Abstract
Oyster mushroom (Pleurotus sajor-caju) cultivated in the laboratory was studied for nutritional constituents, flavor components, antioxidant and antibacterial properties. Nutritional constituents estimated per 100 g dry weight (d.w.) include protein (29.3 g), carbohydrate (62.97 g), crude fat (0.91 g), ash (6.82 g) and crude fiber (12.3 g). Energy value of this mushroom was about 297.5 kcal/100 g d.w. Major mineral components estimated include Ca, Fe, and Mg with highest level of 505.0, 109.5 and 108.7 mg/100 g respectively. Methanolic extract containing significant amounts of phenols and flavonoids showed free radical scavenging potential and antibacterial activities against various spp. of Gram positive and Gram negative bacteria. Compounds responsible for antibacterial activities analyzed by GC-MS include β- Sistosterol, Cholestanol, 1,5-Dibenzoylnaphthalene and 1,2-Benzenedicarboxylic acid. Flavor components extracted by hot extraction method were found to be higher in number and concentration than the cold extraction method. The characteristic flavor component of mushroom i.e. 1-Octen-3-ol was better extracted by hot than the cold.
Keywords: Pleurotus sajor-caju, Volatile components, Phenolics, Flavonoids, Antioxidant property, Antibacterial property
Introduction
The mushroom industry is a global, expanding industry, with world production greater than two million tonnes annually. The chief mushroom varieties cultivated are Agaricus bisporus, Lentinus edodes and Pleurotus ostreatus (Ragunathan and Swaminathan 2003). Oyster mushroom has occupied the second position among globally cultivated edible mushrooms (Banik and Nandi 2004). It is one of the mushrooms which are easy to cultivate on a variety of substrates making it suitable for commercial exploitation. Pleurotus spp. are saprophytic fungi, commonly known as oyster mushrooms, which develop in nature on woody substrate like dead tree trunks, since they have the ability to degrade lignocellulose (Confortin et al.2008). Unlike other microbial sources of single cell proteins (SCP), mushrooms have high consumer preference due to their unique flavor and taste. Mushrooms possess nutritional and medicinal properties and often are referred as neutraceuticals (Schachter et al.2005). They are considered to be good source of digestible proteins (10–40%), carbohydrates (3–21%) and dietary fiber (3–35%) on dry weight basis. They are limiting in sulfur containing amino acids like cysteine and methionine (Mallavadhani et al.2006). Essential minerals such as iron, copper, zinc and manganese, which play important role in biological systems, are present in oyster mushrooms (Kalac and Svoboda 2000). Its nutritive value in terms of protein and minerals make it compatible with FAO’s slogan “Mushroom the Health Food”. Recently, mushrooms have become an attractive functional food mainly because of their chemical composition and this can be explained by the antioxidant property to scavenge free radicals, which are responsible for oxidative damage of lipids, proteins and nucleic acids (Wong and Chye 2009; Wong et al.2009). Mushrooms are considered to be a good source of phenolic antioxidants, such as variegatic acid and diboviquinone. It is also reported that ethanolic extract of the dried mushroom showed antibacterial activity against Gram positive and Gram negative bacteria (Turkoglu et al.2007). The quality of mushrooms depends on their taste, aroma, texture, and color. The aroma is the most important organoleptic characteristic of mushroom products (Yang et al.2001). The principal compounds producing the aroma of raw mushrooms are aliphatic alcohols and ketones (Misharina et al. 2009a, b). The qualitative and quantitative compositions of volatile substances significantly depend on the mushroom species, culture conditions etc.
The present investigation aims to evaluate nutrients, volatiles, phenols and flavonoids compositions of oyster mushroom (Pleurotus sajor-caju) grown on paddy straw as substrate. The hot and cold extraction methods for extraction of volatiles were standardized. Free radicals scavenging potential and antibacterial activities of methanolic extract of oyster mushroom and compounds causing antibacterial properties were also studied.
