Abstract
Objective:
Individuals with multiple alcohol-dependent (AD) relatives are at increased risk for substance use disorders (SUDs). Prospective, longitudinal studies of high-risk (HR) individuals afford the opportunity to determine potential risk markers of SUDs. The current study assessed the effect of familial risk and genetic variation on Iowa Gambling Task (IGT) performance and tested for an association between IGT performance and SUD outcomes.
Method:
Individuals from multiplex AD families (n = 63) and low-risk (LR; n = 45) control families, ages 16–34 years, were tested using a computerized version of the IGT. SUD outcomes were assessed at approximately yearly intervals. 5-HTTLPR and COMT genotypes were available for the majority of participants (n = 86).
Results:
HR offspring showed poorer performance overall on the IGT and especially poor performance on the final trial block (Block 5), indicating a failure to improve decision making with previous experience. The 5-HTTLPR short-allele homozygote participants performed worse than long-allele carriers, with HR S/S carriers exhibiting particularly poor performance. There was no main effect of COMT on IGT performance and no significant COMT by Risk interaction. Significantly more individuals in the HR than LR group met criteria for SUD. Importantly, disadvantageous performance on IGT Block 5 was significantly associated with an earlier age at SUD onset.
Conclusions:
This is the first study to show that both familial risk of SUD and 5-HTTLPR variation impact performance on the IGT. Poorer IGT performance was associated with earlier onset of SUD, suggesting that HR individuals who fail to appropriately attend to long-term costs and benefits during a decision-making task are especially at risk for developing SUD in adolescence and young adulthood.
Individuals with a family history of alcohol dependence (AD) are at increased risk for developing substance use disorders (SUDs) (Cloninger et al., 1981; Goodwin et al., 1973). Those with a multiplex AD background have especially early SUD onset (Hill et al., 2008). Deficits in decision making often characterize individuals with SUD. The Iowa Gambling Task (IGT) has frequently been used to assess decision-making ability (Bechara et al., 1994). The IGT is a sensitive measure of decision making that simulates a real world decision situation requiring evaluation of the magnitude and timing of rewards and punishments under uncertain conditions. Higher short-term rewards are associated with larger punishments, making the choice of lower short-term rewards more advantageous in the long term (Bechara et al., 1994). Substance-misusing adults demonstrate maladaptive performance on the IGT, including lower net scores (Barry and Petry, 2008; Bechara and Martin, 2004; Bechara et al., 2001) and lesser improvement over trial blocks (Bechara and Martin, 2004).
To date, three studies have examined IGT performance in high-risk (HR) offspring. The first study did not find overall performance differences but did find that males with a family history of alcoholism paid greater attention to monetary gains and less attention to losses compared with controls (Lovallo et al., 2006). Dolan et al. contrasted IGT performance in individuals with and without SUD who were either family history positive or negative and found that adults with SUD performed worse than controls regardless of family history status (Dolan et al., 2008). In a functional magnetic resonance imaging (fMRI) version of the IGT, HR individuals showed greater activation in the left dorsal anterior cingulate cortex (dACC) and left caudate nucleus compared to low-risk (LR) controls, despite equivalent task performance, indicating that neural regulation of decision-making processes mediated by the dACC and caudate nucleus may be altered in HR individuals (Acheson et al., 2009). Given that decision-making deficits are observed in both adults with SUD and their HR offspring, decision-making ability may be a potential risk marker of SUD outcomes in HR individuals.
Neurobehavioral deficits observed in HR offspring are thought to be at least partially determined by inherited genetic variation (Hill, 2010). Decision making involves the amygdala, ventromedial prefrontal cortex, and anterior cingulate (McClure et al., 2004), and functional coupling among these regions is associated with variation in the serotonin transporter–linked polymorphic region (5-HTTLPR) (Rao et al., 2007). In combination with rs25531, 5-HTTLPR is functionally triallelic and influences serotonin transporter gene expression and serotonin concentration in the synaptic cleft (Heils et al., 1996); the short (S) and long-G (LG) alleles are associated with lower serotonin transporter activity than the long-A (LA) allele. 5-HTTLPR low-activity allele carriers have been shown to have impaired performance on the IGT (Homberg et al., 2008; van den Bos et al., 2009; Verdejo-García et al., 2013), although some studies have noted that long-allele carriers show impairment on early trial blocks (Gu et al., 2013; Stoltenberg and Vandever, 2010; Stoltenberg et al., 2011).
Variation in 5-HTTLPR has also been associated with AD, such that short-allele carriers have increased odds of being diagnosed with AD and other SUDs, although some results are equivocal (Cao et al., 2013; Feinn et al., 2005). Thus, heritable variation in 5-HTTLPR may contribute to decision-making deficits in HR offspring.
The catechol-O-methyltransferase (COMT) gene, rs4680, which is involved in the inactivation of dopamine in the synaptic cleft, also may influence decision-making abilities. A polymorphism in the COMT gene results in a valine (Val) to methionine (Met) amino acid substitution (Val158Met) and is associated with more extracellular dopamine in the prefrontal cortex (Lachman et al., 1996). Met allele homozygote participants show better performance on cognitive tasks, such as measures of working memory, although healthy Met-Met subjects have been found to have inflexible processing of affective stimuli and poorer performance on the IGT (van den Bos et al., 2009). Accordingly, variation in COMT may also influence decision-making abilities in individuals with family histories of alcoholism.
