to the WT (Fig 5).
Figure 5.
Western blot for a1adreno-receptor protein in heart homogenates. A. Summary of densitometry results corrected to β actin expression; Y axis – arbitrary units, p*<0.05, n=3/group. B. Blot image.
To further study cardiac alpha receptor expression, we also examined isolated adult CASQ2Δ/Δ cardiomyocytes by immunofluorescence. Confocal microscopy imaging confirmed ADRA1 immunostaining of isolated cardiomyocytes (Fig 6). As could be expected, receptor expression appeared to be lower compared to the expression of beta adrenergic receptors.
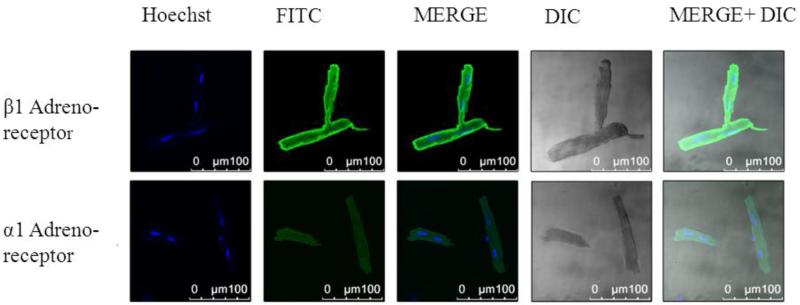
Figure 6.
Confocal microscopy in an isolated CASQ2Δ/Δ cardiomyocyte. Cells were stained for either 1adreno-receptor (upper panels) or for 1 adreno-receptor (lower panel).Abbreviations: Hoechst, nucleus staining; FITC, green fluorescence staining; MERG, Hoechst+ FITC; DIC- light microscopy; MERG+ DIC, Hoechst+ FITC+ light microscopy.
Discussion
We used mice with CASQ2 knock-out as a model for the optimization of CPVT therapy. The recessively inherited CPVT2 is associated with early disease onset, severe arrhythmia, and adverse prognosis1, 8.The principal therapies for CPVT are phenotype driven and include avoiding exercise and, beta adrenergic blockade. The response to beta-blockers is incomplete and often declines with time because of an escape phenomenon3,18. The options in unresponsive patients include additional drugs - primarily flecainide, implanting a defibrillator (ICD), and sympathetic nervous denervation17. Although very effective in mice, calcium channel blockers have a limited benefit in humans, even when combined with beta adrenergic blockers. CaMKII inhibition and attenuating the degradation of mutant protein have shown promising results in vitro but may not yet be applied in clinical practice. Given the success of sympathetic denervation we examined whether drugs affecting the adrenergic pathway have a protective effect against CPVT.
Pharmacological testing conducted in mice and isolated cardiomyocytes identified a novel approach to treat CPVT. Alpha sympathetic blockade alone or alpha blocker combined with a beta blocker effectively eliminate arrhythmia in CASQ2 knockout mice (Table 1, Fig 1) and prevent abnormal calcium release in isolated cardiomyocytes from the affected animals (Table 2, Fig 3).
Administration of the alpha adrenergic agonist phenylephrine induced typical arrhythmia in CASQ2Δ/Δ mice, confirming the cause-and-effect relationship between alpha receptor activation and CPVT (Fig. 2).Importantly, arrhythmia could be abolished by verapamil and was not present in CASQ2Δ/Δ mice injected with saline. Reproducing the protective effect of alpha blockade in isolated cardiomyocytes indicates a postsynaptic mechanism of action. Confocal imaging of isolated murine cardiomyocytes (Fig. 6) confirmed a low-level expression of the alpha adrenergic receptor on the sarcolemma. Although alpha receptors are less abundant compared to beta receptors, their activation has a major role in causing CPVT in mice. Interestingly, there was an increase in adreno-receptor expression in CASQ2 knockout or missense mutant mice (Fig. 5), suggesting that increased receptor responsiveness to sympathetic stimulation could contribute to arrhythmia susceptibility. Table 2 provides some insights as to the role played by alpha blockade in calcium transients. Noradrenaline increased the amplitude, the rise and decay rates of calcium transients in the wild type but not in the CASQ2Δ/Δ cardiomyocytes. While the initial phase of CASQ2Δ/Δ transients was not affected, some of them were followed by abnormal calcium release waves which never occurred in the wild type (Fig. 3). Phentolamine had no effect on calcium transient parameters under resting conditions. It attenuated the physiological changes induced by noradrenaline in wild-type cardiomyocytes and the pathological waves in cells from CPVT mice. Ability of phentolamine to prevent abnormal calcium release caused by pure beta adrenergic stimulation (Fig. 4), suggesting that these 2 pathways interfere at the post-receptor level.