Materials and methods
Raw material
Spawn of P. sajor-caju was obtained from All India Coordinated Mushroom Improvement Project, College of Agriculture, Pune (MS), India. Cultivation of oyster mushroom was carried out in the laboratory as per the procedure suggested by (Chadha and Sharma 1995). Paddy straw used as substrate was soaked in water for 12–14 h followed by draining of excess water for 5–6 h. Drained paddy straw was autoclaved at 121 °C for 20 min. Beds were prepared by putting alternate layers of sterilized paddy straw and spawn in pre-sterilized perforated HDPE bags (15 × 27 inch) and closed by tying with a thread. Beds were kept for mycellial growth for 21 days. Then these bags were removed and beds were kept open for 4–5 days (25–30 °C and 70–80% RH) with 3–4 times watering a day. After 4–5 days developed fruiting bodies were harvested. In the similar manner 5–6 flushes were collected form each bed.
Nutritional constituents of mushroom
The nutritional components like moisture content (AOAC 1995), carbohydrate (Ouzouni et al.2009), crude fat (AOAC 1995), crude fiber (Maynard 1970) and crude protein (Shashirekha et al.2002) of the fruiting bodies were determined. Ash content was analyzed by weighing the samples before and after ignition at 550 °C for 6 h (Ranganna 2000).
Energy value of mushroom
The energy value of fruiting bodies was calculated on the basis of their content of crude protein, fat and carbohydrates (Ragunathan and Swaminathan 2003).
Determination of metal contents
Dried mushroom were ground to powder and stored in pre-cleaned PET bottles. Deionized water with conductivity 0.1 mS, from a Milli- Q system was used to prepare all aqueous solutions. All mineral acids and oxidants (HNO3 and Perchloric acid) used were of the highest quality (Merck, Darmstadt, Germany). A blank digest was analyzed in the same way. The acid digestion sample was prepared as follows: 2 g of dried sample was soaked in 20 ml of concentrated HNO3 in a crucible for 18–20 h. Then the crucible was heated on a hot plate at 70 °C till orange brown colored residue obtained and cooled to room temperature. Perchloric acid (5 ml) was added to it and again it was heated till the residue became white. This residue was dissolved in deionized water and the volume was made up to 100 ml and filtered through Whatman No. 4 filter paper. Analysis of minerals was carried out using atomic absorption spectrophotometer of Perkin-Elmer Optima 2000 ICP-OES.
Estimation of phosphorus
Phosphorus was estimated by the method suggested by Gasim et al. (2008). In this method 2 ml of acid digested sample was added to 2 ml of 2 N HNO3. 1 ml of freshly prepared molybdate-vanadate reagent was added to this mixture and final volume was adjusted to 10 ml with deionized water. Then it was kept for 20 min at room temperature and absorbance was noted at 420 nm by using an UV–VIS Spectrophotometer.
Estimation of potassium and sodium
The acid digested sample was atomized into an oxyacetylene flame of a digital flame photometer (EI – 1382) and the concentration of Na and K was noted (Ranganna 2000).
Preparation of extracts for antioxidant and antibacterial properties
Biologically active substances from 10 g oven-dried (50–60 °C for 8–10 h) fruiting bodies were extracted three times with 100 ml methanol for 24 h at 30 ± 2 °C on a orbital shaking incubator (Remi, CIS 24BL). The extract was filtered through Whatman no. 4 filter paper. The organic solvent in the extract was removed by a rotary evaporator; the extract was kept in the dark at 4 °C and used for analysis of antimicrobial and antioxidant activities within one week (Barros et al.2007). This extract was mixed with HPLC grade methanol and analyzed for its composition by GC-MS.
Antioxidant activity
Radical-scavenging ability was determined by a spectrophotometric method based on the reduction of a methanol solution of 1, 1-Diphenyl-2- picrylhydrazyl (DPPH) (Louli et al.2004). The reaction mixture containing 1.5 ml DPPH solution (1 mg of DPPH in 40 ml methanol) and 1.5 ml sample was shaken vigorously and left to stand for 20 min in dark. The absorbance of reaction mixture was measured at 517 nm by a spectrophotometer (Hitachi, U-1800). The radical scavenging activities were calculated as given in the equation below:
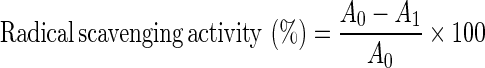 |
Where A0 is the absorbance of the blank and A1 is the absorbance of the sample.
