Abstract
Study objective
Acute upper gastrointestinal (GI) hemorrhage is a common presentation in hospital-based emergency departments (EDs). A novel diagnostic approach is to use video capsule endoscopy to directly visualize the upper GI tract and identify bleeding. Our objective was to evaluate and compare the relative costs and benefits of video capsule endoscopy compared to other strategies in low to moderate risk ED patients with acute upper GI hemorrhage.
Methods
We constructed a model using standard decision analysis software to examine the cost-effectiveness of four available strategies for a base-case patient who presents to the ED with either mild or moderate risk scenarios (by Glasgow-Blatchford Score) for requiring invasive hemostatic intervention (i.e., endoscopic, surgical, etc.) The four available diagnostic strategies were (1) direct imaging with video capsule endoscopy performed in the ED, (2) risk stratification using the Glasgow-Blatchford score, (3) nasogastric tube placement and, finally, (4) an admit-all strategy.
Results
In the low-risk scenario, video capsule endoscopy was preferred strategy (cost $5,691, 14.69 QALYs) and more cost effective than the remaining strategies including nasogastric tube strategy (cost $8,159, 14.69 QALYs), risk stratification strategy (cost $10,695, 14.69 QALYs) and admit-all strategy (cost $22,766, 14.68 QALYs). In the moderate risk scenario, video capsule endoscopy continued to be preferred strategy (cost $9,190, 14.56 QALYs) compared to nasogastric tube (cost $9,487, 14.58 QALYs, ICER $15,891) and more cost effective than admit-all strategy (cost, $22,584, 14.54 QALYs.)
Conclusion
Video capsule endoscopy may be cost-effective for low and moderate risk patients presenting to the ED with acute upper GI hemorrhage.
Keywords: Gastrointestinal Disease, Emergency Medicine, Gastrointestinal Hemorrhage, Cost-Effectiveness, Risk-Stratification, Diagnostic Tests, Capsule Endosocpy
INTRODUCTION
Acute upper gastrointestinal (GI) hemorrhage, clinically manifesting as hematemesis, melena or a combination of both, is a common presentation in hospital-based emergency departments (EDs) in the U.S. and around the world. According to data from the Healthcare Cost and Utilization Project, there were 863,000 U.S. hospital admissions for GI hemorrhage in 2008, which included both upper and lower GI bleeding.1 The mean length of stay for patients discharged from the hospital with a diagnosis of GI hemorrhage is 4.5 days and the mean hospital charges are $26,210 per admission. Acute upper GI hemorrhage is a particularly severe manifestation of GI hemorrhage and is associated with a mortality rate ranging from 15 to 20%.2
One reason that care for patients with acute upper GI hemorrhage is challenging is that ED physicians do not have the ability to rule out active upper bleeding. As a result, many patients with an ultimately benign clinical course are admitted to the hospital and incur considerable costs. Without an ED-based endoscopy, alternative ways to risk-stratify patients with signs of upper GI hemorrhage include placing a nasogastric (nasogastric) tube, which is uniformly uncomfortable and disliked by patients 3, to identify fresh or coffee grounds blood, or, using clinical decision rules (CDRs), such as the Rockall Risk Score and Glasgow-Blatchford Score, or, simply admitting everyone for endoscopy. A novel approach is to use video capsule endoscopy in the ED to directly visualize the upper GI tract and identify presence or absence of blood. In three prior ED-based pilot studies, video capsule endoscopy has demonstrated excellent patient tolerance and high sensitivity for detecting acute upper GI hemorrhage.4-6
Given the duration needed and the cost necessary to compare all four strategies in a traditional clinical study, we performed a cost-effectiveness analysis (CEA). Our objective was to evaluate and compare the relative costs and benefits of using video capsule endoscopy compared to other strategies in ED patients for presenting with acute upper GI hemorrhage.
Methods
Overview
Our model examined the cost-effectiveness of strategies to evaluate a 65 year-old patient who presents to the ED with suspected hematemesis from an upper GI bleed. Age 65 years was chosen because that is the mean age of patients presenting to the ED with upper GI bleeds with a moderate to high risk of hemorrhage (Glasgow-Blatchford score >0) which represents over 90% of all patients with suspected upper GI bleeds.7-10 We tested two scenarios with mild and moderate risks of requiring invasive hemostasis intervention (i.e, endoscopic, surgical, etc.) Our model assumed that the suspected upper GI bleed requires further evaluation and that the four diagnostic strategies were available: (1) direct imaging with video capsule endoscopy performed in the ED, (2) risk stratification using the Glasgow-Blatchford score, (3) nasogastric tube placement and finally, (4) an admit-all strategy in which every patient suspected of an upper GI hemorrhage is admitted to hospital. For each strategy, test characteristics were incorporated to identify whether a high risk lesion (a lesion requiring invasive hemostatic intervention) was present on subsequent traditional endoscopy.
Model Structure and Scenarios
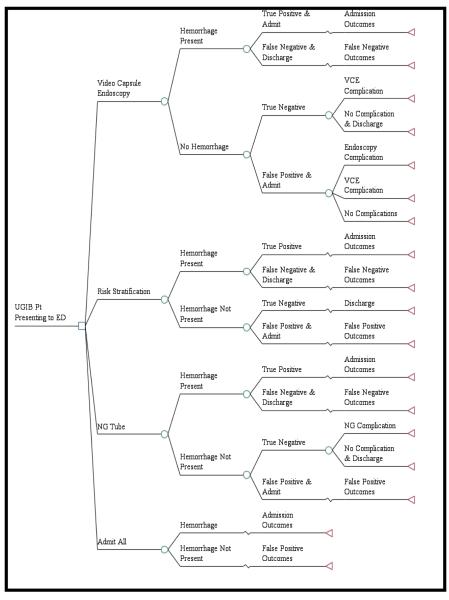
We constructed our model using standard decision analysis software (TreeAge 2013, Williamstown, MA) commonly used to evaluate decision models and perform sensitivity analyses (Figure 1a). The model estimated costs, quality adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs) for a one year time horizon using a societal perspective. We used a standard willingness-to-pay (WTP) threshold of $50,000 per QALY to compare with the ICERs from each strategy.11,12 The optimal value for a WTP threshold is still uncertain, but some advocates have suggested an increase to a $100,000/QALY threshold.11 However, since there is no current consensus, we used the more conservative $50,000/QALY threshold to evaluate our results.11 This study did not constitute human subjects’ research and was exempt from institutional review board review.
Figure 1a.