HR offspring have earlier onset of alcohol use (Hill et al., 2000). This tendency to start drinking earlier has been shown to increase the likelihood of developing AD. One large epidemiologic study found that the age of onset of regular drinking predicted the likelihood of AD, with those initiating drinking before age 14 having a fourfold increase in risk versus those starting at age 20 or later (Grant and Dawson, 1997). Among those who become alcohol dependent, early and late onset of dependence appears to be related to differences in age of onset for regular drinking (Chen et al., 2011). Observations from our family studies (Hill et al., 2008, 2011) indicate that having an ultra-high-risk familial background is associated with significantly earlier onset of SUD and greater likelihood of ever developing an SUD.
These results indicate that age at first use of alcohol is a powerful predictor of lifetime alcohol abuse and dependence. Although offspring of alcoholics are at increased risk for early onset of drinking and development of SUD, not all HR adolescents go on to develop alcohol or other drug problems. Currently, the cognitive and behavioral factors that influence the likelihood that HR individuals will develop SUD in young adulthood are not well understood. Prospective, longitudinal studies of HR offspring afford the opportunity to determine potential risk markers of later SUD.
Decision-making during adolescence and young adulthood, when alcohol and other drug use behaviors first emerge, may confer risk or resilience for the development of SUD. The current study sought to compare performance on the IGT between ultra-high-risk offspring from multiplex, alcohol-dependent families and LR controls from a prospective family study. The goals of the study were to determine (a) whether HR offspring would display worse performance on the IGT overall; (b) if so, whether it might be the result of a failure to benefit from experience on earlier trials; (c) whether performance on the IGT is related to SUD outcome, including an earlier onset to develop SUD; and (d) if there may be preliminary support for an association between the 5-HTTLPR or COMT polymorphism and IGT performance.
Given previous reports of gender differences and Gender by 5-HTTLPR interactions on the IGT (He et al., 2010; Stoltenberg and Vandever, 2010), we also examined whether gender, a Gender by Risk interaction, or a Gender by Genotype interaction affected IGT performance. Finally, given findings of an association between working memory and IGT performance (Fridberg et al., 2013; Pecchinenda et al., 2006), we examined the effect of working memory on decision-making abilities.
Method
Participants
The present report is based on an analysis of data for 108 third-generation offspring who are part of an ongoing family study that selected families through their parents’ generation. The offspring were evaluated during childhood, adolescence, and young adulthood at approximately annual intervals. A total of 63 HR offspring from paternal multiplex families were studied (30 females and 33 males) along with 45 LR offspring (22 females and 23 males). Because of concerns about possible population stratification, the current analysis was limited to an all-White sample to identify potential genetic markers of risk for SUD. The study has ongoing approval from the University of Pittsburgh Institutional Review Board. All participants provided consent at each visit. Children provided assent with parental consent.
High-risk families.
HR offspring were drawn from families selected to be part of a larger family study of alcoholism based on the presence of two adult alcoholic brothers. These brothers are the fathers or uncles of the HR children in the present analyses (Aston and Hill, 1990; Hill et al., 1990). In-person structured interviews using the Diagnostic Interview Schedule (DIS) (Robins et al., 1981) had been performed for the majority of all living and available relatives of the proband by risk-status-blind interviewers, with two family history reports used for deceased or unavailable relatives. Families had not been included if primary, recurrent major depression, bipolar disorder, schizophrenia, or a primary SUD other than AD was present, for either the proband pair of AD brothers or their first-degree relatives.
Low-risk families.
LR community control families consisting of two brothers and their parents were identified through an index case who responded to a newspaper advertisement requesting participants who were interested in a study of heritable aspects of personality. Families were chosen on the basis of having the same structural characteristics as the HR families (at least two adult brothers) and an absence of axis I psychopathology based on the outcome of a DIS interview that provided Diagnostic and Statistic Manual (DSM) and Feighner criteria for alcoholism (Feighner et al., 1972). LR families were included if all first- and second-degree relatives of the index case were free of alcohol and other drug dependence.
Measures
Socioeconomic status.
Socioeconomic (SES) status was assessed using the Hollingshead Four-Factor Index of Socioeconomic Status (Hollingshead, 1975). The SES status closest to the time of IGT testing was selected using the participants’ current occupation and education.
Substance use disorder outcome measures.
SUD outcome was determined using age-appropriate clinical diagnoses obtained during childhood/adolescence (yearly before age 19) with the Schedule for Affective Disorders and Schizophrenia (K-SADS) (Chambers et al., 1985) and with the Composite International Diagnostic Interview (CIDI) (Janca et al., 1992) and CIDI–Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989) biennially during young adulthood.
Iowa Gambling Task.
The version of the IGT used in this study was acquired from Psychological Assessment Resources (Lutz, FL) using the gain/loss structure provided. In this task, the subject selects 100 cards from four decks (A, B, C, and D). After each card selection, an output is given: gain or a gain and loss of money. Two decks (A and B) are not advantageous as the final loss is higher than the final gain. Decks C and D, however, are advantageous because draws from this deck have smaller punishments. The final objective of the task is to make the greatest gain or as much money as possible. The trials are separated into five 20-card blocks. Overall net score and block net scores are calculated by subtracting the number of cards selected from the disadvantageous decks from the number of cards selected from the advantageous decks, with higher scores indicating better performance. T scores, normed for age, were used in analyses (Bechara, 2007).
Working memory score.
Participants were administered the Wechsler Memory Scale, Third Edition (Wechsler, 1997). The Working Memory Index is composed of scaled scores for the Letter–Number Sequencing and Spatial Span subtests.