Alpha adrenergic signaling affects vascular tone, cardiac function and metabolism. Chronic activation of myocardial alpha receptors leads to cardiac hypertrophy, increased energy expenditure followed by apoptosis and worsening heart failure ,produces detrimental effects like cardiac hypertrophy13, 19. On the other than, short-term activation may be cardio protective and even mediate preconditioning20, 21.The role of alpha adrenergic signaling in CPVT and its relative importance in humans as compared to mice is not yet clear. The clinically documented antiarrhythmic effect of sympathetic denervation (on top of beta blockade) suggests that alpha adrenergic innervation does play a pathogenic role. In our study reserpine was ineffective, but this drug, despite causing “chemical sympathectomy” by depleting a neurotransmitter from nerve endings cannot neutralize circulating catecholamines.
Because alpha stimulation had no apparent interaction with beta receptor, PKA activation, L-type or ryanodine channel, we hypothesized that its activity may be mediated through phospholipase C and stimulation of the IP3 (inositol triphosphate) receptor in the perinuclear sarcoplasmic reticulum4. Intracellular Ca2+ stores include SR and the cisternae surrounding the nuclear envelope. The junctional SR is located in the proximity of t-tubules, L type calcium channels and contains the ryanodine receptors and the SR Ca2+ pump (SERCA). The free [Ca2+] inside the perinuclear stores can be ~ 1mM but is not directly involved in excitation-contraction coupling. IP3 is the primary Ca2+ release channel in the smooth muscle, but even cardiac myocytes use local Ca2+ release from the perinuclear stores via IP3 receptors/channels in response to neurohumoral stimuli. The channel agonist IP3 is generated by phospholipase C cleavage of membrane phosphatidyl inositol bisphosphate (PIP2) into IP3 and 1,2- diacylglycerol. IP3-induced Ca2+sparks have lower amplitude and longer rise and descent times. In the ventricular myocyte there are about 50× less IP3 receptors than RyR2 receptors and their location is mainly in the perinuclear envelope and not in the subsarcolemmal SR22. Yet, junctional SR appears to be contiguous with the nuclear envelope in cardiomyocytes. While not directly participating in calcium induced calcium release, opening of IP3channels may increase the basal Ca2+ level in the sarcoplasm thus affecting the susceptibility to arrhythmia in CPVT mice23.
IP3 inhibitor 2APB was shown to prevent abnormal Ca2+ release in cardiomyocytes from the diabetic heart24. We have tried, so far without success, to attenuate stress induced arrhythmia in CASQ2Δ/Δ mice using parenteral 2APB (n=5, data not shown).
Alpha adreno-receptor blockers do not have an established role in treatment of human arrhythmia. Experimental studies suggest that alpha receptors modulate electrophysiological properties of the ventricular myocardium25 and are involved in ventricular arrhythmia of ischemia reperfusion, catecholamine stimulation and heart failure26, 27, as well as neutrally mediated atrial arrhythmia28. Our study demonstrates the presence of alpha adrenergic activity in the cardiomyocytes and favors the potential use of alpha blocking agents in catecholamine- induced arrhythmia.
Nonselective -blockers, propranolol and the peripherally acting nadolol18,29 are the most commonly used agents in CPVT. While very high concentrations of propranolol might produce some alpha-receptor blockage, nadolol is devoid of this activity 30. Reserpine, depleting the sympathetic nerve endings was ineffective in this study but might be tried in combination with beta blockade to antagonize circulating catecholamines. Labetalol comprises 1 with 2 and 1 blocking activity, thus closely mimicking sympathetic denervation by pharmacological means. That makes labetalol an excellent candidate to treat resistant CPVT cases and to avoid invasive procedures such as left cardiac sympathetic denervation and possibly ICD implantation.
Conclusions
The pathway by which alpha adreno-receptor stimulation participates in CPVT pathogenesis warrants further investigation. Another question is whether this result is applicable to the more common form of the disease, CPVT1. While the exact mechanism of protection by alpha blockers remains unclear, their effect against CPVT2 in a genetic murine model, which recapitulates the human disease, is rather remarkable. If reproduced in human studies, it may become an important non-invasive therapeutic option for CPVT patients.
Acknowledgments
Funding: This work was funded by Israel Science Foundation grants 876/2005 and 763/10.