Model Schematic
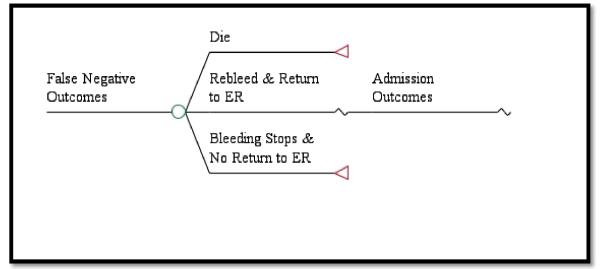
Patients who underwent evaluation with video capsule endoscopy could either have a hemorrhage present or not. If bleeding was suspected after use of the video capsule endoscopy, all patients were assumed to be admitted to the hospital and had a traditional upper endoscopy performed. For patients with a true positive hemorrhage, a potential re-bleed and second endoscopy could occur to evaluate for a source of the bleed and to attempt to control any hemorrhage. A second re-bleed could occur, but at that point, the patient would be taken to surgery for a duodenal or gastric suture. If not controlled, they were assumed to die. Potential outcomes included complications from surgery, endoscopy, nasogastric tube and video capsule endoscopy. Patients with a false negative interpretation of the video capsule endoscopy and who were discharged home could either die, re-bleed and return to the ED, or have the bleeding spontaneously stop without any clinical sequelae without necessitating a return to the hospital. Patients that presented to the ED with a suspected re-bleed then experience the admission outcomes as seen in the true positive branches of our model (Figure 1b).
Figure 1b.
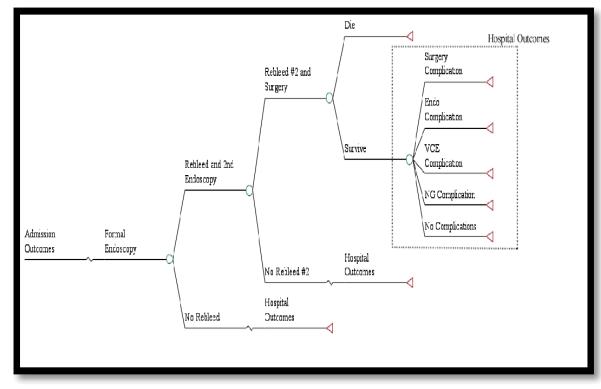
Admission Outcomes
Patients without a hemorrhage had both true negative and false positive outcomes. Those with a true negative and no hemorrhage could either have a complication from the video capsule endoscopy or no complication and subsequent discharge. Those with a false positive and who were admitted to the hospital had a subsequent endoscopy to identify that there was no bleeding source (Figure 1c). Possible outcomes included complications from either the endoscopy or video capsule endoscopy, or no complications at all.
Figure 1c.
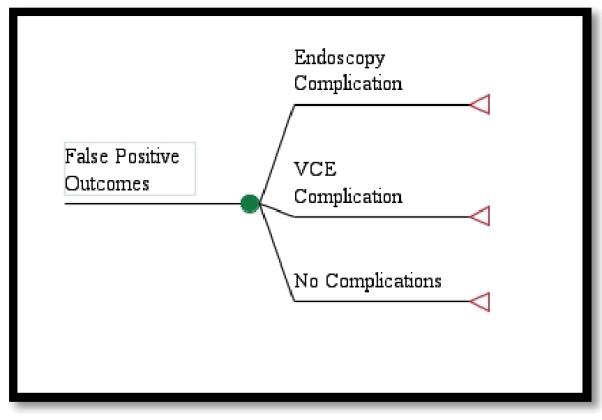
False Positive Outcomes
Risk stratification with Glasgow-Blatchford score resulted in no ED endoscopic testing and admission of the patient if indicated. We assumed that all patients with a Glasgow-Blatchford score of zero were discharged home. In the nasogastric tube branches of our model, patients suspected of having a hemorrhage had a nasogastric tube placed in the ED to evaluate for fresh or coffee grounds blood. If not present, the patient was discharged home and if present, the patient was admitted to the hospital. Lastly, the admit-all strategy was based upon clinician gestalt and all patients with a suspected upper GI hemorrhage were admitted to the hospital for evaluation and subsequent upper endoscopy. Patients allocated to the risk stratification, nasogastric and admit-all strategies had the same potential admission outcomes, false negative outcomes and false positive outcomes as patients allocated to the video capsule endoscopy strategy.
Model Parameters/Input Parameters
Clinical Probabilities
Probabilities and clinically-plausible ranges were obtained from published studies and when necessary, assumptions were made (Table 1). When available, multiple studies were combined to determine mean probabilities for particular events. In the base-case scenario, patients had a Glasgow-Blatchford score of zero with a risk of hemorrhage requiring intervention of 3.4%.13 The additional scenarios tested included a moderate (26.5%) risk of hemorrhage requiring intervention.13 We did not include a test case with a high (89%) risk of hemorrhage because we presumed these patients would all need admission and traditional endoscopy as an inpatient. In the real world, very few high-risk patients will be safe for ED discharge to home no matter what the initial diagnostic test shows.
Table 1.
Base-Case Estimates of Probabilities and Range of Values Used in Sensitivity Analyses
| Variable | Base-Case Estimate | Range | Reference |
|---|---|---|---|
| Complication from EGD | 0.002 | 0.0002 – 0.01 |
Sharma (2007), Ginzburg (2007) Zubarik, 1994 |
| Complication from NG tube placement |
0.001 | 0.0001 – 0.006 | Gough (1986)…Aronchich (1984) |
| Complication from VCE | 0.0001 | 0 – 0.014 | Li, 2008 De Franchis, 2008 |
Hemorrhage Risk
|
0.034 0.27 0.89 |
0 - 1 | Blatchford (2000) |
| Mortality following discharge for False Negative |
0.001 | 0 – 0.03 |
Stanley (2009)
Courtney, 2004 Chandra, 2012 |
| Mortality from Surgery | 0.04 | 0 – 0.25 | Kuwubara. J Clin Med Res. 2011 |
| Re-bleed after initial bleeding stops |
0.12 | 0 – 0.3 | Jairath, 2012 |
| Re-bleed #2 after first rebleed stops |
0.306 | 0.1 – 0.5 | Jairath, 2012 |
| Re-bleed and Return to ED | 0.2 | 0 – 0.3 | Assumption |
| Sensitivity: NG tube | 0.45 | 0.3 – 0.6 |
Aljebreen (2004), Cuellar (1990), Witting (2004)) |
| Sensitivity: Risk Stratification Low Risk (GBS=0) |
0.99 | 0.7 - 1 |
Blatchford (2000)
Chandra (2012) Courtney, 2004 Chandra, 2012 |
| Sensitivity: VCE | 0.78 | 0.7 – 0.9 |
Rubin (2010)
Meltzer (2013) |
| Specificity: NG tube | 0.72 | 0.5 – 0.9 |
Aljebreen (2004), Cuellar (1990), Witting (2004) |
| Specificity: Risk Stratification Low Risk (GBS=0) |
0.32 | 0.1 – .5 |
Blatchford (2000)
Chandra (2012) Courtney, 2004 Chandra, 2012 |
| Specificity: VCE | 0.92 | 0.7 - 1 |
Rubin (2010)
Meltzer (2013) |
EGD, esophagogastroduodenoscopy; GBS, Glasgow-Blatchford bleeding score; NG, nasogastric; VCE, video capsule endoscopy;
Diagnostic Test Assumptions
Video capsule endoscopy had a sensitivity of identifying a hemorrhage of 0.78 and a specificity of 0.92.4,14 nasogastric tube placement had a sensitivity of 0.45 and a specificity of 0.72.15-17Risk stratification for all patients using a Glasgow-Blatchford score had a sensitivity of 0.99 and a specificity of 0.30.10,13 For the admit-all strategy, while not a diagnostic test, we assumed that the process of admitting a patient was a surrogate for a test and those appropriately admitted (those with a hemorrhage) had the same probability as those requiring intervention for their hemorrhage.
Clinical Probabilities
Complications were assumed to occur at a rate of 0.2%, 0.1% and 0.01% for endoscopy, nasogastric tube placement and video capsule endoscopy, respectively.18,19 Although small, we assumed a rate of death following a discharge despite the patient bleeding of 0.1%.7,10,20 The probability of re-bleeding after initial hemostasis was 12% and of these patients, there was a 30.6% probability of re-bleeding.8 Patients who re-bled for a second time will have surgery to perform a duodenal or gastric suture and there is a risk of mortality from the procedure of 4%.21
Costs
We used 2012 Medicare data for diagnosis related groups (DRGs) and relative value units (RVUs) as surrogates for facility and professional costs, respectively (Table 2). Professional costs were based on the 2012 National Physician FeStudy objective: Acute upper gastrointestinal (GI) hemorrhage is a common presentation in hospital-based emergency departments (EDs). A novel diagnostic approach is to use video capsule endoscopy to directly visualize the upper GI tract and identify bleeding. Our objective was to evaluate and compare the relative costs and benefits of video capsule endoscopy compared to other strategies in low to moderate risk ED patients with acute upper GI hemorrhage.
Table 2.
Cost estimates of the facility & professional fees used in decision model of the video capsule.
| Variable | Facility Fees |
Professional Fees |
Total Base- Case Estimate ($) |
Range($) | CPT & DRG Codes |
Reference |
|---|---|---|---|---|---|---|
| Admit but no bleed found |
20,132 | 314.79 | 20,447 | 10,000 -30,000 |
99222-3, 384 |
PPRVU2012, AORV29 |
| Admission for GI Bleed |
15,287 | 268 | 15,555 | 10,000 – 30,000 |
99222-3, 379 |
PPRVU2012, AORV29 |
| Admission for a GI bleed and complication from EGD |
26,865 | 268 | 27,134 | 20,000 – 40,000 |
99222-3, 379 |
PPRVU2012, AORV29 |
| Admission with Surgical Complication |
72,587 | 500 | 73,087 | 50.000 – 100.000 |
99222-3, 377 |
PPRVU2012, AORV29 |
| Admission with VCE Complication |
26,865 | 268 | 27,134 | 20,000 – 40,000 |
99222-3, 379 |
PPRVU2012, AORV29 |
| Admission without a GI bleed found and complication from EGD |
34,500 | 315 | 34,815 | 30,000 – 40,000 |
99222-3, 384 |
PPRVU2012, AORV29 |
| Complication from NG tube |
34,815 | 315 | 35,129 | 20,000 – 40,000 |
99222-3, 384 |
PPRVU2012, AORV29 |
| ED Visit | 2,000 | 168 | 2,168 | 500 -4,000 | 99285 | Assumption, PPRVU2012 |
| Endoscopy | - | 148 | 148 | 50-500 | 43235 | PPRVU2012 |
| Mortality | 108,874 | 500 | 109,375 | 50,000 – 200,000 |
||
| Surgery after 2nd Rebleed |
1,396 | 1,396 | 500 – 2,500 |
44602 | PPRVU2012 | |
| VCE Performed | - | 766 | 766 | 250 – 1,500 |
91111 | PPRVU2012 |
| VCE Equipment | - | - | 700 | 250 – 1,500 |
N/A | Assumption |
CPT, Current Procedural terminology; DRG, Diagnosis Related Group; ED, emergency department; EGD, esophagogastruoduodenoscopy; GI, gastrointestinal; NG, nasogastric; VCE, video capsule endoscopy;
Methods
We constructed a model using standard decision analysis software to examine the cost-effectiveness of four available strategies for a base-case patient who presents to the ED with either mild or moderate risk scenarios (by Glasgow-Blatchford Score) for requiring invasive hemostatic intervention (i.e., endoscopic, surgical, etc.) The four available diagnostic strategies were (1) direct imaging with video capsule endoscopy performed in the ED, (2) risk stratification using the Glasgow-Blatchford score, (3) nasogastric tube placement and, finally, (4) an admit-all strategy.
Results
In the low-risk scenario, video capsule endoscopy was preferred strategy (cost $5,691, 14.69 QALYs) and dominated all of remaining strategies including nasogastric tube strategy (cost $8,159, 14.69 QALYs), risk stratification strategy (cost $10,695, 14.69 QALYs) and admit-all strategy (cost $22,766, 14.68 QALYs). In the moderate risk scenario, video capsule endoscopy continued to be preferred strategy (cost $9,190, 14.56 QALYs) and dominated admit-all strategy (cost, $22,584, 14.54 QALYs) but no longer dominated nasogastric tube (cost $9,487, 14.58 QALYs, ICER $15,891).
Conclusion
Video capsule endoscopy may be cost-effective for low and moderate risk patients presenting to the ED with acute upper GI hemorrhage.e Schedule for outpatient treatment as well as the mean length of stay for inpatient treatment. Current Procedural Terminology (CPT) code 99222 (initial hospital care) was used for hospital day one and CPT code 99232 (subsequent hospital care) was used for each subsequent complete or partial hospital day.22 CPT code 99285 was used for an ED visit.22 We assumed that complications were one standard deviation beyond the mean charges and mortality was two standard deviations beyond mean charges.23 The following DRGs were used for patients with a GI bleed: 377 (GI Hemorrhage with major complication or co-morbidity (MCC)) and 379 (GI Hemorrhage wo CC/MCC). For patients with false positive admissions, we used DRG 384 (Uncomplicated peptic ulcer w/o MCC). Since DRGs bundle facility costs, we added professional costs into existing DRGs. For each endoscopy CPT code 43235 was used ($148). Surgery used CPT code 44602 ($1,396) and capsule endoscopy used CPT code 91111 ($765). Additionally, although equipment is already bundled in the DRG, we added an additional cost for the video capsule endoscopy equipment of $700 so that we could evaluate how the cost of the equipment would affect decision-making.