DNA isolation and genotyping.
Genomic DNA was extracted from whole blood with a second aliquot prepared for Epstein Barr Virus (EBV) transformation and cryopreservation (Hill et al., 2004). Polymerase chain reaction (PCR) conditions were as described in Hill et al. (2012). Genotyping was completed on a Biotage PSQ 96MA Pyrosequencer (Biotage AB, Uppsala, Sweden). Each polymorphism was analyzed by PCR amplification incorporating a biotinylated primer. Thermal cycling included 45 cycles at an annealing temperature of 60 °C. The Biotage workstation was used to isolate the biotinylated single strand from the double-strand PCR products. The isolated product was sequenced using the complementary sequencing primer.
Single nucleotide polymorphism (SNP) genotyping quality control involved ongoing monitoring of SNP signals provided by Qiagen software. Output is provided using three categories for each SNP: pass, fail, and check. Data analysis was performed for only those signals meeting the “pass” criterion. Signals that failed or were returned as needing further checking were rerun. If after three attempts the SNP did not meet the pass criterion, it was eliminated from the analysis. Eighty-six subjects (HR = 55) in the current sample had available 5-HTTLPR and COMT genotyping.
5-HTTLPR.
The 5HTT–linked polymorphic region was amplified using primer sequences described by Wendland et al. (2006) to reveal the long (L) and short (S) variant. To test for the more accurate triallelic characterization (Hu et al., 2006), the rs25531 SNP was digested with the restriction endonuclease Hpall and visualized with agarose gel electrophoresis. Genotypes were determined using the L and S variation along with the rs25531 A or G nucleotide (LA, LG, SA, or SG). The three genotypes were LL (LALA), LS (LALG or LAS), and SS (LGLG, LGS, SASG, or SS). Analyses were based on the recessive model that presumes that SS individuals differ from those with 0 or 1 copy of the S allele. This model appears to be most highly associated with increased odds of developing an SUD (Cao et al., 2013).
Statistical analyses
Linear mixed-model analyses were used to assess risk group differences for overall IGT performance and for net scores from each block of trials as well as the relationship between genetic variation in 5-HTTLPR and COMT and IGT performance. Analyses were also performed for Total Money earned by familial risk status and by genetic variation. All analyses were performed using age, sex, and socioeconomic status as covariates. Because each family had been assigned a unique identification (ID) code, the Family ID could be used as a random effect to control for multiple siblings from the same family in these analyses.
Kaplan–Meier survival analyses were used to assess the relationship between risk and the age at onset of an SUD, IGT net scores and age of SUD onset, and genetic variation and age at SUD onset. To conduct the survival analyses, IGT net scores were recoded into categorical variables by using a median-split: Net scores equal to or above 50 were considered advantageous, whereas those below 50 were considered disadvantageous. All analyses were conducted in IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY).
Results
The HR offspring and LR controls did not significantly differ in gender composition, age at IGT assessment, age at study entry, age at last follow-up, length of follow-up, or socioeconomic status (Table 1). Of the 86 subjects with available genotyping, there were 18 5-HTTLPR L homozygotes, 47 heterozygotes, and 21 S homozygotes. A test of Hardy–Weinberg equilibrium showed that there was no departure from equilibrium across the sample, χ2(1) = 0.77, p = .38, or within the HR group, χ2(1) = 0.001, p = .98.
Table 1.
Sample demographics, Iowa Gambling Task (IGT) net scores, substance use disorder (SUD) outcomes, and 5-HTT-linked polymorphic region (5-HTTLPR) and catechol-O-methyltransferase (COMT) genotype in high-risk (HR) offspring and low-risk (LR) controls. Group comparisons were made using t tests for continuous variablesa and chi-square for frequency datab.
| Variable | HR (n = 63) M (SE) | LR (n = 45) M (SE) | p |
| Male/femaleb | 33/30 | 23/22 | n.s. |
| Age at study entrya | 11.38 (2.94) | 11.78 (2.55) | n.s. |
| Age at last follow-upa | 26.27 (4.92) | 25.09 (5.55) | n.s. |
| Length of follow-upa | 14.89 (5.69) | 13.31 (5.82) | n.s. |
| Age at IGT testinga | 25.35 (4.97) | 24.47 (5.60) | n.s. |
| Socioeconomic statusa | 38.79 (1.49) | 42.41 (1.76) | n.s. |
| IGT Total Net Scorea | 49.27 (8.86) | 53.33 (9.24) | .023 |
| IGT final block scorea | 47.27 (10.65) | 52.49 (11.54) | .017 |
| WMS working memorya | 21.94 (0.51) | 22.84 (0.73) | n.s. |
| Freq. (%) M (SE) | Freq. (%) M (SE) | ||
| Substance use disorderb | 39 (62%) | 9 (20%) | <.001 |
| Age at SUD onseta | 18.87 (3.02) | 20.56 (4.33) | n.s. |
| 5-HTTLPR genotypesb | |||
| LL | 10 (18%) | 8 (26%) | |
| LS | 27 (49%) | 20 (64%) | n.s. |
| SS | 18 (33%) | 3 (10%) | |
| COMT genotypesb | |||
| Val/Val | 16 (29%) | 7 (23%) | |
| Val/Met | 33 (60%) | 17 (54%) | n.s. |
| Met/Met | 6 (11%) | 7 (23%) |
Notes: n.s. = nonsignificant; freq. = frequency; WMS = Wechsler Memory Scale.