Ascorbic acid was determined according to the method of Klein and Perry (1982). Each dried methanolic extract (100 mg) was re-extracted in 10 ml of metaphosphoric acid (10 mg/ml) for 45 min at room temperature and filtered through a disposable membrane filter (Pore size-0.45 μm) (Millipore, Inc. USA). The filtrate (1 ml) was mixed with 9 ml of 2, 6- dichlorophenolindophenol and the absorbance was measured at 515 nm. Content of ascorbic acid was calculated on the basis of the calibration curve of standard L-ascorbic acid.
Assay for total flavonoids
Total flavonoids content was determined by the method suggested by Meda et al. (2005). In this method, 0.5 ml of 2% aluminium trichloride (AlCl3) in methanol was mixed with same volume of the methanolic extracts (0.1 mg/ml) of mushroom. Absorbance was noted after 10 min of reaction at 415 nm. The concentration of total flavonoids was calculated from the standard quercetin graph.
Assay for total phenolics
The amount of total phenolics was determined by using Folin–Ciocalteu method (Amin et al.2006). A 0.5 ml of the methanolic extract was transferred into a test tube and 125 μl of Folin-Ciocalteu reagent (Sigma) was added and mixed. The mixture was allowed to stand at room temperature for 10 min. 125 μl of 20% (w/v) sodium carbonate was added to the mixture and mixed gently and this was left at room temperature for another 60 min. The absorbance of the mixture was recorded at 760 nm using a spectrophotometer (Hitachi, U-1800). Total phenolic compounds were calculated from a standard calibration curve of tannic acid (Fluka).
Antibacterial activity
The antibacterial activity of Pleurotus sajor-caju extracts was tested against Gram-positive bacteria Bacillus cereus (NCIM-2156), Staphylococcus aureus (NCIM-5021), Micrococcus luteus (NCIM-2103) and Gram-negative bacteria Escherichia coli (NCIM-2089), Salmonella typhimurium (NCIM-2501). All strains were procured from National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory, Pune (MS), India. Agar well diffusion method was used to determine antimicrobial activity (Amin et al. 2006). Bacterial cultures were grown at 37 °C for 24 h in nutrient broth. The microbial suspensions (absorbance 0.4 at 550 nm) were prepared in sterile 0.85% NaCl solution and mixed with soft agar (1.5%). This was over layered on sterile nutrient agar plates. Wells (7.0 mm in diameter) were made and 150 μl of the dried extract dissolved in dimethyl-sulfoxide (DMSO) was added to the wells and same volume (150 μl) of pure DMSO was used as a control. The inoculated plates were incubated for 24 h, diameter of inhibition zone was measured and expressed in milimeter.
Possible presence of components in the methanolic extract of P. sajor-caju responsible for antibacterial activities was detected by using QP- 2010 gas chromatography coupled with mass spectroscopy (Shimadzu). Conditions for this analysis were described under determination of volatile components.
Volatile extraction and analysis
Cold extraction
Chilled mushrooms (10 g) were macerated in a prechilled mortar and pestle (kept in ice bath) by using 10 ml of chilled water. Pentadecane (SD Fine chemicals, Mumbai) at the concentration of 5000 μg per 100 g of mushroom was added as an inner standard to this paste (Wojtasiak 2004; Misharina et al. 2009) and filled in a stoppered glass bottle. Chilled diethyl ether (10 ml) was added to it. The stoppered bottle was shaken to mix well all the contents and kept in a refrigerator (5 °C) for 2 h. The content was extracted with 10 ml of diethyl ether for another 2 times followed by 2 h of cold storage each time. After this, all the solvent was pooled and concentrated to 2 ml at room temperature. This was analyzed for presence of flavor components using GC-MS.
Hot extraction
The extract for analysis of volatiles was prepared by using the method of Wojtasiak (2004). Ten grams of mushroom sample was macerated by using a porcelain mortar and pestle in 100 ml distilled water and Pentadecane (SD Fine chemicals, Mumbai) at the concentration of 5000 μg per 100 g of mushroom was added to it as an inner standard (Wojtasiak 2004; Misharina et al. 2009a, b). This was allowed to stand for 15–20 min for enzyme activation (Venkateshwarlu et al.1999) and then steam distilled at 50 °C for 2 h and condensed fraction was collected into a well stoppered flask. This condensate (about 50 ml) was extracted 3 times with equal concentration of dichloromethane and the pooled solvent was dried over anhydrous Na2SO4. It was further concentrated to 2 ml at room temperature and analyzed for flavour components by using GC-MS.
