Abstract
Purpose
Dasatinib inhibits src family kinases and has anti-angiogenic properties. We conducted a phase I study of dasatinib, capecitabine, oxaliplatin, and bevacizumab (CapeOx/bevacizumab), with an expansion cohort in metastatic colorectal cancer (CRC).
Methods
Patients were enrolled in a dose escalation cohort to establish the maximum tolerated dose (MTD) and the recommended phase II dose (RP2D). Using a “3+3” design, twelve patients with advanced solid tumors received dasatinib (50mg twice daily or 70mg daily), capecitabine (850mg/m2 twice daily, days 1-14), oxaliplatin (130mg/m2 on day 1) and bevacizumab (7.5mg/kg on day1), every 3 weeks. Ten patients with previously untreated metastatic CRC were then enrolled in an expansion cohort. Activated src (srcact) expression was measured by immunohistochemistry, using an antibody that selectively recognizes the active conformation of src (clone 28).
Results
Twenty-two patients were enrolled between June 2009 and May 2011. Two DLTs were observed in the 50mg bid dasatinib cohort, and one DLT was observed in the 70mg daily dasatinib cohort. The MTD and RP2D for dasatinib was 70mg daily. The most common treatment-related adverse events were fatigue (20; 91%) and diarrhea (18; 82%). Biomarker analysis of srcact expression demonstrated that the overall response rate (ORR) was 75% (6/8) for patients with high srcact expression (IHC≥ 2), compared to 0% (0/8) for patients with low srcact expression (IHC 0 or 1); (p =0.007).
Conclusions
The RP2D of dasatinib is 70 mg daily in combination with CapeOx/bevacizumab. High levels of srcact expression may predict those patients most likely to benefit from dasatinib.
Keywords: dasatinib, bevacizumab, metastatic colorectal cancer, src
Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer death worldwide [9]. First line treatment for metastatic disease often includes 5-fluorouracil (5-FU), oxaliplatin, and the VEGF inhibitor bevacizumab. When these therapies are given in combination, response rate (RR) is nearly fifty percent, progression free survival (PFS) is 9-11 months, and overall survival (OS) is approximately two years [15,29]. To improve these results, new therapies—and biomarkers to identify patients likely to benefit—are needed.
Src is a non-receptor protein tyrosine kinase involved in cell adhesion, invasiveness, motility, and angiogenesis [27,35]. Src either assumes a closed, “inactive” conformation, or an open, “activated” state. When src is activated on the plasma membrane inner surface, it interacts with several proteins, including E-cadherin, Focal Adhesion Kinase (FAK), integrins, and STAT-3 [39]. Both src activation and increased src protein expression are directly associated with tumor invasiveness and metastasis [36]. Src expression increases in primary colon tumors compared to benign polyps, and further increases in metastatic lesions compared to primary tumors [3,4,33,34]. In CRC, src expression is associated with adverse clinical outcomes [1].
Dasatinib [SPRYCEL®] is a broad spectrum ATP-competitive inhibitor of BCR-ABL, ephrin (EPH), c-KIT, PDGF receptor β (PDGFRβ), and src family kinases. Dasatinib is approved by the United States Food and Drug Administration (FDA) for the treatment of adults with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL) [32]. In solid tumors, preclinical studies suggest that the combination of dasatinib and oxaliplatin has additive, and possibly synergistic activity. CRC tumors treated with the combination of dasatinib and oxaliplatin demonstrate markedly decreased microvessel activity and increased oxidative stress [21,23]. In addition, colon cancer cell lines with defective src kinases are particularly sensitive to oxaliplatin-induced apoptosis [11]. Src inhibition also decreases VEGF expression in vitro [7]. Based on this observation, dasatinib may prevent increases in plasma VEGF during treatment with bevacizumab, [37] and may enhance the durability of benefit from anti-angiogenic therapies.
In this study, we assessed the safety and tolerability of dasatinib, capecitabine (oral 5-FU), oxaliplatin, and bevacizumab (CapeOx/bevacizumab). The primary objective was to define the maximal tolerated dose (MTD) and the recommended phase II dose (RP2D) of the combination in patients with advanced solid tumors. We then established the safety and tolerability of dasatinib and CapeOx/bevacizumab in a cohort of patients with previously untreated metastatic CRC. Finally, we evaluated the pharmacodynamic properties of dasatinib and CapeOx/bevacizumab on circulating angiogenesis factors, and explored the relationship between activated src (srcact) protein expression and response to therapy.
Patients and Methods
Study Design
This phase I study consisted of two parts. In part 1 (dose escalation), we identified the MTD and RP2D of dasatinib (Bristol-Myers Squibb, Princeton, NJ, USA), capecitabine (Genentech, South San Francisco, CA, USA), oxaliplatin (Sanofi-Aventis, Bridgewater, NJ, USA) and bevacizumab (Genentech, South San Francisco, CA, USA) in patients with advanced solid tumors. A standard phase I “3 + 3” design was used. The MTD was defined around toxicities in the first 21-day cycle; the RP2D was selected based upon toxicities occurring in all cycles. The dose and schedule of each therapy are listed in Table 1. In part 2 (expansion cohort), we assessed the safety, tolerability, and clinical activity of dasatinib, capecitabine, oxaliplatin, and bevacizumab in ten patients with previously untreated metastatic CRC. Dosing for each agent was based on the RP2D from the dose escalation cohort. Treatment was continued for all patients until disease progression, intercurrent illness that prevented further treatment, unacceptable toxicity, patient withdrawal from the study, or general or specific changes in the patient's condition that rendered further treatment inappropriate per judgment of the investigator or treating physician. We then explored the correlation between blood and paraffin biomarkers and clinical outcomes in both cohorts.