Abbreviations
- ADRA
alpha adrenergic receptor
- CASQ2Δ/Δ
homozygous calsequestrin knock-out mice
- CASQ2D307H
mice homozygous for D307H calsequestrin mutation
- CPVT
Catecholaminergic polymorphic ventricular tachycardia
- IP
intraperitoneal
- PVCs
premature ventricular contractions
- SR
sarcoplasmic reticulum
- VT
ventricular tachycardia
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None declared
References
- 1.Lahat H, Eldar M, Levy-Nissenbaum E, Bahan T, Friedman E, Khoury A, Lorber A, Kastner DL, Goldman B, Pras E. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13-21. Circulation. 2001;103:2822–2827. doi: 10.1161/01.cir.103.23.2822. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 4.Katz G, Arad M, Eldar M. Catecholaminergic polymorphic ventricular tachycardia from bedside to bench and beyond. Curr Probl Cardiol. 2009;34:9–43. doi: 10.1016/j.cpcardiol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Lehnart SE, Mongillo M, Bellinger A, et al. Leaky Ca2+ release channel/ryanodine receptor. 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldar M, Pras E, Lahat H. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med. 2003;13:148–151. doi: 10.1016/s1050-1738(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 8.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin. 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 9.Katz G, Khoury A, Kurtzwald E, Hochhauser E, Porat E, Shainberg A, Seidman JG, Seidman CE, Lorber A, Eldar M, Arad M. Optimizing catecholaminergic polymorphic ventricular tachycardia therapy in calsequestrin-mutant mice. Heart Rhythm. 2010;7:1676–1682. doi: 10.1016/j.hrthm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin. 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knollmann BC, Chopra N, Hlaing T, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papay RS, Shi T, Piascik MT, Naga Prasad SV, Perez DM. alpha(1)A-adrenergic receptors regulate cardiac hypertrophy in vivo through interleukin-6 secretion. Mol Pharmacol. 2013;83:939–948. doi: 10.1124/mol.112.084483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon R, Chaudhry M. Effect of alpha1-adrenergic receptors in cardiac pathophysiology. Am Heart J. 2006;152:842–850. doi: 10.1016/j.ahj.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzwald-Josefson E, Hochhauser E, Katz G, Porat E, Seidman JG, Seidman CE, Chepurko Y, Shainberg A, Eldar M, Arad M. Exercise training improves cardiac function and attenuates arrhythmia in CPVT mice. J Appl Physiol. 2012;113:1677–1683. doi: 10.1152/japplphysiol.00818.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota T, Yamazaki N, Sudo J, Monma Y, Kaku T, Okuyama T, Tanabe T. Protective effects of adrenoceptor-blocking agents on myocardial injury induced by epinephrine in mice. J Toxicol Sci. 1990;15:1–13. doi: 10.2131/jts.15.1. [DOI] [PubMed] [Google Scholar]
- 16.Katz G, Shainberg A, Hochhauser E, Kurtzwald-Josefson E, Issac A, El-Ani D, Aravot D, Afek A, Seidman JG, Seidman CE, Eldar M, Arad M. The role of mutant protein level in autosomal recessive catecholamine dependent polymorphic ventricular tachycardia (CPVT2). Biochem Pharmacol. 2013;86:1576–1583. doi: 10.1016/j.bcp.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace. 2012;14:175–183. doi: 10.1093/europace/eur277. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 19.Jensen BC, O'Connell TD, Simpson PC. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. J Mol Cell Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Ashraf M. Activation of alpha1-adrenergic receptor during Ca2+ pre conditioning elicits strong protection against Ca2+ overload injury via protein kinase C signaling pathway. J Mol Cell Cardiol. 1998;30:2423–2435. doi: 10.1006/jmcc.1998.0802. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O'Connell TD. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- 23.Escobar AL, Perez CG, Reyes ME, Lucero SG, Kornyeyev D, Mejia-Alvarez R, Ramos-Franco J. Role of inositol 1,4,5-trisphosphate in the regulation of ventricular Ca(2+) signaling in intact mouse heart. J Mol Cell Cardiol. 2012;53:768–779. doi: 10.1016/j.yjmcc.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fauconnier J, Lanner JT, Zhang SJ, Tavi P, Bruton JD, Katz A, Westerblad H. Insulin and inositol 1,4,5-trisphosphate trigger abnormal cytosolic Ca2+ transients and reveal mitochondrial Ca2+ handling defects in cardiomyocytes of ob/ob mice. Diabetes. 2005;54:2375–2381. doi: 10.2337/diabetes.54.8.2375. [DOI] [PubMed] [Google Scholar]
- 25.Rivard K, Trepanier-Boulay V, Rindt H, Fiset C. Electrical remodeling in a transgenic mouse model of alpha1B-adrenergic receptor overexpression. Am J Physiol Heart Circ Physiol. 2009;296:H704–718. doi: 10.1152/ajpheart.00337.2008. [DOI] [PubMed] [Google Scholar]
- 26.Kurz T, Yamada KA, DaTorre SD, Corr PB. Alpha 1-adrenergic system and arrhythmias in ischaemic heart disease. Eur Heart J. 1991;12(Suppl F):88–98. doi: 10.1093/eurheartj/12.suppl_f.88. [DOI] [PubMed] [Google Scholar]
- 27.Priori SG, Napolitano C, Schwartz PJ. Cardiac receptor activation and arrhythmogenesis. Eur Heart J. 1993;14(Suppl E):20–26. doi: 10.1093/eurheartj/14.suppl_e.20. [DOI] [PubMed] [Google Scholar]
- 28.Richer LP, Vinet A, Kus T, Cardinal R, Ardell JL, Armour JA. Alpha-adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1175–1180. doi: 10.1152/ajpregu.00840.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright JM, Dobosiewicz MR, Clarke PB. alpha-and beta-Adrenergic receptors differentially modulate the emission of spontaneous and amphetamine-induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology. 2012;37:808–821. doi: 10.1038/npp.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stauber RE, Heinemann A, Trauner M, Krejs GJ. Divergent effects of propranolol and nadolol in isolated mesenteric arteries from normal and portal hypertensive rats. Eur J Clin Invest. 1996;26:676–680. doi: 10.1111/j.1365-2362.1996.tb02152.x. [DOI] [PubMed] [Google Scholar]