Analysis of volatile components
The volatile components were analyzed using QP- 2010 gas chromatography coupled with mass spectroscopy (Shimadzu). The ionization voltage was 70ev. Gas chromatography was conducted by temperature programming made with Rtx-5Ms column (60 m × 250 μm i.d. × 0.25 μm film thickness). Carrier gas was He (1 ml−1) and the chromatographic conditions were as follows: initial oven temperature was maintained at 50 °C for 5 min and subsequently programmed from 50 °C to 150 °C at a rate of 3 °C min−1 and at a rate of 10 °C min−1 from120 to 280 °C where it was held for another 10 min. Injector Temperature: 250 °C; Mass range: 40–650 amu; Solvent delay: 4 min.; electron impact at 70ev. The volatile compounds were identified by comparison of retention time and fragmentation pattern, as well as with mass spectra in NIST spectral library stored in the computer software (version 1.10 beta, Shimadzu).
Results and discussion
Chemical composition
Proximate composition of mushrooms is depicted in Table 1. When the nutritional value of mushrooms is evaluated, perhaps the most important factor is their dry matter/moisture content, which directly affects the nutrient content of mushrooms (Ouzouni et al.2009). As shown in Table 1 the moisture content of the oyster mushroom species was 90.02 ± 2.58%. This moisture variability is dependent on the parameters such as environmental temperature, relative humidity during growth and relative amount of metabolic water that may be produced or utilized during storage (Crisan and Sands 1978).
Table 1.
Chemical composition of P. sajor-caju
| Component | Concentration* |
|---|---|
| Moisturea | 90.0 ± 2.58 |
| Proteinb | 29.3 ± 1.03 |
| Crude fatb | 0.9 ± 0.06 |
| Ashb | 6.8 ± 0.48 |
| Total carbohydrateb | 62.9 ± 2.56 |
| Crude fiberb | 12. 3 ± 0.26 |
| Energyc | 297.5 ± 9.31 |
| Ca d | 505.0 ± 4.64 |
| Fe d | 109.5 ± 3.02 |
| Mg d | 108.7 ± 2.12 |
| Na d | 70.0 ± 1.89 |
| K d | 40.0 ± 2.21 |
| Zn d | 35.0 ± 1.82 |
| P d | 32.8 ± 2.03 |
| Ni d | 32.7 ± 2.65 |
| Mn d | 27.2 ± 1.01 |
| Pb d | 2.6 ± 2.01 |
| Cu d | 16.1 ± 0.86 |
| Cr d | 3.1 ± 0.92 |
| Co d | 1.6 ± 0.43 |
*Values are mean ± SD of three determinations
a Fresh weight basis (g/100 g)
b Dry weight basis (g/100 g)
c Dry weight basis (kcal/100 g)
d Dry weight basis (mg/100 g)
The total concentration of protein calculated showed 29.3 g/100 g dry matter. Amount of crude protein in different Pleurotus spp. varies from 25.6 to 44.3 g/100 g dry matter according to different types of substrate used for cultivation (Raghunathan and Swaminathan 2003). While Yang et al. (2001) reported that winter mushroom (strain yellow) contains highest protein content (26.7) than other winter (strains white), shiitake (strains 271 and Taichung 1) and Oyster mushrooms (abalone and tree oyster mushrooms). Mushrooms proved to be a good source of protein compared to other green vegetables.
Carbohydrate content of mushroom mainly includes glucose, mannitol, α-trehalose and glycogen as a reserve polysaccharide. Chitin is a water-insoluble structural polysaccharide (80–90% of dry matter in mushroom cell walls) which is indigestible for humans and apparently decreases the availability of other mushroom components. In addition to chitin it also contains other insoluble fiber compounds like hemicelluloses, pectic substances. The high proportion of insoluble fiber seems to be nutritionally desirable (Kalac 2009). The data showed total carbohydrate and crude fiber were 62.97 g/100 g and 12.30 g/100 g on dry weight basis respectively. While Ragunathan and Swaminathan (2003) reported that amount carbohydrate and crude fiber present in different Pleurotus spp. ranges from 40.1 to 46.2 g/100 g and 11.40 to 212 21.6 g/100 g of dry matter respectively.
