Abstract
Objective:
Individuals suffering from alcohol use disorders tend to show impairments in inhibitory control, and these deficits may be exacerbated in the presence of craving-inducing alcohol cues. Imbalances between neural reward and control networks can influence the trajectory of alcohol use disorders such that individuals for whom the reward (craving) network strongly overpowers the control (inhibition) network tend to have worse outcomes. Brain activation related to inhibitory control can be examined using the stop-signal task (SST), which requires balancing speed and accuracy in the context of frequent go and infrequent stop stimuli. Further, brain areas related to cue-induced craving can be studied using visual cue tasks comparing neural responses to alcohol and control images. This study aims to explore the interaction of inhibitory control and cue-elicited craving using a single functional neuroimaging task.
Method:
We developed a novel task involving presentation of alcohol and control cues concurrently with a standard SST paradigm and administered it to 53 heavy drinkers (29 women).
Results:
Successful response inhibition during alcohol compared to control picture trials was associated with significant activation in anterior cingulate, supplementary motor, and frontal inferior regions, and this activation was differentially related to alcohol use symptom severity across several self-report measures.
Conclusions:
Results suggest that recruitment of compensatory error detection and inhibitory control resources may be required for successful inhibition in the presence of alcohol cues among more severe drinkers. These preliminary findings support the construct validity of the task and indicate several methodological alterations to the task’s design that should be implemented in future studies.
Alcohol dependence represents a significant public health concern in the United States (Rehm et al., 2009), with 18% of Americans experiencing some form of alcohol use disorder (AUD) during their lifetime (Hasin et al., 2007). Unfortunately, current treatments for AUDs have proven to be only modestly effective (Miller et al., 2001). One factor that may contribute to relapse and the difficulty in treating alcohol dependence involves a general loss of inhibitory control and, more specifically, a loss of control over alcohol consumption. For example, heavy drinkers tend to demonstrate impulsive responding and decreased performance on inhibitory control tasks (Bjork et al., 2004; Lawrence et al., 2009; Mitchell et al., 2005), suggesting that their failure to control problem drinking may be compromised by a general response inhibition deficit. In addition to problems with response inhibition, heavy substance users also show altered neural reward processing that appears to promote craving in response to drug relevant cues (Maas et al., 1998; Sinha and Li, 2007; Wrase et al., 2007).
In recent years, the etiology of substance dependence has been conceptualized as an imbalance between the incentive reward network (Kalivas and Volkow, 2005), which underlies the urge to use a substance, and the control network (Bechara, 2005), which influences whether impulses are acted on (Hutchison, 2010; Hutchison et al., 2008). The incentive network includes structures involved in reward and reinforcement (i.e., ventral tegmental area, nucleus accumbens, thalamus, insula, and amygdala; Filbey et al., 2009; Karoly et al., 2013a; Koob and Le Moal, 2001; McFarland and Kalivas, 2001), and the control network includes structures related to executive functioning (i.e., inferior frontal gyrus [IFG], orbitofrontal cortex, and the dorsolateral prefrontal cortex; Boettiger et al., 2009; Claus et al., 2011; Karoly et al., 2013a). The extent to which the reward network dominates the control network is likely to influence whether an individual will act on the craving for or urge to use a substance. Further, it has been demonstrated that the imbalance between these two networks may be one of the strongest determinants of loss of control over substance use (Baler and Volkow, 2006; Bickel et al., 2007; Goldstein and Volkow, 2002).
In addition, the balance of reward processing and inhibitory control appears to become increasingly dysregulated as the dependence cycle progresses from recreational use to the dependent state (Hutchison, 2010; Karoly et al., 2013a). Thus, to better understand the progression of substance use disorders, it is necessary to consider the interaction between these two substance-related neural adaptations: (a) compromised inhibitory control and (b) changes in reward processing such that drug cues become more salient and levels of cue-induced craving increase. Characterizing interrelated neural mechanisms that underlie these changes could be useful for informing pharmacological treatments and designing more efficacious relapse-prevention programs.
Several behavioral tasks have been widely used to study inhibitory control at the neural level. Such tasks generally require individuals to balance competing cognitive demands and respond to stimuli both quickly and accurately. Specifically, brain activation related to inhibition/suppression of a pre-potent manual response can be examined using the stop-signal task (SST; Logan, 1994), in which subjects perform intermixed stop-and-go trials and are asked to inhibit habitual “go” responding whenever an infrequent “stop” signal is presented. Successful performance involves simultaneous self-monitoring of stop accuracy and go speed and requires strategic adaptation of responses to adequately balance the conflicting demands of the stop-and-go trials (Verbruggen and Logan, 2008). Neuroimaging research has made progress identifying several neural correlates of stop-signal inhibition among healthy individuals. Functional magnetic resonance imaging (fMRI) studies implementing this paradigm have shown that response inhibition involves the right IFG (R-IFG), insula, basal ganglia, pre-supplementary motor area (pre-SMA), and subthalamic nucleus (Aron and Poldrack, 2006; Aron et al., 2007a; Chevrier et al., 2007; Chikazoe et al., 2007; Li et al., 2008a).
The SST is particularly relevant in the context of substance use disorders, as heavy users tend to show impaired inhibitory control (Ernst and Paulus, 2005), and dependence by definition involves some degree of failure to inhibit pre-potent drug-taking behavior. It has been suggested that successful or unsuccessful response inhibition occurs as a result of the balance between neural inhibition and activation mechanisms (Burle et al., 2004; Jaffard et al., 2007; Narayanan and Laubach, 2006), and it is possible that this balance may be dysregulated among chronic substance users. Thus, brain-imaging studies have used the SST in an effort to delineate the neural correlates of impaired response inhibition among substance users. In one study, the rostral anterior cingulate cortex demonstrated less activation during stop-signal inhibition for cocaine users compared with controls (Li et al., 2008b). In heavy smokers, hyperresponsivity in the dorsal–medial prefrontal cortex was observed during both correct and incorrect inhibition trials (de Ruiter et al., 2012). Altered neural processing during inhibition was also observed among alcohol-dependent individuals compared with controls (Li et al., 2009). In a study of individuals with and without a family history of alcoholism, intravenous alcohol infusion during this task significantly reduced stop-trial minus go-trial activation in the right prefrontal cortex among family history–negative but not family history–positive subjects (Kareken et al., 2013).
Given the importance of the interaction between the reward and control networks, one of the most important limitations of previous studies is the fact that the two networks are often tested independently of one another. Thus, additional work is needed to concurrently examine both networks and determine whether alcohol dependence influences their interaction. Ideally, this would involve a task paradigm involving cue-exposure presented simultaneously with a cognitively demanding inhibition task. The present study evaluated a new version of the previously established SST, called the Stop Signal Alcohol Cue Task (SSACT), in which a standard stop-signal paradigm is combined with the presentation of visual alcohol cues and control (nonalcoholic beverage) cues, in an effort to further explicate the nature of the relationship between cue-induced craving and response inhibition among heavy drinkers.
Method
Participants
Participants (n = 53) were 28 women and 25 men ages 21–53 years (M = 28.30, SD = 6.9). Subjects were recruited from two university research sites. Data from 14 subjects were collected at the University of Colorado in Boulder, CO, and data from the remaining 39 subjects were collected at the Mind Research Network/University of New Mexico in Albuquerque, NM (Table 1). Subjects from the two sites did not differ significantly in terms of age, gender, or self-reported alcohol use severity, but the New Mexico sample was composed of significantly more subjects who identified their ethnicity as Hispanic. All participants were required to provide informed consent before participation. All study procedures were officially approved by the University of New Mexico’s Human Research Protections Office, and this research was approved by all relevant institutional review boards.
Table 1.
Sample demographics
| University of Colorado site n or M (SD) (n = 14) | Mind Research Network site n or M (SD) (n = 39) | Test statistic | p | |
| Gender | χ2(1, N = 53) = 2.24 | .14 | ||
| Male | 8 | 16 | ||
| Female | 6 | 23 | ||
| Age, in years | 27.86 (6.14) | 29.04 (7.61) | t(51) = -0.28 | .78 |
| Race | χ2(4, N = 53) = 6.18 | .19 | ||
| White | 14 | 26 | ||
| African American | 0 | 1 | ||
| Asian or Pacific Islander | 0 | 2 | ||
| American Indian/Native American | 0 | 3 | ||
| Mixed | 0 | 7 | ||
| Ethnicity | χ2(1, N = 53) = 5.57 | .02 | ||
| Hispanic or Latino | 0 | 12 | ||
| Non-Hispanic or Latino | 14 | 27 | ||
| AUDIT total score | 11.57 (5.45) | 10.28 (5.66) | t(51) = 0.74 | .46 |
| AUDIT consumption | 7.36 (2.02) | 6.64 (1.83) | t(51) = 1.22 | .23 |
| TLFB total drinks | 155.32 (95.6) | 94.38 (63.82) | t(50) = 2.655 | .011 |
| TLFB drinks per drinking day | 5.01 (2.66) | 4.46 (2.72) | t(50) = 0.660 | .512 |
Notes: AUDIT = Alcohol Use Disorders Identification Test; TLFB = Timeline Followback.
The minimum drinking criterion required for participation in the study was at least two drinks twice per week for women or at least three drinks twice per week for men. No maximum drinking criteria were imposed for participation in this study. A total of 23% of the sample (12 subjects) scored 16 or higher on the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993), which is suggestive of high levels of problematic or hazardous drinking. Subjects included in this study reported a wide range of alcohol consumption levels, with an overall mean AUDIT score of 10.62 (SD = 5.58; range: 4–24).
Individuals were excluded from participating in the study if they reported having suffered a serious medical illness in the past 6 months, were currently taking insulin, or met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994), criteria for a psychotic spectrum disorder. Because the risk to fetuses associated with MRI is unknown, women were excluded from participating in the scan if they were pregnant (as indicated by a urine pregnancy screen) or if they reported that they were trying to become pregnant.
Self-report measures
Demographics questionnaire.
A basic demographics questionnaire was administered to all subjects to collect information on age, sex, marital status, socioeconomic status, occupation, income, education, race, and ethnicity.
Alcohol Use Disorders Identification Test.
The AUDIT (Saunders et al., 1993) is a measure used to assess both current problems (occurring within the last 3 months) and lifetime problems associated with alcohol use. The AUDIT consists of 10 questions covering alcohol consumption, drinking behavior, adverse psychological reactions to alcohol, and alcohol-related problems. The maximum score on this measure is 40. In our sample, the AUDIT demonstrated good internal consistency reliability (α =. 828).
Sixty-day Timeline Followback (TLFB).
The TLFB (Sobell et al., 1979) is an interviewer-administered assessment used to obtain estimates of daily alcohol and other drug use. The TLFB has demonstrated good psychometric characteristics within a variety of drinker groups and can generate variables that provide a wide range of information about an individual’s drinking behavior (e.g., pattern, variability, and magnitude of drinking). This instrument requires participants to recall from memory the number of drinks consumed for each day over the prior 60 days. The total number of drinks consumed over 60 days (TLFB-TD) represents one measure of alcohol consumption that can be generated from the TLFB, and this metric has been used as an outcome in previous neuroimaging research (Karoly et al., 2013b).
Procedure
After answering initial screening questions over the phone, eligible participants completed a battery of self-report measures. Next, participants completed the MRI scanning session. Before entering the scanner, participants were presented with detailed instructions for the SSACT and were then given an opportunity to practice the task and ask the research assistant for assistance or clarification if necessary.
Functional magnetic resonance imaging
Stop-Signal Alcohol-Cue Task.
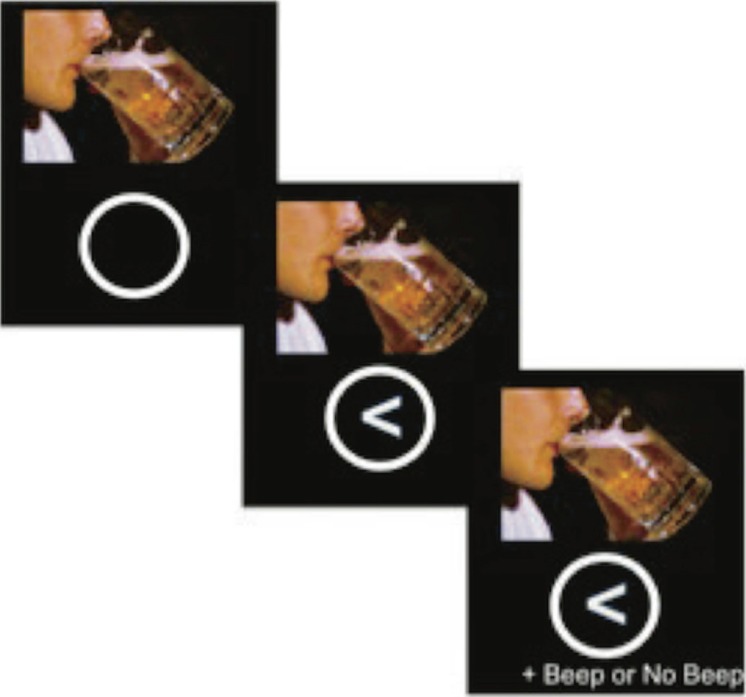
This task is comprised of three 6-minute functional runs. All subjects received the three runs in the same order. Each run contained 132 trials, including 98 go and 34 stop trials. An auditory tone served as the inhibitory stop cue. Fifty percent of both go and stop trials were presented concurrently with an alcohol picture, and 50% were presented with a picture of a “control” beverage (e.g., juice, Gatorade). The go cue was either a left-facing or a right-facing arrow, which appeared immediately after the alcohol or control picture was presented (Figure 1). Fifty percent of trials included a left-facing arrow, and 50% included a right-facing arrow. Because all subjects were right-handed, they were instructed to respond to left-facing arrows by pressing a button with their index finger and to right-facing arrows by pressing a button with their middle finger. The trials were balanced so that each run included an equal number of each trial type. That is, an equal number of go-right-alcohol, go-left-alcohol, go-right-control, and go-left-control trials, and an equal number of stop-right-alcohol, stop-left-alcohol, stop-right-control, and stop-left-control trials were distributed evenly within each run and between each of the three runs. Thus, trial orders differed between Run 1, Run 2 and Run 3, but all subjects received these same three runs in the same order. Alcohol and control images were taken from a previously developed alcohol-cue task in which they were standardized and matched based on perceptual and affective visual features (Pulido et al., 2010).
Figure 1.
Image of a Stop Signal Alcohol Cue Task (SSACT) trial. For each trial, the picture cue (either an image of an alcoholic beverage or an image of a nonalcoholic control beverage) appears directly above a white circle. The “go” cue, a left-facing or right-facing arrow, appears inside the white circle 500 ms later. If the trial is a “stop” trial, the stop cue (an auditory beep) is presented up to 700 ms after the arrow appears. If the trial is a go trial, no auditory cue occurs. Each trial lasts for 2,000 ms.
The SSACT was programmed to include a staircase-tracking algorithm designed to dynamically adjust task difficulty for each subject based on that individual’s cumulative stop-trial accuracy, with the goal of constraining performance such that subjects fail to inhibit on 50% of all stop trials. Briefly, the staircase-tracking procedure involves recalculating each subject’s overall stop-trial accuracy (% correct stop trials) after each stop trial and then altering the difficulty of the task based on overall performance, such that when individuals perform well on the task (i.e., correctly stopping on more than 50% of all stop trials), the next stop trial will be made more difficult through increasing the amount of time between the arrow appearing on the screen (go signal) and the presentation of the auditory tone (stop signal). Conversely, stop trials can be made less difficult (i.e., when subjects are incorrectly responding on more than 50% of all stop trials) through decreasing the amount of time between the arrow presentation and the stop tone.
Image acquisition
A high-resolution, magnetization-prepared rapid gradient-echo (MP-RAGE) anatomical scan and whole-brain functional images were acquired at both scan sites on 3.0 Tesla Siemens Trio MRI whole-body scanners (Erlangen, Germany) during three 6-minute runs of the SSACT using identical sequences. An echo-planar gradient-echo pulse sequence (repetition time = 2,000 ms; echo time = 29; flip angle = 75; field of view = 240 mm) was acquired with a 12-channel head coil, and images were acquired parallel to the ventral surface of the orbitofrontal cortex. Each volume consisted of 33 axial slices (64 × 64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap).
Imaging analysis
Images were slice-time and motion corrected and then normalized using the Statistical Parametric Mapping Version 8 (SPM8) software (Wellcome Department of Imaging Neuroscience; London, United Kingdom). Subjects with excessive motion (>3 mm translational or 0.053 radians rotational movement) were excluded from all analyses. Our initial sample consisted of 60 individuals: 4 subjects were excluded based on excessive motion, and 3 subjects were excluded because they were outliers on task performance. Thus, 53 subjects (29 women) were included in the present analyses.
First-level modeling and task contrasts
A general linear model using SPM8’s canonical hemodynamic response function modeled go trials (GO) and correctly inhibited stop trials (correct response; CR) for both alcohol and control picture trials for each individual. Use of the CR-GO (correct reject minus go) contrast is typical for standard SST analyses, as it represents a straightforward measure of successful stop-signal inhibition (Filbey et al., 2012). In the present study, this contrast was created for CR-GO (collapsing across picture type), and two related contrasts were created to specifically examine alcohol and control conditions (i.e., CRAlcohol-GOAlcohol and CRControl-GOControl). Additional exploratory contrasts unique to our version of the task were created for the CRAlcohol-CRControl and GOAlcohol-GOControl conditions in order to examine any potential differences in activation in response to alcohol versus control pictures within trial types. For the purposes of these analyses, and in accordance with typical SST analysis procedures (e.g., Aron and Poldrack, 2006), we did not discriminate between correct and incorrect GO trials. Thus, five different task contrasts were modeled. It should be noted that additional contrasts can be modeled using other available trial types built into this task (e.g., false alarm trials). However, taking into account previous findings (e.g., Aron and Poldrack, 2006; Filbey et al., 2012; Sharp et al., 2010) and the nature of our conceptual question concerning inhibitory control in the presence of cue-elicited craving, we determined that CR trials are of greatest interest for comparison across alcohol and control trials, and thus represent the focus of the present study.
Second-level analyses
To validate the purely stop-signal aspect of the SSACT, it was necessary to verify expected activation based on results from previous studies using the standard SST. To do this, a one-sample t test was conducted in SPM8 using the standard stop-signal contrast (CR-GO, collapsing across alcohol and control picture trials) from all subjects, across the whole brain. We examined the same contrast for alcohol picture trials only (CRAlcohol-GOAlcohol) and for control picture trials only (CRControl-GOControl). In addition to these standard SST contrasts, the SSACT also allows for potentially meaningful comparisons to be conducted within trial type (i.e., comparing alcohol and control trials for single trial type). To test our hypothesis that alcohol use severity (measured as a continuous variable) predicts differential activation during successful response inhibition for alcohol trials compared with control trials, we performed multiple regression analyses, examining the within-trial-type alcohol minus control contrast for correct reject trials only (CRAlcohol-CRControl). Additionally, the GOAlcohol-GOControl contrast was modeled as a control, given that GO trials presumably do not involve inhibition. All analyses were corrected for family-wise error rate (Nichols and Hayasaka, 2003), and a significance threshold of p =. 001 was used.
To focus specifically on brain areas most directly related to our hypotheses, we tested two regions of interest (ROIs)— the R-IFG and left IFG (L-IFG). These ROIs were chosen based on previous research suggesting that the R-IFG (and to a lesser extent the L-IFG) plays a major role in inhibitory control broadly defined (see Aron et al., 2004, 2014; Swick et al., 2008), as well as specifically in the context of the SST (e.g., Hampshire et al., 2010). We used the bilateral IFG ROIs identified in Hampshire et al. (2010) (all coordinates in the Montreal Neurological Institute space): L-IFG (x = 36, y = 16, z = 4) and R-IFG (x = 42, y = 18, z = 6). To examine the relationship between activation associated with L-IFG and R-IFG and self-reported alcohol use, we conducted a series of ordinary least squares multiple regression analyses using SPSS (Version 21). Effect sizes for L-IFG and R-IFG were extracted from our contrast of interest (CRAlcohol-CR-Control) and from our “control contrast” (GOAlcohol-GOControl) and regressed on each alcohol use measure, controlling for subject age and gender. Each predictor was tested in a separate model. Due to previous research supporting conflicting hypotheses regarding the possible inhibitory roles of R-IFG (Aron et al., 2004, 2014) versus L-IFG (Swick et al., 2008), the two ROIs were tested as separate dependent variables rather than combining them to form a single bilateral IFG value. The CRAlcohol-CRControl contrast was selected to highlight the effects of the presence of alcohol cues on successful inhibitory processing, and we hypothesized that individuals should differ in the degree to which alcohol cues affect IFG recruitment based on severity of alcohol use problems and/or frequency of consumption. The GOAlcohol-GOControl contrast was selected as a control comparison because we hypothesized that alcohol use would not predict differential neural recruitment for alcohol GO trials compared with control GO trials, because executing GO trials should not involve the recruitment of inhibitory control regions.
Finally, given the exploratory nature of this study and the fact that no comparable fMRI tasks examining cue reactivity and inhibitory control simultaneously have been conducted to date, multiple regression analyses were also performed across the whole brain using SPM8. CRAlcohol-CRControl was again selected as the contrast of interest, and GOAlcohol-GO-Control served as a control contrast. Each self-report measure was tested in a separate regression model.
Results
Stop-trial performance
The average overall stop-trial performance was 60% (SD = 19%) correct; this indicates that our staircase-tracking procedure did not adjust target duration quickly enough to force subjects to the desired 50% success rate. Stop-trial performance was consistent across picture types such that, on average, subjects achieved 60% correct on alcohol stop trials and 60% correct on control stop trials, suggesting that picture type did not influence stopping performance.
Whole-brain task contrasts
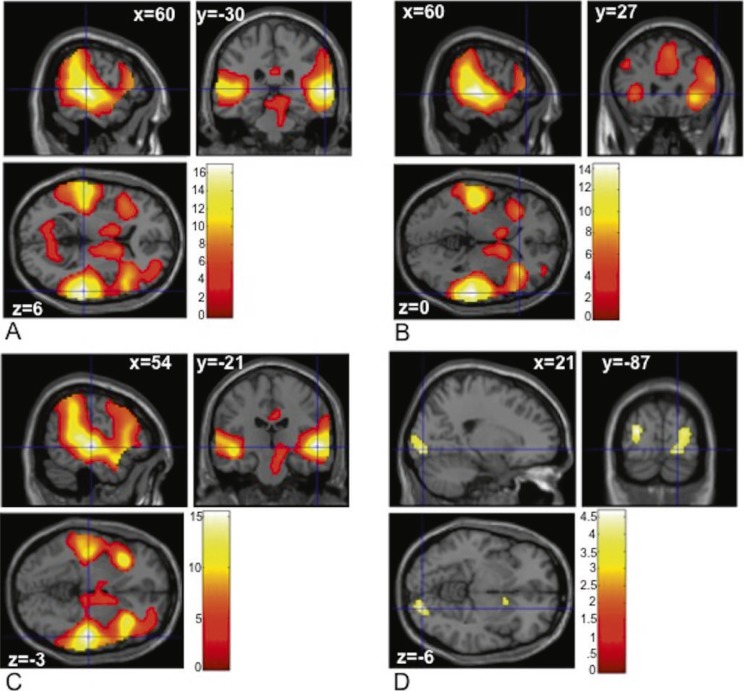
Successful inhibition (CR-GO) was associated with significant activation of right insula and inferior and superior frontal areas, as well as bilateral superior and middle temporal regions (Table 2). When only alcohol picture trials were included in this analysis (CRAlcohol-GOAlcohol), significant activation emerged in bilateral insula, inferior frontal, and superior and middle temporal regions. Similar activation patterns were observed when only control picture trials were tested (CRControl-GOControl), and results of a two-sample t test comparing CRAlcohol-GOAlcohol activation to CRControl-GOControl activation demonstrated no significant differences between the two contrasts. Thus, it appears that the SSACT produces results consistent with previous SSTs across both the alcohol and control trials. Finally, significant activation differences in right occipital, lingual, and calcarine regions emerged for correctly inhibited alcohol trials compared with correctly inhibited control trials (CRAlcohol-CRControl). Results from all whole-brain t tests are depicted in Figure 2.
Table 2.
Brain regions showing significant activation in CR-GO, CRAlcohol-GOAlcohol, CRControl-GOControl, and CRAlcohol-CRControl contrasts
| Contrast | Region | Voxel T value | MNI coordinates |
Cluster size, voxels | Cluster level p (FWE-corr) | Voxel level p (FEW-corr) | ||
| x | y | z | ||||||
| CR-GO | R/L STG/MTG, R IFG/SFG/insula | 16.91 | 60 | -30 | 6 | 14,233 | <.001 | <.001 |
| L cerebellum | 8.46 | -18 | -75 | -33 | 538 | .001 | <.001 | |
| CRA-GOA | R STG/MTG/MFG/IFG/insula | 14.40 | 60 | -27 | 0 | 7,379 | <.001 | <.001 |
| L STG/MTG/IFG/insula | 12.56 | -57 | -27 | 6 | 2,750 | <.001 | <.001 | |
| CRC-GOC | R STG/MTG/MFG/IFG/insula | 15.49 | 54 | -21 | -3 | 8,490 | <.001 | <.001 |
| L STG/MTG/IFG insula | 12.69 | -36 | 18 | -6 | 3,402 | <.001 | <.001 | |
| L cerebellum | 8.11 | -18 | -75 | -36 | 602 | <.001 | <.001 | |
| CRA-CRC | R MOG/lingual/calcarine/SOG | 4.28 | 21 | -87 | -6 | 202 | .034 | .178 |
Notes: CR = correct response; GO = go trials; CRA-GOA = CRAlcohol-GOAlcohol; CRC-GOC = CRControl-GOControl; CRA-CRC = CRAlcohol-CRControl; MNI = Montreal Neurological Institute space; FWE-corr = family-wise error corrected; R = right; L = left; STG = superior temporal gyrus; MTG = middle temporal gyrus; IFG = inferior frontal gyrus; SFG = superior frontal gyrus; MFG = middle frontal gyrus; MOG = middle occipital gyrus; SOG = superior occipital gyrus.
Figure 2.
Task contrasts examined across the whole brain. (A) Brain regions showing more activation in correct reject (CR) compared with GO trials (CR - GO). (B) Brain regions showing more activation in CR alcohol compared with GO alcohol trials (CRAlcohol - GOAlcohol). (C) Brain regions showing more activation in CR control compared with GO control trials (CRControl - GOControl). (D) Brain regions showing more activation in CR alcohol picture trials compared with CR control picture trials (CRAlcohol-CRControl). The color bar represents voxel T value.
Inferior frontal gyrus: Relationship between activation and self-report drinking severity.
Using ROI values extracted from our main contrast of interest (CRAlcohol-CRControl), AUDIT total score (AUDtot) was significantly related to R-IFG activation, b = 0.368, t(52) = 2.706, p = .009. When AUDtot was replaced with TLFB-TD (n = 52; TLFB data not available for one subject) in the same model, TLFB-TD was significantly related to R-IFG activation, b = 0.389, t(51) = 2.822, p =.007. Similarly, when L-IFG was tested as the dependent variable, AUDtot was significantly associated with L-IFG activation, b = 0.372, t(52) = 2.733, p = .009. However, TLFB-TD was not a significant predictor when it was substituted for AUDtot in the model predicting L-IFG activation. When the same regression models were tested using R-IFG and L-IFG effect sizes extracted from the GOAlcohol-GOControl contrast, neither AUDtot nor TLFB-TD was significantly associated with activation in R-IFG or L-IFG.
Whole-brain exploratory analyses: Relationship between activation and self-report drinking severity.
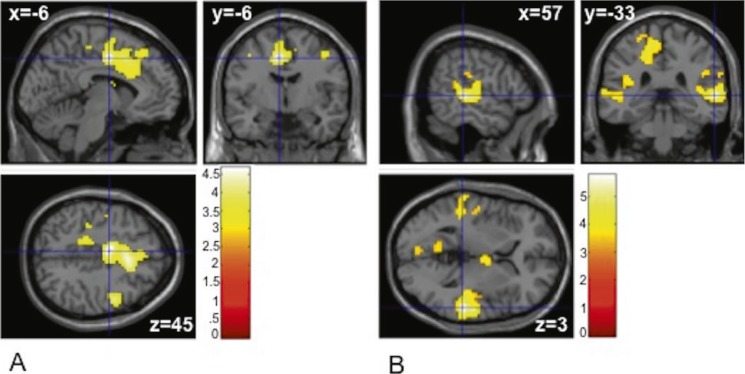
When we examined the relationship between self-report measures and activation across the whole brain using the CRAlcohol-CRControl contrast, AUDtot was associated with significant activation in bilateral cingulate and SMA (x = -6, y = -6, z = 45; voxel T = 4.94, 870 voxels). For the same contrast, TLFB-TD predicted significant activation in five clusters, which included bilateral superior temporal regions, anterior and middle cingulate, and left SMA (Table 3). These results are depicted in Figure 3. Neither self-report measure was significantly associated with activation related to the GOAlcohol-GOControl contrast.
Table 3.
Brain regions associated with AUDIT total and TLFB-TD scores in the CRAlcohol-CRControl contrast
| Alcohol Measure | Region | Voxel T value | MNI coordinates |
Cluster size, voxels | Cluster level p (FWE-corr) | Voxel level p (FEW-corr) | ||
| x | y | z | ||||||
| AUDIT | R/L cingulate/SMA | 4.94 | -6 | -6 | 45 | 870 | <.001 | <.001 |
| TLFB | R STG/MTG | 5.78 | 57 | -33 | 3 | 455 | .001 | .003 |
| L paracentral/SMA | 4.52 | -9 | -30 | 60 | 492 | .001 | .105 | |
| R/L ant/mid cingulate | 4.30 | 3 | 27 | 30 | 176 | .047 | .185 | |
| L STG/MTG/RO | 4.15 | -63 | -33 | 6 | 246 | .017 | .263 | |
| R/L calcarine/cuneus | 3.74 | 9 | -78 | 15 | 237 | .019 | .332 | |
Notes: AUDIT = Alcohol Use Disorders Identification Test; TLFB-TD = Timeline Followback total number of drinks consumed; MNI = Montreal Neurological Institute space; FWE-corr = family-wise error corrected; R = right; L = left; SMA = supplementary motor area; STG = superior temporal gyrus; MTG = middle temporal gyrus; ant/mid = anterior/middle; RO = Rolandic operculum.
Figure 3.
Multiple regression: Alcohol Use Disorders Identification Test (AUDIT) and Timeline Followback (TLFB) scores and brain activation across the whole brain. (A) Brain regions showing activation associated with AUDIT total score in the CRAlcohol - CRControl contrast. (B) Brain regions showing activation associated with TLFB total drinks in the CRAlcohol -CRControl contrast. The color bar represents voxel T value.
Discussion
The SSACT was developed to examine the simultaneous functioning of inhibitory control and reward processes in the context of heavy alcohol use, with the goal of better understanding the neural basis of their interaction. Activation in response to the CR-GO contrast demonstrated expected patterns based on prior research using the standard SST paradigm (Hampshire et al., 2010; Li et al., 2006). Because substantive interpretation of the CR-GO contrast is particularly straightforward (i.e., CR-GO is a measure of successful response inhibition), the CR-GO contrast has been widely examined in prior SST studies. In our study, successful inhibition appears to elicit a large area of activation that encompasses, most notably, the IFG. This is largely consistent with prior research, which has converged on the idea that successful stopping seems to depend on fronto-basal-ganglia circuitry, emphasizing the crucial role of the IFG (Aron et al., 2007b). However, we also report significant CR-GO activation in temporal areas, which has not been demonstrated in previous research and appears to be unique to our version of the task. This superior temporal activation, observed in the CR-GO contrast as well as in the CRAlcohol-GOAlcohol and CRControl-GOControl contrasts, included primary auditory cortex (i.e., Brodmann areas 41 and 42; Morosan et al., 2001), suggesting that this activation is likely related to processing the auditory tone, which is present in CR trials but not in GO trials. Consistent with this explanation is the lack of activation in primary auditory cortex in the CRAlcohol-CRControl contrast.
In addition, ROI analyses demonstrated differential R-IFG and L-IFG activation during CR but not GO trials, during alcohol compared with control trials, and demonstrated that this difference depends on the level of self-reported alcohol use. Whole-brain analysis showed that higher scores on alcohol use measures predicted more activation during successful inhibition for alcohol trials compared with control trials in the anterior cingulate, supplementary motor, and temporal regions. This relationship was not observed when alcohol GO and control GO trials were compared, which was expected given that inhibitory processes should not be activated in response to either type of GO trial. Taken together, these results suggest that for subjects with greater alcohol use, increased error detection and/or inhibitory control mechanisms may be needed in order to correctly inhibit responding while an alcohol picture is being viewed. Given that these analyses have focused on new contrasts unique to our version of the task, results should be regarded as preliminary. However, initial findings are promising and are generally consistent with the hypothesis that chronic substance users may need to recruit additional frontal brain networks to assist with difficult cognitive tasks, perhaps to compensate for compromised neural functioning associated with substance-related adaptations (see Desmond et al., 2003; Eldreth et al., 2004).
There are several potential confounds inherent in the current version of this task. The first concerns the use of visual go signals and auditory stop signals. Some level of ambiguity regarding the interpretation of the present results is introduced by the fact that the alcohol images—which are intended to distract from the stop task, elicit alcohol craving, and activate the reward system—are presented visually, whereas the stop cue—which should be activating the inhibitory control system—is an auditory stimulus. It is possible that because reward and control stimuli are presented using two different sensory modalities, subjects are able to focus sufficient attention on both and are not sufficiently distracted by the visual cue. Given that the purpose of the task is to simultaneously tax the control system and activate the reward system, a problem may arise if the subjects’ ability to perform the inhibition task is not affected by the presence of the alcohol cue (i.e., if the visual cues are not adequately distracting). Further, in the present study, significant activation was observed in primary auditory cortex during stop trials compared to go trials. This activation likely reflects the presence of the tone in stop trials (and its absence in go trials) and is not likely related to differences in stop and go processing. Thus, future versions of the task may benefit from incorporating a visual stop signal, forcing both stop and go responses to rely exclusively on visual processing.
Importantly, the staircase-tracking procedure did not succeed in forcing stop-trial performance to the desired level of 50% correct. For each subject, the staircase algorithm varies the amount of time between the presentation of the arrow (go signal) and the presentation of the tone (stop signal) on each trial, based on cumulative stop-trial performance. However, our staircase algorithm appeared to adjust this value too slowly, such that on average, trials did not become sufficiently difficult to force performance down to 50% correct. In addition, the algorithm was programmed to reset at the beginning of each run rather than taking performance from the previous run(s) into account. The reset feature also made the algorithm less effective and likely contributed to the inability of the task to become sufficiently difficult. The algorithm will be reprogrammed to more quickly increase task difficulty in order to reach the desired 50% stop-trial performance level on future versions of the task.
In addition, because of the placement of the arrow in a circle below each picture, it may be possible for subjects to concentrate their visual attention on the arrows (and on the circle, which serves as the warning cue indicating that the arrow is about to appear) and ignore the content of the images. This problem can be easily addressed in future versions of the task through removing the circle around the arrow and superimposing the arrow on top of the alcohol and control images, such that each image will itself serve as the warning cue. The appearance of the arrow on the image will require that visual attention remain on the image for the duration of all trials. It is possible that subjects could still ignore the content of the images, but the placement of the arrow superimposed on each picture would at least ensure that subjects visually attend to the images on all trials. Future versions of the task may also benefit from adding a brief image rating session following the MRI scan, in which subjects are asked to rate all alcohol and control images on dimensions such as arousal, valence, and reward value. This would provide useful quantitative information about the effectiveness of the pictures in eliciting craving and reward responses. Further, the images appeared only 500 ms before the go signal, and this amount of time could be increased in future versions (i.e., to 2,000 ms), to allow sufficient time to activate neural reward processing before imposing the inhibitory demands of the stop cue.
Finally, this study included individuals spanning a wide range of alcohol consumption levels and clinical severity. In the future, it would be beneficial to administer this task to a more clinically severe population (i.e., only individuals meeting diagnostic criteria for alcohol dependence), as this would be consistent with previous visual-cue research and may elicit more robust differences in craving- and reward-related brain areas across the two visual cue conditions. Although the results of the present investigation should be seen as preliminary, the fact that significant differences in activation were observed based on alcohol use severity, even among this nonclinical population, is promising. Further research using this task should focus on clinical populations to corroborate these initial findings and ultimately illuminate the relationship between inhibitory control and reward processes in AUDs.
Conclusions and future directions
The results of our preliminary analysis of the SSACT suggest that this paradigm may be useful for studying the relationship between inhibitory control and cue-induced craving among heavy drinkers. One possible goal of research in this area may be to determine a consistent “neural signature” that is common across heavy alcohol users and to then use this information to inform potential pharmacological treatments for AUDs based on affected brain areas and/or relevant pathways and neurotransmitter systems. The SSACT may provide a rare opportunity to observe this type of distinct activation pattern because of the high level of detail afforded by the many unique contrasts inherent in the task. For instance, studies could benefit from examining additional task contrasts, including those focused on false alarm responding. Additional research using this task among a larger, more clinically severe sample will likely serve to expand on these promising initial findings.
In addition to lending support to the overall construct validity of the SSACT, the present study has implicated several crucial changes to the overall task design that should be implemented in subsequent iterations. First, it will be useful to ask participants to rate all visual cues on various dimensions such as valence, reward value, and arousal, to determine whether the alcohol pictures are perceived as rewarding in comparison with the control pictures; this would also allow for parametric modeling of brain activation patterns based on individualized ratings. Importantly, signal arrows should be superimposed on the cue images to focus visual attention, images should be presented for at least 2,000 ms before go signal presentation, and a visual stop signal should be used rather than the auditory stop tone. Finally, the staircase-tracking algorithm should be adjusted to achieve the desired stop-trial performance of 50% correct. With the addition of these relatively minor methodological changes, future research using this task will be well equipped to examine the neural circuitry underlying the complex relationship between cue-induced craving and response inhibition.
Acknowledgments
The authors acknowledge Dr. Carmen Pulido and Dr. Susan Tapert for their assistance with development and standardization of the alcohol and control cue images used in the SSACT.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant R01AA012238 (awarded to Kent E. Hutchison).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience. 2007a;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. The Journal of Neuroscience. 2007b;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. The Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences. 2014 doi: 10.1016/j.tics.2013.12.003. Advance online publication. Retrieved from doi:10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:145S8–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence, 90, Supplement 1. 2007:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Kelley EA, Mitchell JM, D’Esposito M, Fields HL. Now or later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacology, Biochemistry, and Behavior. 2009;93:291–299. doi: 10.1016/j.pbb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain and Cognition. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of Cognitive Neuroscience. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcoholism: Clinical and Experimental Research. 2011;35:1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Oosterlaan J, Veltman DJ, van den Brink W, Goudriaan AE. Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug and Alcohol Dependence. 2012;121:81–89. doi: 10.1016/j.drugalcdep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. NeuroImage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Morgan M, Forester GR, Hutchison K. Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addiction Biology. 2012;17:1046–1056. doi: 10.1111/j.1369-1600.2011.00328.x. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Filbey F. The incentive salience of alcohol: Translating the effects of genetic variant in CNR1. Archives of General Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffard M, Benraiss A, Longcamp M, Velay JL, Boulinguez P. Cueing method biases in visual detection studies. Brain Research. 2007;1179:106–118. doi: 10.1016/j.brainres.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W, II, Oberlin BG, Harezlak J, O’Connor SJ. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology. 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Harlaar N, Hutchison KE. Substance use disorders: A theory-driven approach to the integration of genetics and neuroimaging. Annals of the New York Academy of Sciences. 2013a;1282:71–91. doi: 10.1111/nyas.12074. [DOI] [PubMed] [Google Scholar]
- Karoly HC, Stevens CJ, Thayer RE, Magnan RE, Bryan AD, Hutchison KE. Aerobic exercise moderates the effect of heavy alcohol consumption on white matter damage. Alcoholism: Clinical and Experimental Research. 2013b;37:1508–1515. doi: 10.1111/acer.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology. 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. The Journal of Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008b;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcoholism: Clinical and Experimental Research. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Sinha R, Lee T-W. Subcortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008a;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? Journal of Studies on Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Pulido C, Brown SA, Cummins K, Paulus MP, Tapert SF. Alcohol cue reactivity task development. Addictive Behaviors. 2010;35:84–90. doi: 10.1016/j.addbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience. 2008;9:102–112. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]