Life Expectancy and Disutilities
To determine QALYs, life expectancies and utilities were input into the model (Table 3). The initial life expectancy estimate was obtained from the National Vital Statistics report for 65 year-old patients across all races, ethnicities and genders.24 Since life expectancy represents years that have not yet been lived and exist only in the future, we employed a standard discount rate of 3% to account for the present value of the remaining life years.25
Table 3.
Assumptions for Utility Calculations
| Variable | Base Case Estimate |
Range | Reference |
|---|---|---|---|
| Age (in years) | 65 | 20 – 80 | 31 |
| Discount Rate | 3% | 2 – 4% | 22 |
| Short Term Disutility for Complication from EGD |
0.25 | 0.1 – 0.5 | Assumption |
| Short Term Disutility for Complication from NG Tube Placement |
0.04 | 0 – 0.1 | Assumption |
| Short Term Disutility for Complication from Surgical Complication |
0.3 | 0.1 – 0.5 | Assumption |
| 31Short Term Disutility for Complication from VCE Complication |
0.01 | 0 – 0.1 | Assumption |
| Short Term Disutility for False Negative Discharge |
0.15 | 0.1 – 0.3 | Assumption |
| Short Term Disutility for Hospital Admission for GI Bleed |
0.02 | 0 – 0.05 | Tengs (2000), Sanderock (2002) |
| Short Term Disutility for Hospital Admission without GI Bleed |
0.01 | 0 – 0.05 | Ward (2012) |
| Short Term Disutility for NG Tube Placement |
0.008 | 0 – 0.05 | Assumption |
| Utility of GI Bleed (Active) |
0.77 | 0.7 – 0.9 | Gerson |
EGD, esophagogastruoduodenoscopy; GI, gastrointestinal; NG, nasogastric; VCE, video capsule endoscopy.
Health states were assigned utilities to reflect their corresponding qualities of life. Normal states of health were assigned a value of 1 while death was assigned a value of 0. Disease states ranged in value between 0 and 1 depending upon qualities of life and could either temporarily or permanently reduce quality of life. To reflect disruption of normal life activities, we used a disutility to reflect a decrement in normal quality of life. Short-term disutilities reflect temporary disruption of quality of life (e.g. treatment of a fracture) and are reflected in a subtraction from the overall QALY whereas a long-term disutility represents a permanent change in quality of life (e.g. chronic illness) and are multiplied by the overall quality of life. Given the paucity of literature on the pertinent short-term disutilities, we made assumptions of their values and ran wide sensitivity analyses to assess the robustness of our assumptions on model results. Short-term disutilities were calculated by subtracting the utility from a perfect state of health and dividing by the number of periods in one year for which the patient was affected. For example, we assumed that the utility for a nasogastric tube placement was 0.6, so the disutility is 0.4. We assumed that this would affect the patient for 1 week, so we divided 0.4 by 52 periods and calculated a short-term disutility of 0.008. Additional short-term disutilities were calculated for complication from endoscopy (0.25), complication from nasogastric tube placement (0.04), complication from video capsule endoscopy (0.01), false negative discharge despite a GI bleed (0.15), post-surgical complication (0.3) and GI bleed (0.02).
Sensitivity Analyses
We performed one-way sensitivity analyses using incremental cost-effectiveness ratios (ICER) for all variables in the model in order to evaluate their effect on the decision strategy. Using all variables that altered the decision strategy, two-way sensitivity analyses were conducted to evaluate for potential interaction amongst the variables. We also used probabilistic sensitivity analyses and Monte Carlo Simulation to evaluate the uncertainty caused by parameter assumptions on the model decision strategies.26,27 Monte Carlo simulation creates samples from predetermined probability distributions from probability distributions to assess how a specific assumption affects model output. We ran 1,000 simulations using a validated random number generator with beta distributions given the uncertainty for each variable.28 Input parameters for the distributions came from existing studies.
Both cost-effective and dominant strategies are reported. “Cost-effective” and “dominant” are terms that are applied to the relationship of different strategies. Strategies that are not “dominant” over each-other can be “cost-effective” if the ICER is below the WTP threshold, $50,000/QALY in this study. For example, a strategy that yields a higher cost and higher number of QALYs can be “cost-effective”. A dominant strategy is one in which it is both less costly and more effective (i.e. a higher number of QALYs). A preferred strategy is one in which more than one strategy is cost-effective, but the ICER is lower for the respective strategy.
Results
Main Results
In the base-case scenario with a low-risk by Glasgow-Blatchford score of requiring intervention, video capsule endoscopy had a cost of $5,691 and an effectiveness of 14.69 QALYs (Table 4) and dominated all of the remaining strategies including the nasogastric tube strategy (cost of $8,159 and effectiveness of 14.69 QALYs), the risk stratification strategy (cost of $10,695 and 14.69 QALYs) and the admit-all strategy (cost of $22,766 and 14.68 QALYs). In the moderate risk group, video capsule endoscopy continued to be the preferred strategy (cost of $9,190 and 14.56 QALYs) but no longer dominated nasogastric tube (cost of $9,487 and 14.58 QALYs with an ICER of $15,891). However, the admit-all strategy was dominated (cost of $22,584 and 14.54 QALYs).
Table 4.
Base-case results of a 65 year-old presenting to the Emergency Department with unexplained hematemesis.
| Strategy | Total Cost ($) |
Total Effectiveness (QALYs) |
Incremental Cost ($) |
Incremental Effectiveness (QALYs) |
Incremental C/E Ratio, ICER ($/QALY) |
Dominance |
|---|---|---|---|---|---|---|
| Low Risk | ||||||
| VCE | $5,690.65 | 14.69 | ||||
| NG Tube | $8,159.47 | 14.69 | $2,468.82 | −0.01 | −$379,852.46 | (Dominated) |
| Risk Stratification |
$16,385.75 | 14.69 | $10,695.10 | −0.01 | − $1,283,894.59 |
(Dominated) |
| Admit All | $22,766.39 | 14.68 | $17,075.73 | −0.01 | − $1,462,307.88 |
(Dominated) |
| Moderate Risk | ||||||
| VCE | $9,190.04 | 14.56 | ||||
| NG Tube | $9,486.55 | 14.58 | $296.51 | 0.02 | $15,891.13 | |
| Admit All | $22,584.67 | 14.54 | $13,098.12 | −0.04 | −$294,524.27 | (Dominated) |
EGD, esophagogastruoduodenoscopy; GI, gastrointestinal; NG, nasogastric; ICER, Incremental Cost-Effectiveness Ratio; LP, Lumbar Puncture; QALY, quality adjusted life year; VCE, video capsule endoscopy.
Sensitivity Analyses
One-way sensitivity analyses were performed across all input variables to evaluate their direct effects on the decision strategies and the degrees to which each independent variable had to be modified in order to change the decision strategy (Table 5). Results of the one-way sensitivity analyses are presented by probability of need for intervention following an upper GI hemorrhage (low, moderate and high risk for intervention). The decision strategy changes at a probability threshold of 31% for a hemorrhage requiring intervention. Below 31%, video capsule endoscopy is preferred and above this, nasogastric tube is dominant.
Table 5.
Sensitivity analysis.
| Sensitivity Analysis Range | Probability of Hemorrhage & Intervention | |||||
|---|---|---|---|---|---|---|
| Base-Case Assumption |
Low | High | Low Risk (Glasgow- Blatchford score 0) |
Mod. Risk (Glasgow- Blatchford score 1-5) |
High Risk (Glasgow- Blatchford score ≥6) |
|
| Cost | ||||||
| Admit for GI Bleed |
$15,556 | $10,000 | $30,000 | VCE | nasogastric dom. ≥ $19,912 |
NG |
| Admit and No Bleed Found |
$20,448 | $10,000 | $30,000 | VCE | VCE pref. ≥ $18,413 |
NG |
| Cost of VCE Professional Services |
$766 | $250 | $1,500 | VCE | nasogastric dom. ≥ $1,062 |
NG |
| Cost of VCE Equipment |
$600 | $250 | $1,500 | VCE | nasogastric dom. ≥ $896 |
NG |
| Clinical Probabilities & Test Characteristics | ||||||
| Mortality from Discharge |
0.001 | 0% | 3% | VCE | VCE dom until 1.6% then pref. |
NG dom. until 2.1% then pref. |
| Mortality from Surgery |
4.1% | 0% | 25% | VCE dom until 9% then pref. |
VCE pref until 2.4% then dom. |
NG pref until 1.7% then dom. |
| Hemorrhage Requiring Intervention |
27% | 0% | 100% | <----------≥31% NG dominant----------> | ||
| Re-bleed & Return to ER |
20% | 0% | 30% | VCE | VCE pref. ≥ 5% |
NG |
| Sensitivity: NG | 45% | 30% | 60% | VCE | VCE pref. ≥ 38% |
NG |
| Sensitivity: VCE | 78% | 70% | 90% | VCE | NG dom. ≥ 86% |
NG |
| Specificity: NG | 72% | 50% | 90% | NG pref ≥ 85% |
NG dom. ≥ 74% |
NG |
| Specificity: VCE | 92% | 80% | 100% | VCE | VCE pref. ≥ 91% |
NG |
| Utility | ||||||
| Age of Patient | 65 | 20 | 80 | VCE | VCE dom ≥ 80 |
NG |
| Disutility: NG Tube Placement |
0.008 | 0 | 0.05 | VCE pref until .0015 then dom. |
VCE pref until .027 then dom. |
NG |
Low-Risk
In the low-risk patient, video capsule endoscopy is the dominant strategy. However, when the probability of mortality from surgery is above 9%, video capsule endoscopy goes from the dominant strategy to the preferred strategy. For the specificity of nasogastric tube, video capsule endoscopy is the dominant strategy until 85% then nasogastric tube is the preferred strategy. Lastly, when evaluating the disutility of nasogastric tube placement, video capsule endoscopy is preferred until 0.0015 and then video capsule endoscopy is dominant.
Moderate-Risk
In the moderate risk patient, video capsule endoscopy is preferred when the cost of a GI bleed admission is below $19,912 and above this threshold, nasogastric tube is dominant. For a false positive admission in which no intervenable hemorrhage is identified, the nasogastric tube strategy is preferred until $18,413 and then video capsule endoscopy is preferred. For the cost of video capsule endoscopy professional fees, video capsule endoscopy is preferred until $1,062 above which nasogastric tube is dominant. For the added societal cost of the video capsule endoscopy equipment, video capsule endoscopy is preferred until $896 and then nasogastric tube is dominant. Evaluating clinical probabilities and test characteristics, video capsule endoscopy is dominant until a probability of mortality from a false negative discharge of 1.6% and above this, is the preferred strategy. For the mortality following surgery, video capsule endoscopy is preferred until 2.4% and then dominant. If patients re-bleed and return to the ED above 5%, video capsule endoscopy is the preferred strategy. If the sensitivity of nasogastric tube for detecting hemorrhage is above 38%, video capsule endoscopy is preferred and if the sensitivity of video capsule endoscopy is above 86%, nasogastric tube is dominant. If the specificity of nasogastric is above 74%, nasogastric is dominant and if the sensitivity of video capsule endoscopy is above 91%, video capsule endoscopy is preferred. Evaluating the age of patients, video capsule endoscopy is preferred until age 80 and above this, video capsule endoscopy is dominant. The only disutility to affect the decision strategy was nasogastric tube placement and above a disutility of 0.027 QALYs, video capsule endoscopy switches from preferred to the dominant strategy.
Variables with no effect on decision strategy
Numerous variables did not have an effect on the strategy regardless of level of risk for intervention (Table 6). For costs, these variables include the costs for the admission for a GI bleed with complication, the ED visit, an endoscopy complication with and without a GI bleed, the endoscopy procedure, the mortality, a nasogastric tube complication, the surgery, a surgery complication and a video capsule endoscopy complication. For clinical probabilities and test characteristics, the variables that did not have an effect included the probability of complication from endoscopy, the nasogastric tube insertion and the video capsule endoscopy, the probability of first and second re-bleed and the probability of surgery complication. The test characteristics that did not have an effect on decision strategy included the sensitivity and specificity of risk stratification. When evaluating the discount rate, the tested ranges did not affect the decision strategy. Lastly, multiple disutilities and their test ranges affected the decision model. The tested disutilities included those for admission to the hospital, complication from endoscopy, nasogastric tube and video capsule endoscopy, false negative and discharge from the ED, GI bleed and post-surgical complication.
Table 6.
Variables with no effect on decision strategy.
| Sensitivity Analysis Range | |||
|---|---|---|---|
| Base-Case Assumption |
Low | High | |
| Cost | |||
| Admit for GI Bleed with Complication |
$27,134 | $20,000 | $40,000 |
| ED Visit | $2,168 | $500 | $4,000 |
| EGD Complication in Patient with GI Bleed |
$27,134 | $20,000 | $40,000 |
| EGD Complication in Patient with no GI Bleed |
$34,815 | $30,000 | $40,000 |
| EGD | $148 | $50 | $500 |
| Mortality | $109,375 | $50,000 | $200,000 |
| NG Complication | $35,129 | $30,000 | $40,000 |
| Surgery | $1,396 | $500 | $2,500 |
| Surgery Complication | $73,087 | $50,000 | $100,000 |
| VCE Complication | $27,134 | $20,000 | $40,000 |
| Clinical Probabilities & Test Characteristics | |||
| Complication from EGD | 0.2% | 0% | 1% |
| Complication from NG tube | 0.1% | 0% | 0.6% |
| Complication from VCE | 0.01% | 0% | 1.4% |
| Re-bleed | 11.9% | 0% | 50% |
| Re-bleed #2 | 30.6% | 10% | 50% |
| Sensitivity: Risk Stratification | 99% | 25% | 75% |
| Specificity: Risk Stratification | 32% | 10% | 50% |
| Surgery Complication | 10.5% | 0% | 30% |
| Utilities | |||
| Discount Rate | 3% | 2% | 4% |
| Disutility: Admit | 0.01 | 0 | 0.05 |
| Disutility: Complication from EGD | 0.25 | 0.1 | 0.5 |
| Disutility: Complication from NG Tube |
0.04 | 0 | 0.1 |
| Disutility: Complication from VCE | 0.01 | 0 | 0.1 |
| Disutility: False Negative and Discharge |
0.15 | 0.1 | 0.3 |
| Disutility: GI Bleed | 0.02 | 0 | 0.05 |
| Disutility: Post-Surgical Complication |
0.3 | 0.1 | 0.5 |
Two-Way Sensitivity Analyses
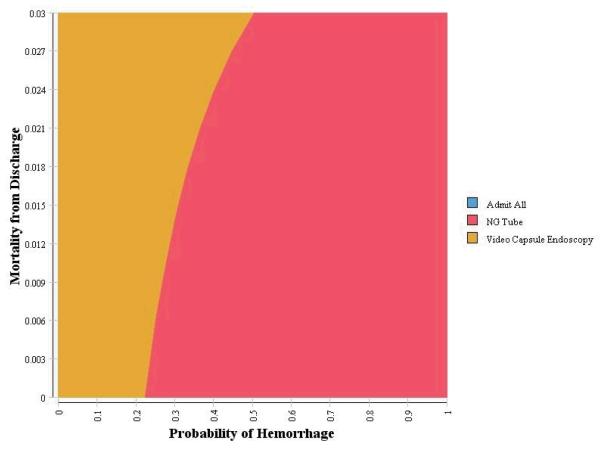
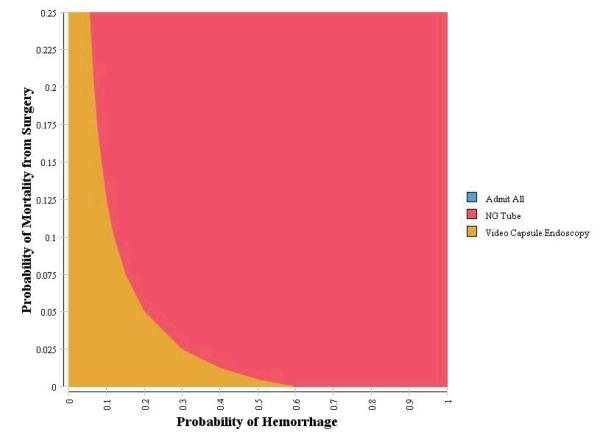
Two-way sensitivity analyses were conducted using the clinical probability and test characteristic variables identified in the one-way sensitivity analyses as having an effect on the decision strategy using a WTP of $50,000/QALY. Two non-linear relationships were identified with the probability of hemorrhage: mortality from discharge and mortality from surgery. First, as the probability of hemorrhage increases, there is a non-linear increase in the threshold to switch from video capsule endoscopy to nasogastric. Second, as the probability of hemorrhage increases, there is a non-linear decrease in the threshold to switch from video capsule endoscopy to nasogastric for probability of mortality from surgery (Figure 2a and 2b).
Figure 2a.
Sensitivity Analysis
Figure 2b.
Sensitivity Analysis
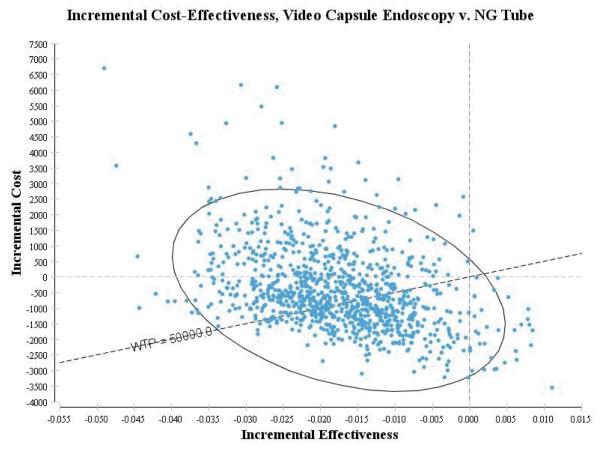
Probabilistic Sensitivity Analysis
Results of a 1,000 replication Monte Carlo simulation for a Glasgow-Blatchford score of 0 demonstrated that the video capsule endoscopy strategy had a mean cost of $5,643 (95% CI $4,052.23, $9,435.88) and mean effectiveness of 14.69 QALYs (95% CI 14.68, 14.7), risk stratification strategy with a mean cost of $16,382 (95% CI $16,298, $16,483) and mean effectiveness of 14.69 QALYs (95% CI 14.67, 14.70), the nasogastric strategy had a mean cost of $8,277.79 (95% CI of $7,176, $9,401) and a mean effectiveness of 14.69 (95% CI 14.68, 14.70) and the admit-all strategy had a mean cost of $22,767 (95% CI $22,737, $22,790) with a mean effectiveness of 14.68 QALYs (95% CI 14.67, 14.69). For a Glasgow-Blatchford score of 1-5, the video capsule endoscopy strategy had a mean cost of $9,124 (95% CI $7,412, $12,165) with a mean effectiveness of 14.57 QALYs (95% CI 14.53, 14.6). The nasogastric strategy had a mean cost of $9,544.7 ($8,707, $10,455) and mean effectiveness of 14.58 QALYS (14.56, 14.61) and admit-all strategy had a mean cost of $22,589 (95% CI $22,408, $22,771) with mean effectiveness of 14.54 QALYs (95% CI 14.5, 14.57). A figure depicting the simulations for patients with a moderate risk of hemorrhage is shown in Figure 3.
Figure 3.
Probability of Sensitivity Analysis
DISCUSSION
In this study, we explored the cost-effectiveness of various ED-based approaches to risk-stratify patients with signs and symptoms of acute upper GI hemorrhage and found that using video capsule endoscopy was the dominant strategy for both low-risk and moderate-risk populations. This finding is primarily driven by the favorable test characteristics of video capsule endoscopy compared to the other strategies, where many patients without need for intervention can be safely discharged home without incurring the costs and potential complications associated with a hospital admission.
Video capsule endoscopy is a new strategy that is not currently used in EDs but has the potential to change the management paradigm of acute upper GI hemorrhage in the ED. Advantages of video capsule endoscopy include patient tolerance of the procedure, the ability to obtain immediate results (using the Real-Time Viewer at the patient’s bedside)29, and the ability to avoid the risks of hospitalization and esophagogastroduodenoscopy which requires conscious sedation.18 Because video capsule endoscopy is not currently available in EDs and is not standard practice, barriers to adoption may include the cost of the equipment, training ED physicians to read it – or establishing a secure infrastructure to transmit images to on-call GI specialists, and the time required to use video capsule endoscopy in a busy ED.
Developing a decision-analytic model is an important step prior to initiating a larger study and prior to widespread implementation of this technology. Our model shows that video capsule endoscopy is cost-effective for low to moderate risk patients despite increased upfront costs compared to using clinical decision rules or using nasogastric tubes because use of video capsule endoscopy in the ED can potentially lead to more patients being safely discharged from the ED. The hospital admission is the single most expensive decision made by an emergency physician. In 2011, 236,000 patients received an esophagogastroduodenoscopy in the hospital with an average hospital stay of 4 days costing $23,549 per patient.1 By comparison, the national average Medicare fee for the video capsule endoscopy is $750 per patient.
We do not know if the results of video capsule endoscopy will impact admission decisions in a real-world setting. Further studies with larger sample sizes will be needed to confirm the sensitivity of the test for both the presence of fresh and/or coffee ground blood and for detecting high-risk bleeding lesions. Video capsule endoscopy has not been tested as a means to guide clinical decisions compared to standard of care. In addition, in a prior study, physicians did not discharge patients from the ED despite a reassuring traditional esophagogastroduodenoscopy and, likewise, physicians may not choose to discharge a patient after a reassuring video capsule endoscopy.30 Finally, the video capsule endoscopy also does not replace the need for traditional esophagogastroduodenoscopy when hemostasis or biopsy is needed.
In the low risk patients, defined by a Glasgow-Blatchford score of zero, video capsule endoscopy was shown to be the preferred method of risk-stratification. Using clinical decision rules as a strategy to discharge low-risk patients has been validated in the United Kingdom.7 However, survey studies have shown that there is very poor uptake by US physicians of this rule and some limitations to its use in US settings.10,14,31 As a result, the Blatchford score has demonstrated limited impact on clinical care. In addition, the low specificity of the Blatchford score means that most patients with suspected upper GI hemorrhage are typically still admitted. Finally, an endoscopic view (using traditional esophagogastroduodenoscopy) of patients who qualify as very low risk may still be desired by physicians and patients.
The nasogastric tube is notorious for being the single most disliked procedure by ED patients.3 By contrast, the video capsule endoscopy was well-tolerated by 96% of ED patients.14 In addition to being well-tolerated, the use of video capsule endoscopy could allow videos to be transmitted electronically to an off-site gastroenterologist when advanced interpretation is needed. This feature of video capsule endoscopy could be beneficial in rural EDs or in community hospitals that do not have gastroenterology services immediately available. Similar to other diagnostic modalities such as radiography, electrocardiogram and ultrasound, a video capsule endoscopy could be initially interpreted by emergency physicians but then formally read by GI specialists while emergency physicians develop comfort with the procedure and the interpretation. A potential scenario is that emergency physicians provide a “wet read” regarding the presence or absence of fresh blood while gastroenterology physicians provide an interpretation to more detailed endpoints.
There were several limitations to our study. First and most importantly, this was a decision model that used assumptions from published data; however, the model used clinical estimates where published data were not directly available. Some assumptions were based on studies with varying sample populations, particularly studies of video capsule endoscopy in the ED. Since our analyses used risk percentages and test characteristics that were based on studies with varying degrees of uncertainty in the form of their confidence intervals, To minimize the effect of this uncertainty, we used sensitivity analyses (one-way, two-way and probabilistic sensitivity analyses) to attempt to adjust for this uncertainty. In particular we used probabilistic sensitivity analysis to adjust for the relative imprecision of the estimates for sensitivity and specificity of video capsule endoscopy. However, not all of the variables had data to be able to use in the probabilistic sensitivity analysis limiting our understanding of the effect of uncertainty on our estimates. We also restricted the analysis and admission decisions specifically to upper GI hemorrhages. In clinical practice, patients presenting with upper GI hemorrhages may be admitted to the hospital for other reasons (i.e. individual or system preferences) that are not directly related to the hemorrhage itself such as concerning symptoms, or concurrent active or co-morbid disease. In addition, there is limited available literature that describes the risk of death or serious negative outcome for a missed GI bleed in a patient discharged from the Emergency Department which may have resulted in cost being primary driver of model. In addition, for our model, the assumption that high-risk patients would be discharged from the ED is clinically highly unlikely so we elected to exclude this scenario from our analysis. The use of decision modeling may also not reflect actual clinical decisions by providers due to differences in risk tolerance, local standards of care, and the availability of video capsule endoscopy testing. Charge was obtained from Medicare charge databases for DRGs and CPT codes and served as a proxy for hospital and physician costs. These data are reflective of care provided to disabled patients and those above 65 years-old. Our base-case scenario involved a 65 year-old patient and the costs used may not be an exact reflection of the actual costs for each of these patients. Lastly, alternative data sources may serve as a better proxy for patient-specific conditions and costs.
In conclusion, there is a need for new diagnostic methods for upper GI hemorrhage without having a specialist at the bedside. Many investigators have sought a better way to risk stratify patients with suspected upper GI hemorrhage both with and without an esophagogastroduodenoscopy. 32-36 We have shown that video capsule endoscopy may be cost-effective for low and moderate risk patients presenting to the ED with acute upper GI hemorrhage. The high sensitivity of video capsule endoscopy makes this test a promising target for future studies including a randomized controlled trial comparing its use to standard of care. Future studies will determine the utility of video capsule endoscopy to safely guide clinical decision making and determine how the use of video capsule endoscopy compares with the current standard of care in the acute ED setting.
Figure 1d.
False Negative Outcomes
Acknowledgments
Source of support: Investigator-initiated Grant from Given Imaging, LTD.
Footnotes
Meetings: To be presented at American College of Emergency Physicians Scientific Assembly. October 2013, Seattle WA.
Author contributions: All authors (ACM, IMG, MAW, JMP) contributed significantly to study conception, data analysis, manuscript preparation including writing and editing for this piece of scholarship.
References
- 1.AHRQ Weighted national estimates from HCUP nationwide emergency department sample (NEDS) 2008.