Crude fat in mushrooms includes several classes of lipid compounds, free fatty acids, mono, di and triglycerides, sterols, sterol esters and phospholipids (Crisan and Sands 1978). The results showed that fat content of mushroom was 0.91 g/100 g dry matter. Crude fat in different Pleurotus spp. grown on different substrates varied from 0.95 to 3.16 g/100 g dry matter (Ragunathan and Swaminathan 2003).
Data depicted in Table 1 showed that ash content of mushroom was 6.82 g/100 g on a dry weight basis. Concentration of ash in different Pleurotus spp. ranges from 5.40 to 8.40 g/100 g dry matter depending upon different substrates used for cultivation (Ragunathan and Swaminathan 2003). As compared with vegetables, mushrooms proved to be good sources of many mineral elements. The main constituents in the mushrooms ash are K and P (Mattila et al.2002).
The energy value for Oyster mushroom calculated from the proximate composition was 325.85 ± 9.31 kcal/100 g dry matters. According to Ragunathan and Swaminathan (2003) energy value of different Pleurotus spp. ranges from 272 to 316 kcal/100 g dry matter.
Mushrooms are important sources of essential elements and play a vital role in the proper development of human body. However, high amounts of certain minerals are also toxic for most of the organisms (Gursoy et al.2009). Ten elements (Cu, Mn, Co, Zn, Fe, Ca, K, P, Na and Mg) and three heavy metals (Ni, Pb and Cr,) were determined in P. sajor-caju. Element concentrations of the mushroom are presented in Table 1. According to the results, the most abundant elements are calcium and magnesium, respectively. These are followed by Fe. On the other hand, cobalt was the lowest element presented in this table (1.60 mg/100 g d.w.). In the case of heavy metals, the most abundant was nickel (Table 1). Amount of this metal was 32.7 mg/100 g. When compared with nickel, the amount of lead was much lower (2.25 mg/100 g). The lowest heavy metal was determined as Cr (3.1 mg/100 g). The amount of these heavy metals are well within the limits as per FAO/WHO guideline 2001 except lead (Pb) and chromium (Cr) which are slightly higher than recommended values for leafy vegetables. The mean metal concentrations across all the samples of Oyster mushroom were in the order: Ca > Fe > Mg > Na > K > Zn > P > Ni > Mn > Pb > Cu > Cr > Co. The results of nutritionally valuable minerals show that mushroom species contained high amounts of potassium, calcium, magnesium, iron and manganese. This is in good agreement with the report of analysis of cultivated mushrooms, such as A. bisporus, L. edodes and P. ostreatus (Mattila et al.2002). Most of them contain little sodium, phosphorus or copper. Minerals in the diet are required for metabolic reactions, transmission of nerve impulses, rigid bone formation and regulation of water and salt balance (Kalac and Svoboda 2000). P. sajor-caju is a good source of minerals including iron, potassium, phosphorus, magnesium, manganese, zinc and calcium (Table 1). The results obtained for trace elements in analyzed P. sajor-caju seem acceptable for human consumption at nutritional levels.
Antioxidant components
Recently, there has been increasing interest in discovering natural antioxidants, especially those of plant origin (Jayakumar et al.2009). Natural antioxidants derived from plants, chiefly phenolics, are of considerable interest as dietary supplements or food preservatives (Jayakumar et al.2006). Hence, an attempt was made to determine the putative antioxidant components of the methanolic extract of P. sajor-caju. The amount of total phenols, ascorbic acid, and flavonoids in the mushroom extract was determined. Phenolics are important constituents with scavenging ability due to their hydroxyl groups and hence may contribute directly to the antioxidative action. In the present study, the total phenolic content of P. sajor-caju was found to be high i.e., (52.20 mg/g) (Table 2), compared to the reported values in other mushrooms such as P. cystidiosus (10.24 mg/g), P. ostreatus (15.7 mg/g) (Yanga et al.2002), G. lucidum (47.25 mg/g), G. tsugae (51.28 mg/g) (Mau et al.,2001), M. rotunda (16.98 mg/g), M. crassipes (18.59 mg/g) (Gursoy et al.2009).
