Abstract
Dopamine D2-autoreceptors play a key role in regulating the activity of dopamine neurons and control the synthesis, release and uptake of dopamine. These Gi/o-coupled inhibitory receptors play a major part in shaping dopamine transmission. Found at both somatodendritic and axonal sites, autoreceptors regulate the firing patterns of dopamine neurons and control the timing and amount of dopamine released from their terminals in target regions. Alterations in the expression and activity of autoreceptors are thought to contribute to Parkinson’s disease as well as schizophrenia, drug addiction and attention deficit hyperactivity disorder (ADHD), which emphasizes the importance of D2-autoreceptors in regulating the dopamine system. This review will summarize the cellular actions of dopamine autoreceptors and discuss recent advances that have furthered our understanding of the mechanisms by which D2-receptors control dopamine transmission.
Keywords: Psychostimulants, cocaine, VTA, Substantia Nigra, GPCR
Introduction
The dopamine system is comprised of three major divisions that include the mesocorticolimbic, striatonigral and tuberoinfundibular systems. These systems play critical roles in neuronal actions that range from cognition, locomotion, and reward processing to neuroendocrine function (Missale et al., 1998, Beaulieu and Gainetdinov, 2011). While the majority of dopamine receptors are located on non-dopamine neurons, dopamine receptors (autoreceptors) are also present on dopamine neurons themselves. These autoreceptors play a key role in regulating the dopamine system by providing feedback inhibition that controls cell firing and the synthesis, release, and uptake of dopamine. This review will focus on the cellular actions of dopamine autoreceptors in the mesocorticolimbic and striatonigral systems and discuss the role that these receptors play in regulating activity in the ventral tegmental area (VTA) and substantia nigra (SNc) and controlling the release of dopamine in projection areas.
Dopamine D2-autoreceptors – Location and Behavioral Functions
Dopamine receptors (D1–D5) are members of the large, rhodopsin-like (Class A), seven transmembrane superfamily of G-protein coupled receptors (GPCRs). The five mammalian receptor subtypes are divided into two major groups that form the D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors. Members of the D1-family are located on non-dopamine neurons and stimulate neuronal signaling via Gαs/olf to activate adenylyl cyclase (AC) and increase cAMP levels. In axon terminal regions, the activation of D1-receptors leads to increases in excitability and promotes transitions to the up-state via increases in NMDA receptor, L-type calcium channel and sodium channel currents (Surmeier et al., 2010).
Dopamine D2-like receptors are inhibitory. These receptors couple to Gαi/o to inhibit AC and calcium channels, and activate inhibitory G-protein activated inwardly rectifying potassium channels (GIRK) (Neve et al., 2004, Beaulieu and Gainetdinov, 2011). The majority of D2-like receptors are found on non-dopamine neurons and mediate numerous brain functions, playing major roles in regulating locomotor activity, cognition and motivation (Missale et al., 1998, Beaulieu and Gainetdinov, 2011). As such, D2-receptors are important pharmacological targets for treatment of a variety of psychiatric diseases (Missale et al., 1998, Beaulieu and Gainetdinov, 2011). D2-receptors are found at a high density in the striatum, nucleus accumbens, and olfactory tubercle, and to a lower extent in the hippocampus, amygdala, hypothalamus and cortical regions (Missale et al., 1998, Schmitz et al., 2003, De Mei et al., 2009, Beaulieu and Gainetdinov, 2011).
Autoreceptors on dopamine neurons are comprised of the D2-subtype of dopamine receptors. These autoreceptors are located on the soma and dendrites of midbrain dopamine neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) as well as on their axon terminals in projection areas (Missale et al., 1998, Romanelli et al., 2010, Beaulieu and Gainetdinov, 2011). As feedback regulators, autoreceptors modulate activity directly through the activation of a potassium conductance and indirectly through downstream control of the expression of tyrosine hydroxylase and the plasma membrane dopamine transporter to modulate dopamine dependent transmission. Activation of these receptors decreases both excitability of dopamine neurons and the release of dopamine. Thus, autoreceptors are key regulators of dopamine dependent transmission. While both D2- and D3- receptors are present on dopamine neurons (Rivet et al., 1994, Levant, 1997, Koeltzow et al., 1998, Beaulieu and Gainetdinov, 2011), the D3-receptor likely plays only a minor functional role as an autoreceptor, and the vast majority of autofeedback inhibition is thought to be mediated through the D2-receptor.
Numerous studies have shown that activation of D2-autoreceptors leads to a reduction in locomotion and alters the motivating and reinforcing properties of drugs of abuse including psychostimulants like cocaine and amphetamine (Jackson and Westlind-Danielsson, 1994, Missale et al., 1998). As such, studies that have examined mice genetically lacking D2-receptors (D2-null) have found these animals to have altered extracellular levels of dopamine (Benoit-Marand et al., 2001, Rouge-Pont et al., 2002, Schmitz et al., 2002, Lindgren et al., 2003), and show reduced activity and decreased reinforcement to the rewarding effects of drugs of abuse including ethanol, cocaine and morphine (Baik et al., 1995, Maldonado et al., 1997, Phillips et al., 1998, Cunningham et al., 2000, Chausmer et al., 2002). Detailed reviews of the behavioral actions mediated by autoreceptors can be found in (Jackson and Westlind-Danielsson, 1994, Missale et al., 1998, Beaulieu and Gainetdinov, 2011).
As D2-receptors are found on both the terminals of dopamine neurons and post-synaptically on non-dopamine neurons (heteroreceptors), historically it has been difficult to separate the physiological and behavioral role of autoreceptors from that of heteroreceptors. To distinguish between these two populations, two groups have recently generated conditional knock out mice in which D2-receptors have been deleted only from dopamine neurons (autoreceptor-null) (Bello et al., 2011, Anzalone et al., 2012). These knock out mice exhibit normal levels of D2-heteroreceptors, yet lack D2-autoreceptor mediated inhibition of dopamine release from dopamine terminals and hyperpolarizations measured in the cell body (Bello et al., 2011, Anzalone et al., 2012). Importantly, these animals are hyperactive and exhibit increased sensitivity to cocaine (Bello et al., 2011, Anzalone et al., 2012). This confirms the role of autoreceptors in regulating locomotor and reward-driven behaviors. As different genetic strategies were used to generate the two lines of autoreceptor null mice, there are several differences in the behavior and physiological actions between the two animals (Bello et al., 2011, Anzalone et al., 2012), however, both approaches confirm a key role of autoreceptors in regulating the dopamine system. This work largely supports past clinical observations that altered autoreceptor function correlates with changes in impulsivity and novelty seeking behaviors (Zald et al., 2008, Buckholtz et al., 2010).
Short and Long Isoforms of the D2 Receptor and the Identity of the Autoreceptor
Alternative splicing of the mRNA encoding the D2-receptor results in two isoforms of the receptor, short (D2S) and long (D2L), which differ by 29 amino acids within the third intracellular loop (Bunzow et al., 1988, Dal Toso et al., 1989, Giros et al., 1989, Grandy et al., 1989, Monsma et al., 1989). As the third intracellular loop is involved in G-protein coupling, D2S and D2L can couple to distinct G-proteins (Montmayeur et al., 1993, Senogles, 1994, Guiramand et al., 1995). However, attempts to assign specific signaling roles for each isoform have been difficult due to conflicting results depending on the assay and cell line used (Neve et al., 2004). The D2S receptor has a higher affinity for dopamine and several benzamides (Castro and Strange, 1993, Malmberg et al., 1993) and more effectively inhibits adenylyl cyclase (Montmayeur and Borrelli, 1991). However, differences in the ability of D2S and D2L to activate subsequent downstream signaling cascades such as MAP kinases and Akt/GSK-3β have made it difficult to conclusively assign distinct signaling roles for the two isoforms (Neve et al., 2004), (Romanelli et al., 2010)
The physiological roles for the long and short forms of the D2-receptor have been predicted based on studies using selective D2L knockout mice (Usiello et al., 2000, Wang et al., 2000). In these animals, D2S receptor expression levels are up regulated, replacing the expression of lost D2L receptors (Usiello et al., 2000, Wang et al., 2000). Biochemical and behavioral studies have confirmed that D2L knock-out mice exhibit a loss of responses thought to be associated with post-synaptic striatal dopamine D2-receptors, including D2-receptor antagonist induced catalepsy (Usiello et al., 2000) as well as post-synaptic inhibition of cAMP-regulated phosphoprotein, 32 kDa (DARPP-32), phosphorylation (Lindgren et al., 2003). Mice lacking D2L receptors also exhibit increased consumption of both sugar water and ethanol, suggesting that imbalances in the ratio of D2S to D2L could contribute to increased alcohol drinking (Bulwa et al., 2011). Importantly, despite upregulation of D2S, D2L knock-out mice still exhibit autoreceptor-mediated actions on dopamine soma (hyperpolarization) and pre-synaptic terminals (inhibition of dopamine release) as well as quinpirole-mediated inhibition of locomotor activity and D2-receptor mediated inhibition of tyrosine hydroxylase activity (Usiello et al., 2000, Wang et al., 2000, Centonze et al., 2002, Rouge-Pont et al., 2002, Lindgren et al., 2003). These findings suggest that the D2L isoform is the primary heteroreceptor within the striatum and support earlier findings that the long form is expressed more strongly on medium spiny neurons than on dopamine terminals (Khan et al., 1998). As viral expression of both D2S and D2L in the striatum is sufficient to restore methamphetamine induced locomotor activity (Neve et al., 2013), post-synaptic differences between the two receptors are likely due to the target expression rather than differences in signaling.
While these same studies are often taken to suggest that the D2S isoform is the likely autoreceptor, both D2S and D2L receptors may function as autoreceptors in dopamine cells. In individually isolated SNc neurons, D2L mRNA is expressed in a greater proportion of cells than D2S and the expression of either isoform is able to equally suppress neuronal firing (Jang et al., 2011). Furthermore, in D2 receptor knockout mice, viral mediated restoration of either D2S or D2L in SNc neurons is sufficient to restore D2-receptor mediated inhibitory GIRK currents and D2-receptor mediated somatodendritic transmission (Neve et al., 2013). In conclusion, while it may be that D2L is the principle heteroreceptor, both D2S and D2L may serve as autoreceptors on dopamine neurons to regulate cellular activity and control pre-synaptic dopamine release.
Actions of Dopamine Autoreceptors
Autoreceptors regulate the activity of dopamine cells through several mechanisms. Pre-synaptic autoreceptors on nerve terminals regulate dopamine transmission by inhibiting the probability of vesicular dopamine release (Suaud-Chagny et al., 1991, Benoit-Marand et al., 2001, Phillips et al., 2002), decreasing dopamine synthesis (Kehr et al., 1972, Wolf and Roth, 1990) and altering the uptake of dopamine (Figure 1) (Cass and Gerhardt, 1994, Wu et al., 2002, Truong et al., 2004). Somatodendritic D2-autoreceptors on VTA and SNc neurons inhibit the excitability of dopamine neurons and modulate the firing rate by activating a hyperpolarizing potassium GIRK current (Aghajanian and Bunney, 1977, Lacey et al., 1987, Mercuri et al., 1997, Beckstead et al., 2004, Courtney et al., 2012). Autoreceptor feedback actions have been discussed in several reviews (Missale et al., 1998, Neve et al., 2004, Feuerstein, 2008, De Mei et al., 2009, Beaulieu and Gainetdinov, 2011). The next two sections will summarize what is known about the mechanisms underlying this feedback control and discuss recent work examining how local synaptic mechanisms tightly regulate the duration and extent by which D2-autoreceptors are activated.
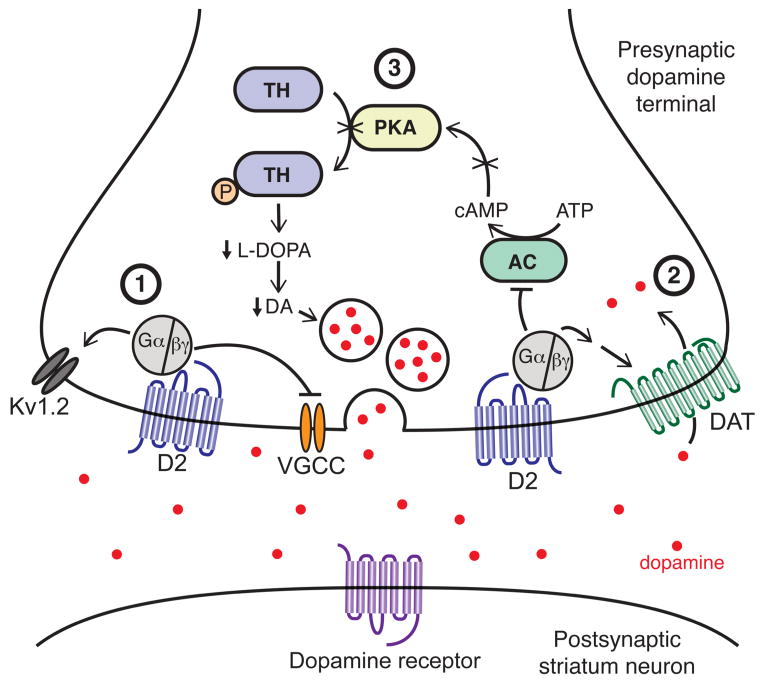
Fig 1.
Schematic illustrating D2-autoreceptor signaling and regulation of dopamine transmission at axon terminals in the striatum. At presynaptic terminals D2-receptors regulate the release, uptake and synthesis of dopamine. (1) Autoreceptors inhibit the probability of dopamine vesicle release by activating Kv1.2 channels and also potentially secondarily by inhibiting calcium entry through of voltage-gated calcium channels (VGCC). (2) Presynaptically D2-receptors increase the rate of dopamine uptake by increasing the plasma membrane expression of DAT and also by direct interactions that increase the activity of existing DATs. (3) Long-term activation of D2-autoreceptors leads to decreased PKA-mediated phosphorylation of TH leading to decreased synthesis and packaging of dopamine in vesicles.
Role of D2-Autoreceptors in Regulating Dopamine Release in the Striatum
Dopamine autoreceptors are present on most axon terminals (Sesack et al., 1994, Delle Donne et al., 1996). The actions of autoreceptors have been best characterized in the striatum and nucleus accumbens. Pre-synaptic feedback inhibition of dopamine release likely plays a major role in regulating dopamine transmission, as dopamine release and signaling is well known to be inhibited by D2-receptor agonists and enhanced by D2-receptor antagonists in vivo (Seeman and Lee, 1975, Stamford et al., 1991, Kennedy et al., 1992).
The selective activation of autoreceptors in vivo has long been thought to arise from these receptors having a much higher affinity for dopamine than heteroreceptors. However, despite this assumption, the affinity of autoreceptors for dopamine has yet to be directly determined with a functional assay. While D2 receptors can exist in both high and low affinity states (Sibley et al., 1982), the high affinity state is found only in membrane preparations in the absence of sodium without the addition of GTP. In sodium-containing solutions with normal concentrations of GTP, agonist binding is in the low affinity (uncoupled) state. As such, studies in intact cells have found that by far the greatest proportion of functionally relevant D2-receptors are in the low affinity state (Sibley et al., 1983, Schoffelmeer et al., 1994, Ford et al., 2009). Accumulating evidence has begun to question the biological actions of the high affinity state of the autoreceptor (Skinbjerg et al., 2012, van Wieringen et al., 2013). Rather than a difference in binding affinity, a large receptor reserve of D2-autoreceptors in the striatum (Yokoo et al., 1988) may instead account for the inhibition of dopamine release that is induced by low concentrations of dopamine agonists.
1) Regulation of Dopamine Release
The major action of D2-autoreceptors is to regulate the exocytotic release of dopamine from axon terminals (Palij et al., 1990, Kennedy et al., 1992, Benoit-Marand et al., 2001, Phillips et al., 2002, Rouge-Pont et al., 2002, Schmitz et al., 2002, Benoit-Marand et al., 2011, Anzalone et al., 2012). Following release, dopamine activates these autoreceptors to decrease the probability of dopamine release to subsequent presynaptic stimulation. The duration of this inhibition varies between in vivo and in vitro studies, but generally occurs over a range from several hundred milliseconds up to several seconds (Benoit-Marand et al., 2001, Phillips et al., 2002), (Schmitz et al., 2002). This transient inhibition may limit excess dopamine release during prolonged bursts of action potentials (Benoit-Marand et al., 2001).
Many Gi/o-coupled GPCRs inhibit transmitter release at pre-synaptic terminals in the central and peripheral nervous system (Wu and Saggau, 1997). A common mechanism by which these receptors regulate transmitter release is via G-protein βγ-mediated inhibition of voltage-gated calcium channels (Herlitze et al., 1996, Ikeda, 1996). D2-receptors inhibit both the P/Q- and N-type calcium channels (Cardozo and Bean, 1995) that are responsible for the calcium entry triggering dopamine release (Phillips and Stamford, 2000). However, several Gi/o coupled GPCRs have been found to inhibit transmitter release independent of Ca2+ channel inhibition in dopamine neurons, including D2-receptor mediated inhibition of glutamate co-release (Congar et al., 2002) and kappa-opioid receptor inhibition of somatodendritic dopamine release (Ford et al., 2007). Other work has shown that autoreceptor inhibition of dopamine release also occurs at steps downstream from calcium entry (Congar et al., 2002). This has led to the suggestion of a novel mechanism mediated through a hyperpolarization via voltage-dependent potassium channels (Kv1.2) (Fulton et al., 2011, Martel et al., 2011). Blocking Kv1.2 channels or using Kv1.2-null mice reduces quinpirole-dependent inhibition of dopamine release in the striatum (Fulton et al., 2011, Martel et al., 2011). Thus, autoreceptors were concluded to induce a local inhibition of voltage-dependent conductance that suppressed dopamine release. Whether this is a conserved mechanism within dopamine neurons remains unknown. Different mechanisms likely regulate dopamine release at axon and dendritic terminals since autoreceptor-mediated inhibition of somatodendritic release occurs by the activation of the G-protein activated inwardly rectifying potassium channels (GIRK), not Kv1.2 channels (Beckstead et al., 2007, Ford et al., 2007). Autoreceptor inhibition therefore likely occurs via different pathways at different terminals.
2) Regulation of Uptake
Following release, clearance of dopamine from the extracellular space is primarily determined by uptake by the dopamine transporter (DAT) (Schmitz et al., 2003, Ford et al., 2010). D2-autoreceptors also regulate dopamine transmission by increasing the activity of DAT (Cass and Gerhardt, 1994, Dickinson et al., 1999, Mayfield and Zahniser, 2001, Schmitz et al., 2002, Wu et al., 2002, Benoit-Marand et al., 2011). This occurs at least partially via an increase in DAT cell surface expression after D2-receptor activation (Mayfield and Zahniser, 2001), and possibly through changes in the voltage dependence of uptake (Sonders et al., 1997), but see (Prasad and Amara, 2001).
While autoreceptors can increase DAT activity when saturating amounts of dopamine are applied, it is not clear whether levels of dopamine in vivo rise to high enough levels to engage this mechanism during periods of physiologically relevant activity. D2-antagonists do not alter the clearance of evoked endogenous dopamine when driven by a single stimulus (Benoit-Marand et al., 2001, Benoit-Marand et al., 2011), and the selective loss of D2-autoreceptors does not alter DAT mediated dopamine uptake (Bello et al., 2011), yet see (Anzalone et al., 2012). This suggests that the tonic background firing of dopamine neurons does not raise the concentration of extracellular dopamine to levels high enough to activate D2-autoreceptor mediated DAT regulation (Kennedy et al., 1992, Beckstead et al., 2007). Autoreceptor mediated acceleration of uptake may therefore only occur during periods of excessive D2-receptor activation or during prolonged trains of stimulation (Benoit-Marand et al., 2011).
3) Regulation of Tyrosine Hydroxylase
A third mechanism by which terminal D2-receptors can change dopamine transmission is through the inhibition of tyrosine hydroxylase (Kehr et al., 1972, Wolf and Roth, 1990). This is a slow, long-lasting mechanism that differs from the sub-second feedback inhibition of dopamine release that occurs during burst firing. Reductions in TH activity likely occur via an inhibition of AC and a reduction in cAMP-PKA mediated phosphorylation in the regulatory domain of TH (Onali and Olianas, 1989, Harada et al., 1996, O’Hara et al., 1996, Lindgren et al., 2001, Anzalone et al., 2012). Down-regulation of tyrosine hydroxylase following prolonged autoreceptor activation leads to reductions in the filling of pre-synaptic dopamine vesicles (Pothos et al., 1998) and alters the distribution and expression of the vesicular monoamine transporter (VMAT) (Truong et al., 2004). Chronic changes in extracellular dopamine levels at axon terminals therefore likely regulate dopamine synthesis and transmission through several overlapping mechanisms.
Role of Autoreceptors in Regulating Dopamine Cell Excitability via Somatodendritic Transmission
Midbrain autoreceptors modulate the firing of dopamine neurons (Bunney et al., 1973, Aghajanian and Bunney, 1977, Lacey et al., 1987, Mercuri et al., 1997) by activating an inwardly rectifying GIRK conductance (Lacey et al., 1987, Kim et al., 1995). While dopamine neurons in the VTA and SNc express both Kir 3.2 (GIRK2) and Kir3.3 (GIRK3) channels (Davila et al., 2003), D2-receptors couple primarily to GIRK2-containing channels (Beckstead et al., 2004). The activation of GIRK channels at resting membrane potentials leads to efflux of potassium, which hyperpolarizes dopamine cells (Figure 2).
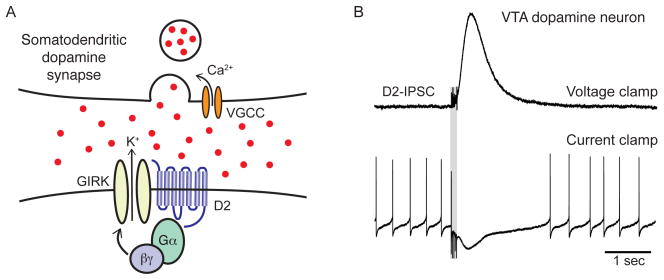
Fig 2.
Somatodendritic activation of D2-receptors mediates an inhibitory post-synaptic current that hyperpolarizes dopamine cells and causes a pause in firing. (A) Schematic illustrating the somatodendritic activation of D2-autoreceptors and GIRK2 channels in the VTA and SNc. GIRK2 channel activation and the associated efflux of potassium occurs though a G-protein βγ-mediated membrane delimited mechanism. (B) A train of extracellular stimuli evokes the local release of dopamine (modified from Courtney et al., 2012). The high concentration of dopamine activating D2-receptors leads to rapid activation of a D2-IPSC that is sufficient to cause a temporary pause in the firing of a VTA dopamine neuron.
Multiple classes of neurotransmitters and neuromodulators, including catecholamines, activate GIRK channels to evoke slow inhibitory post-synaptic potentials (Luscher and Slesinger, 2010). Upon agonist binding, Gi/o coupled GPCRs undergo a conformational change, leading to Gα-subunit exchange of GDP for GTP. In a membrane-delimited manner, the activation of Gα liberates Gβγ subunits that bind to GIRK channels and allow them to open (Pfaffinger et al., 1985, Logothetis et al., 1987, Reuveny et al., 1994, Wickman et al., 1994, Huang et al., 1995). In the resting state, Gi/o G-proteins likely form a pre-coupled macromolecular complex with GIRK channels (Riven et al., 2006). Channel opening occurs via local rearrangement of Gβγ and rotation of GIRK cytoplasmic domains (Riven et al., 2006, Whorton and MacKinnon, 2013). Depending on the receptor, the metabotropic activation of GIRK currents proceeds after a lag of ~50–100 ms (Surprenant and North, 1988, Sodickson and Bean, 1996, 1998, Ford et al., 2009).
Dopamine neuron firing evokes local dopamine release in the VTA and SNc from somatodendritic sites (Geffen et al., 1976, Wilson et al., 1977, Cheramy et al., 1981, Groves and Linder, 1983, Rice et al., 1994, Nirenberg et al., 1996a, Jaffe et al., 1998, Beckstead et al., 2004, Ford et al., 2010, Courtney et al., 2012), and dopamine neurons make lateral synaptic connections between dendrites (Wilson et al., 1977). Somatodendritically released dopamine leads to a robust hyperpolarization of most dopamine neurons and drives a temporary pause in their firing (Beckstead et al., 2004, Beckstead and Williams, 2007, Ford et al., 2010, Bello et al., 2011, Courtney et al., 2012). Autoreceptor feedback is therefore an important regulator in controlling activity and firing patterns of dopamine cells (Beckstead et al., 2004, Courtney et al., 2012). One notable exception to this form of lateral inhibition, however, occurs in mesocortical VTA dopamine neurons that project to the prefrontal cortex. These cells exhibit reduced levels of D2-receptors and GIRK channels and are therefore insensitive to autoreceptor-mediated feedback inhibition (Chiodo et al., 1984, Lammel et al., 2008). Heterogeneity among dopamine neurons in regards to differential expression of DAT, VMAT2 and D2 receptors in subpopulations of TH+ dopamine neurons (Li et al., 2013) may further add to the complexity of somatodendritic signaling by autoreceptors.
The fact that dopamine neurons can release dopamine from somatodendritic sites has been well established (Geffen et al., 1976, Wilson et al., 1977, Cheramy et al., 1981, Groves and Linder, 1983, Kalivas and Duffy, 1991, Benoit-Marand et al., 2011). The mechanisms that have been proposed to regulate somatodendritic release, however, differ depending on the techniques employed. Unlike axon terminal dopamine release, somatodendritic release is thought to exhibit weak dependence upon entry of extracellular calcium and limited sensitivity to pharmacological manipulations (Adell and Artigas, 2004). There are, however, many conflicting reports. With the use of electrochemical techniques such as fast-scan cyclic voltammetry that use a carbon fiber to measure the extracellular concentration of dopamine, there are reports of both calcium-independent (Hoffman and Gerhardt, 1999, Chen and Rice, 2001, 2002, Chen et al., 2006) and calcium-dependent (Jaffe et al., 1998, Ford et al., 2010) forms of release. While differences in the electrochemical methods used to measure extracellular dopamine may have contributed to the this variability, species differences among the animal models account for a significant portion of these differences (Courtney et al., 2012). In rats and mice, both the extracellular concentration of dopamine detected with fast-scan cyclic voltammetry and the physiological activation of somatodendritic D2-autoreceptor mediated inhibitory post-synaptic currents (D2-IPSCs) are steeply dependent on the concentration of extracellular calcium (Courtney et al., 2012). This differs from measurements made in the VTA and SNc of the guinea pig, in which somatodendritic dopamine release was significantly less sensitive to changes in extracellular calcium (Chen and Rice, 2001, Courtney et al., 2012). Therefore, in both rats and mice, dopamine transmission may be regulated by similar mechanisms at both axon and dendritic terminals (Beckstead et al., 2004, Ford et al., 2009, Ford et al., 2010, Courtney et al., 2012).
At somatodendritic sites, both D2-receptors and DATs are found at extrasynaptic sites (Sesack et al., 1994, Nirenberg et al., 1996b). The presence of extrasynaptic autoreceptors has led to the conclusion that dopamine transmission occurs via a spill-over mechanism involving volume transmission (Fuxe and Agnati, 1991). Support for this hypothesis comes from the ability to easily detect electrically evoked dopamine within the extracellular space of the VTA and SNc with carbon-fiber electrochemistry. The long-lasting presence of dopamine, often for several seconds, suggests that low concentrations of dopamine may diffuse for extended distances from somatodendritic release sites (Rice and Cragg, 2008). The role that volume transmission plays in the physiological activation of D2-receptors is not clear. Studies that have examined D2-receptor IPSCs suggest that dopamine transmission likely occurs in a much more localized manner (Beckstead et al., 2004, Beckstead and Williams, 2007, Ford et al., 2009, Ford et al., 2010, Courtney et al., 2012, Gantz et al., 2013). Unlike the repetitive stimulations that are often required to produce slow IPSCs mediated by GABA, D2-IPSCs are readily evoked even by a single stimulus (Beckstead et al., 2004, Ford et al., 2009, Gantz et al., 2013). This suggests that pooling of dopamine in the extracellular space is not required for somatodendritic autoreceptor activation. Furthermore, following a single stimulus, D2-receptor IPSCs activate following a lag of ~50–70ms (Beckstead et al., 2004, Ford et al., 2009). The rapid activation of these autoreceptor currents can only be mimicked by rapid application of a saturating concentration of dopamine (Ford et al., 2009). This indicates that somatodendritic autoreceptors are functionally active at somatodendritic sites in the low affinity state. While low dopamine concentrations (nM to μM) can be detected in the extracellular space with electrochemistry, the physiological concentration at autoreceptors that mediates somatodendritic transmission is at least several orders of magnitude greater (≥ 10 μM) (Ford et al., 2009). The time course of D2-IPSCs is therefore limited by the time course of D2-receptor/G-protein/GIRK signaling and not the duration of the extracellular diffusion of dopamine (Ford et al., 2009, Ford et al., 2010). Thus, dopamine transmission in the VTA and SNc likely occur in a more localized manner rather than a volume transmission mode of signaling.
Recently, spontaneous vesicular release of dopamine has been reported to evoke small spontaneous autoreceptor mediated synaptic events (Gantz et al., 2013). These spontaneous IPSCs are kinetically similar to electrically evoked events suggesting that each is the result of a limited number or single quanta of dopamine activating a local set of post-synaptic D2-receptors (Gantz et al., 2013). As both evoked (Beckstead et al., 2004, Courtney et al., 2012) and spontaneous (Gantz et al., 2013) release of dopamine transiently inhibit the firing of dopamine neurons, these local connections have significant functional effects in regulating the activity of dopamine neurons. Thus, dopamine release from somatodendritic sites can regulate the tonic level of dopamine firing as well as drive pauses in firing that follow bursts.
Plasticity and Regulation of Autoreceptors by Rewards and Drugs of Abuse
The midbrain dopamine system plays a key part in reward related behaviors. Differences in the activity of dopamine neurons are associated with increased vulnerability to drug self-administration (Marinelli and White, 2000). As this increase in vulnerability and impulsivity correlates with reduced midbrain autoreceptor inhibition levels in the VTA (Marinelli and White, 2000, Buckholtz et al., 2010), alterations in autoreceptor function may predispose individuals to increased risk-taking behaviors. This is supported by work showing that rats exhibiting increased responses to novel situations show higher levels of dopamine neuron firing during psychostimulant withdrawal (McCutcheon et al., 2009).
A common property of all drugs of abuse is that they increase the extracellular concentration of dopamine at both axon and somatodendritic terminals (Nestler, 2005, Luscher and Malenka, 2011). Exposure to these drugs of abuse is known to induce long-term changes in the function and morphology of dopamine neurons, and these adaptive changes may contribute to the development of addiction (Luscher and Malenka, 2011, Lammel et al., 2013). While alterations in excitatory glutamatergic inputs to dopamine neurons play a central role in addiction (Saal et al., 2003, Kalivas et al., 2009), alterations in autoreceptor mediated inhibition in the VTA are also known to occur following repeated psychostimulant and alcohol exposure (Henry et al., 1989, Wolf et al., 1993, Jones et al., 2000, Marinelli et al., 2003, Perra et al., 2011, Madhavan et al., 2013).
Unlike the long-term changes in excitatory transmission that occur within the VTA, less is currently known about the mechanisms underlying autoreceptor plasticity. Prolonged activation of D2-receptors via either low frequency electrical stimulation or exogenous application of dopamine drives long-term depression (LTD) at somatodendritic dopamine synapses (Beckstead and Williams, 2007). This form of LTD occurs through a calcium-dependent post-synaptic mechanism involving autoreceptor desensitization (Beckstead and Williams, 2007, Perra et al., 2011). This form of plasticity may promote burst firing in dopamine neurons and may account for changes in dopamine cell activity that occurs following chronic exposure to drugs of abuse. Following withdrawal from chronic ethanol, autoreceptors exhibit increased sensitivity for D2-agonists as well as less desensitization (Perra et al., 2011). This occurs due to the down-regulation of calcium release from intracellular stores and activation of calmodulin-dependent protein kinase II (CaMKII) (Perra et al., 2011). As both drugs of abuse and natural rewards increase dopamine firing and release (Phillips et al., 2003, Tobler et al., 2005, Bromberg-Martin and Hikosaka, 2009, Cohen et al., 2012), changes in chronic food availability also promote D2-receptor desensitization in the midbrain (Branch et al., 2013). Post-synaptic LTD of autoinhibition may therefore be a common mechanism regulating dopamine neuron activity.
Lastly, somatodendritic D2-receptor transmission can undergo long-lasting increases in activity. A single dose of cocaine induces long-term potentiation of spontaneous D2-IPSCs in the SNc dopamine cells (Gantz et al., 2013). This suggests that, like the plastic changes that occur at excitatory glutamatergic synapses on dopamine neurons (Ungless et al., 2001), somatodendritic synapses are also dynamically regulated as a result of even a single exposure to cocaine.
Conclusion
Dopamine receptors have long been known to regulate the activity of the mesocorticolimbic and striatonigral systems, playing a critical role in shaping the duration and extent of dopamine transmission. Recent cellular, synaptic and behavioral studies have begun to untangle the diverse roles that autoreceptors play in regulating dopamine release within the dopamine system. Future studies aimed at furthering our understanding of how dopamine feedback inhibition shapes the profile of dopamine signaling will provide further insight into the behavioral actions directed toward natural rewards and drugs of abuse that are mediated by the dopamine system.
Highlights.
D2-autoreceptors provide feedback inhibition that regulates the activity of the dopamine system
Axonal autoreceptors control the synthesis, release, and uptake of dopamine
Midbrain autoreceptors mediate transmission controlling dopamine neuron firing
This review summarizes the actions of D2-autoreceptors in regulating dopamine signaling
Acknowledgments
Supported by NIH grants DA026417 and DA035821 and the Mt. Sinai Health Care Foundation. I thank Aphroditi Mamaligas for the schematic illustrations along with members of the Ford lab and J.T. Williams for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, Borrelli E. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Ford CP, Phillips PE, Williams JT. Presynaptic regulation of dendrodendritic dopamine transmission. Eur J Neurosci. 2007;26:1479–1488. doi: 10.1111/j.1460-9568.2007.05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J Neurosci. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Ballion B, Borrelli E, Boraud T, Gonon F. Inhibition of dopamine uptake by D2 antagonists: an in vivo study. J Neurochem. 2011;116:449–458. doi: 10.1111/j.1471-4159.2010.07125.x. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013;33:13861–13872. doi: 10.1523/JNEUROSCI.5099-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–126. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulwa ZB, Sharlin JA, Clark PJ, Bhattacharya TK, Kilby CN, Wang Y, Rhodes JS. Increased consumption of ethanol and sugar water in mice lacking the dopamine D2 long receptor. Alcohol. 2011;45:631–639. doi: 10.1016/j.alcohol.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK, Roth RH. Comparison of effects of L-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurones. Nature: New biology. 1973;245:123–125. doi: 10.1038/newbio245123a0. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Cardozo DL, Bean BP. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. J Neurophysiol. 1995;74:1137–1148. doi: 10.1152/jn.1995.74.3.1137. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–263. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Castro SW, Strange PG. Differences in the ligand binding properties of the short and long versions of the D2 dopamine receptor. J Neurochem. 1993;60:372–375. doi: 10.1111/j.1471-4159.1993.tb05863.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Usiello A, Gubellini P, Pisani A, Borrelli E, Bernardi G, Calabresi P. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:723–726. doi: 10.1016/S0893-133X(02)00367-6. [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology. 2002;163:54–61. doi: 10.1007/s00213-002-1142-y. [DOI] [PubMed] [Google Scholar]
- Chen BT, Moran KA, Avshalumov MV, Rice ME. Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca channels contrasted with strong regulation of axonal dopamine release. J Neurochem. 2006;96:645–655. doi: 10.1111/j.1471-4159.2005.03519.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Synaptic regulation of somatodendritic dopamine release by glutamate and GABA differs between substantia nigra and ventral tegmental area. J Neurochem. 2002;81:158–169. doi: 10.1046/j.1471-4159.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congar P, Bergevin A, Trudeau LE. D2 receptors inhibit the secretory process downstream from calcium influx in dopaminergic neurons: implication of K+ channels. J Neurophysiol. 2002;87:1046–1056. doi: 10.1152/jn.00459.2001. [DOI] [PubMed] [Google Scholar]
- Courtney NA, Mamaligas AA, Ford CP. Species differences in somatodendritic dopamine transmission determine D2-autoreceptor mediated inhibition of ventral tegmental area neuron firing. J Neurosci. 2012;32:13520–13528. doi: 10.1523/JNEUROSCI.2745-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacology, biochemistry, and behavior. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH. The dopamine D2 receptor: two molecular forms generated by alternative splicing. The EMBO journal. 1989;8:4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci. 2003;23:5693–5697. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9:53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Donne KT, Sesack SR, Pickel VM. Ultrastructural immunocytochemical localization of neurotensin and the dopamine D2 receptor in the rat nucleus accumbens. J Comp Neurol. 1996;371:552–566. doi: 10.1002/(SICI)1096-9861(19960805)371:4<552::AID-CNE5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ. Presynaptic receptors for dopamine, histamine, and serotonin. Handb Exp Pharmacol. 2008:289–338. doi: 10.1007/978-3-540-74805-2_10. [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol. 2007;97:883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Phillips PE, Williams JT. The time course of dopamine transmission in the ventral tegmental area. J Neurosci. 2009;29:13344–13352. doi: 10.1523/JNEUROSCI.3546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Thibault D, Mendez JA, Lahaie N, Tirotta E, Borrelli E, Bouvier M, Tempel BL, Trudeau LE. Contribution of Kv1.2 voltage-gated potassium channel to D2 autoreceptor regulation of axonal dopamine overflow. The Journal of biological chemistry. 2011;286:9360–9372. doi: 10.1074/jbc.M110.153262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF. Volume transmission in the brain : novel mechanisms for neural transmission. New York: Raven Press; 1991. [Google Scholar]
- Gantz SC, Bunzow JR, Williams JT. Spontaneous inhibitory synaptic currents mediated by a G protein-coupled receptor. Neuron. 2013;78:807–812. doi: 10.1016/j.neuron.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz JC. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, Fischer JB, Burke-Howie KJ, Bunzow JR, Server AC, et al. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989;86:9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- Guiramand J, Montmayeur JP, Ceraline J, Bhatia M, Borrelli E. Alternative splicing of the dopamine D2 receptor directs specificity of coupling to G-proteins. The Journal of biological chemistry. 1995;270:7354–7358. doi: 10.1074/jbc.270.13.7354. [DOI] [PubMed] [Google Scholar]
- Harada, Wu J, Haycock JW, Goldstein M. Regulation of L-DOPA biosynthesis by site-specific phosphorylation of tyrosine hydroxylase in AtT-20 cells expressing wild-type and serine 40-substituted enzyme. J Neurochem. 1996;67:629–635. doi: 10.1046/j.1471-4159.1996.67020629.x. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Gerhardt GA. Differences in pharmacological properties of dopamine release between the substantia nigra and striatum: an in vivo electrochemical study. J Pharmacol Exp Ther. 1999;289:455–463. [PubMed] [Google Scholar]
- Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacology & therapeutics. 1994;64:291–370. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JY, Jang M, Kim SH, Um KB, Kang YK, Kim HJ, Chung S, Park MK. Regulation of dopaminergic neuron firing by heterogeneous dopamine autoreceptors in the substantia nigra pars compacta. J Neurochem. 2011;116:966–974. doi: 10.1111/j.1471-4159.2010.07107.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J Neurochem. 1991;56:961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr W, Carlsson A, Lindqvist M, Magnusson T, Atack C. Evidence for a receptor-mediated feedback control of striatal tyrosine hydroxylase activity. The Journal of pharmacy and pharmacology. 1972;24:744–747. doi: 10.1111/j.2042-7158.1972.tb09104.x. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95:7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Nakajima Y, Nakajima S. G protein-coupled inward rectifier modulated by dopamine agonists in cultured substantia nigra neurons. Neuroscience. 1995;69:1145–1158. doi: 10.1016/0306-4522(95)00326-e. [DOI] [PubMed] [Google Scholar]
- Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, White FJ. Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci. 1998;18:2231–2238. doi: 10.1523/JNEUROSCI.18-06-02231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain structure & function. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci U S A. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Herrera-Marschitz M, Haycock J, Hokfelt T, Fisone G. Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur J Neurosci. 2001;13:773–780. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan A, Argilli E, Bonci A, Whistler JL. Loss of D2 dopamine receptor function modulates cocaine-induced glutamatergic synaptic potentiation in the ventral tegmental area. J Neurosci. 2013;33:12329–12336. doi: 10.1523/JNEUROSCI.0809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- Malmberg A, Jackson DM, Eriksson A, Mohell N. Unique binding characteristics of antipsychotic agents interacting with human dopamine D2A, D2B, and D3 receptors. Mol Pharmacol. 1993;43:749–754. [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology. 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel P, Leo D, Fulton S, Berard M, Trudeau LE. Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PloS one. 2011;6:e20402. doi: 10.1371/journal.pone.0020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Zahniser NR. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol. 2001;59:113–121. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, White FJ, Marinelli M. Individual differences in dopamine cell neuroadaptations following cocaine self-administration. Biol Psychiatry. 2009;66:801–803. doi: 10.1016/j.biopsych.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, McVittie LD, Gerfen CR, Mahan LC, Sibley DR. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342:926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Borrelli E. Transcription mediated by a cAMP-responsive promoter element is reduced upon activation of dopamine D2 receptors. Proc Natl Acad Sci U S A. 1991;88:3135–3139. doi: 10.1073/pnas.88.8.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Guiramand J, Borrelli E. Preferential coupling between dopamine D2 receptors and G-proteins. Mol Endocrinol. 1993;7:161–170. doi: 10.1210/mend.7.2.7682286. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Neve KA, Ford CP, Buck DC, Grandy DK, Neve RL, Phillips TJ. Normalizing dopamine D2 receptor-mediated responses in D2 null mutant mice by virus-mediated receptor restoration: Comparing D2 and D2. Neuroscience. 2013;248C:479–487. doi: 10.1016/j.neuroscience.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Journal of receptor and signal transduction research. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996a;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996b;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara CM, Uhland-Smith A, O’Malley KL, Todd RD. Inhibition of dopamine synthesis by dopamine D2 and D3 but not D4 receptors. J Pharmacol Exp Ther. 1996;277:186–192. [PubMed] [Google Scholar]
- Onali P, Olianas MC. Involvement of adenylate cyclase inhibition in dopamine autoreceptor regulation of tyrosine hydroxylase in rat nucleus accumbens. Neurosci Lett. 1989;102:91–96. doi: 10.1016/0304-3940(89)90313-3. [DOI] [PubMed] [Google Scholar]
- Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL, Humphrey PP. Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res. 1990;509:172–174. doi: 10.1016/0006-8993(90)90329-a. [DOI] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:993–1002. doi: 10.1038/npp.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stamford JA. Differential recruitment of N-, P- and Q-type voltage-operated calcium channels in striatal dopamine release evoked by ‘regular’ and ‘burst’ firing. Brain Res. 2000;884:139–146. doi: 10.1016/s0006-8993(00)02958-9. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Przedborski S, Davila V, Schmitz Y, Sulzer D. D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. J Neurosci. 1998;18:5575–5585. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BM, Amara SG. The dopamine transporter in mesencephalic cultures is refractory to physiological changes in membrane voltage. J Neurosci. 2001;21:7561–7567. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: Rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008 doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA. Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro. Exp Brain Res. 1994;100:395–406. doi: 10.1007/BF02738400. [DOI] [PubMed] [Google Scholar]
- Riven I, Iwanir S, Reuveny E. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron. 2006;51:561–573. doi: 10.1016/j.neuron.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Rivet JM, Audinot V, Gobert A, Peglion JL, Millan MJ. Modulation of mesolimbic dopamine release by the selective dopamine D3 receptor antagonist, (+)-S 14297. European journal of pharmacology. 1994;265:175–177. doi: 10.1016/0014-2999(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Romanelli RJ, Williams JT, KAN . Dopamine Receptor Signaling: Intracellular Pathways to Behavior. In: Neve KA, editor. The Dopamine Receptors. Portland OR: Humana Press; 2010. pp. 137–174. [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Mulder AH, Ronken E, Stoof JC, Drukarch B. Dopamine displays an identical apparent affinity towards functional dopamine D1 and D2 receptors in rat striatal slices: possible implications for the regulatory role of D2 receptors. Synapse. 1994;17:190–195. doi: 10.1002/syn.890170308. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Senogles SE. The D2 dopamine receptor isoforms signal through distinct Gi alpha proteins to inhibit adenylyl cyclase. A study with site-directed mutant Gi alpha proteins. The Journal of biological chemistry. 1994;269:23120–23127. [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, De Lean A, Creese I. Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. The Journal of biological chemistry. 1982;257:6351–6361. [PubMed] [Google Scholar]
- Sibley DR, Mahan LC, Creese I. Dopamine receptor binding on intact cells. Absence of a high-affinity agonist-receptor binding state. Mol Pharmacol. 1983;23:295–302. [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A. Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochemical pharmacology. 2012;83:193–198. doi: 10.1016/j.bcp.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: interactions among multiple receptors. J Neurosci. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. Differential effects of dopamine agonists upon stimulated limbic and striatal dopamine release: in vivo voltammetric data. Br J Pharmacol. 1991;102:45–50. doi: 10.1111/j.1476-5381.1991.tb12130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Ponec J, Gonon F. Presynaptic autoinhibition of the electrically evoked dopamine release studied in the rat olfactory tubercle by in vivo electrochemistry. Neuroscience. 1991;45:641–652. doi: 10.1016/0306-4522(91)90277-u. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res. 2010;183:149–167. doi: 10.1016/S0079-6123(10)83008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, North RA. Mechanism of synaptic inhibition by noradrenaline acting at alpha 2-adrenoceptors. Proc R Soc Lond B Biol Sci. 1988;234:85–114. doi: 10.1098/rspb.1988.0039. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Truong JG, Newman AH, Hanson GR, Fleckenstein AE. Dopamine D2 receptor activation increases vesicular dopamine uptake and redistributes vesicular monoamine transporter-2 protein. European journal of pharmacology. 2004;504:27–32. doi: 10.1016/j.ejphar.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- van Wieringen JP, Booij J, Shalgunov V, Elsinga P, Michel MC. Agonist high- and low-affinity states of dopamine D(2) receptors: methods of detection and clinical implications. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:135–154. doi: 10.1007/s00210-012-0817-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-betagamma G-protein complex. Nature. 2013;498:190–197. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Fifkova E. Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp Brain Res. 1977;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264:249–255. [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo H, Goldstein M, Meller E. Receptor reserve at striatal dopamine receptors modulating the release of [3H]dopamine. European journal of pharmacology. 1988;155:323–327. doi: 10.1016/0014-2999(88)90523-7. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]