- 2.Crooks C, Card T, West J. Reductions in 28-day mortality following hospital admission for upper gastrointestinal hemorrhage. Gastroenterology. 2011;141:62–70. doi: 10.1053/j.gastro.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer AJ, Richman PB, Kowalska A, et al. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33:652–658. [PubMed] [Google Scholar]
- 4.Rubin M, Hussain SA, Shalomov A, et al. Live view video capsule endoscopy enables risk stratification of patients with acute upper GI bleeding in the emergency room: A pilot study. Dig Dis Sci. 2010:1–6. doi: 10.1007/s10620-010-1336-9. [DOI] [PubMed] [Google Scholar]
- 5.Gralnek IM, Ching JYL, Maza I, et al. Capsule endoscopy in acute upper gastrointestinal hemorrhage - A prospective cohort study. Endoscopy. 2012 doi: 10.1055/s-0032-1325933. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer AC, Ali MA, Kresiberg RB, et al. Video capsule endoscopy in the emergency department: A prospective study of acute upper gastrointestinal hemorrhage. Ann Emerg Med. 2013 doi: 10.1016/j.annemergmed.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: Multicentre validation and prospective evaluation. Lancet. 2009;373:42–47. doi: 10.1016/S0140-6736(08)61769-9. [DOI] [PubMed] [Google Scholar]
- 8.Jairath V, Barkun AN. Improving outcomes from acute upper gastrointestinal bleeding. Gut. 2012;61:1246–1249. doi: 10.1136/gutjnl-2011-300019. [DOI] [PubMed] [Google Scholar]
- 9.Chen IC, Hung MS, Chiu TF, et al. Risk scoring systems to predict need for clinical intervention for patients with nonvariceal upper gastrointestinal tract bleeding. Am J Emerg Med. 2007;25:774–779. doi: 10.1016/j.ajem.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Chandra S, Hess EP, Agarwal D, et al. External validation of the glasgow-blatchford bleeding score and the rockall score in the US setting. Am J Emerg Med. 2012;30:673–679. doi: 10.1016/j.ajem.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Shiroiwa T, Fukuda T, Tsutani K. Health utility scores of colorectal cancer based on societal preference in japan. Quality of Life Research. 2009;18:1095–1103. doi: 10.1007/s11136-009-9513-z. [DOI] [PubMed] [Google Scholar]
- 12.Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Quality of Life Research. 2003;12:599–607. doi: 10.1023/a:1025119931010. [DOI] [PubMed] [Google Scholar]
- 13.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318–1321. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 14.Meltzer AC, Ali MA, Kresiberg RB, et al. Video capsule endoscopy in the emergency department: A prospective study of acute upper gastrointestinal hemorrhage. Annals of Emergency Medicine. 2012 doi: 10.1016/j.annemergmed.2012.11.008. (in press) [DOI] [PubMed] [Google Scholar]
- 15.Witting MD, Magder L, Heins AE, et al. Usefulness and validity of diagnostic nasogastric aspiration in patients without hematemesis. Ann Emerg Med. 2004;43:525–532. doi: 10.1016/j.annemergmed.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc. 2004;59:172–178. doi: 10.1016/s0016-5107(03)02543-4. [DOI] [PubMed] [Google Scholar]
- 17.Cuellar RE, Gavaler JS, Alexander JA, et al. Gastrointestinal tract hemorrhage. the value of a nasogastric aspirate. Arch Intern Med. 1990;150:1381–1384. doi: 10.1001/archinte.150.7.1381. [DOI] [PubMed] [Google Scholar]
- 18.Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 19.De Franchis R, Eisen GM, Laine L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595–1603. doi: 10.1002/hep.22227. [DOI] [PubMed] [Google Scholar]
- 20.Courtney AE, Mitchell RMS, Rocke L, et al. Proposed risk stratification in upper gastrointestinal haemorrhage: Is hospitalisation essential? Emergency Medicine Journal. 2004;21:39–40. doi: 10.1136/emj.2003.012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwabara K, Matsuda S, Fushimi K, et al. Reappraising the surgical approach on the perforated gastroduodenal ulcer: Should gastric resection be abandoned? J Clin Med Res. 2011;3:213–222. doi: 10.4021/jocmr608w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Services CfMaM . In: National physician fee schedule relative value file. Services USDoHaH, editor. 2009. [Google Scholar]
- 23.Ward MJ, Sodickson A, Diercks DB, et al. Cost-effectiveness of lower extremity compression ultrasound in emergency department patients with a high risk of hemodynamically stable pulmonary embolism. Acad Emerg Med. 2011;18:22–31. doi: 10.1111/j.1553-2712.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- 24.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: Final data for 2006. Natl Vital Stat Rep. 2009:1–134. [PubMed] [Google Scholar]
- 25.Shepard D. In: Cost-effectiveness in Health and Medicine. J Ment Health Policy Econ. Gold MR, Siegel JE, Russell LB, et al., editors. Oxford University Press; New York: 1996. [Google Scholar]
- 26.Briggs A, Sculpher M. An introduction to markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 27.Ades AE, Claxton K, Sculpher M. Evidence synthesis, parameter correlation and probabilistic sensitivity analysis. Health Econ. 2006;15:373–381. doi: 10.1002/hec.1068. [DOI] [PubMed] [Google Scholar]
- 28.Kelton WD, Sadowski RP, Sturrock DT. Simulation with Arena. 4th Edition McGraw-Hill Higher Education; Boston; London: 2007. [Google Scholar]
- 29.Spada C, Riccioni ME, Costamagna G. Rapid® access real-time device and rapid access software: New tools in the armamentarium of capsule endoscopy. Expert Review of Medical Devices. 2007;4:431–435. doi: 10.1586/17434440.4.4.431. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkman DJ, Zaman A, Fennerty MB, et al. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: An effectiveness study. Gastrointest Endosc. 2004;60:1–8. doi: 10.1016/s0016-5107(04)01287-8. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer AC, Burnett S, Pinchbeck C, et al. Rockall and blatchford scores to identify emergency department patients with suspected upper gastrointestinal bleeding who do not need endoscopic intervention. Annals of Emergency Medicine. 2011;Vol. 58(Issue 4):S277. doi: 10.1016/j.jemermed.2012.11.021. Supplement. [DOI] [PubMed] [Google Scholar]
- 32.Bjorkman DJ, Zaman A, Fennerty MB, et al. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: An effectiveness study. Gastrointest Endosc. 2004;60:1–8. doi: 10.1016/s0016-5107(04)01287-8. [DOI] [PubMed] [Google Scholar]
- 33.Chu A, Ahn H, Halwan B, et al. A decision support system to facilitate management of patients with acute gastrointestinal bleeding. Artif Intell Med. 2008;42:247–259. doi: 10.1016/j.artmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Kollef MH, Canfield DA, Zuckerman GR. Triage considerations for patients with acute gastrointestinal hemorrhage admitted to a medical intensive care unit. Crit Care Med. 1995;23:1048–1054. doi: 10.1097/00003246-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Lee JG, Turnipseed S, Romano PS, et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: A randomized controlled trial. Gastrointest Endosc. 1999;50:755–761. doi: 10.1016/s0016-5107(99)70154-9. [DOI] [PubMed] [Google Scholar]
- 36.Cappell MS, Medscape Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7:214–229. doi: 10.1038/nrgastro.2010.24. [DOI] [PubMed] [Google Scholar]