Table 2.
Concentration (mg/g) of total phenols, ascorbic acid and total flavonoids in a methanolic extract of P. sajor-caju
| Compound | Concentration* |
|---|---|
| Total phenola (expressed as tannic acid equivalent) | 52.2 ± 1.64 |
| Ascorbic acida | 8.3 ± 0.73 |
| Flavonoidsa (expressed as quercetin equivalent) | 4.7 ± 0.05 |
| Free radical scavenging propertyb | 88.0 ± 0.68 |
*Values are mean ± SD of three determinations
amg/g
bPercentage
Ascorbic acid was reported to interact directly with radicals such as O−2 and OH- in plasma, thus preventing damage to red cell membranes. It also assists α-tocopherol in inhibition of lipid peroxidation (LPO) by recycling the tocopherol radical (Beyer, 1994). In the present study, the ascorbic acid contains of P. sajor-caju extract was high (8.28 mg/g) (Table 2).
Flavonoids occur throughout the entire plant kingdom. The most widely distributed flavonoids, flavone and flavonols are mainly hydroxylated in the β-ring at the 3′- and 4′- positions (rutin) followed by the 4′- position (naringenin) (Jayakumar et al.2006). Many flavonoids and related compounds are reported to possess strong antioxidative characteristics. Hydroxylation of the β -ring is an important factor governing the antioxidative activity of these compounds, although it is not a prerequisite for manifestation of the activity. Interestingly in the present study, a high concentration of quercetin (4.70 mg/g) was noted in the mushroom P. sajor- caju (Table 2).
All the mushroom species showed appreciable DPPH free radical scavenging activities at the selected scope of concentrations (Wong et al.2009). The free radical scavenging properties of the P. sajor-caju was found 88.07%. Wong and Chye (2009) reported free radical scavenging effect of 83.04% in petroleum ether extract of Pleurotus porrigens. Free radical scavenging effect has been known as an established phenomenon in inhibiting lipid oxidation, which otherwise can be deleterious to the cellular components and cellular function (Puttaraju et al.2006). Consumption of Oyster mushrooms might be beneficial to protect human body against oxidative damage due to the presence of radical scavenging activity (Wong et al.2009).
Antibacterial activity
The antibacterial effect of methanolic extract of P. sajor-caju was tested against Gram-positive and Gram-negative bacteria. As summarized in Table 3, the inhibition zones of P. sajor-caju which were obtained against all tested bacteria were in the range of 18–25 mm. The highest inhibitory activity was noticed against S. aureus (NCIM-5021) (25 mm, diameter of inhibition zone). On the other hand, the weakest inhibitory activity was determined against B. cereus (NCIM-2156) (18 mm, diameter of inhibition zone). Turkoglu et al. (2007) reported that Laetiporus sulphureus showed poor antimicrobial activity (6 mm, diameter of inhibition zone) against Gram positive bacteria. Since many plant phenolics have been found to be responsible for several biological properties, including antimicrobial properties (Chanwitheesuk et al.2007; Yaltirak et al.2009), it was expected that the antimicrobial activity of this plant species would be related to its antioxidant compounds.
Table 3.
Antimicrobial activity in a methanolic extract of P. sajor-caju
| Test bacteria | Inhibition zone diameter (mm)* |
|---|---|
| Escherichia coli | 21 ± 1.32 |
| Salmonella typhimurium | 29 ± 2.34 |
| Bacillus cereus | 18 ± 2.02 |
| Staphylococcus aureus | 25 ± 2.56 |
| Micrococcus luteus | 23 ± 2.86 |
*Values are mean ± SD of three determinations
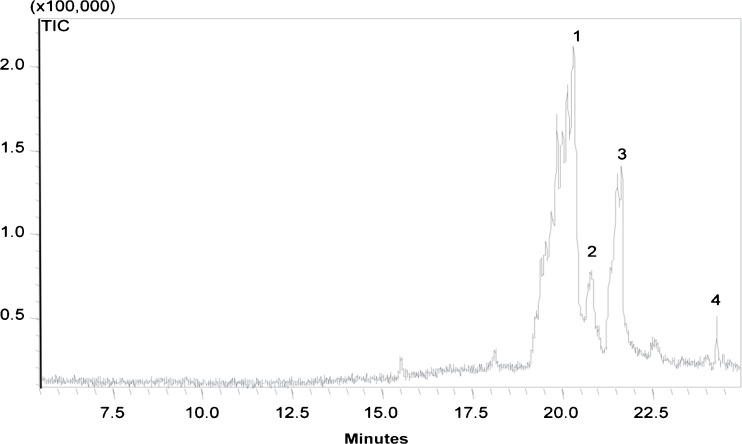
Compounds present in the methanolic extract of P. sajor-caju detected by GC-MS are shown in Fig. 1. Four different compounds tentatively identified using the spectral library are β- Sistosterol (Sanches et al.2005), Cholestanol (Salmi et al.2008), 1, 5-Dibenzoylnaphthalene (Findlay et al.1987) and 1, 2-Benzenedicarboxylic acid (Sani and Pateh 2009). These compounds responsible for possible antimicrobial activities are yet not reported to be present in P. sajor-caju. But earlier findings on various plant extracts showed that β- Sistosterol had antibacterial activity mostly against Gram positive bacteria (Sanches et al.2005), Cholestanol had antibacterial activity against pathogenic bacteria (Salmi et al. 2008), 1,5-Dibenzoylnaphthalene had antimicrobial activity (Findlay et al.1987) and 1,2-Benzenedicarboxylic acid had antibacterial property (Sani and Pateh 2009).
Fig. 1.
Chromatogram of methanolic extract of P. sajor-caju
Volatile components
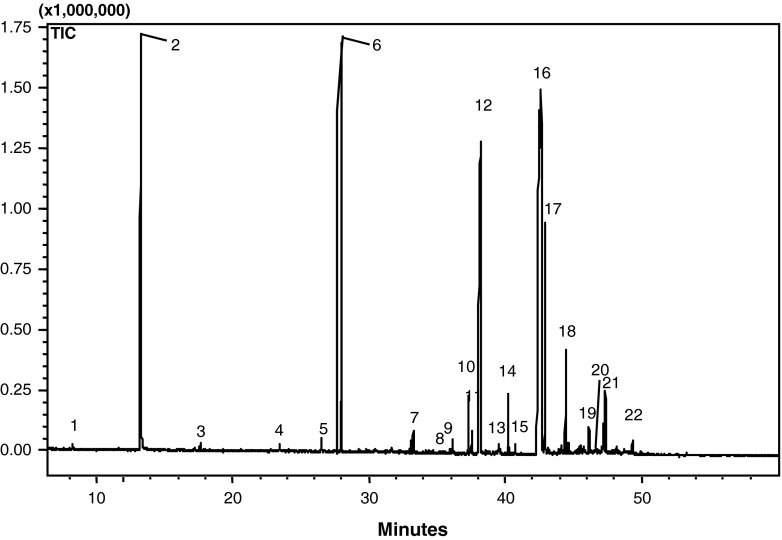
Volatile compounds present in P. sajor-caju detected by GC-MS are reported in Table 6. In this study, volatile compounds of mushrooms were determined as estimated approach by the use of gas chromatography and mass spectrophotometer library catalog (GC-MS). One of the most important consumer acceptances is the flavor of the foods (Caglarırmak 2007). The study was focused on the standardization of the extraction of the 1-octen-3-ol which is a characteristic aroma compound of mushrooms (Wojtasiak 2004) and quantification of volatiles components present therein. In this work, extraction of volatile compounds was carried out by following cold and hot extraction methods by using Pentadecane as an inner standard. The most of abundant volatile compounds in cold extraction and their concentrations (per 100 g of mushrooms) are as follows: octadecanoic acid, methyl ester 104.14 μg; hexadecanoic acid 60.92 μg and 1-octen-3-ol 42.18 μg (Table 4 and Figure not shown). Flavour components found in hot extraction of P. sajor-caju are shown in Table 4 and Fig. 2. The volatile compounds present in higher amounts are 1-octen-3-ol (5000 μg), hexadecanoic acid (6118.52 μg) and octadecenoic acid (13132.5 μg). It was found that hot extraction method was more effective than the cold extraction method to extract more number of volatile components and also in higher concentrations. As per our literature survey the hot extraction method combined with steam distillation, used in the present work for extraction of flavour components in Pleurotus sajor-caju is first of its kind.
Table 4.
Volatile compounds found by cold extraction and hot extraction in P. sajor-caju
| Extraction | Peak | Compound | RTa | RIb | Concentration (μg/100 g) |
|---|---|---|---|---|---|
| Cold extraction | 1 | 1-Octen-3-ol* | 13.250 | 1042 | 42.18 |
| 2 | Pentadecane (Inner standard) | 27.970 | 1742 | 5000 | |
| 3 | n-Hexadecanoic acid* | 39.183 | 2079 | 60.92 | |
| 4 | Octadecanoic acid, methyl ester* | 43.300 | 2202 | 104.14 | |
| Hot extraction | 1 | Hexanal* | 8.375 | 794 | 19.97 |
| 2 | 1-Octen-3-ol* | 13.350 | 1042 | 5000 | |
| 3 | 8-Octanoic acid, | 17.683 | 1308 | 49.93 | |
| 4 | 1-Octanol | 23.450 | 1605 | 33.29 | |
| 5 | Nonanoic acid, 9-oxo-, methyl ester | 26.508 | 1699 | 96.54 | |
| 6 | Pentadecane (Inner standard) | 27.958 | 1742 | 5000 | |
| 7 | Methional* | 33.058 | 1897 | 83.22 | |
| 8 | Tetradecanoic acid, | 33.258 | 1903 | 106.52 | |
| 9 | Guai-1(10)-en-11-ol | 36.142 | 1990 | 93.21 | |
| 10 | Azulene | 31.242 | 2024 | 56.59 | |
| 11 | Hexadecenoic acid, methyl ester* | 37.550 | 2033 | 153.13 | |
| 12 | n-Hexadecanoic acid* | 38.208 | 2053 | 6118.52 | |
| 13 | Hexadecadienoic acid, methyl ester* | 39.517 | 2093 | 99.87 | |
| 14 | Oleic acid, methyl ester | 40.250 | 2115 | 66.58 | |
| 15 | Heptadecanoic acid | 40.742 | 2130 | 56.59 | |
| 16 | Octadecenoic acid* | 42.650 | 2188 | 13132.51 | |
| 17 | Octadecanoic acid, methyl ester* | 42.950 | 2197 | 1674.44 | |
| 18 | Nonadecenoic acid* | 44.442 | 2242 | 589.22 | |
| 19 | Eicosanoic acid | 46.158 | 2294 | 243.01 | |
| 20 | 13-Methylpentadec-14-ene-1,13-diol | 47.208 | 2326 | 159.79 | |
| 21 | Methyl decenoate | 47.358 | 2330 | 369.51 | |
| 22 | Docosanoic acid | 49.350 | 2391 | 86.55 |
Fig. 2.
Chromatogram of hot extraction of volatile components of P. sajor-caju
Conclusion
Pleurotus sajor-caju is found to be a rich source of protein, carbohydrates, crude fiber and important major minerals like Ca, Fe, Mg, Na, K and P with some minor minerals but contains very less amount of fat. This species contains significant amount of antioxidant components like phenols, ascorbic acid and flavonoids having free radical scavenging potential and antibacterial activities. Therefore, Pleurotus sajor-caju which is normally consumed as a vegetable provides all important major and minor nutrients with antioxidant properties which can be used as a nutritious food to combat various deficiency diseases and malnutrition. The major consumer acceptance of Oyster mushroom is due to its characteristic flavor which is mainly contributed by 1-octen-3-ol. Hot extraction method combined with steam distillation was found to be better for extracting this characteristic component compared to cold extraction method.
Acknowledgements
We are thankful for the sincere supports from The Coordinator, Department of Food Science and Technology and Common Facility Center (CFC), Shivaji University, Kolhapur (Maharashtra), India.
References
- Amin I, Norazaidah Y, Hainida EKI. