Abstract
Purpose
This phase II single-institution trial of adjuvant thalidomide after cytoreductive surgery (CS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with appendiceal and colorectal malignancies sought to detect an improvement in progression-free survival (PFS) from 7 to 12 months.
Methods
Eligible patients received CS, HIPEC, and baseline imaging, followed by pretreatment thalidomide counseling. All participants were then started on a 28-day regimen of thalidomide, 100 mg by mouth at bedtime, followed by 200 mg for 4 weeks, followed by 300 mg as the final maintenance dose, as tolerated.
Results
Twenty-seven eligible patients (median age 52 years; 52%appendiceal/48%colorectal) were enrolled on this trial and included in the analysis, and 26 were evaluable for response. Eighteen patients demonstrated stable disease on adjuvant thalidomide, while eight showed evidence of progression. Approximately 30 % of the patients withdrew due to toxicity. Grade 3/4 toxicities included neurological disorders (16 %), nausea (12 %), vomiting (8 %), and thromboembolism (8 %). Median overall survival (OS) and PFS were 43.0 and 9.3months, respectively, and median follow-up was 40.4 months. Multivariate modeling showed significant improvements in PFS and OS for appendiceal patients and those with R0 or R1 resections. On an intent-to-treat analysis, the PFS of the study group was 9 months.
Conclusions
Based on these findings, thalidomide cannot be recommended as adjuvant therapy after CS and HIPEC for gastrointestinal malignancies. Further research is needed to identify active agents in this population.
Keywords: Peritoneal carcinomatosis, Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, Thalidomide
Introduction
Peritoneal surface disease (PSD) is seen at an initial presentation of colon cancer in 10 to 15 % of cases [1, 2] and is frequently present with appendiceal tumors. PSD is often associated with a poor quality of life due to symptoms related to mass lesions, obstruction, and/or ascites. Often, PSD can be the sole manifestation of metastatic disease [2, 3].
Standard surgical resection is considered palliative and thereby is limited in scope for patients having gastrointestinal cancers with trans-serosal tumor invasion presenting with malignant ascites or peritoneal tumor studding. This remains the case even in the absence of distant visceral metastatic disease. There is a poor response to debulking alone since persistence of microscopic disease with rapid recurrence is common. Sugarbaker et al. pioneered intraperitoneal chemotherapy combined with cytoreductive surgery (CS) for patients with pseudomyxoma peritonei from mucinous tumors. Their study demonstrated histologic changes of atrophy, degeneration, and reduction in the number of foci of atypical neoplastic epithelium after six cycles of intraperitoneal 5-fluorouracil (5-FU) and mitomycin C [4].
We have reported our experience with CS and hyperthermic intraperitoneal chemotherapy (HIPEC) for both appendiceal neoplasms and colorectal carcinoma [5, 6]. The 5-year overall survival (OS) rate for appendiceal and colorectal tumors was 53 and 26 %, respectively. Unfortunately, the rate of disease progression and recurrence is still high, with a median progression-free survival (PFS) at 1 year of only 40 % and a median time to progression of 7 months (Shen, 2002, unpublished data) after CS and HIPEC for PSD from colorectal cancer. These recurrences are the result of the growth of residual disease, which was present at the conclusion of CS and HIPEC. Currently, there is no standard adjuvant therapy given in combination with CS and HIPEC for patients with PSD from colorectal and appendiceal cancer. We hypothesized that by adding an adjuvant antiangiogenic agent to CS and HIPEC in these high-risk patients, we might improve their time to progression and potentially impact their survival by preventing the growth of these micrometastases.
Traditional anticancer therapies have targeted the tumor cell with agents designed to inhibit the cell’s ability to proliferate. There are several potential advantages to targeting tumor vasculature, a concept known as antiangiogenic therapy [7]. By focusing on the tumor’s blood supply, histologic differences between the tumors, as well as clonal differences within the tumors, may be avoided. This may overcome tumor resistance to therapy as well as the result in therapies that can be more universally applied across tumor types [8, 9]. In order for the tumors to grow from a microscopic focus into a macroscopic lesion, blood vessel formation is required. By impacting on the ability of tumor neovessels to form, it is hoped that the progression of microscopic foci will be halted.
Thalidomide (THALOMID®, Celgene Corporation, Summit, NJ) is an antiangiogenic agent that has been shown in preclinical models to prevent the development of neovascularization [10, 11]. The oral dose of 200–300 mg/day has been otherwise well tolerated in various clinical trials with the most common side effects being somnolence, dizziness, constipation, and peripheral neuropathy. Antiangiogenic agents are likely to show their greatest effect on the development of early neovasculature during the growth of micrometastases. This trial sought to address this clinical scenario by evaluating the effect of oral thalidomide in patients following CS and HIPEC for PSD from colorectal and appendiceal cancer.