Table 1. Dose escalation schema and dose limiting toxicity events.
| Cohort Level | No. of patients in dose cohort (n) | Dasatinib po Days 1-21 | Capecitabine (mg/m2) po bid Days 1-14 | Oxaliplatin (mg/m2) IV Day 1 | Bevacizumab (mg/kg) IV Day 1 | No. of patients with DLT (n) | DLT events |
|---|---|---|---|---|---|---|---|
| -1 | 8* | 70mg daily | 850 | 130 | 7.5 | 1 | • Grade 3 fatigue |
| 1 | 4 | 50mg twice daily | 850 | 130 | 7.5 | 2 | • Grade 2 intolerable nausea† |
| • Grade 3 anorexia† | |||||||
|
| |||||||
| Expansion Cohort | 10 | 70mg daily | 850 | 130 | 7.5 | ||
2 subjects withdrew from the study and were not evaluable for DLT due to clinical progression <2 weeks and oxaliplatin-related infusion reaction.
Unable to receive 85% of scheduled doses for capecitabine and dasatinib.
This multi-center study was conducted at Duke University Medical Center (Durham, NC) and Rocky Mountain Cancer Centers (Denver, CO) after approval by the Institutional Review Boards of both centers. Subject accrual occurred between June 2009 and May 2011. All patients provided informed written consent prior to any study-related procedure. This study was conducted in accordance with guidelines of the Helsinki Declaration. This study is registered with ClinicalTrials.gov (NCT00920868).
Patients
Eligibility in part 1 (dose escalation) required a histologically confirmed metastatic or unresectable solid tumor malignancy. Eligibility in part 2 (expansion cohort) required a histologically documented adenocarcinoma of the colon or rectum that was metastatic or recurrent, and radiographically measurable. No prior treatment for metastatic disease was permitted in the colorectal expansion cohort. Prior adjuvant treatment with a 5-FU/LV or capecitabine based regimen was allowed if it was completed at least six months before study registration. Prior adjuvant therapy can have included oxaliplatin and/or bevacizumab only if completed at least twelve months before study registration. Inclusion criteria for all subjects included age ≥18, Karnofsky performance status (KPS) ≥70%, life expectancy of at least three months, adequate organ and marrow function, and negative pregnancy test in women of child-bearing age. Exclusion criteria for all subjects included anti-cancer therapy within 28 days prior to start of treatment, systolic blood pressure >150 and/or diastolic blood pressure > 100 mmHg, significant cardiovascular disease, history of fistula, history of gastrointestinal perforation, serious non-healing wound, proteinuria at the time of screening, and any other concurrent severe and/or poorly controlled medical condition that could compromise safety of treatment. Patients with known central nervous system (CNS) metastases were excluded.
Safety
The National Cancer Institute Common Toxicity Criteria version 3.0 (NCI-CTC; version 3.0) was used to grade adverse events. The following adverse events were considered DLT in cycle 1: grade 4 neutropenia or thrombocytopenia lasting over 7 days; neutropenic fever grade ≥ 3; nausea, vomiting or diarrhea grade ≥ 3 and lasting ≥ 4 days despite adequate supportive measures; inability to receive at least 85% of scheduled doses of each study drug due to treatment-related toxicity; other non-hematologic toxicity ≥ grade 3; any treatment-related death or treatment-related hospitalization. Anorexia, hand-foot syndrome, hypertension, and neuropathy were considered dose-limiting only if they reached grade 4 or were considered unmanageable.
Clinical and Radiographic Assessment
All patients completed an extensive medical history, physical examination and clinical assessment at baseline, weekly during cycle one, then every three weeks before each treatment. These assessments included vital signs, performance status, complete blood count (CBC), biochemistries (including creatinine, AST, ALT, bilirubin, alkaline phosphatase, albumin, magnesium, calcium, sodium, potassium, chloride, bicarbonate, BUN, glucose, phosphorus, total protein, LDH), and prothrombin time/ partial thromboplastin time/international normalized ratio (PT/PTT/INR). Urine protein to creatinine ratio was obtained every three weeks (each cycle). A 12-lead EKG was obtained at baseline and repeated every three cycles. Serum beta-human chorionic gonadotropin for women of child bearing potential was obtained at baseline and repeated every three cycles. Cardiac ejection fraction and TSH were measured at baseline and every six months.
Enrolled patients were considered evaluable for toxicity if they received any treatment. Patients were evaluable for DLT and MTD determinations if they completed cycle one or experienced a DLT in cycle one. Patients not evaluable for DLT and MTD were replaced. Radiographic disease assessments by either contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis were completed within four weeks prior to the start of therapy and repeated every three cycles (9 weeks). Tumors were evaluated using RECIST criteria (version 1.0).
Biomarkers
Src Immunohistochemistry
Sixteen evaluable patients had pretreatment tumor samples available for analysis. Activated src expression (srcact) was assessed using immunohistochemistry (IHC). Formalin-fixed, paraffin-embedded samples were deparaffinized with three changes of xylene and then rehydrated through a series of ethanol/water solutions (100%, 95%, 70% ethanol, distilled water). Endogenous peroxidase blocking was performed. Antigen retrieval was achieved using citrate buffer, pH 6.1 (Dako, Cat# S1699) at 100 °C for 20 minutes. Primary antibody binding was performed at room temperature for 45 minutes using a 1:100 dilution of antibody directed against the activated conformation of src (Abnova, clone 28). Binding was detected using the Envision+ anti-mouse polymer (Dako, Cat# K4001) for 30 minutes. DAB+ (Dako, Cat# K3468) was utilized for substrate visualization. A GI pathologist who was blinded to patient outcomes graded membranous, cytoplasmic, and nuclear srcact expression using a standard semi-quantitative method. Samples were assigned an IHC score from 0 to 3 (0=none; 1= weak/faint; 2= moderate; 3= strong/circumferential) (see figure 1). Although membranous and cytoplasmic staining were generally concordant (data not shown), we analyzed results from membranous staining, as this was considered most biologically relevant.
Figure 1. (A): Control sample from benign epithelium (IHC Grade 0); (B)-(E) were obtained from tumor specimens of enrolled subjects: B) IHC Grade 0; C) IHC Grade 1; D) IHC Grade 2; E) IHC Grade 3.

Multiplex ELISA assays
EDTA plasma was obtained at baseline and after the third cycle of treatment. Samples were available from fourteen evaluable patients. IL-6, HGF, PAI-1, PDGF-β, PlGF, VEGFR1, VEGFR2, TGFβ-1 and TGFβ-2 were measured using the SearchLight multiplex platform (Aushon Biosystems, Inc., Billerica, MA) according to the manufacturer's protocol. The TβR3 assay was performed as previously described [25]. Briefly, samples were thawed on ice, centrifuged at 20,000 × g for five minutes to remove any residual precipitate and appropriately diluted before placement onto SearchLight plates. Samples and standards were incubated at room temperature for one hour while shaking. Plates were washed three times using an automated plate washer (Biotek Instruments, Inc., Model EL×405, Winooski, VT), biotinylated secondary antibody was added, and plates were then incubated for an additional thirty minutes. After three more washes, streptavidin-HRP was added to the plates, plates were incubated for thirty minutes, washed again, and SuperSignal substrate was added. Images of the plates were taken within ten minutes, followed by image analysis using the SearchLight array analyst software (Version 2.1). Analyte concentrations were calculated based on a 6-point standard curve performed on each plate. Patient samples were tested in duplicate and the mean value was used for analysis.
Statistical Analysis
Analyses were descriptive and exploratory. No formal hypotheses were tested. Toxicities were tabulated by type and grade for all enrolled patients according to dose received. The number and percent of patients achieving complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were summarized. Overall response rate (ORR) was defined as the rate of CR plus PR. Clinical benefit was defined as CR, PR, or SD greater than six months. PFS was defined as the time between enrollment and progression of disease by RECIST or death while on protocol; patients who withdrew from the study for reasons other than progression or death were censored at the time of discontinuation from the study. Kaplan-Meier analyses and plots were used to estimate the PFS of patients treated in the colorectal expansion cohort.
The primary endpoint of the exploratory biomarker analysis was the correlation between membranous srcact expression and ORR. 2-sided Fisher's Exact test was used to evaluate the association between ORR and srcact expression. In addition, changes in plasma angiogenesis factors between baseline and the end of cycle three were described. To evaluate on-treatment changes, L-ratio was calculated using the formula Log2(post-treatment level/baseline level) for each analyte. To determine the significance of L-ratio changes, signed-rank tests were conducted. All biomarker analyses were considered exploratory and not adjusted for multiple testing; for this reason all biomarker p values were considered descriptive.
Results
Patient characteristics
Patient demographics are summarized in Table 2. A total of twenty-two patients were enrolled and received at least one dose of dasatinib, capecitabine, oxaliplatin, and bevacizumab. The median age in this study was 61 years (range 40-74). Of the twenty-two patients enrolled in this study, fifteen patients discontinued treatment due to disease progression, six patients discontinued treatment due to adverse events, and one patient was censored due to resection of liver metastases. Twelve patients were enrolled in the dose escalation cohort. In this cohort, two patients were excluded from assessment of DLT; one patient discontinued therapy due to early disease progression and another patient experienced an oxaliplatin infusion reaction. In the dose escalation cohort, four patients had received no prior treatments for metastatic disease, and four patients had received three or more prior treatments. Various tumor types were included in the dose escalation cohort, including CRC, pancreatic cancer, cholangiocarcinoma, adenocarcinoma of the small bowel, and uterine cancer.
Table 2. Baseline demographics and patient characteristics.
| Demographic/characteristic | Dose Escalation Cohort (n=12) | Expansion Cohort (n=10) | Total (n=22) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 8 (67) | 6 (60) | 14 (64) |
| Female | 4 (33) | 4 (40) | 8 (36) |
| Age, years | |||
| Mean | 59.3 | 61.0 | 60.1 |
| Median | 59.9 | 61.7 | 61.7 |
| Range | 40-74 | 48-71 | 40-74 |
| Race/ethnicity, n (%) | |||
| White | 9 (75) | 9 (90) | 18 (82) |
| African-American | 3 (25) | 1 (10) | 4 (18) |
| Performance status, KPS (%) | |||
| 100 | 2 | 4 | 6 |
| 90 | 7 | 6 | 13 |
| 80 | 3 | 0 | 3 |
| Number of prior treatments for metastatic disease, n (%) | |||
| 0 | 4 | 10** | 14 |
| 1 | 1 | 0 | 1 |
| 2 | 3 | 0 | 3 |
| ≥3 | 4 | 0 | 4 |
| Tumor type, n (%) | |||
| Colon | 5 (42) | 8 (80) | 13 (59) |
| Rectal | 2 (17) | 2 (20) | 4 (18) |
| Pancreatic | 2 (17) | 0 (0) | 2 (9) |
| Other* | 3 (24) | 0 (0) | 3 (14) |
Other consists of 1 patient each with the following: cholangiocarcinoma, duodenal cancer, and uterine cancer
6 patients received prior adjuvant chemotherapy
Ten patients were then enrolled in the colorectal expansion cohort. Six of these patients had received prior adjuvant chemotherapy. In the colorectal expansion cohort, KRAS mutation status was tested in eight patients; one patient had a tumor with a KRAS mutation.
Dose escalation and MTD determination
The dose escalation schema and corresponding DLT events are listed in Table 1. Dose findings were based on the overall safety and tolerability of the investigational drug combination. In cohort 1 (dasatinib 50mg twice daily), two subjects experienced DLT events and were unable to receive 85% of scheduled doses of dasatinib and capecitabine. These DLT events included grade two intolerable nausea (n=1) and grade three anorexia (n=1). In cohort -1 (n=8), dasatinib was reduced from 50mg twice daily to 70mg once daily. Two subjects in cohort -1 were not evaluable due to early clinical progression and an oxaliplatin-related infusion reaction, respectively. One DLT event occurred in this cohort level (grade three fatigue), and this dose level was established as the MTD and RP2D.
Safety
Table 3 summarizes treatment-related toxicities for subjects in the dose escalation cohort, the colorectal expansion cohort, and overall. All treatment-related adverse events causing grade three or greater toxicity are shown. All patients experienced at least one adverse event of any grade related to therapy; twelve patients (55%) experienced grade three or greater toxicity. The most common non-hematological adverse events of any grade were fatigue (91%), diarrhea (82%), anorexia (77%), neuropathy (64%), edema (41%), and hypertension (23%). The category of edema included swelling and/or fluid retention in the limbs, trunk, viscera, genitalia, head and neck, or lymphatic system. Most adverse events were mild to moderate (grade one or two) and resolved with supportive clinical care and protocol-specified dose holdings and reductions. The most common grade three or greater adverse events were neutropenia (23%), fatigue (18%), and diarrhea (14%). There was one treatment-related death related to gastrointestinal perforation.
Table 3. Summary of treatment-related adverse events*.
| AE type reported | Dose Escalation Cohort N=12, n (%) | Expansion Cohort N=10, n (%) | Total N=22, n (%) | |||
|---|---|---|---|---|---|---|
| All events | Grade ≥3 | All events | Grade ≥3 | All events | Grade ≥3 | |
| Patients with AEs | 12 (100) | 5 (42) | 10 (100) | 7 (70) | 22 (100) | 12 (55) |
| AE by preferred term | ||||||
| Neutropenia | 4 (33) | 4 (33) | 5 (50) | 1 (10) | 9 (41) | 5 (23) |
| Fatigue | 12 (100) | 2 (17) | 8 (80) | 2 (20) | 20 (91) | 4 (18) |
| Diarrhea | 11 (92) | 2 (17) | 7 (70) | 1 (10) | 18 (82) | 3 (14) |
| Anemia | 2 (17) | 1 (8) | 2 (20) | 1 (10) | 4 (18) | 2 (9) |
| Anorexia | 11 (92) | 2 (17) | 6 (60) | - | 17 (77) | 2 (9) |
| GI perforation | 1 (8) | 1 (8) | 1 (10) | 1 (10) | 2 (9) | 2 (9) |
| Dehydration | 2 (17) | 1 (8) | 1 (10) | - | 3 (14) | 1 (5) |
| Edema | 6 (50) | - | 3 (30) | 1 (10) | 9 (41) | 1 (5) |
| Hemorrhage/bleeding | 1 (8) | 1 (8) | 3 (30) | - | 4 (18) | 1 (5) |
| Hypertension | - | - | 5 (50) | 1 (10) | 5 (23) | 1 (5) |
| Hyponatremia | - | - | 1 (10) | 1 (10) | 1 (5) | 1 (5) |
| Hypophosphatemia | - | - | 1 (10) | 1 (10) | 1 (5) | 1 (5) |
| Neuropathy | 5 (42) | - | 9 (90) | 1 (10) | 14 (64) | 1 (5) |
| Neutropenic fever | 1 (8) | 1 (8) | - | - | 1 (5) | 1 (5) |
| Pleural effusion | 1 (8) | - | 2 (20) | 1 (10) | 3 (14) | 1 (5) |
| Proteinuria | - | - | 1 (10) | 1 (10) | 1 (5) | 1 (5) |
Includes all treatment-related adverse events with grade 3 or higher toxicity
Efficacy
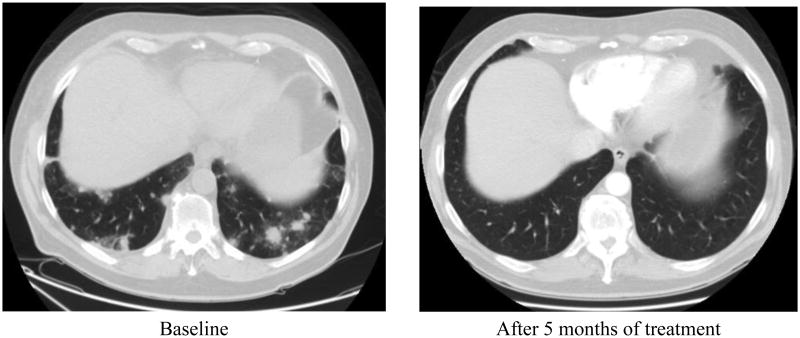
In the dose escalation cohort, clinical activity was observed. A patient with previously untreated metastatic cholangiocarcinoma had a 93% decrease in target lesions (see Figure 2). Another patient with treatment-refractory metastatic rectal cancer had stable disease for over thirty-three months. Of the ten evaluable patients in this cohort, one patient had a partial response and six patients had stable disease (ORR= 10%). In this cohort, six patients (60%) experienced clinical benefit (CR+PR+SD>6 months). Due to toxicity, two patients withdrew from the study prior to first radiographic assessment and were not evaluable for response.
Figure 2. Patient with metastatic cholangiocarcinoma to lung.
In the colorectal expansion cohort, further signs of clinical activity were observed. Median PFS was 13.4 months (95% C.I., 1.2 months-not reached). One patient had a complete response, six patients had a partial response and two patients had stable disease greater than six months (ORR= 70%; clinical benefit= 90%).
Tumor srcact analysis
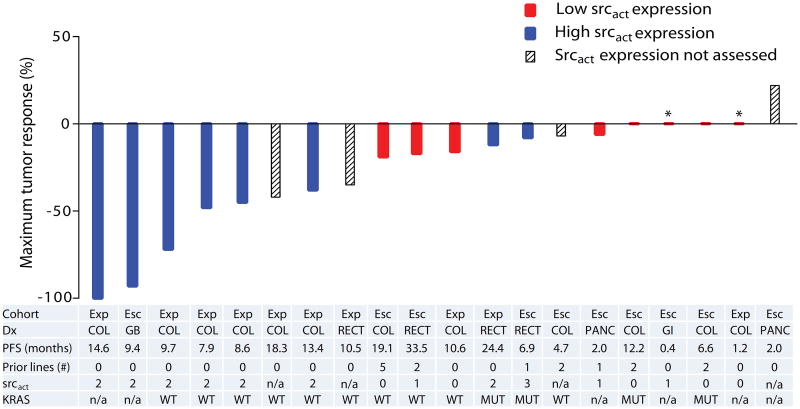
A waterfall plot (see figure 3) demonstrates the maximum tumor response by srcact expression level, for all evaluable patients enrolled in this study. Cohort, diagnosis, PFS, number of prior therapies, srcact expression, and KRAS mutation status are also listed. Srcact expression was assessed in tumors from sixteen evaluable patients. Overall, eight tumors had low levels of membranous srcact expression (IHC= 0 or 1), and eight tumors had high levels of srcact expression (IHC≥2). Benign colonic epithelium did not demonstrate membranous srcact expression (IHC=0). For patients with high srcact expression, the ORR was 75% (6/8 patients), compared to 0% for the eight patients with low srcact expression (2-sided Fisher's exact p-value=0.007).
Figure 3. Clinical activity of dasatinib, capecitabine, oxaliplatin, and bevacizumab.
* Clinical progression prior to first restaging
Best response by srcact status for 20 evaluable subjects. Cohort: Exp (expansion), Esc (dose escalation); Dx (Diagnosis): COL (colon cancer), GB (gallbladder cancer), GI (adenocarcinoma of the small bowel), PANC (pancreatic cancer), RECT (rectal cancer); Prior lines (number of prior lines of treatment for metastatic disease); srcact (activated src expression): 0=none; 1= weak/faint; 2= moderate; 3= strong/circumferential; KRAS: n/a (not assessed), MUT (mutation detected), WT (no mutation detected).
Within the colorectal expansion cohort, srcact expression was assessed in tumors from eight evaluable patients. Six of eight patients had high srcact expression. For the six patients with high srcact expression, the ORR was 83%, and the PFS range was 7.9 to 24.4 months. For the two patients with low srcact expression, the ORR was 0% (0/2), and PFS was 10.6 months and 1.2 months, respectively.
Analysis of plasma angiogenesis factors
To evaluate biomarker responses to treatment, each subject's baseline biomarker profile was used as his or her reference control. Plasma samples were collected at baseline and on-treatment (end of cycle three). In total, ten biomarkers (IL-6, HGF, PAI-1, PDGF-β, PlGF, VEGFR1, VEGFR2, TβR3, TGFβ-1 and TGFβ-2) were analyzed. In the 14 evaluable subjects, there was a statistically significant increase in soluble TβR3 (betaglycan) (P=0.0166). No statistically significant changes were noted in the other biomarkers evaluated (P>0.05).
Discussion
Src activation is implicated in chemotherapy resistance, and preclinical studies support the role of src inhibitors in restoring sensitivity to 5-FU and platinum-based chemotherapy [5,11,16]. Src family kinases (SFKs) are also implicated in angiogenesis. Inhibition of SFKs decreases VEGF, IL-8, and FGF, and inhibits endothelial cell proliferation and migration [13,20,22]. Dasatinib also inhibits BCR-ABL, EPH, cKIT, PDGFRβ and blocks downstream signaling from multiple receptor tyrosine kinases, including c-MET, IGF-1R, EGFR, and VEGFR-2.
In this phase I study, we demonstrated that dasatinib could be safely combined with CapeOx/bevacizumab, albeit at a lower dose than anticipated. The RP2D for dasatinib in this combination was 70mg daily, which is lower than the FDA approved dose for chronic phase CML (100mg po daily) and lower than the RP2D from several other dasatinib-chemotherapy combinations [6,10,13,24,30]. It is unclear whether this lower dose reflects overlapping toxicity with CapeOx/bevacizumab, or lower tolerance for combination therapy in a heavily pretreated patient population. To better understand the optimal dose of dasatinib for patients with CRC, results of ongoing phase I studies of dasatinib plus bevacizumab,[19] and dasatinib plus 5-FU, leucovorin, and oxaliplatin (FOLFOX) [24] will be helpful.
Mild to moderate (grade 1-2) adverse events were commonly encountered in this study, but more severe events (grade 3-5) were uncommon. One subject experienced a grade 5 (fatal) gastrointestinal perforation during cycle 2 of therapy. Perforation is a well-described adverse event associated with bevacizumab and other anti-VEGF therapies; the contribution of dasatinib to this isolated event is unclear. The most common treatment related adverse event was fatigue, although only 18% of subjects experienced grade 3 or greater fatigue. Fatigue was manageable with dose interruption or reduction and use of glucocorticoids. Diarrhea, neutropenia, and anorexia were also commonly experienced by study participants, but these events were typically mild to moderate in severity. Of note, edema was not a significant problem for most subjects on this study. Edema, when it occurred, was manageable with diuretics and dose reductions.
In this study, the combination of dasatinib and CapeOx/bevacizumab demonstrated signs of clinical activity. A patient with metastatic gallbladder cancer had a significant response to therapy (see figure 1), and remained on study for over 9 months. Although the ORR was only 10% in the dose escalation cohort, the clinical benefit rate, which includes disease stability greater than six months, was 60%. These results suggest that this combination may have sustained clinical activity in some patients with chemotherapy refractory disease. Activity was also observed in patients receiving first-line treatment of metastatic CRC. In the colorectal expansion cohort, 90% of patients had either a partial response or prolonged disease stability. The two patients with stable disease as best response had PFS of 10.6 months and 20.4 months, respectively. It is possible that dasatinib enhanced anti-tumor activity and duration of response.
Prior studies of dasatinib in unselected patients with advanced solid tumors have demonstrated mostly disappointing clinical activity [2,14,17,31]. The identification of biomarkers for dasatinib therapy has been limited by low numbers of responders, the multiple targets inhibited by dasatinib, the uncertain relevance of these targets in different diseases, and the technical challenges of measuring the activated target(s) [28]. In this trial, we evaluated membranous srcact expression as a possible biomarker of treatment benefit. The antibody used in this analysis, clone 28, has been reported to perform well in FFPE samples [18]. Despite previous reports that increased src expression is associated with poor prognosis,[1,38] in our study, high srcact expression was associated with improved response to treatment. This small non-randomized study cannot distinguish between biomarkers that are prognostic of outcome versus predictors of benefit from a specific treatment. However, the improved treatment response in tumors with high srcact expression suggests this biomarker may merit further testing.
We measured the pharmacodynamic effects of dasatinib and CapeOx/bevacizumab on several circulating biomarkers. Of the biomarkers we analyzed, only soluble TβR3 demonstrated a statistically significant change between baseline and the end of cycle 3. Soluble TβR3 is shed from the cell surface, where it binds TGF-β and mediates changes in the tumor microenvironment. TβR3 is also thought to have an important role in anti-tumor immunologic responses. Soluble TβR3 suppresses the development of CD4+CD25+ regulatory T cells (TRegs), and promotes immunologic activation against tumor antigens [12]. Based on its role in mediating anti-tumor immunity, increased soluble TβR3 is associated with favorable outcomes. In this study, 11 of 14 patients had measurable increases in soluble TβR3. Dasatinib, which inhibits several SFKs, including Lck, Yes, Fyn, and Hck, [26] is known to inhibit TRegs in a dose dependent manner [8]. Further studies are needed to explore the relationship between dasatinib, soluble TβR3, and anti-tumor immunologic responses.
This study has several limitations. The non-randomized trial design limits our ability to make conclusions about clinical activity. In addition, only two patients in the colorectal expansion cohort had low srcact expression, which limits correlation between srcact expression and clinical outcomes. Similarly, due to small sample size, plasma based biomarker analyses were underpowered. For these reasons, all biomarker analyses should be considered highly exploratory.
In conclusion, we have demonstrated that the combination of dasatinib—at 70mg daily—plus CapeOx/bevacizumab is safe and generally well tolerated. This regimen appears active and our data suggest that tumor staining for srcact expression may identify those patients most likely to benefit. Our finding that dasatinib may modulate levels of soluble TβR3 suggests a novel immunologic mechanism for dasatinib's activity. These findings merit follow up in future clinical studies of dasatinib and other src inhibitors.
Acknowledgments
We gratefully acknowledge the invaluable contributions of the patients and their families. We would also like to acknowledge the Duke University GI Oncology clinical trials team.
Role of the funding source: This was an investigator initiated study supported by Bristol-Myers Squibb, New Jersey, USA. The study was independently managed and analyzed. The final responsibility for the manuscript and the decision to submit for publication was made by the investigators. This work was also supported by National Institute of Health Grant 5K24-CA113755-05 (H Hurwitz).
Footnotes
Conflict of Interest Statement: A.B. Nixon, H.E. Uronis, M.A. Morse and H. I. Hurwitz currently have research funding with Bristol-Myers Squibb (BMS). M.A. Morse has received honoraria for speaking from Bristol-Myers Squibb. All other authors have declared no conflict of interest with BMS.
References
- 1.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 2.Araujo JC, Trudel GC, Saad F, Armstrong AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev V, Efstathiou E, Oudard S, Morris MJ, Sizer B, Goebell PJ, Bono JSD, Paliwal P, Durham S, Cheng S, Logothetis C. Overall survival (OS) and safety of dasatinib/docetaxel versus docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC): Results from the randomized phase III READY trial. J Clin Oncol. 2013;(suppl 6) abstr LBA8. [Google Scholar]
- 3.Bolen JB, Veillette A, Schwartz AM, DeSeau V, Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987;84:2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989;83:2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceppi P, Papotti M, Monica V, Lo Iacono M, Saviozzi S, Pautasso M, Novello S, Mussino S, Bracco E, Volante M, Scagliotti GV. Effects of Src kinase inhibition induced by dasatinib in non-small cell lung cancer cell lines treated with cisplatin. Mol Cancer Ther. 2009;8:3066–3074. doi: 10.1158/1535-7163.MCT-09-0151. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, Specht J, Gradishar W, Strauss L, Rybicki A, Wu X, et al. Dasatinib plus capecitabine for advanced breast cancer: safety and efficacy data from phase 1 study CA180-004. Cancer Res. 2009;69:S676–677. doi: 10.1158/1078-0432.CCR-12-0652. [DOI] [PubMed] [Google Scholar]
- 7.Ellis LM, Staley CA, Liu W, Fleming RY, Parikh NU, Bucana CD, Gallick GE. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998;273:1052–1057. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 8.Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Gotz M, Dohner H, Bunjes D, Schmitt M. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol. 2009;144:195–205. doi: 10.1111/j.1365-2141.2008.07433.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. [accessed on 7 June 2013];GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10. [Internet]. Available from: http://globocan.iarc.fr.
- 10.Fornier MN, Morris PG, Abbruzzi A, D'Andrea G, Gilewski T, Bromberg J, Dang CT, Dickler MN, Norton L, Hudis C. Dasatinib (D) in combination with weekly (w) paclitaxel (P) for patients (pts) with metastatic breast carcinoma (MBC): A phase I/II study. J Clin Oncol. 2010;28 abstr 1156. [Google Scholar]
- 11.Griffiths GJ, Koh MY, Brunton VG, Cawthorne C, Reeves NA, Greaves M, Tilby MJ, Pearson DG, Ottley CJ, Workman P, Frame MC, Dive C. Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem. 2004;279:46113–46121. doi: 10.1074/jbc.M408550200. [DOI] [PubMed] [Google Scholar]
- 12.Hanks BA, Holtzhausen A, Gimpel P, Jamieson R, Campbell OM, Sun L, Augustine CK, Tyler DS, Osada T, Morse M, Ling LE, Lyerly HK, Blobe GC. Effect of the loss of the type III TGFβ receptor during tumor progression on tumor microenvironment: Preclinical development of TGFβ inhibition and TGFβ-related biomarkers to enhance immunotherapy efficacy. J Clin Oncol. 2012;30(1) abstr 10563. [Google Scholar]
- 13.Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, Antonia S, Gray J, Litschauer S, Tetteh L, Neuger A, Song L, Rawal B, Schell MJ, Bepler G. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1387–1394. doi: 10.1200/JCO.2009.25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold CI, Chadaram V, Peterson BL, Marcom PK, Hopkins J, Kimmick GG, Favaro J, Hamilton E, Welch RA, Bacus S, Blackwell KL. Phase II trial of dasatinib in patients with metastatic breast cancer using real-time pharmacodynamic tissue biomarkers of Src inhibition to escalate dosing. Clin Cancer Res. 2011;17:6061–6070. doi: 10.1158/1078-0432.CCR-11-1071. [DOI] [PubMed] [Google Scholar]
- 15.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 16.Ischenko I, Camaj P, Seeliger H, Kleespies A, Guba M, De Toni EN, Schwarz B, Graeb C, Eichhorn ME, Jauch KW, Bruns CJ. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27:7212–7222. doi: 10.1038/onc.2008.326. [DOI] [PubMed] [Google Scholar]
- 17.Johnson FM, Bekele BN, Feng L, Wistuba I, Tang XM, Tran HT, Erasmus JJ, Hwang LL, Takebe N, Blumenschein GR, Lippman SM, Stewart DJ. Phase II study of dasatinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:4609–4615. doi: 10.1200/JCO.2010.30.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakatsu H, Sakai T, Takagaki Y, Shinoda Y, Saito M, Owada MK, Yano J. A new monoclonal antibody which selectively recognizes the active form of Src tyrosine kinase. J Biol Chem. 1996;271:5680–5685. doi: 10.1074/jbc.271.10.5680. [DOI] [PubMed] [Google Scholar]
- 19.Kim G, Annunziata CM, Sarosy GA, Minasian LM, Prindiville SA, Zujewski J, Otten L, Squires J, Houston ND, Kohn EC. Phase I study of dasatinib in combination with bevacizumab in advanced solid tumors. J Clin Oncol. 2010;28(1) abstr TPS163. [Google Scholar]
- 20.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 21.Kim MP, Park SI, Kopetz S, Gallick GE. Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Cell Tissue Res. 2009;335:249–259. doi: 10.1007/s00441-008-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YM, Lee YM, Kim HS, Kim JD, Choi Y, Kim KW, Lee SY, Kwon YG. TNF-related activation-induced cytokine (TRANCE) induces angiogenesis through the activation of Src and phospholipase C (PLC) in human endothelial cells. J Biol Chem. 2002;277:6799–6805. doi: 10.1074/jbc.M109434200. [DOI] [PubMed] [Google Scholar]
- 23.Kopetz S, Lesslie DP, Dallas NA, Park SI, Johnson M, Parikh NU, Kim MP, Abbruzzese JL, Ellis LM, Chandra J, Gallick GE. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopetz S, Wolff RA, Glover K, Henry L, Eng C, Chang DZ, Overman M, Gallick G, Abbruzzese J. Phase I study of Src inhibition with dasatinib in combination with 5-fluoruracil, leucovorin, oxaliplatin (FOLFOX) and cetuximab in metastatic colorectal cancer; Paper presented at the 2008 GI Cancers Symposium; Orlando, FL. 2008. abstract 325. http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=53&abstractID=10340. [Google Scholar]
- 25.Liu Y, Starr MD, Bulusu A, Pang H, Wong NS, Honeycutt W, Amara A, Hurwitz HI, Nixon AB. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Medicine. 2013;2:234–242. doi: 10.1002/cam4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 27.Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman TJ. Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene. 1997;15:3083–3090. doi: 10.1038/sj.onc.1201496. [DOI] [PubMed] [Google Scholar]
- 28.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011;16:566–578. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 30.Secord AA, Teoh DK, Barry WT, Yu M, Broadwater G, Havrilesky LJ, Lee PS, Berchuck A, Lancaster J, Wenham RM. A phase I trial of dasatinib, an SRC-family kinase inhibitor, in combination with paclitaxel and carboplatin in patients with advanced or recurrent ovarian cancer. Clin Cancer Res. 2012;18:5489–5498. doi: 10.1158/1078-0432.CCR-12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma MR, Wroblewski K, Polite BN, Knost JA, Wallace JA, Modi S, Sleckman BG, Taber D, Vokes EE, Stadler WM, Kindler HL. Dasatinib in previously treated metastatic colorectal cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2012;30:1211–1215. doi: 10.1007/s10637-011-9681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SPRYCEL [package insert] Bristol-Myers Squibb; Princeton, NJ: [accessed August 7, 2012]. Retrieved. from http://packageinserts.bms.com/pi/pi_sprycel.pdf. [Google Scholar]
- 33.Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Termuhlen PM, Curley SA, Talamonti MS, Saboorian MH, Gallick GE. Site-specific differences in pp60c-src activity in human colorectal metastases. J Surg Res. 1993;54:293–298. doi: 10.1006/jsre.1993.1046. [DOI] [PubMed] [Google Scholar]
- 35.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 36.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, Bast RC, Mills GB, Gallick GE. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88:73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 37.Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY, Shellito P, Czito BG, Wong TZ, Paulson E, Poleski M, Vujaskovic Z, Bentley R, Chen HX, Clark JW, Jain RK. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, Clarke RB, Dive C, Bundred NJ. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer. 2006;95:1410–1414. doi: 10.1038/sj.bjc.6603444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]