For COMT, there were 23 Val homozygotes, 50 heterozygotes, and 13 Met homozygotes. There was no departure from equilibrium across the sample, χ2(1) = 2.75, p = .10, or within the HR group, χ2(1) = 3.20, p = .07. There were no significant differences in allele frequency by risk group, but there were marginally more 5-HTTLPR short allele homozygotes in the HR group, χ2 = 5.73, p = .06. Working Memory Index scores did not differ by risk, F(1, 67.58) = 0.105, p = .75, or genotype, F(1, 83.25) = 0.07, p = .80.
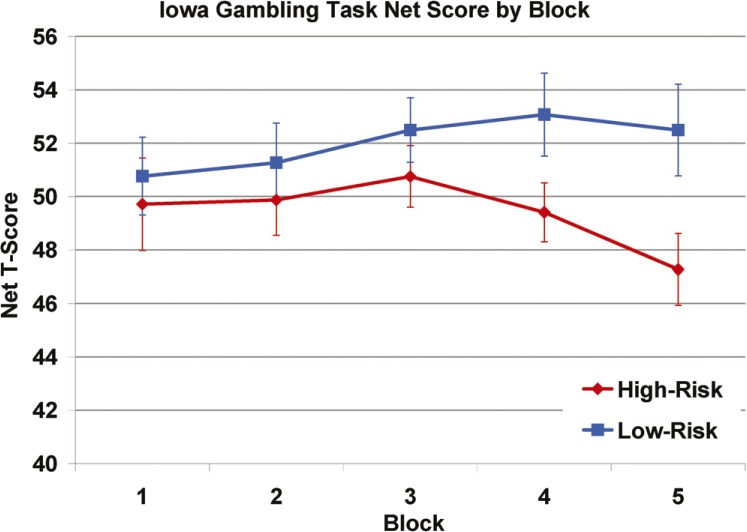
Consistent with our hypotheses, HR offspring had significantly lower Total Net Scores on the IGT overall, F(1, 59.80) = 4.17, p = .046, and on Block 5, F(1, 56.30) = 5.54, p = .022, than LR controls (Figure 1). These effects remained significant after accounting for the Working Memory Index score. HR offspring chose significantly fewer cards from deck D, the advantageous deck that offers frequent small gains, F(1, 106) = 8.937, p = .003, and marginally more cards from deck A, the disadvantageous deck with more frequent small losses, F(1, 106) = 3.522, p = .063, compared with LR controls.
Figure 1.
Iowa gambling task net score by block: High-risk offspring (n = 63) had significantly lower net scores on the Iowa Gambling Task overall and on Block 5 than low-risk controls (n = 45).
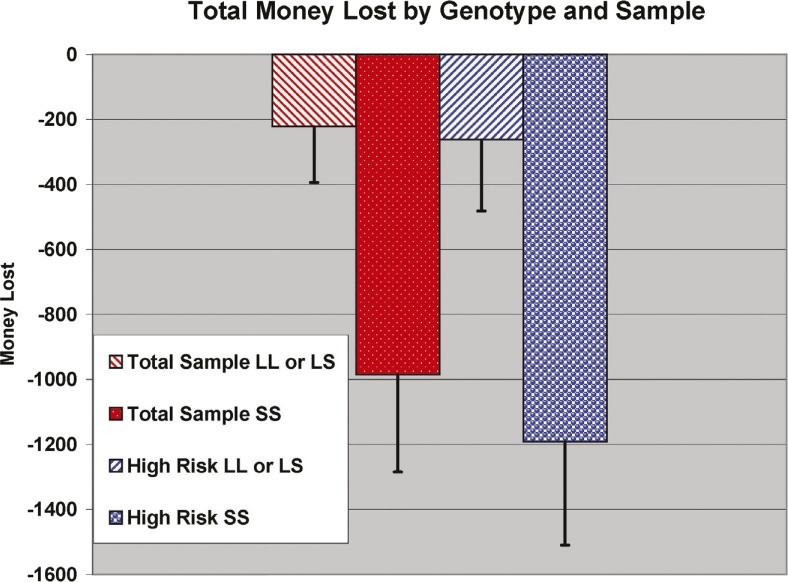
Across the entire sample, and without consideration of familiar risk, we first tested the effect of variation in the 5-HTTLPR polymorphism covarying age, sex, and SES. Those participants who were homozygous for the short allele had significantly lower IGT net scores, F(1, 73.91) = 5.39, p = .023, and earned less Total Money, F(1, 68.12) = 4.95, p = .029, than those carrying one or two L alleles (Figure 2). It was not possible to adequately test the interaction of familial risk status and 5-HTTLPR genotype because of the small number of control individuals (n = 3) with the SS genotype. However, analysis of genotypic variation within the HR group revealed a significant difference for IGT total scores, F(1, 43.48) = 4.42, p = .041 (Table 2).
Figure 2.
Total Money lost by genotype and sample: Both across all participants and within the high-risk group, 5-HTTLPR short-allele homozygotes had significantly lower Iowa Gambling Task net scores and earned less Total Money than those carrying one or two L alleles.
Table 2.
Linear mixed model analysis of Iowa Gambling Task (IGT) performance by familial risk and 5-HTTLPR LL/LS vs. SS genotype carriers (adjusted means and standard deviations)a
| 5-HTTLPR and IGT Net Score |
5-HTTLPR and IGT Total Money |
|||||||
| Total sample |
High-risk |
Total sample |
High-risk |
|||||
| Variable | M (SD) | p | M (SD) | p | M (SD) | p | M (SD) | p |
| LL and LS | 51.78 (1.24) | 0.45 | 50.77 (1.63) | 0.52 | -231.40 (173.48) | 0.35 | -255.06 (225.51) | 0.14 |
| SS | 46.89 (2.14) | 45.72 (2.34) | -970.79 (303.69) | -1,217.39 (325.73) | ||||
Means are adjusted for multiple siblings in the family.
Analysis of Total Money won by genotype within the HR group also showed significant effects, F(1, 43.05) = 6.03, p = .018. There was no main effect of COMT on IGT performance (p = .89) and no significant COMT × Risk interaction (p = .44). Because genotypes were available for only a subset of individuals with IGT performance (N = 86), the power to detect genotypic variance was rather low. However, power analysis within the HR group did increase to 0.70.
A main effect of gender on the IGT overall net score was not seen, F(1, 103.08) = 0.933, p = .34, although females performed more poorly than males on IGT Block 5, F(1, 105.42) = 4.61, p = .034. When risk was added to the model, the effect of gender was not significant, F(1, 103.96) = 1.65, p = .21. A Gender by 5-HTTLPR interaction for the IGT overall net score, F(1, 82.00) = 2.50, p = .12, or Block 5 score, F(1, 81.77) = 0.031, p = .86, was not seen. Because gender did not explain any variance in IGT performance above and beyond risk status or 5-HTTLPR genotype, it was excluded from further analyses.
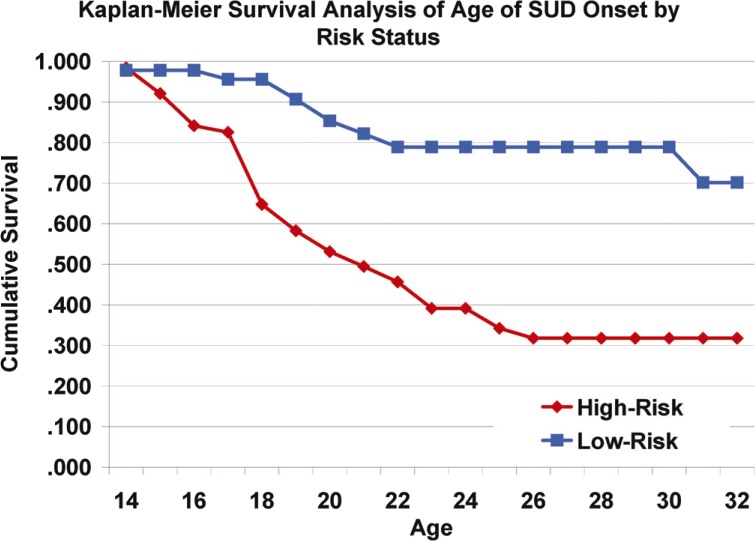
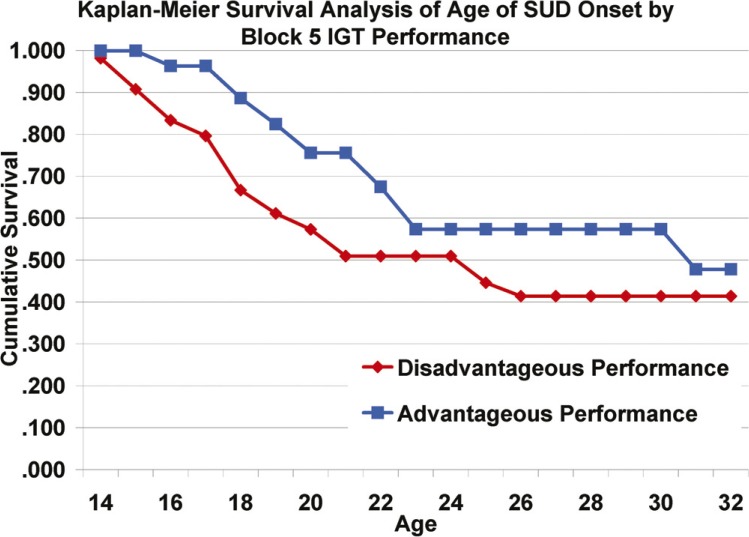
Survival analysis for HR and LR groups showed significantly earlier onset of SUD for the HR offspring (Tarone–Ware χ2 = 16.78, p < .001) (Figure 3). Although SUD survival was not significantly influenced by overall IGT net scores (Tarone–Ware χ2 = 0.62, p = .43), disadvantageous performance on Block 5 was associated with earlier age at SUD onset (Tarone–Ware χ2 = 4.833, p = .028) (Figure 4). In addition, there was a marginally significant main effect of 5-HTTLPR genotype on SUD survival (Tarone–Ware χ2 = 2.87, p = .09), such that short-allele homozygotes had earlier age at SUD onset.
Figure 3.
Kaplan–Meier survival analysis of age at onset of substance use disorder by risk status: Significantly more high-risk offspring (n = 39, 62%) met criteria for substance use disorder than low-risk controls (n = 9, 20%), and high-risk offspring had earlier ages at substance use disorder onset.
Figure 4.
Kaplan–Meier survival analysis of age at onset of substance use disorder by Block 5 Iowa Gambling Test performance: Individuals who performed disadvantageously (net score < 50; n = 54) on Block 5 of the Iowa Gambling Test had significantly earlier ages at substance use disorder onset than those who performed advantageously (net score ≥ 50; n = 54). This effect remained significant after accounting for familial risk status. The effect of Block 5 performance is most prominent for those experiencing early-onset SUD.
A post hoc Cox survival analysis was performed to assess the effect of Block 5 performance as a predictor of SUD survival using familial risk group status and genotype as covariates. The overall survival model indicated a highly significant relationship between these predictors and SUD onset (χ2 = 16.508, p = .001). In addition, main effects of Block 5 performance (Wald = 4.79, p = .029) and familial risk status (Wald = 7.396, p = .007) remained significant. In this model, there was no main effect of 5-HTTLPR variation (Wald = 0.96, p = .33).
The mean age at IGT testing was higher than the mean age at SUD onset. In an attempt to disentangle the impact of family history of alcoholism and personal exposure to alcohol and/or other drugs, we repeated the analysis of IGT net score by risk group within the subsample of participants who did not meet criteria for SUD before they completed the IGT task (n = 60; 24 HR, 36 LR). Unaffected HR offspring performed significantly worse than unaffected LR controls on the IGT overall, F(1, 42.41) = 4.80, p = .034. Within the sample of unaffected HR offspring, short-allele homozygotes had significantly lower IGT net scores, F(1, 40.90) = 4.91, p = .032, and earned less Total Money, F(1, 40.38) = 5.14, p = .029, than long-allele carriers.
Finally, to determine the relationship between personal exposure to alcohol or other drugs and IGT performance, linear mixed models were used to assess the relationship between presence of an SUD and IGT net score performance. Generalized linear mixed models were used to assess the relationship between the number of years a subject met criteria for an SUD before IGT administration and IGT net score. The IGT overall net score was not related to the presence of an SUD diagnosis, F(1, 105.92) = 0.326, p = .57, or the length of time with an SUD diagnosis, F(1, 42) = .23, p = .63.
Discussion
This is the first study to show that individuals at high risk for developing an SUD have lower net scores on the IGT. The deficient performance of HR offspring suggests that familial risk influences the ability to appropriately weigh long-term rewards and punishments. Unlike LR controls, HR offspring performed especially poorly on the final trial block (Block 5), indicating a failure to learn from prior negative outcomes. These results are not attributable to poorer working memory in HR offspring. Individuals at ultra-high familial risk for AD who were also homozygous for the short allele of the 5-HTTLPR polymorphism showed exceptionally poor IGT performance.
However, these results should be considered preliminary given that genotyping was available for only 86 individuals. Poor performance among HR offspring was observed for the sample as a whole and within the subsample of individuals who had no current or past diagnosis of alcohol or other drug abuse or dependence, suggesting that maladaptive decision making in substance misuse is not solely a consequence of the effects of consuming alcohol or other drugs. For individuals who did meet criteria for SUD, poor performance on the IGT was associated with earlier age of SUD onset above and beyond the effect of familial risk, which in turn predicts poorer long-term prognosis for alcohol and other drug abuse and dependence.
Adults with SUD have consistently been found to have poor performance on the IGT, and the finding that their HR offspring show similar deficits indicates that maladaptive decision making may be part of a behavioral spectrum of risk for SUD. Abnormal sensitivity to rewards may reflect inherited differences in brain function underlying a tendency to value positive and rewarding cues. Neurobiological theories of alcohol and other drug dependence implicate dysfunctions in neural circuitry associated with distorted evaluation and appraisal of positive and negative consequences as well as diminished control over behavior, functions that play a role in decision making (Goldstein and Volkow, 2002; Krawczyk, 2002; Tessner and Hill, 2010).
Accordingly, others have reported that HR offspring who do not meet criteria for SUD show abnormal neural activation in the dorsal anterior cingulate cortex and caudate nucleus while performing the IGT despite similar behavioral performance (Acheson et al., 2009). Similarly, non–substance-misusing adults with a family history of alcoholism show different patterns of activation in the caudate, insula, orbitofrontal cortex, and nucleus accumbens than LR controls when anticipating rewards (Andrews et al., 2011). Inherited abnormalities in neural circuitry associated with risk-prone decision making may be an important factor contributing to the high prevalence of SUD in individuals with a family history of alcoholism.
Decision-making abilities were also related to variation in the serotonin transporter linked polymorphism, 5-HTTLPR, with individuals homozygous for the low-activity short allele showing poorer performance on the IGT, an effect that was most notable in the HR offspring. Serotonin neuronal cell bodies reside in the brainstem and project their axons into the prefrontal cortex, amygdala, striatum, and insular cortex, regions associated with affective processing and decision making (Bechara, 2005). The short allele is linked to reduced serotonin transporter expression (Heils et al., 1996), and low-activity allele carriers have reduced functional coupling between the amygdala and rostral anterior cingulate cortex as well as the amygdala and prefrontal cortex (Pezawas et al., 2005; Roiser et al., 2009). Thus, this polymorphism may affect decision making in HR populations through its association with emotional processing.
Previous findings on the effect of the 5-HTTLPR polymorphism on decision making have been mixed; the short allele has been linked to poor performance on the IGT in healthy females (Homberg et al., 2008; van den Bos et al., 2009), cannabis users (Verdejo-García et al., 2013), individuals with obsessive–compulsive disorder (da Rocha et al., 2008), and within a large sample of Chinese undergraduate students (He et al., 2010). However, samples of healthy adults and schizophrenics carrying the long allele have been found to have impaired performance on the first block of the IGT (Gu et al., 2013; Stoltenberg and Vandever, 2010; Stoltenberg et al., 2011). Thus, additional research is needed to investigate possible mediators and moderators of the relation between this polymorphism and decision making.
A main effect of COMT variation or its interaction with familial risk on IGT performance was not seen. Similarly, previous studies have failed to find a main effect of this polymorphism on IGT performance in cannabis users (Verdejo-García et al., 2013) or in healthy populations (He et al., 2012; Kang et al., 2010). However, van den Bos and colleagues previously reported that healthy female Met homozygotes performed worse on later trial blocks than Val homozygotes, although the effect was no longer significant when the heterozygote genotype was considered (van den Bos et al., 2009). Some evidence suggests that the COMT Met allele is protective for cognitive functions such as working memory, but the Val allele is protective for flexible, emotional processes (van den Bos et al., 2009). Thus, the effect of this allele may not be robust enough to detect on a task such as the IGT that contains both cognitive and emotional decision making, especially in a small sample.
Consistent with previous research (Hill et al., 2008), HR individuals were more likely to develop SUD than LR controls. Importantly, poorer IGT performance on the last trial block was associated with earlier onset of SUD, even after accounting for variance explained by familial risk status. Because individuals with an earlier onset of SUD also had more years of personal exposure to alcohol and other drugs, it is difficult to determine whether especially poor IGT performance is a risk factor for, or a consequence of, early drug and alcohol abuse or dependence. However, neither the presence of SUD before the IGT nor the length of time a participant met criteria for an SUD before IGT administration was related to task performance, indicating that the relation between very early onset of alcohol and other drug use and especially poor IGT performance may be at least partially attributable to premorbid risk.
Our finding that HR offspring who had not developed SUD before IGT evaluation demonstrated poorer performance than LR controls suggests that IGT performance is a premorbid risk factor that can be used to assess potential for later development of SUD. Others have noted that disadvantageous performance on the IGT predicts more frequent heavy drinking in men 2 years after IGT assessment (Goudriaan et al., 2011). Future follow-up of the younger participants in our sample will allow us to address this hypothesis.
One limitation of the current study is the relatively wide age range of the participants who performed the IGT. Although age-corrected T scores were used in our analyses, decision-making abilities are believed to improve in adolescence and young adulthood (Cauffman et al., 2010), and future research is needed on decision-making abilities in HR offspring during specific developmental periods.
The present study found that HR individuals with the SS genotype of the 5-HTTLPR showed poorer performance on the IGT whether assessed as Total Net Scores or in terms of Total Money earned. A limitation to our analysis was that we were unable to test this effect across risk groups because of the small number of SS genotypes among the control sample, with only three cases present. Nevertheless, we did find that within the HR subjects, statistically significant differences in IGT performance were seen by genotype.
Our conclusions regarding the effect of 5-HTTLPR genotype and IGT performance within the HR group are an important preliminary finding. Although the power for the overall relationship between 5-HTTLPR and IGT performance was rather low, within the HR group power increased to 0.70. Selection of multiplex families appears to provide an opportunity for testing genes that may have an especially high penetrance. The 5-HTTLPR polymorphism may be especially relevant to decision making within HR families.
Use of ultra-high-risk AD families can be viewed as either a strength or weakness of our findings. On the positive side, families with increased transmission for AD are ideal for finding genetic variation and endophenotypic characteristics associated with familial risk. However, these families are not representative of AD families in the general population. Follow-up of offspring from these multiplex families indicates an exceptionally high rate of AD and substance use by young adulthood (Hill et al., 2008, 2011). Although these families may not be representative of AD families in the general population, the study of multiplex families provides an efficient means for identifying risk factors and genetic variation that can then be taken to population samples for replication.
In conclusion, these data suggest that HR offspring display deficits in decision-making regardless of whether they have initiated behaviors associated with misuse of alcohol and other drugs. Decision-making abilities are influenced by both familial risk and variation in 5-HTTLPR, and failure to attend appropriately to long-term costs and benefits is associated with SUD outcomes. Inherited abnormalities in neural circuitry associated with risk-prone decision making may lead individuals with a family history of alcoholism to make poor decisions regarding alcohol and other drug consumption, thereby increasing their likelihood of developing SUD in adolescence and young adulthood. Identification of risk factors for SUD is important to the search for ways in which those at highest risk can be identified for possible intervention.
Acknowledgments
The authors acknowledge the contributions of Nicholas Zezza and Scott Stiffler, who completed all of the genotyping for this study, and recognize the many clinical staff members who assessed research participants to determine phenotypic data. We also acknowledge the contribution of the family members who have contributed DNA and have continued to be involved in clinical follow-up.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA018289, AA05909, AA08082, and AA015168 (to Shirley Y. Hill).
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Drug and Alcohol Dependence. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biological Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston CE, Hill SY. Segregation analysis of alcoholism in families ascertained through a pair of male alcoholics. American Journal of Human Genetics. 1990;46:879–887. [PMC free article] [PubMed] [Google Scholar]
- Barry D, Petry NM. Predictors of decision-making on the Iowa Gambling Task: Independent effects of lifetime history of substance use disorders and performance on the Trail Making Test. Brain and Cognition. 2008;66:243–252. doi: 10.1016/j.bandc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A. Iowa Gambling Task Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2007. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Cao J, Hudziak JJ, Li D. Multi-cultural association of the serotonin transporter gene (SLC6A4) with substance use disorder. Neuropsychopharmacology. 2013;38:1737–1747. doi: 10.1038/npp.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46:193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Prescott CA, Walsh D, Patterson DG, Riley BP, Kendler KS, Kuo P-H. Different phenotypic and genotypic presentations in alcohol dependence: Age at onset matters. Journal of Studies on Alcohol and Drugs. 2011;72:752–762. doi: 10.15288/jsad.2011.72.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. British Journal of Addiction. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- da Rocha FF, Malloy-Diniz L, Lage NV, Romano-Silva MA, de Marco LA, Correa H. Decision-making impairment is related to serotonin transporter promoter polymorphism in a sample of patients with obsessive-compulsive disorder. Behavioural Brain Research, 2008;195:159–163. doi: 10.1016/j.bbr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Dolan SL, Bechara A, Nathan PE. Executive dysfunction as a risk marker for substance abuse: The role of impulsive personality traits. Behavioral Sciences & the Law. 2008;26:799–822. doi: 10.1002/bsl.845. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Gerst KR, Finn PR. Effects of working memory load, a history of conduct disorder, and sex on decision making in substance dependent individuals. Drug and Alcohol Dependence. 2013;133:654–660. doi: 10.1016/j.drugalcdep.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Archives of General Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and response inhibition as predictors of heavy alcohol use: A prospective study. Alcoholism: Clinical and Experimental Research. 2011;35:1050–1057. doi: 10.1111/j.1530-0277.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gu H, Liu C, Liu C, Chen M, Zhang Q, Zhai J, Chen C. The combined effects of the 5- HTTLPR and HTR1A rs6295 polymorphisms modulate decision making in schizophrenia patients. Genes, Brain & Behavior. 2013;12:133–139. doi: 10.1111/j.1601-183X.2012.00866.x. [DOI] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Lu Z, Dong Q, Lei X, Bechara A. Serotonin transporter gene-linked polymorphic region (5-HTTLPR) influences decision making under ambiguity and risk in a large Chinese sample. Neuropharmacology. 2010;59:518–526. doi: 10.1016/j.neuropharm.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Lu Z-L, Chen C, Lei X, Bechara A. COMT Val158Met polymorphism interacts with stressful life events and parental warmth to influence decision making. Scientific Reports. 2012;2:677. doi: 10.1038/srep00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hill SY. Neural plasticity, human genetics, and risk for alcohol dependence. International Review of Neurobiology. 2010;91:53–94. doi: 10.1016/S0074-7742(10)91003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biological Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: A prospective study during childhood and adolescence. Psychiatry Research. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Tessner KD, McDermott MD. Psychopathology in offspring from families of alcohol dependent female probands: A prospective study. Journal of Psychiatric Research. 2011;45:285–294. doi: 10.1016/j.jpsychires.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Weeks DE, Jones BL, Zezza N, Stiffler S. ASTN1 and alcohol dependence: Family-based association analysis in multiplex alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2012;159B:445–455. doi: 10.1002/ajmg.b.32048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Zubin J, Steinhauer SR. Personality resemblance in relatives of male alcoholics: A comparison with families of male control cases. Biological Psychiatry. 1990;27:1305–1322. doi: 10.1016/0006-3223(90)90501-r. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Homberg JR, van den Bos R, den Heijer E, Suer R, Cuppen E. Serotonin transporter dosage modulates long-term decision-making in rat and human. Neuropharmacology. 2008;55:80–84. doi: 10.1016/j.neuropharm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Hu X-Z, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janca A, Robins LN, Cottler LB, Early TS. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI Field Trials–Wave II at the St Louis site. British Journal of Psychiatry. 1992;160:815–818. doi: 10.1192/bjp.160.6.815. [DOI] [PubMed] [Google Scholar]
- Kang JI, Namkoong K, Ha RY, Jhung K, Kim YT, Kim SJ. Influence of BDNF and COMT polymorphisms on emotional decision making. Neuropharmacology. 2010;58:1109–1113. doi: 10.1016/j.neuropharm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and Biobehavioral Reviews. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: Studies from the Oklahoma family health patterns project. Alcoholism: Clinical and Experimental Research. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: The modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Pecchinenda A, Dretsch M, Chapman P. Working memory involvement in emotion-based processes underlying choosing advantageously. Experimental Psychology. 2006;53:191–197. doi: 10.1027/1618-3169.53.3.191. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GMV, Kaercher KA, Farah MJ. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biological Psychiatry. 2007;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, Dolan RJ. A genetically mediated bias in decision making driven by failure of amygdala control. Journal of Neuroscience. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Lehmann MK, Anderson C, Nag P, Anagnopoulos C. Serotonin transporter (5-HTTLPR) genotype and childhood trauma are associated with individual differences in decision making. Frontiers in Genetics. 2011;2:33. doi: 10.3389/fgene.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Vandever JM. Gender moderates the association between 5-HTTLPR and decision-making under ambiguity but not under risk. Neuropharmacology. 2010;58:423–428. doi: 10.1016/j.neuropharm.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychology Review. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Homberg J, Gijsbers E, den Heijer E, Cuppen E. The effect of COMT Val158 Met genotype on decision-making and preliminary findings on its interaction with the 5-HTTLPR in healthy females. Neuropharmacology. 2009;56:493–498. doi: 10.1016/j.neuropharm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Fagundo AB, Cuenca A, Rodriguez J, Cuyás E, Langohr K, de la Torre R. COMT val158met and 5-HTTLPR genetic polymorphisms moderate executive control in cannabis users. Neuropsychopharmacology. 2013;38:1598–1606. doi: 10.1038/npp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale, Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]