Materials and Methods
The study was approved by the Institutional Review Board of Wake Forest University Health Sciences and the Protocol Review Committee of the Comprehensive Cancer Center of Wake Forest University. All patients provided written informed consent prior to enrollment. Between December 2002 and July 2006, 27 eligible patients were registered at Wake Forest University Baptist Medical Center in a phase II trial of adjuvant thalidomide after HIPEC for appendiceal or colorectal cancer. Eligible patients were ages ≥18 years old who had undergone CS and HIPEC for PSD secondary to colorectal or appendiceal cancer with pathologic confirmation, including those with residual disease and those with no evidence of disease post-HIPEC. They were also required to participate and comply with the System for Thalidomide Education and Prescribing Safety (S.T.E.P.S.®). Patients were excluded from the study if they had severe comorbid illness, greater than Grade 1 peripheral neuropathy, history of deep vein thrombosis or pulmonary embolism, extra-abdominal disease, or an active second malignancy other than nonmelanoma skin cancer. Patients with a history of other malignancies must have been disease free for at least 5 years.
Primary and Secondary Objectives
The primary objective of the study was to determine time to progression from surgery for patients with PSD from colorectal or appendiceal cancer receiving CS and HIPEC followed by adjuvant thalidomide. Secondary objectives were to estimate PFS and OS probability and obtain toxicity data for patients receiving adjuvant thalidomide after CS and HIPEC for PSD from colorectal or appendiceal cancer.
Surgical Treatment and HIPEC
The specific details of CS and HIPEC have been described in previous publications [12]. The resection status of patients was determined following CS using the following classification: R0, complete removal of all visible tumor with negative microscopic margins; R1, complete removal of all visible tumor with positive microscopic margins; R2a, minimal residual tumor, nodule(s) ≤0.5 cm; R2b, gross residual tumor, nodule(s) >0.5 cm but ≤2 cm; and R2c, extensive disease remaining, nodule(s) >2 cm.
Surgical complications and deaths were identified and classified according to a system proposed by Clavien and associates [13]. Grading of complications was mainly based on the therapy used to treat it and had been shown to significantly correlate with the complexity of surgery and the length of hospital stay. Eligible patients were given thalidomide as a 28-day supply with female participants required to have a negative urine pregnancy test documented before receiving each 28-day supply. Patients started with thalidomide 100 mg per orem (PO) every hours of sleep (qhs) for 4 weeks, then progressed to 200 mg (PO qhs) for 4 weeks, and then progressed to 300 mg (PO qhs) as the final maintenance dose, as tolerated.
Toxicity/Dose Modifications
Toxicity assessments were performed monthly using the Common Toxicity Criteria (CTC) of the National Cancer Institute Version 2.0.With regard to dose modifications, grade 3 nonhematologic or grade 4 hematologic toxicity resulted in a dose reduction to the previous dose level the patient was receiving safely when the toxicity occurred. If toxicity reversed, patients were returned to the dose of drug in the escalation schema they were receiving prior to the toxicity. If toxicity persisted despite dose reduction, patients were taken off the study at the discretion of the principal investigator. Patients who experienced grade 2 or greater peripheral neuropathy had their dose held until they returned to grade 1 toxicity or their baseline. At that point, they restarted thalidomide at the previous dose level that they were receiving safely before the toxicity occurred. If the patients experienced grade 2 or greater peripheral neuropathy at the previous dose, they were removed from the study at the discretion of the principal investigator. Eelectromygraphy was performed at the discretion of the principal investigator. All patients were placed on a bowel regimen to avoid constipation. Patients who experienced grade 3 constipation refractory to stool softeners and laxatives had their dose held for 2 days and then reduced to the previous dose level they had received safely before the toxicity occurred. If the symptoms did not resolve, patients were taken off the study at the discretion of the principal investigator. Patients that experienced unacceptable levels of sedation on the drug were reduced to the previous dose level the patient was receiving safely when the toxicity occurred. It should be noted that thalidomide was given at bedtime. If the sedation persisted despite dose reduction and there was unacceptable somnolence, the patient was taken off the study. Patients were cautioned not to operate a motor vehicle or heavy machinery if they experienced significant sedation.
Assessments
Patients were seen at 3-month intervals following CS and HIPEC (or every 4 weeks following a dose modification until they have returned to a stable dose of thalidomide). Patients returned to the clinic every 28 days to receive a new prescription of thalidomide. Patients received telephone calls in between monthly visits. At these 3-month follow-up time points, the following were performed: routine staging studies (CT or MRI), serum CEA and CA 19-9 level (if preoperative levels were elevated), CBC, hepatic function panel, serum creatinine, PT, and PTT. CBC and hepatic function panel were also drawn monthly while on dose escalation. Female patients receiving thalidomide who were of childbearing potential were required to have a urine pregnancy test done within 24 h of beginning thalidomide, weekly for the first 4 weeks of treatment, and then every 4 weeks if the patients’ periods are regular or every 2 weeks if they are not.
Statistical Design and Analysis
Sample size calculations were based on an improvement of PFS from 7 to 12 months (67 % increase). The present study was conducted as a phase II single arm trial to provide suggestive evidence that thalidomide as adjuvant therapy after CS and HIPEC was likely beneficial and would merit further study in a multicenter phase III trial or that it was not beneficial, in which case, further development for use in this disease would end. In order to detect a difference between 7 and 12-month median PFS, using a one-tailed alpha=0.05 and 90 % power, an accrual goal of 43 patients was selected. This assumed a 24-month accrual period and 12 months of additional follow-up after the last patient had been entered. Final analysis was performed at least 12 months following the entry of the last patient or when all patients had documented progression.
OS was estimated as time to death from any cause using the Kaplan-Meier method, as well as PFS. Logrank tests were used to test for significant differences in OS and PFS, unadjusted for other patient characteristics. Cox proportional hazards regression was used to test for significant associations of patient and clinical characteristics with OS and PFS. A multivariable model with gender, age at HIPEC surgery, resection type, and primary disease site was estimated. The proportionality assumption was tested based on Schoenfeld residuals and demonstrated adequacy. A two-sided P value less than 0.05 was considered to be statistically significant. All analyses were performed using Stata v11.2 (StataCorp LP, College Station, TX).
Results
Patient Characteristics
Twenty-seven eligible patients were enrolled on this single-institution trial and were included in the analysis. Patient characteristics are summarized in Table 1 along with univariate analysis for PFS and OS. One patient was found to have had a previous CS and HIPEC procedure after enrollment and was therefore ruled ineligible for the study and not included in the analysis.
Table 1.
Patient characteristics (n=27) with univariate analysis of baseline characteristics
| Characteristic |
N (%) or mean±SD |
OS P value |
PFS P value |
|---|---|---|---|
| Age at HIPEC surgery | 52.2±10.5 | 0.842 | 0.226 |
| Sex | 0.482 | 0.652 | |
| Women | 14 (52) | ||
| Men | 13 (48) | ||
| Race | 0.341 | 0.998 | |
| White | 23 (85) | ||
| African-American | 4 (15) | ||
| Primary disease site | 0.028 | 0.009 | |
| Appendiceal | 14 (52) | ||
| Colorectal | 13 (48) | ||
| ECOG performance status | 0.914 | 0.880 | |
| 0 | 13 (48) | ||
| 1 | 9 (33) | ||
| 2 | 5 (19) | ||
| Type of resection | 0.062 | 0.008 | |
| R0, R1 | 13 (48) | ||
| R2 | 14 (52) |
SDstandard deviation, OSoverall survival, PFSprogression-free survival, HIPEC hyperthermic intraperitoneal chemotherapy, ECOG Eastern Co-operative Oncology Group
Responses and Survival
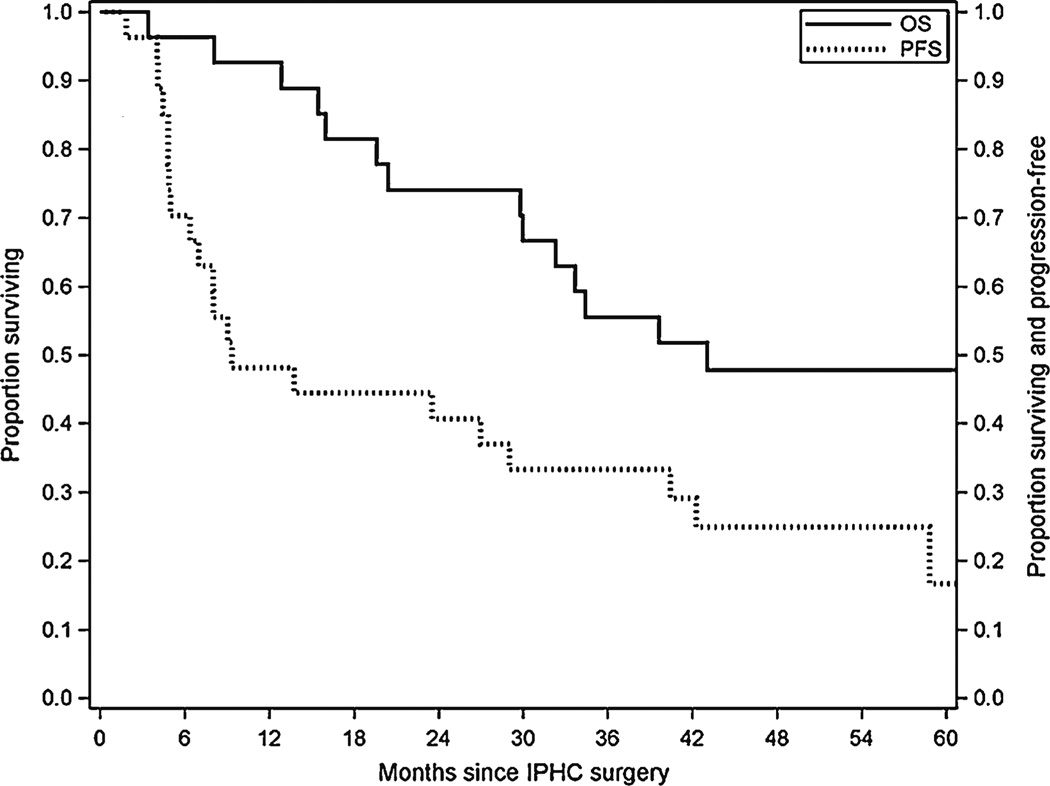
Out of the 27 eligible study patients, 26 were evaluable for response. Eighteen patients had stable disease on adjuvant thalidomide, while eight patients had progressive disease. There were no conclusive partial or complete responses in our study population in the patients with residual disease after CS and HIPEC. The median OS and PFS (Fig. 1) were 43.0 (95 % CI=29.8, N/A) and 9.3 months (95 % CI=5.0, 40.4), respectively. The median follow-up was 40.4 months (range=3.4, 91.7).
Fig. 1.
Kaplan-Meier estimates for overall and progression-free survival
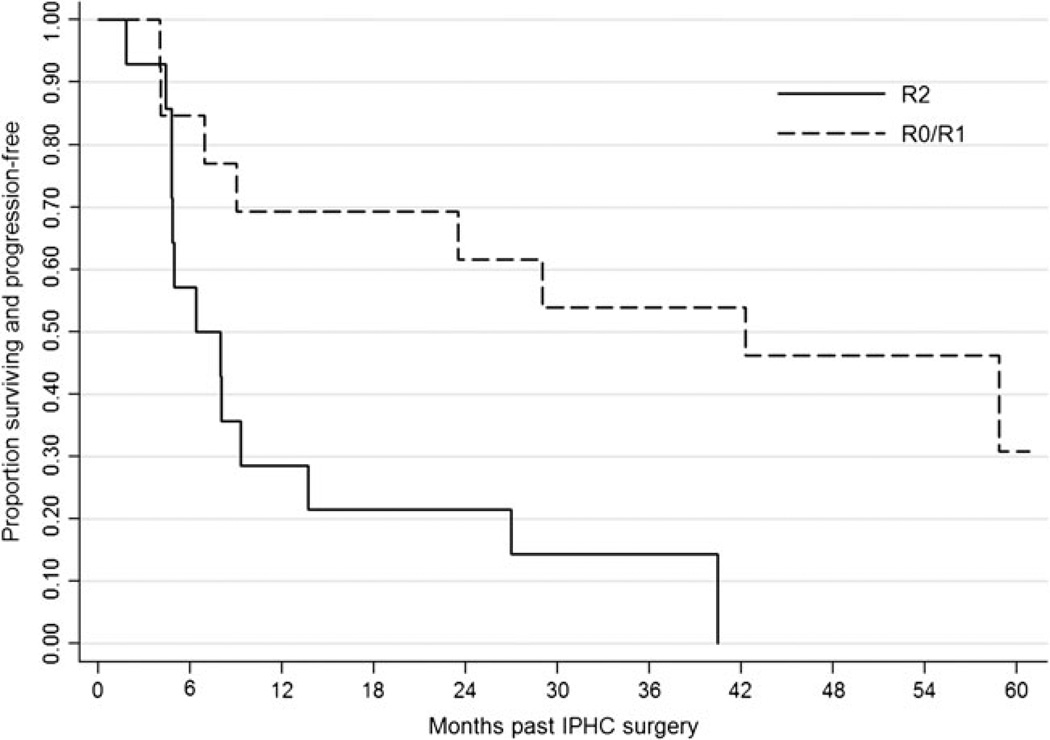
The results from Cox proportional hazards multivariable modeling for PFS and OS are given in Table 2. R0/R1 and R2 patients had a median PFS of 42.3 and 6.4 months, respectively. Median OS was not reached for R0/R1 patients (all OS estimates were >50 %). Median OS for R2 patients was 29.8 months (95 % CI=12.8, 64.9).
Table 2.
Cox proportional hazard multivariable regression for progression-free and overall survival (n=27)
| Characteristic | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Adj HR | 95 % CI | P value | Adj HR | 95 % CI | P value | |
| Primary disease site | ||||||
| Appendiceal | 0.28 | 0.11, 0.73 | 0.009 | 0.27 | 0.09, 0.84 | 0.023 |
| Colorectal (reference) | ||||||
| Type of resection | ||||||
| R0, R1 (reference) | ||||||
| R2 | 4.69 | 1.45, 15.2 | 0.010 | 3.96 | 1.15, 13.7 | 0.030 |
Adj HR adjusted hazard ratio, CI confidence interval, PFS progression-free survival, OS overall survival
The 1-, 3-, and 5-year PFS rates for R0/R1 and R2 patients were 69.2, 53.9, and 30.8 % and 28.6, 14.3, and 0 %, respectively (Fig. 2). The 1-, 3-, and 5-year OS rates for R0/R1 and R2 patients were 100, 69.2, and 61.5 % and 85.7, 42.9, and 34.3, respectively (Online Resource 1).
Fig. 2.
Progression-free survival stratified by resection status
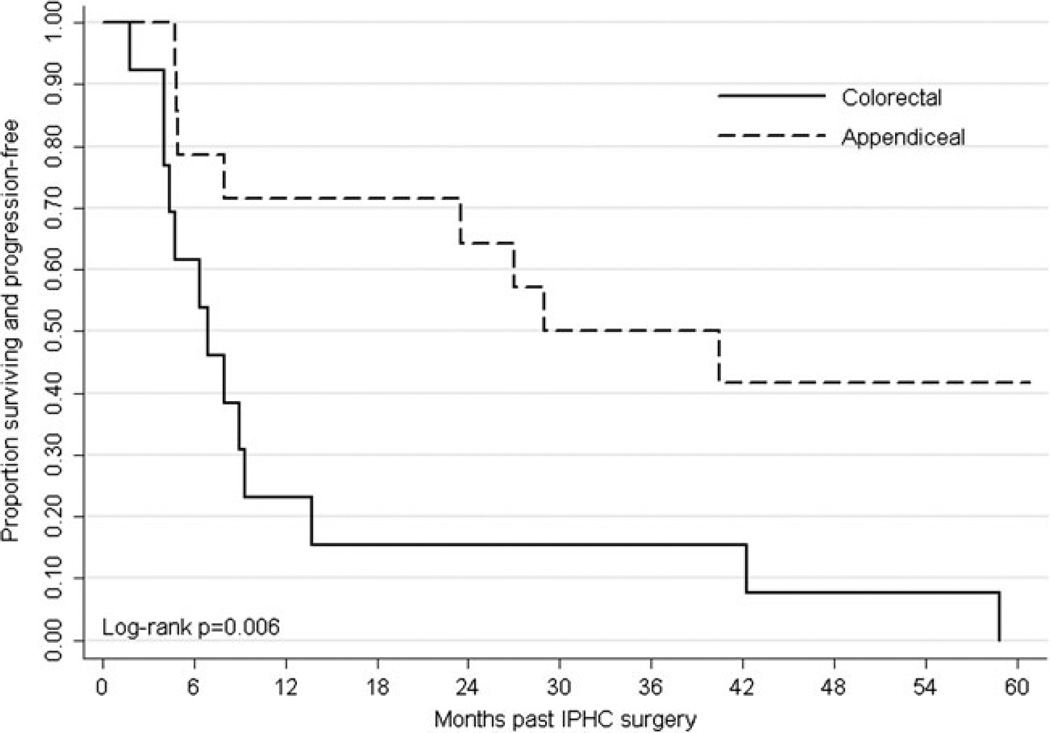
Appendiceal and colorectal patients had a median PFS of 29.0 and 7.0 months, respectively. Median OS time was not reached for appendiceal patients. Colorectal patients had a median OS of 30.0 months. The 1-, 3-, and 5-year PFS rates for appendiceal and colorectal patients were 71.4, 50.0, and 41.7 % and 23.1, 15.4, and 0 %, respectively (Fig. 3). The 1-, 3-, and 5-year OS rates for appendiceal and colorectal patients were 92.9, 78.6, and 71.4 % and 92.3, 30.8, and 23.1 %, respectively (Online Resource 2).
Fig. 3.
Kaplan-Meier estimates for progression-free survival by tumor type
Toxicity
Grade 3/4 toxicities >5 % included thromboembolism (8 %), neurological disorders (16 %), nausea (12 %), and vomiting (8 %) (Table 3). Other grade 3/4 toxicities included constipation, hemorrhage, hypertension, febrile neutropenia, and creatinine, each affecting one patient. Nine patients withdrew due to adverse events (Online Resource 3). Median length of treatment in our study was 155 days (range 26–609). Those patients who withdrew due to drug toxicity compared with those who did not had a median length of treatment of 126 and 179 days, respectively. There were 12 postoperative complications for a morbidity rate of 44 % (12/27). This included two grade 1, six grade 2, and four grade 3 occurrences (Online Resource 4). Twenty-five percent of complications were hematologic related to the HIPEC. There were no postoperative deaths.
Table 3.
Grade 3 and 4 toxicities
| Toxicity | Grade 3 No. |
Grade 4 No. |
|---|---|---|
| Cardiac | 1 | 1 |
| Hypokalemia | 0 | 1 |
| Hypomagnesemia | 1 | 0 |
| Hypoxia | 0 | 1 |
| Thrombosis/embolism | 1 | 1 |
| Constipation | 1 | 0 |
| Dyspnea | 1 | 0 |
| Febrile neutropenia | 1 | 0 |
| Infection (unknown absolute neutrophil count) | 3 | 0 |
| Neuromood | 1 | 0 |
| Neuromotor | 1 | 0 |
| Neurosurgery | 4 | 0 |
| Weight gain | 2 | 0 |
Discussion
Previous studies have demonstrated the benefit of CS and HIPEC in improving disease-free survival and OS in patients with PD from appendiceal and colorectal cancer [5, 14]. Unfortunately, a majority of patients have a disease recurrence despite the use of aggressive CS and HIPEC due to low-volume residual disease. Systemic chemotherapy has been used postoperatively, particularly 5-FU and leucovorin, with reported median survival in the range of only 5–12 months [15, 16]. Traditional systemic chemotherapy for peritoneal malignancies is limited in efficacy due to poor penetration into the peritoneum. In this study, thalidomide was selected for adjuvant therapy based on its antiangiogenic properties, as it could theoretically slow or stop the growth of neovessels associated with residual disease.
The use of thalidomide for peritoneal disease was described in a pilot study of ten patients (seven with ovarian adenocarcinoma, two with papillary serous peritoneal adenocarcinoma of the peritoneum, and one had both) [17]. All patients had failed standard chemotherapy regimens. Doses started at 200 mg/day and increased to 400 mg/day with no concurrent chemotherapy. Two patients went to hospice before completing the study; however, of the eight assessable patients, there was one partial response by the RECIST criteria, and two patients had a biochemical response based on decreasing CA 125 levels. All three responders had peritoneal disease. Another phase I clinical trial also used thalidomide in 17 heavily pretreated patients with recurrent ovarian and primary peritoneal cancers [18]. The initial dose was 100 mg/day with dose escalations up to 1,200 mg/day as tolerated. Grade 1 or 2 side effects had an incidence of 65–76 % while grade 3 or 4 events occurred in 29 % of patients. Three (18 %) patients had a partial response and six (35 %) had stabilization of disease after 6 months.
Experience with thalidomide in gastrointestinal malignancies is limited. A phase II clinical trial examining the effects of thalidomide in refractory progressing metastatic colorectal cancer was published in 2003 [19]. Seventeen patients who had failed the standard chemotherapy regimens were entered. Patients were treated with 200 mg/day with an increase in dose by 200 mg/day every 2 weeks until a final daily dose of 800 mg/day was achieved. One patient was removed from the study due to drug toxicity. There were no objective responses or stable disease. Median OS was 3.6 months. In our series, three of the colorectal patients had received preoperative chemotherapy. Only three (17.6%) of the patients in this study had peritoneal disease. The vast majority had visceral metastatic disease.
Although the primary end point of improved time to progression was not met in our study, the results do show a trend toward longer PFS of 9.3 months compared with the historical value of 7 months. The study did not meet its accrual goal, so one cannot tell if the primary end point would have been reached if all 43 patients had been entered in the study. The trial was closed due to a combination of slow accrual and toxicity from the thalidomide. Thirty-three percent of the patients in this study were taken off the study for apparent grade 3/4 drug toxicities. The most common causes were neurologic side effects (15 %) and thrombosis/embolism (7 %). The toxicity of the study drug may have been exacerbated in this patient population since all subjects had recently undergone a major surgical procedure which may have rendered them more susceptible to the side effects of thalidomide. It is possible that a modified dosing schedule that takes into account the postoperative recovery of these patients may have helped to reduce the patient dropout rate.
The study results did highlight the significant differences in the clinical outcome between patients with appendiceal and colorectal cancer and those patients who received a complete resection of all gross disease compared with those who did not. Appendiceal patients and those with R0/R1 resections had significantly improved PFS and OS. Both factors were independent predictors of PFS and OS in multivariate analysis comparing other demographic and clinicopathologic factors.
Palumbo et al. reviewed the toxicity of thalidomide in patients with multiple myeloma (MM) and reported the frequency and severity of thalidomide side effects are dose-related and time-dependent [20]. Peripheral neuropathy in MM patients usually occurs after prolonged administration of a drug. With regard to the toxicities encountered in our study, 70 % of the patients developed neurologic side effects after 12 months of treatment, although most were grade 1/2. Depression has also been reported by Palumbo et al. [20] and this did occur in one of the patients taken off in our study.
In our study, the median length of treatment was 155 days. Those patients who withdrew due to toxicity had a median length of treatment of 126 days compared with 179 days for those who did not withdraw due to toxicity. No obvious clinical variables were found to predict those who would experience significant toxicity compared with those who did not. The patient numbers were too small to perform logistic regression analysis for significant associations.
Thromboembolic events are one of the most important side effects of thalidomide. The pathogenic mechanisms of deep vein thrombosis (DVT) associated with thalidomide have not been clearly established. Typically the risk of DVT increases when thalidomide is administered with chemotherapy due to endothelial cell injury and the subsequent restoration of endothelial cell PAR-1 expression [21]. When given alone without chemotherapy in newly diagnosed MM patients, the DVT/pulmonary embolism (PE) rate is 3 % [22] compared with our rate of 7 %. The hypercoagulable state induced by surgery and malignancy most likely played a role in the increased incidence in our study population. In MM patients, Palumbo et al. suggested using thromboprophylaxis for those on single-agent thalidomide with other risk factors for DVT/PE. This was not done in our study, although our current practice is to send patients home with two additional weeks of thromboprophylaxis using subcutaneous Lovenox.
Previous studies have shown that PSD from appendiceal cancer typically has a more favorable prognosis and slower progression than PSD associated with colorectal cancer [23, 24]. Our results support this concept as subgroup analysis shows a statistically significant difference in PFS and OS between the two groups, favoring appendiceal cancer. Although there is a different tempo of disease progression between the appendiceal and colorectal groups, both are uniformly fatal without aggressive intervention. As has been demonstrated by the previous studies, PFS and OS were improved in patients undergoing more complete cytoreduction [14, 25, 26].
Over the last several years, the introduction of novel chemotherapy strategies in metastatic colorectal cancer has extended the median OS to approximately 2 years [27]. Patients with PSD represent a challenging population based on the relative inability of chemotherapy to penetrate the peritoneum. A recent study provided evidence that patients with PSD from colorectal cancer had worse outcomes compared with stage IV patients with other metastatic sites in several large cooperative group chemotherapy trials [28]. In addition, the biology of appendiceal tumors is not well understood due to the rarity of the disease. Colorectal cancer with peritoneal-only disease appears to represent a different, more aggressive tumor biology. This can make it difficult to identify the targets for biologic therapy.
Although this study does suggest a possible effect of antiangiogenic therapy in PSD from appendiceal/colorectal cancer, no definitive conclusions can be made. The NSABP C-08 examined the role of adding bevacizumab, another antiangiogenic agent, to modified FOLFOX6 as adjuvant therapy for stage II and III colon cancer in a phase III clinical trial. It was a negative study with the addition of bevacizumab having no significant effect on the primary end point of disease-free survival [29]. Thus, the role of adjuvant therapy using an antiangiogenic approach after CS and HIPEC for metastatic appendiceal and colorectal malignancies remains unclear. Currently, the use of thalidomide in this setting is not recommended outside of a clinical trial. Further trials are needed to address the need for effective adjuvant therapy for patients who undergo CS and HIPEC for PSD from appendiceal and colorectal malignancies.
Acknowledgments
The authors would like to acknowledge the Celgene Corporation for their sponsorship of this clinical trial by providing the drug and funding for this study. The final manuscript was reviewed and approved for publication by the Celgene Corporation.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Contributor Information
Perry Shen, Email: pshen@wakehealth.edu, Surgical Oncology Service, Wake Forest Baptist Health, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Christopher R. Thomas, Piedmont Hematology and Oncology, Winston-Salem, NC, USA
Joyce Fenstermaker, Surgical Oncology Service, Wake Forest Baptist Health, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Mebea Aklilu, Advocate Illinois Masonic Medical Center, Creticos Cancer Center, Chicago, IL, USA.
Thomas P. McCoy, The University of North Carolina at Greensboro School of Nursing, Greensboro, NC, USA
Edward A. Levine, Surgical Oncology Service, Wake Forest Baptist Health, Medical Center Boulevard, Winston-Salem, NC 27157, USA
References
- 1.Dawson LE, Russell AH, Tong D, et al. Adenocarcinoma of the sigmoid colon: sites of initial dissemination and clinical patterns of recurrence following surgery alone. J Surg Oncol. 1983;22:95–99. doi: 10.1002/jso.2930220208. [DOI] [PubMed] [Google Scholar]
- 2.Chu DZ, Lang NP, Thompson C, et al. PSD in nongynecologic malignancy: a prospective study of prognostic factors. Cancer. 1989;63:3643–3667. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16(4) Suppl 6:83–97. [PubMed] [Google Scholar]
- 4.Sugarbaker PH, Landy D, Jaffe G, et al. Histologic changes induced by intraperitoneal chemotherapy with 5-fluorouracil and mitomycin C in patients with PSD from cystadenocarcinoma of the colon or appendix. Cancer. 1990;65:1495–1501. doi: 10.1002/1097-0142(19900401)65:7<1495::aid-cncr2820650708>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Stewart JH, 4th, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13(5):624–634. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 6.Shen P, Stewart JH, 4th, Levine EA. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer with peritoneal surface disease. Curr Probl Cancer. 2009;33:154–167. doi: 10.1016/j.currproblcancer.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333(26):1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 8.Vile R. Cancer therapy. Less blood means more sanguinity. Curr Biol. 1995;5(1):10–13. doi: 10.1016/s0960-9822(95)00004-2. [DOI] [PubMed] [Google Scholar]
- 9.Bikfalvi A. Significance of angiogenesis in tumour progression and metastasis. Eur J Cancer. 1995;31A(7/8):1101–1104. doi: 10.1016/0959-8049(95)00169-j. [DOI] [PubMed] [Google Scholar]
- 10.Or R, Feferman R, Shoshan S. Thalidomide reduces vascular density in granulation tissue of subcutaneously implanted polyvinyl alcohol sponges in guinea pigs. Exp Hematol. 1998;26:217–221. [PubMed] [Google Scholar]
- 11.Kenyon B, Browne F, D’Amato R. Effects of thalidomide and related metabolites in amouse corneal model of neovascularization. Exp Eye Res. 1997;64:971–978. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JH, 4th, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005;12:765–777. doi: 10.1245/ASO.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Calvien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of PSD from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Sadeghi B, Arvieux C, Glehen O, et al. PSD from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Verwaal VJ, Van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with PSD of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 17.Abramson N, Stokes PK, Luke M, et al. Ovarian and papillary-serous peritoneal carcinoma: pilot study with thalidomide. J Clin Oncol. 2002;20:1147–1149. doi: 10.1200/JCO.2002.20.4.1147. [DOI] [PubMed] [Google Scholar]
- 18.Chan JK, Manuel MR, Ciaravino G, et al. Safety and efficacy of thalidomide in recurrent epithelial ovarian and peritoneal carcinoma. Gynecol Oncol. 2006;103:919–923. doi: 10.1016/j.ygyno.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Lago LD, Richter MF, Cancela AI, et al. Phase II trial and pharmacokinetic study of thalidomide in patients with metastatic colorectal cancer. Invest New Drugs. 2003;21:359–366. doi: 10.1023/a:1025485031112. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo A, Facon T, Sonneveld P, et al. Thalidomide for the treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968–3977. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 21.Kaushal V, Kaushal GP, Melkaveri SN, et al. Thalidomide protects endothelial cells from doxorubicin-induced apoptosis but alters cell morphology. J Thromb Haemost. 2004;2:327–334. doi: 10.1046/j.1538-7933.2003.00573.x. [DOI] [PubMed] [Google Scholar]
- 22.Rajkumar SV, Gertz MA, Lacy MQ, et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia. 2003;17:775–779. doi: 10.1038/sj.leu.2402866. [DOI] [PubMed] [Google Scholar]
- 23.Ronnett BM, Yan H, Sugarbaker PH, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous surface disease. Cancer. 2001;92(1):85–91. doi: 10.1002/1097-0142(20010701)92:1<85::aid-cncr1295>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Yan TD, Black D, Savady R, et al. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for PSD from colorectal carcinoma. J Clin Oncol. 2006;24:4011–4019. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 25.Levine EA, Stewart JH, 4th, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–955. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 26.Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.O’Neil BH, Goldberg RM. Innovations in chemotherapy for metastatic colorectal cancer: an update of recent clinical trials. Oncologist. 2008;13:1074–1083. doi: 10.1634/theoncologist.2008-0083. [DOI] [PubMed] [Google Scholar]
- 28.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]