Abstract
Objective:
This study builds on previous work delineating a hierarchical model of family environmental risk in relation to a hierarchical model of externalizing disorders (EXTs) by evaluating for gene–environment interplay in these relationships. The associations between parent–child relationship quality (conflict, bonding, and management) and substance-specific adolescent family environments (parental/sibling tobacco/alcohol use) in relation to young adult EXTs (age ∼22 years nicotine, alcohol, and other drug dependence; antisocial and risky sexual behavior) were evaluated.
Method:
The sample included 533 adopted offspring and 323 biological offspring. Because adopted youth do not share genes with their parents, a significant association between parent–child relationship quality and EXTs would provide evidence against passive gene–environment correlation (rGE). Significant associations between parental tobacco/alcohol use in relation to offspring nicotine/alcohol dependence in the adopted offspring support common environmental influence. Significant associations detected for the biological offspring only suggest common genetic influence.
Results:
For both adoptive and biological offspring, there was a significant association between parent–child relationship quality and EXTs. Parental tobacco/alcohol use was unrelated to EXTs. Sibling tobacco/alcohol use was related to EXTs, but only for the biological siblings. Parental tobacco use was associated with the residual variance in nicotine dependence in adopted offspring.
Conclusions:
Findings replicate a long-term influence of adolescent parent–child relationship quality on adult EXTs. Findings extend previous research by providing evidence against passive rGE in this association. The association between parental tobacco use and adult nicotine dependence appears to be environmentally mediated, but caution is warranted as we found this relationship only for adopted youth.
Substance use problems are moderately correlated across substance type (e.g., alcohol and illicit drug dependence; Hasin et al., 2007), as well as with antisocial behavior (King et al., 2004; Krueger et al., 2002; McGue and Iacono, 2005; Ramrakha et al., 2007), risky sexual behavior (Bailey et al., 1999; Khan et al., 2012), and personality characteristics such as impulsivity or negative emotionality (Chassin et al., 2004; McGue et al., 1999). This clustering of behaviors has been interpreted as an expression of a general liability to externalizing disorders (EXTs; Iacono et al., 2003; Krueger et al., 2002), which is largely influenced by common genetic factors (e.g., Hicks et al., 2011; McGue et al., 2006; Prescott et al., 2006). Nonetheless, past work has identified a nonnegligible degree of substance-specific variance that is not explained by the general EXT liability (Newcomb and McGee, 1991). For example, between 8% and 16% of genetic variance in substance dependence outcomes at age 17 is unique and not shared with other substances or related externalizing behaviors (Hicks et al., 2011). Similar residual genetic influences on substance use disorders have been reported for adults (Kendler et al., 2003). Unique environmental influences on substance use disorders are also evident, such as whether specific substances are available (Baker et al., 2012; Gillespie et al., 2009).
To better understand sources of common and unique influences on substance use disorders, Bailey et al. (2011) proposed a hierarchical model of family environmental risk that follows the hierarchical model of EXT liability (described in Figure 1). Three distinct characteristics of the adolescent family environment were evaluated: the general family environment (measured by reports of parent–child relationship quality), the family smoking environment (measured by parental and sibling smoking), and the family drinking environment (measured by parental and sibling drinking). Bailey et al. found evidence for a relationship between the general adolescent family environment and a general factor of adult EXTs and that substance-specific family smoking and drinking environments during adolescence explained unique variance in adult nicotine and alcohol dependence symptoms (after accounting for EXT liability). Bailey and colleagues concluded that interventions targeting the general, as well as substance-specific, family environments may have beneficial effects on young adult EXTs and that this general/specific analytic plan may be useful for future genetic studies.
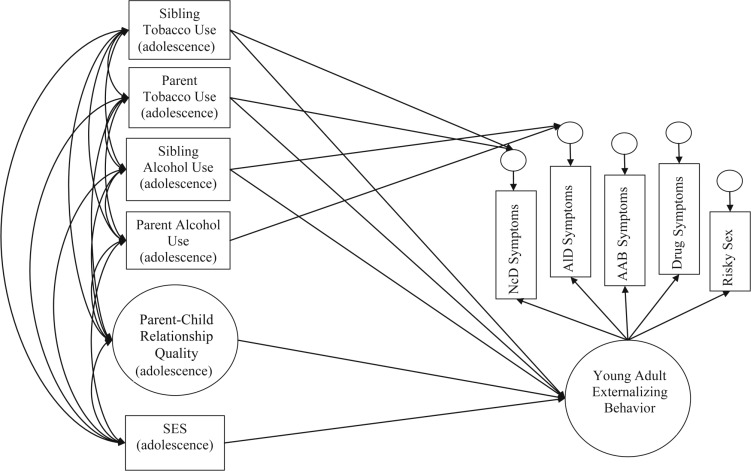
Figure 1.
Conceptual model depicting study hypotheses. The independent variables of parental and sibling tobacco and alcohol use and parent–child relationship quality during adolescence were measured as predictors of adult externalizing behavior. Parent–child relationship quality was measured as a latent factor, indicated by adolescent reports of conflict, bonding, and management (indicators not shown here for clarity of presentation). Socioeconomic status (SES) was included as a covariate in this model. All variables were age-, sex-, and ethnicity-adjusted before analysis. Adult externalizing behavior was also measured as a latent factor, indicated by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, clinical symptoms of nicotine dependence (NcD), alcohol dependence (AlD), illicit drug dependence (DrD), adult antisocial behavior (AAB), and a measure of risky sexual behavior. The associations between all independent variables and adult externalizing behavior were examined, in addition to the relationship between parental and sibling tobacco use with the residual variance of NcD symptoms, and between parental and sibling alcohol use to the residual variance of AlD symptoms.
To better understand the etiology of the relationships reported by Bailey et al. (2011), we aimed to replicate and extend this study by evaluating the proposed model in a sample of adopted youth, who are genetically unrelated to their parents and siblings, and in a sample of youth who are biologically related to their parents and siblings. As adoptees do not share genes with their rearing parents, a significant relationship between parental tobacco use and subsequent adoptee nicotine dependence evidences environmental influences in this association. Conversely, if the association between parental tobacco use and subsequent offspring nicotine dependence is only evident in genetically related parents and offspring, this would provide support for genetic influences in this association. The same rationale is true for the relationship between parental alcohol use and offspring alcohol use disorder and between sibling smoking and drinking in relation to offspring adult substance use disorders.
Using our adoption sample to understand the etiology of the relationship between the general adolescent family environment (i.e., adolescent parent–child relationship quality) and adult EXTs is somewhat more complex. It could be that an adoptee’s report of parent–child relationship quality is influenced by the adoptee’s genetic predispositions. In fact, previous research found that parent–child relationship quality is heritable (Ludeke et al., 2013) and that common genetic influences are evident in the relationship between parent–child relationship quality and EXT in adolescence (e.g., Neiderhiser et al., 1999; Pike et al., 1996), which is often interpreted as gene–environment correlation (rGE).
Passive rGE refers to the situation in which parents provide both gene endowment and rearing environment for their biologically related children, making it impossible to distinguish whether the association between parenting and child outcomes is attributable to genetic or environmental influence (Scarr and McCartney, 1983; also see Dick, 2011). Evocative rGE refers to the situation in which parents may be responding to their children’s genetically influenced EXT traits, again making it impossible to distinguish between genetic or environmental influence in the relationship between parenting behavior and adolescent outcomes. Twin studies are unable to distinguish between passive versus evocative rGE because parents and children share genes. However, because adoptive parents and offspring are not genetically related, a significant association between parent–child relationship quality and EXTs in adoptive families is inconsistent with a passive rGE hypothesis. Adoption studies cannot rule out evocative rGE (adopted children may still evoke a unique adoptive parental response based on their unique genotype).
In total, this study sought to build on previous research (e.g., Bailey et al., 2011; Hicks et al., 2011; Kendler et al., 2003) evaluating common and specific influences on adult substance use and EXTs. We expected to replicate Bailey et al. (2011) by finding substance-specific associations (parental and sibling smoking would be associated with unique variance in adult nicotine dependence; parental and sibling drinking would be associated with unique variance in adult alcohol use disorder), as well as an association between more general aspects of the family environment (parent–child relationship quality) with a general factor of adult EXTs. We extended these findings by testing for common genetic and environmental influences on these associations through the use of a genetically informed, adoption design.
Method
Participants
Participants were from the Sibling Interaction and Behavior Study (McGue et al., 2007), a longitudinal adoption study designed to investigate genetic and environmental influences on substance use and related behavioral problems. To participate, families were required to have at least two adolescent children up to 5 years apart in age and to live within driving distance of the laboratory. Each of the 617 participating families included parents and two offspring: 285 families included two adoptive offspring (genetically unrelated to either parent and to each other [adopt group]), 208 families included two offspring genetically related to the rearing parents and to each other (bio group), and 124 families included one biological offspring and one adoptive offspring (adopt-bio group).
Adoptive family eligibility included having an adopted child between the ages of 11 and 21 years who had been permanently placed into the adoptive home before age 2 (96% were placed before they were 1 year old) and having a second adolescent in the home who was not biologically related to the adopted adolescent. Adoptive families were identified through three large adoption agencies. Biological families were ascertained from public birth certificates of children and recruited to match the age and sex of the adopted children. Participation rates for nonadoptive (57%) and adoptive (63%) families were not significantly different. A brief telephone interview was administered to the majority (73%) of nonparticipating families. There were no significant differences in parental demographics—including marital status, education, occupation, and parent-reported behavioral disorders in their children—for participating and nonparticipating adoptive families. The only difference for nonadoptive families was that participating mothers were more likely to be college educated than nonparticipating mothers (McGue et al., 2007). Altogether, these results suggest that any effects of participation bias are likely minimal.
Comparing the parent’s education and marital status to 2000 Census data showed that the study sample was generally representative of the region from which it was drawn (McGue et al., 2007). However, adoptive parents tended to have slightly higher socioeconomic status and lower rates of EXTs than nonadoptive parents (see McGue et al., 2007, for a detailed sample description). A total of 1,158 adolescents returned at Time 2 (94% retention), and 1,109 adolescents have participated in the ongoing Time 3 assessment (currently 90% retention). Of the 1,109 adolescents who visited at Time 3, 624 were adopted (25% domestic, 75% international) and 485 were nonadopted. The overall mean ages at each time point were as follows: Time 1 Mage = 14.9 years (SD = 1.9), Time 2 Mage = 18.3 (SD = 2.1), and Time 3 Mage = 22.4 (SD = 1.9).
Our primary aim was to test the conceptual model (Figure 1) across adolescents who were genetically related versus unrelated to all family members. Therefore, we excluded offspring who were genetically related to their parents but not their siblings as target participants in our modeling (however, we did use their data to obtain sibling report of tobacco and alcohol use). Also, because of the variability in ages at each assessment (e.g., Time 1 range: 11–21 years), we selected environmental data from the first assessment (Time 1 or Time 2) that the participant was at least 16.5 years of age or older (n = 202 from Time 1, n = 654 from Time 2) to better approximate Bailey et al.’s (2011) ages of assessment, for a total sample size of 856. Exclusion criteria for this sub-sample and accompanying ns are described in Figure 2. Of the 856 participants, 533 were adopted (and not genetically related to their parents or sibling) and 323 were the biological offspring of their parents (with a full biological sibling). Just over half of the participants were female (54.3%). Young adult EXTs were assessed at Time 3 for all participants. Thus, the measures of the adolescent family environment and young adult EXTs were assessed at mean ages of 18.2 years (SD = 1.0) and 22.3 years (SD = 1.5), respectively. A total of 796 of the 856 eligible participants for this subsample visited at Time 3 (93% retention). Demographics of the selected subsample are further described in Table 1.
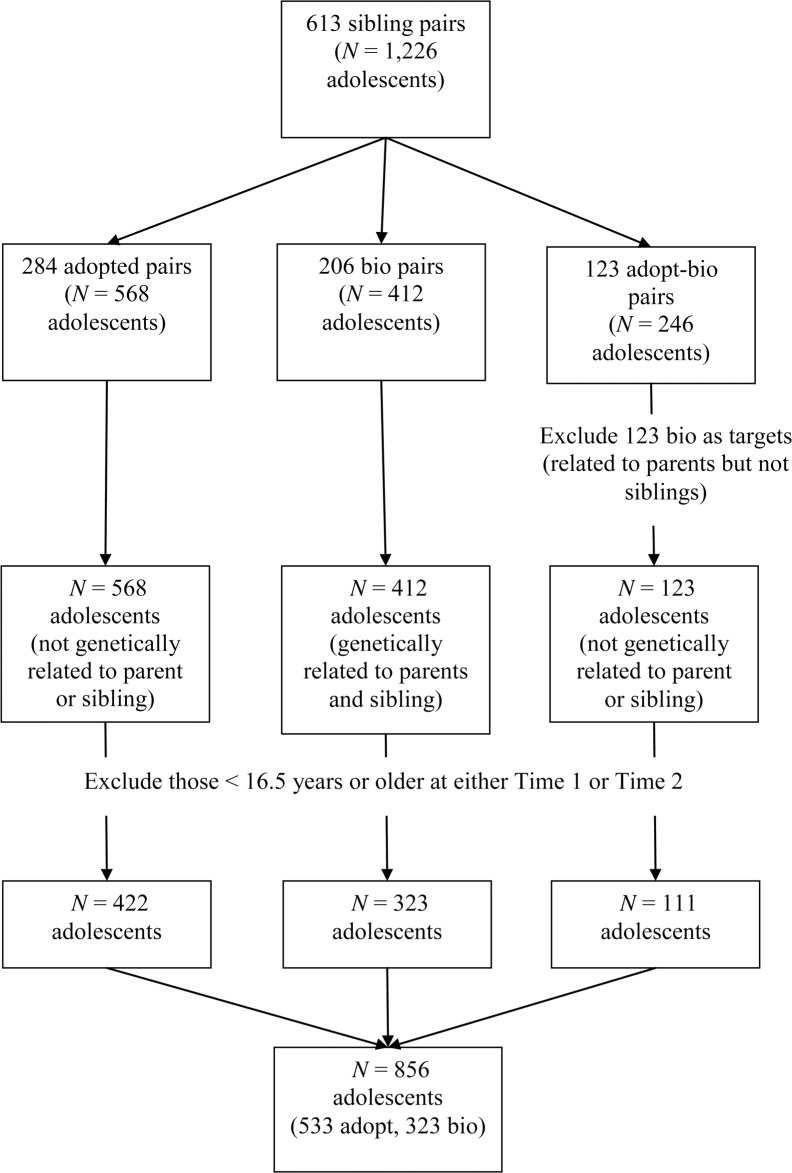
Figure 2.
Exclusion and inclusion criteria. The Sibling Interaction and Behavior Study has 613 sibling pairs (1,226 individuals) available for data analysis. This is broken up into three family types (see second row). For the present study’s analyses, bios from adopt-bio pairs were excluded as targets in the analysis. This was so that we could compare adolescents who were genetically related to parents and siblings with adolescents who were genetically unrelated to parents and siblings in one simultaneous model. We further excluded those who were 16.5 years or younger at either Time 1 or Time 2 (we used Time 1 or Time 2 data to assess independent variables and Time 3 to assess dependent variables; see Figure 1). This left us with 856 target adolescents for the analysis (533 adopt, 323 bio). Adopted = adolescents who were not genetically related to parents or siblings; bio = adolescent biological offspring of parents with a full biological sibling; adopt-bio = families that include one biological offspring and one adoptive offspring.
Table 1.
Demographics of the Sibling Interaction and Behavior Study subsample across adoptive status
| Variable | Adopt (n = 533) | Bio (n = 323) | Significant difference |
| Age independent variables were assessed, M (SD) | 18.24 (1.02) | 18.13 (1.01) | n.s. |
| Age dependent variables were assessed, M (SD) | 23.00 (1.54) | 22.96 (1.49) | n.s. |
| % Female | 56% | 52% | n.s. |
| % European ancestry | 20% | 95% | p < .001 |
| % Asian ancestry | 68% | 0% | p < .001 |
| % Other ancestry | |||
| (African, Native, mixed) | 12% | 5% | p = .01 |
| SES, M (SD) | .12 (.71) | -.26 (.81) | p < .001 |
Notes: This table denotes demographics across adopt and bio groups. Adopt = families that included two adoptive offspring; bio = families that included two offspring genetically related to the rearing parents and to each other; n.s. = not significant; SES = socioeconomic status (range: -2.26–1.32).
Measures
Parental and sibling tobacco and alcohol use during adolescence.
As demonstrated on the left side of Figure 1, independent variables included parental and sibling tobacco and alcohol use during the target’s adolescence (Mage = 18). Tobacco and alcohol use were measured using the Substance Abuse Module (given to those 16 or older; Robins et al., 1987)—an expanded version of the Composite International Diagnostic Interview (Robins et al., 1988)—or the Diagnostic Interview for Children and Adolescents–Revised DSM-III-R version (given to those 15 and younger; Reich and Welner 1988), and the Computerized Substance Assessment (given to all adolescents who came to the laboratory for an assessment; Han et al., 1999).
Sibling tobacco use was measured by sibling’s report of tobacco use quantity (answered on a scale of 0 = never to 6 = 2 or more packs/20 or more cigars/2 or more tins of snuff per day) and frequency (0 = never to 6 = every day or nearly every day) for the past 12 months (standardized and summed, α = .91). Sibling alcohol use was measured by the sibling’s report of alcohol use quantity (answered on a scale of 0 = none to 10 = ≥10 drinks) and frequency (answered on a scale of 0 = never to 5 = every day or nearly every day) for the past 12 months (standardized and summed, α = .89).
Parental tobacco use was measured using mother’s and father’s report of tobacco use quantity (number of tobacco items used on a typical day, including cigarettes, cigars, pipes, chews; answered on a scale of 0 = none to 99 = indicating too many count, capped at 50) and frequency (number of days in a month they typically smoked, answered on a scale of 0 = none to 30 = every day) for the past 12 months. Parental alcohol use was measured by mother’s and father’s report of alcohol use quantity (number of drinks drank per day, ranging from 0 = zero to 30 = indicating too many to count) and frequency (how often they drank, answered on a scale of 0 = none to 10 = three or more times per day) for the past 12 months. Mother and father variables were standardized, averaged, and summed to create the parental tobacco and alcohol use composites; α’s were .75 for parental tobacco use and .65 for parental alcohol use.
Parent–child relationship quality.
Parent–child relationship quality during the target’s adolescence (Mage = 18; Figure 1) was measured with three scales using items from the Parent Environment Questionnaire (PEQ; Elkins et al., 1997), which asks adolescents to rate items that describe their relationships with their parents on a scale of 1 (very true) to 4 (very false). Items were selected based on a close conceptual match to items used in Bailey et al. (2011). Each item was standardized and used in a summed scale (ratings of mother and father from the PEQ were averaged at the item level). Family management (α = .76) was measured using six PEQ items (e.g., “My [father/mother] doesn’t know much about how I spend my spare time”). Family conflict (α = .85) was measured using four to six PEQ items, depending on whether Time 1 or Time 2 data were used (e.g., “My [father/mother] and I often get into arguments”). Family bonding (α = .85) was measured using six PEQ items (e.g., “I don’t feel very close to my father/mother”).
Young adult externalizing disorders.
As demonstrated on the right side of Figure 1, dependent variables included the target adolescent’s EXTs in young adulthood (Mage = 22). DSM-IV (American Psychiatric Association, 1994) symptom counts of alcohol dependence, nicotine dependence, and illicit drug dependence (illicit drug with the highest symptom count) were assessed using the Substance Abuse Module modified to cover DSM-IV criteria. Adult antisocial behavior (Antisocial Personality Disorder, Criterion A) was assessed using a version of the DSM-III-R Structured Clinical Interview for Personality Disorders (Spitzer et al., 1987), modified to cover DSM-IV criteria. Coded interviews were reviewed by pairs of individuals, and symptoms were assigned based on a review of the severity and frequency of reported behavior. Kappa coefficients were greater than .90 for all substance use disorder diagnoses and .79 for adult antisocial behavior. Risky sexual behavior was measured using number of lifetime sexual partners from the Life Events Interview.
Analysis plan
All analyses were conducted using structural equation modeling in Mplus Version 6.12 (Muthén and Muthén, 1998–2012). Because of the diversity of the Sibling Interaction and Behavior Study (Table 1) and consistent with prior research (e.g., Burt et al., 2007; Samek et al., 2013), age, sex (1 = male, 2 = female), and ethnicity (1 = European ancestry, 2 = other) were regressed out of study variables before analysis. The modeling (based on Bailey et al., 2011) is described in Figure 1; a latent factor of EXTs was estimated using the indicators of risky sex, adult antisocial behavior, nicotine dependence, alcohol dependence, and illicit drug dependence. The associations between parental/sibling tobacco/alcohol use and parent–child relationship quality (shown to the left in Figure 1) with the latent EXT factor and the residual variance in nicotine dependence and alcohol dependence (shown on the right side of Figure 1) were evaluated. Socioeconomic status (measured as a standardized composite of mother and father’s education level, highest occupational prestige rating, and family income) was included as a covariate in the modeling.
Maximum likelihood estimation with robust standard errors was used to account for the nonnormal distribution of clinical symptoms. To account for the fact that adolescents were nested within families, we used the COMPLEX specification and clustered analyses by family ID. Missing data were handled using full-information maximum likelihood estimation (Enders and Bandalos, 2001). Group differences across genetic relatedness were tested using the chi-square difference test. Model fit was evaluated using the chi-square fit statistics, root mean square error of approximation (RMSEA), standardized root mean square residual (SRMR), and the comparative fit index (CFI). Standard interpretation is that RMSEA < .05, SRMR < .05, and CFI > .90 indicate good fit (Kenny 2012; Kline, 2005).
Results
Preliminary information
Descriptive information on dependent variables (DSM-IV clinical symptoms and number of lifetime sexual partners) is presented in Table 2. There were no significant differences in the means of outcome variables or clinical diagnoses across adopt or bio groups. Correlations and descriptive statistics for all age-, sex-, and ethnicity-corrected variables are presented in Table 3. For both adopt and bio adolescents, there were moderate to large correlations among EXT variables (risky sex, nicotine dependence, alcohol dependence, other drug dependence symptoms, and adult antisocial behavior), suggesting the presence of an underlying EXT factor in both groups. Of note, although adoptees had greater socioeconomic status than biological offspring (Table 1), childhood socioeconomic status was not significantly correlated with early adult EXT outcomes for either the adopt or bio groups (Table 3).
Table 2.
Descriptive information for DSM-IV clinical symptoms and number of lifetime sexual partners at the second follow-up (Mage = 22.3, SD = 1.5)
| Variable | Percentage of those who met criteria for DSM-IV disorders |
|||
| Adopt M (SD) (n = 533) | Bio M (SD) (n = 323) | Adopt % (n = 533) | Bio % (n = 323) | |
| AAB criteria | 1.28 (1.39) | 1.16 (1.32) | 13.9 | 11.8 |
| NcD criteria | 0.93 (1.51) | 0.83 (1.47) | 17.3 | 17.0 |
| AlD criteria | 0.64 (1.20) | 0.68 (1.29) | 7.5 | 7.7 |
| DrD criteria | 0.68 (1.53) | 0.62 (1.42) | 9.6 | 7.4 |
| No. partners | 2.84 (3.24) | 2.90 (3.54) | – | – |
Notes: There were no significant differences in mean level of symptoms or proportion of those meeting criteria for a diagnosis across adopt (adopted adolescents who were not biologically related to parents or siblings) and bio (adolescent biological offspring of parents with a full biological sibling) groups, as calculated by the chi-square difference test on 1 df. Criteria for meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), disorders included three or more symptoms for adult antisocial behavior (AAB), and three or more symptoms for nicotine dependence (NcD), alcohol dependence (AlD), and illicit drug dependence (DrD). No. partners = number of lifetime sexual partners.
Table 3.
Correlations and descriptive statistics for adopted adolescents (n = 533, shown above the diagonal) and bio adolescents (n = 323, shown below the diagonal)
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | M | SD | % Valid | |
| 1. Fam. manag. | _ | -.55* | -.69* | -.11* | -.00 | -.12* | -.10* | -.13* | -.30* | -.19* | -.12* | -.12* | .04 | -0.00 | 1.00 | 94.6 |
| 2. Fam. conflict | -.55* | – | .66* | .01 | -.03 | .05 | .05 | .16* | .37* | .24* | .16* | .19* | -.01 | 0.04 | 1.04 | 94.6 |
| 3. Fam. bonding | .69* | -.55* | – | .05 | -.05 | .04 | .04 | .14* | .27* | .24* | .10† | .12* | -.02 | 0.05 | 1.03 | 94.6 |
| 4. Sibling tobacco | -.15* | .09 | -.03 | – | .11† | .60* | .09† | .06 | .10† | .09 | .08 | .04 | .01 | 0.08 | 1.78 | 96.1 |
| 5. Parental tobacco | .00 | -.03 | -.01 | .23* | – | .02 | .06 | -.08* | -.06 | .03 | -.07* | -.04 | -.20* | -0.19 | 2.84 | 99.4 |
| 6. Sibling alcohol | -.12† | .11† | -.04 | .66* | .16* | – | .16* | .05 | .14* | .06 | .07 | .06 | .11* | 0.03 | 1.99 | 96.1 |
| 7. Parental alcohol | -.00 | .09 | -.03 | .13† | .20* | .25* | – | .06 | .06 | .08† | .06 | .12* | .17* | -0.26 | 2.66 | 99.4 |
| 8. No. of partn. | -.22* | .19* | -.14* | .22* | .10 | .31* | .14* | – | .33* | .18* | .27* | .17* | .03 | 0.01 | 0.96 | 91.7 |
| 9. AAB sxs | -.28* | .30* | -.19* | .36* | .21* | .29* | .11† | .46* | – | .46* | .44* | .55* | .02 | 0.00 | 1.02 | 92.1 |
| 10. NcD sxs | -.17* | .22* | -.17* | .23* | .05 | .20* | .11 | .25* | .38* | – | .37* | .45* | .02 | 0.03 | 1.00 | 92.1 |
| 11. AlD sxs | -.02 | .19* | -.03 | .18* | -.04 | .20* | . 11† | .25* | .40* | .35* | – | .38* | .02 | -0.00 | 0.97 | 92.1 |
| 12. DrD sxs | -.19* | .19* | -.20* | .24* | .07 | .16* | .12* | .20* | .43* | .33* | .47* | – | -.01 | 0.01 | 1.02 | 91.9 |
| 13. SES | .24* | -.16* | .18* | -.16* | -.20* | -.10 | .09 | .07 | -.11† | -.10 | -.06 | -.00 | – | 0.12 | 0.71 | 100.0 |
| M 0.04 | -0.07 | -0.09 | -0.06 | 0.61 | 0.08 | 0.30 | -0.01 | -0.00 | -0.06 | 0.00 | -0.01 | -0.26 | – | – | ||
| SD 1.00 | 0.97 | 0.99 | 1.85 | 4.04 | 2.01 | 3.11 | 1.06 | 0.95 | 0.99 | 1.05 | 0.96 | 0.81 | – | – | ||
| % Valid | 92.0 | 92.0 | 92.0 | 96.3 | 98.5 | 96.3 | 98.5 | 91.6 | 91.0 | 91.0 | 91.0 | 91.0 | 100.0 |
Notes: Adopted adolescents = those not genetically related to parents or siblings; bio adolescents = those genetically related to the rearing parents and to each other; fam. manag. = family management; fam. conflict = family conflict; fam. bonding = family bonding; no. of partn. = number of lifetime sexual partners; AAB sxs = adult antisocial behavior (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) symptoms; NcD sxs = nicotine dependence symptoms; AlD sxs = alcohol dependence symptoms; DrD sxs = illicit drug dependence symptoms; SES = socioeconomic status. Mothers’ and fathers’ tobacco and alcohol scores were moderately to substantially correlated (rs ranged from .22 to .46); therefore, they were collapsed into the same (parental) variables. All variables were adjusted for age, sex, and ethnicity before analysis. Correlations were obtained from MPLUS; twins were clustered by family ID to inflate standard error and control for shared family variance.
Correlations that are significant are denoted by †p < .10;
p < .05.
Structural equation model results
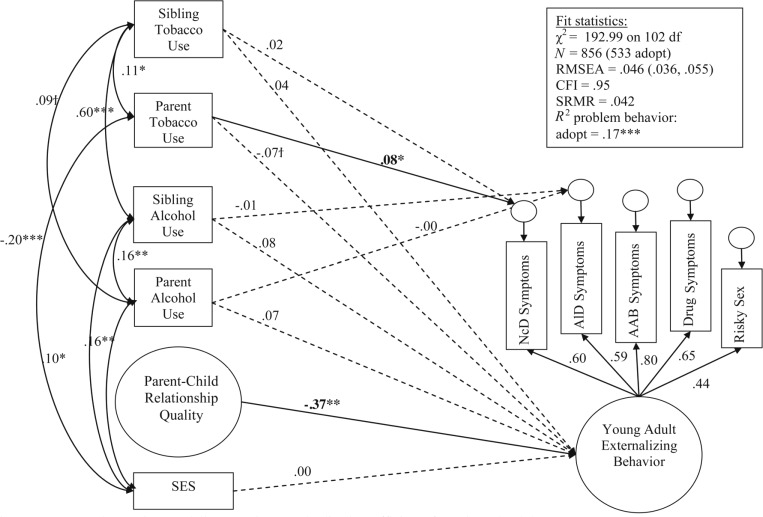
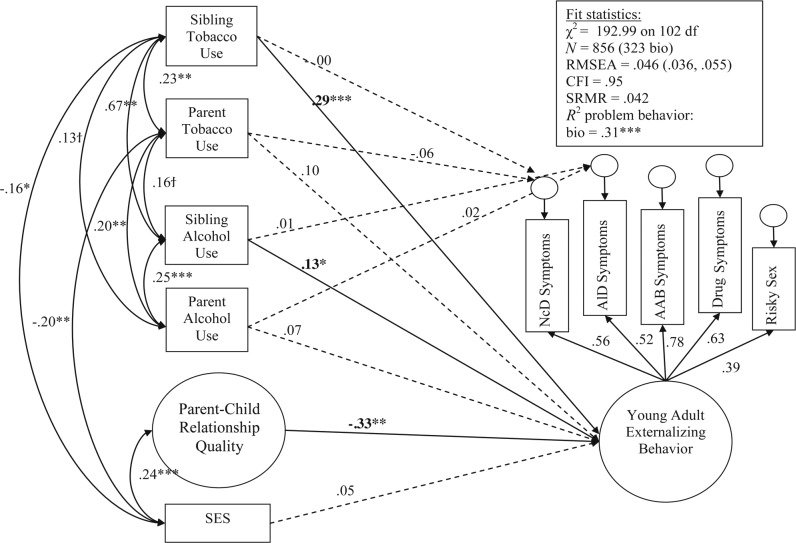
Figure 1 describes the structural equation model we evaluated. Overall, the model fit well, χ2(102) = 192.99 (CFI = .95; RMSEA = .04; SRMR = .04). Constraining all paths to be equal across adopt and bio offspring resulted in a significantly worse-fitting model, χ2(27) = 74.09, p < .001. We then fit a series of models that constrained paths one at a time to identify group differences. To ease the clarity of presentation, the results for adopted offspring are shown in Figure 3, and the results for bio offspring are shown in Figure 4. Table 4 provides the correlations among independent variables separately for adopt and bio offspring, as well as any significant differences in those correlations. Please note that factor loadings for parent–child relationship quality are not shown for clarity of presentation, but were .70 or greater for all indicators across adopt and bio groups (M = .79), suggesting adequate fit of the latent factor of parent–child relationship quality.
Figure 3.
Structural equation modeling results: Standardized coefficients for adopted adolescents. Significant paths are denoted by †p < .10; *p < .05; **p < .01; ***p < .001. All independent variables (left side of diagram) were correlated and assessed in adolescence for clarity of presentation. Correlations that were not significantly different from zero are not shown (see Table 3 for all correlations). Also, nonsignificant paths between independent and dependent variables were dashed. SES = socioeconomic status; RMSEA = root mean square error of approximation; CFI = comparative fit index; SRMR = standardized root mean square residual; adopt = adolescents who were not genetically related to parents or siblings; NcD = nicotine dependence; ALD = alcohol dependence; AAB = adult antisocial behavior.
Figure 4.
Structural equation modeling results: Standardized coefficients for bio adolescents. Significant paths are denoted by †p < .10; *p < .05; **p < .01; ***p < .001. All independent variables (left side of diagram) were correlated and assessed in adolescence; for clarity of presentation, correlations that were not significantly different from zero are not shown (see Table 3 for all correlations). Also, nonsignificant paths between independent and dependent variables were dashed. SES = socioeconomic status; RMSEA = root mean square error of approximation; CFI = comparative fit index; SRMR = standardized root mean square residual; bio = adolescent biological offspring of parents with a full biological sibling; NcD = nicotine dependence; AlD = alcohol dependence; AAB = adult antisocial behavior.
Table 4.
Correlations among independent variables from structural equation modeling analysis for adopted and bio adolescents
| Variable | Adopt (n = 533) | Bio (n = 323) | χ2 (on 1 df) |
| Sibling tobacco use: parental tobacco use | .11* | .23** | 2.48 |
| Sibling tobacco use: sibling alcohol use | .60*** | .67*** | 1.03 |
| Sibling tobacco use: parental alcohol use | .09† | .13† | 0.47 |
| Sibling tobacco use: general family environment | -.07 | -.09 | 0.03 |
| Sibling tobacco use: SES | .01 | -.16* | 5.25* |
| Parental tobacco use: sibling alcohol use | .02 | .16† | 2.80† |
| Parental tobacco use: parental alcohol use | .06 | .20** | 4.82* |
| Parental tobacco use: general family environment | .04 | .02 | 0.00 |
| Parental tobacco use: SES | -.20*** | -.20*** | 0.75 |
| Sibling alcohol use: parental alcohol use | .16** | .25*** | 1.83 |
| Sibling alcohol use: general family environment | -.07 | -.09 | 0.00 |
| Sibling alcohol use: SES | .10* | -.10 | 6.18* |
| Parental alcohol use: general family environment | -.07 | -.04 | 0.05 |
| Parental alcohol use: SES | .16* | .09 | 0.17 |
| General family environment: SES | .03 | .24*** | 6.90** |
Notes: Adopt = families that included two adoptive offspring genetically unrelated to one another and adoptive parents; bio = families that included two offspring genetically related to the rearing parents and to each other; SES = socioeconomic status.
Significance is denoted by †p < .10;
p < .05;
p < .01;
p < .001.
Significant differences across correlations are denoted by the chi-square difference test; a χ2 of 3.84 on 1 df is needed to be significant at p = .05.
In general, the factor structure of young adult externalizing was similar across adopt and bio samples, as shown by the similar magnitude of loadings for clinical symptoms and risky sexual behavior (right side of Figures 3 and 4). The association between parent–child relationship quality in adolescence and the EXT factor in adulthood was significant and did not differ across adopt and bio groups, χ2(1) = 0.29, p = .59, providing evidence against passive rGE for this association. Sibling tobacco and alcohol use during adolescence had a significant association with the young adult EXT factor in the bio group only (Figure 4); the association between sibling tobacco use in adolescence and the young adult EXT factor was significantly greater in the bio group than in the adopt group, χ2(1) = 5.61, p = .02, suggesting genetic influences in this association. Neither sibling tobacco or alcohol use predicted the residual variance in adult nicotine or alcohol dependence symptoms. Of note, parental tobacco and alcohol use during adolescence was not related to the young adult EXT factor for either the adopt or bio group. However, parental tobacco use was related to the residual variance in young adult nicotine dependence, but only for the adopt group (Figure 3). The difference between adopt and bio groups was significant, χ2(1) = 4.81, p = .03. This suggests at least some environmental influence of parental tobacco use in adolescence and young adult nicotine dependence.
To test whether findings would be affected by including autoregressive effects of adolescent smoking and drinking, we included the adolescent’s report of smoking and drinking as independent variables (in addition to parental and sibling smoking and drinking, the general family environment, socioeconomic status, and the intercorrelations among these predictors; Figure 1) in a post-hoc analysis. We evaluated whether these autoregressive effects predicted young adult EXT, as well as the residual variance in young adult nicotine and alcohol dependence. We found that (a) adolescent smoking and drinking significantly predicted variance in young adult EXTs for both adopt and bio groups (βs = .17–.48, ps < .01), (b) adolescent smoking predicted unique variance in nicotine dependence for both groups (βs = .38–.47, ps < .001), and (c) adolescent drinking did not predict unique variance in alcohol dependence for either group. Of note, study results were not affected by the inclusion of autoregressive effects; the general family environment was still significantly associated with young adult EXTs in both groups (βs = -.18–.23, ps < .05), parental smoking predicted unique variance in young adult nicotine dependence for adoptees only (β = .07, p < .05), and sibling smoking was significantly associated with young adult EXTs for biological offspring only (β = .20, p < .01).
Discussion
Substance use problems are consistently correlated across substance type (Hasin et al., 2007) and with other related externalizing behaviors (King et al., 2004; Krueger et al., 2002), including risky sexual behaviors (Khan et al., 2012). Previous twin and adoption research has confirmed that common genetic factors contribute to these associations (e.g., Haberstick et al., 2011; Krueger et al., 2002), yet unique genetic and environmental influences exist on each of these phenotypes (Hicks et al., 2011; Kendler et al., 2003). The primary aim of this study was to build on previous research (e.g., Bailey et al., 2011; Baker et al., 2012; Gillespie et al., 2009) to better understand the influence of substance-specific family environments in relation to unique adult nicotine and alcohol dependence after accounting for the relationship between parent–child relationship quality and general EXT liability. To do this, we examined the relationships between general and specific characteristics of the adolescent family environment in relation to general and specific facets of young adult EXTs, as proposed by Bailey et al. (2011). Furthermore, we investigated genetic and environmental influences on these relationships by comparing results across a group of adolescents who were adopted and not biologically related to their parents or siblings with a group of adolescents who were the biological offspring of their parents and had a full biological sibling.
Passive rGE refers to the situation in which parents provide both gene endowment and rearing environment for their biologically related children, making it impossible to distinguish whether the association between parenting and child outcomes is attributable to genetic or environmental influence (Scarr and McCartney, 1983; also see Dick, 2011). Of note, we replicated the long-term influence of the adolescent parent–child relationship on young adult EXTs demonstrated by Bailey et al. (2011) in both adopted and biological offspring samples. This association was not significantly different across adopt and bio groups, providing evidence inconsistent with a passive rGE explanation. Nonetheless, the relationship between family environment and adult EXTs could still be due to evocative rGE (i.e., adolescents may evoke less bonding, more conflict, and poorer management practices from their parents because of their EXT-influenced genotype). Prior research has supported both common genetic (Neiderhiser et al., 1999; Pike et al., 1996) and environmental factors (Burt et al., 2007; Klahr et al., 2011) for this association. Thus, the relationship between adolescent parent–child relationship quality and young adult EXTs is likely attributable to a combination of evocative rGE and environmental influences but not passive rGE.
Following previous research demonstrating an environmentally mediated relationship between parental and adolescent smoking (Keyes et al., 2008), we found that parental tobacco use during adolescence and adult nicotine dependence were linked for adopted adolescents (who do not share genes with their parents), suggesting environmental mediation. Nonetheless, caution is warranted in interpreting this finding, as we did not see a significant relationship between parental tobacco use and child nicotine dependence for adolescents who are biologically related to parents; one would expect to see a consistent relationship across groups. We are a bit puzzled by these results. It could be that the effect of parental tobacco use on child nicotine dependence is quite small, making it difficult to detect using smaller samples (the sample size for the biologically related adolescents was nearly half the sample size as the adopted adolescents in this study). Notably, the reliabilities of parental tobacco and alcohol use during the last 12 months at the adolescent assessment were also low (α’s ranged from .65 to .75), which may have affected this lack of association for the biological offspring.
Perhaps more telling is the influence of the offspring’s age when parental tobacco use was measured; we aimed to have comparable ages of assessment as the original Bailey et al. (2011) study, which assessed adolescent family environments at ages 10–18 and adult EXT outcomes at age 24. Indeed, the mean age at which the adolescent family environments were assessed in this study was 18.2 years; however, the ages ranged from 16.5 to 21.3 years of age (64% were ages 17–19), and about 70% of the adolescents lived at home at the time of their adolescent assessment. We conducted a follow-up analysis and confirmed a similar pattern of results across those who lived at home or did not live at home at the time of the adolescent assessment, with the exception that parental smoking during adolescence was not significantly associated with the unique influence on adult nicotine dependence in the adopted sample whether they lived at home or did not live at home. The magnitude of the effect in both groups (lived at home vs. did not live at home) was roughly equivalent to the original study results; thus, this may be a power issue in detecting a relatively small effect size. Nonetheless, perhaps the relationship between parental tobacco use and adult nicotine dependence would be more prominent if parental tobacco use was assessed at an earlier age in the offspring’s adolescence (i.e., ages 10–17) rather than just in the last 12 months of the adolescent assessment. Very few adolescents in this sample were less than age 17 (65 adoptees and 54 biological offspring); thus, we did not have the power to adequately test this hypothesis.
Turning to sibling effects, we found that neither sibling tobacco nor alcohol use during adolescence predicted unique variance in later nicotine or alcohol dependence. Instead, sibling tobacco and alcohol use were related to subsequent EXTs, but only for the adolescents with full biological siblings. This suggests that these associations were at least in part attributable to shared genes, particularly the relationship between sibling tobacco use and EXT (because the group difference was significant). This is not surprising given that adolescent siblings are often similar in their adolescent substance use (Low et al., 2012; Rowe and Gulley, 1992; Samek and Rueter, 2011; Whiteman et al., 2013) and that the link between adolescent substance use and subsequent substance use disorders in adulthood has been shown to be primarily influenced by genetic factors (Agrawal et al., 2009; Sartor et al., 2009). Of note, the overall pattern of family environmental effects in relation to adult EXTs differed for parental and sibling environments, demonstrating the need to partial these unique family environments in applications of the hierarchical model of family environmental risk as proposed by Bailey et al. (2011).
There were several limitations to this study. The first limitation concerns generalizability; although this sample was generally representative of the population from which it was drawn in terms of parental socioeconomic status, ethnicity, and marital status, the majority of participants lived in two-parent households during adolescence and with parents who identified predominantly as having European ancestry. Adopted adolescents had significantly higher socioeconomic status and were more likely to be of Asian than European ancestry compared with biological offspring (Table 1). Previous research has shown that those of Asian ancestry are more likely to have a version of the ALDH2 allele important to metabolizing the effects of alcohol. Thus, those with this version are less likely to report alcohol problems but not other substance use problems (Irons et al., 2007, 2012). Nonetheless, socioeconomic status and ethnic ancestry (European vs. Asian) were not significantly associated with EXT outcomes in this study (including alcohol dependence). Thus, even though adopted adolescents were more likely to have higher childhood socioeconomic status than biological off-spring, and those with Asian ancestry are more likely to have a copy of the ALDH2 allele related to a lower likelihood of problematic drinking behavior, differences in socioeconomic status and ethnic ancestry across samples did not likely affect study results. Nonetheless, replication across additional genetically informed designs variable in ethnic ancestry and socioeconomic status would be beneficial to evaluate the generalizability of the present study’s findings.
A second limitation is that siblings in this study were up to 5 years apart in age. The relationship between sibling substance use in adolescence and adult EXTs may be stronger for siblings closer in age compared with those further apart, although we were underpowered to detect such influences here. A third limitation concerns the adolescent assessment; we selected the assessment based on the first time adolescents were 16.5 years of age to closely match Bailey et al. (2011). Results may likely differ if parental and sibling smoking and drinking were measured earlier rather than in late adolescence. The fourth limitation is that modeling did not account for peer and other important social factors in adolescence (such as the substance-specific effect of parental attitudes toward substance use), demonstrating the need for continued research in the evaluation of general versus specific environmental effects of adolescence on adult EXTs. The fifth limitation notes that, although we controlled for autoregressive effects of adolescent smoking and drinking, we did not control for autoregressive effects of adolescent externalizing due to the complexity of the modeling; thus, the analysis was not fully prospective. To balance these limitations, the study has a number of important strengths, including a genetically informed design, a longitudinal sample, expert ratings of diagnostic outcomes, and multiple reporters.
The results of this study shed light on the degree to which the link between adolescent family environments and EXTs is genetically or environmentally mediated. We found that parent–child relationship quality in adolescence was related to adult EXTs for both adolescents who were biologically related to family members and those who were biologically unrelated to family members, which suggests that this relationship cannot be due to passive rGE. Sibling tobacco and alcohol use during adolescence were related to young adult EXTs, but only for biologically related siblings, suggesting at least some genetic influences on this association. Notably, a substance-specific effect of parental tobacco use on young adult nicotine dependence was found for parents and children who were not biologically related, suggesting at least some environmental influence in this relationship. However, we are hesitant in drawing conclusions based on this finding because we did not see a significant relationship between parental tobacco use and nicotine dependence for biologically related parents and children. Results suggest that, for the most part, characteristics of the adolescent family environment relate to adult EXTs generally rather than specifically to nicotine and alcohol use disorders.
Footnotes
Funding for this study was provided by National Institute on Drug Abuse Grants DA05147, DA 024411, DA008093, and DA009679 and National Institute on Alcohol Abuse and Alcoholism Grant AA09367. Diana R. Samek was also supported by postdoctoral training grant MH0 17069 from the National Institute of Mental Health. The National Institutes of Health had no further role in study design, collection, analysis, interpretation of the data, writing the report, or the decision to submit this article for publication.
References
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, Heath AC. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcoholism: Clinical and Experimental Research. 2009;33:2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bailey JA, Hill KG, Meacham MC, Young SE, Hawkins JD. Strategies for characterizing complex phenotypes and environments: General and specific family environmental predictors of young adult tobacco dependence, alcohol use disorder, and co-occurring problems. Drug and Alcohol Dependence. 2011;118:444–451. doi: 10.1016/j.drugalcdep.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SL, Pollock NK, Martin CS, Lynch KG. Risky sexual behaviors among adolescents with alcohol use disorders. Journal of Adolescent Health. 1999;25:179–181. doi: 10.1016/s1054-139x(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Baker JH, Maes HH, Kendler KS. Shared environmental contributions to substance use. Behavior Genetics. 2012;42:345–353. doi: 10.1007/s10519-011-9516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. Environmental contributions to adolescent delinquency: A fresh look at the shared environment. Journal of Abnormal Child Psychology. 2007;35:787–800. doi: 10.1007/s10802-007-9135-2. [DOI] [PubMed] [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Dick DM. Gene-environment interaction in psychological traits and disorders. Annual Review of Clinical Psychology. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: Evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. Relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling: A Multidisciplinary Journal. 2001;8:430–457. [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: A multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, Hewitt JK. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011;106:215–224. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: Univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior Genetics. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Irons DE, Iacono WG, Oetting WS, McGue M. Developmental trajectory and environmental moderation of the effect of ALDH2 polymorphism on alcohol use. Alcoholism: Clinical and Experimental Research. 2012;36:1882–1891. doi: 10.1111/j.1530-0277.2012.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons DE, McGue M, Iacono WG, Oetting WS. Mendelian randomization: A novel test of the gateway hypothesis and models of gene-environment interplay. Development and Psychopathology. 2007;19:1181–1195. doi: 10.1017/S0954579407000612. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kenny DA. 2012. Measuring model fit. Retrieved from http://davidakenny.net/cm/fit.htm.
- Keyes M, Legrand LN, Iacono WG, McGue M. Parental smoking and adolescent problem behavior: An adoption study of general and specific effects. American Journal of Psychiatry. 2008;165:1338–1344. doi: 10.1176/appi.ajp.2008.08010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MR, Berger AT, Wells BE, Cleland CM. Longitudinal associations between adolescent alcohol use and adulthood sexual risk behavior and sexually transmitted infection in the United States: Assessment of differences by race. American Journal of Public Health. 2012;102:867–876. doi: 10.2105/AJPH.2011.300373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Klahr AM, Rueter MA, McGue M, Iacono WG, Burt SA. The relationship between parent-child conflict and adolescent antisocial behavior: Confirming shared environmental mediation. Journal of Abnormal Child Psychology. 2011;39:683–694. doi: 10.1007/s10802-011-9505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York, NY: Guilford Press; 2005. [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Low SA, Shortt JW, Snyder J. Sibling influences on adolescent substance use: The role of modeling, collusion, and conflict. Development and Psychopathology. 2012;24:287–300. doi: 10.1017/S0954579411000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludeke S, Johnson W, McGue M, Iacono WG. Genetic amplification and the individualization of the parent-child relationship across adolescence. Psychological Medicine. 2013;43:413–422. doi: 10.1017/S0033291712001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. American Journal of Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Keyes M, Sharma A, Elkins I, Legrand L, Johnson W, Iacono WG. The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS) Behavior Genetics. 2007;37:449–462. doi: 10.1007/s10519-007-9142-7. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Iacono WG. Personality and substance use disorders: II. Alcoholism versus drug use disorders. Journal of Consulting and Clinical Psychology. 1999;67:394–404. doi: 10.1037//0022-006x.67.3.394. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén L. Mplus Version 6.12. Los Angeles, CA: Authors; 1998–2012. [Google Scholar]
- Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Relationships between parenting and adolescent adjustment over time: Genetic and environmental contributions. Developmental Psychology. 1999;35:680–692. doi: 10.1037//0012-1649.35.3.680. [DOI] [PubMed] [Google Scholar]
- Newcomb MD, McGee L. Influence of sensation seeking on general deviance and specific problem behaviors from adolescence to young adulthood. Journal of Personality and Social Psychology. 1991;61:614–628. doi: 10.1037//0022-3514.61.4.614. [DOI] [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depressive symptoms and antisocial behavior: A multivariate genetic analysis. Developmental Psychology. 1996;32:590–603. [Google Scholar]
- Prescott CA, Madden PAF, Stallings MC. Challenges in genetic studies of the etiology of substance use and substance use disorders: Introduction to the special issue. Behavior Genetics. 2006;36:473–482. doi: 10.1007/s10519-006-9072-9. [DOI] [PubMed] [Google Scholar]
- Ramrakha S, Bell ML, Paul C, Dickson N, Moffitt TE, Caspi A. Childhood behavior problems linked to sexual risk taking in young adulthood: A birth cohort study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1272–1279. doi: 10.1097/chi.0b013e3180f6340e. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z. Diagnostic Interview for Children and Adolescents–Revised: DSM-III-R version (DICA-R) St. Louis, MO: Washington University; 1988. [Google Scholar]
- Robins LN, Babor T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis, MO: Authors; 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, … Towle LH. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rowe DC, Gulley BL. Sibling effects on substance use and delinquency. Criminology. 1992;30:217–234. [Google Scholar]
- Samek DR, Keyes MA, Iacono WG, McGue M. Peer deviance, alcohol expectancies, and adolescent alcohol use: Explaining shared and nonshared environmental effects using an adoptive sibling pair design. Behavior Genetics. 2013;43:286–296. doi: 10.1007/s10519-013-9595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samek DR, Rueter MA. Considerations of elder sibling closeness in predicting younger sibling substance use: Social learning versus social bonding explanations. Journal of Family Psychology. 2011;25:931–941. doi: 10.1037/a0025857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: Evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-III-R (SCID) New York, NY: New York State Psychiatric Institute, Biometrics Research; 1987. [Google Scholar]
- Whiteman SD, Jensen AC, Maggs JL. Similarities and differences in adolescent siblings’ alcohol-related attitudes, use, and delinquency: Evidence for convergent and divergent influence processes. Journal of Youth and Adolescence. 2013;43:687–697. doi: 10.1007/s10964-013-9971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]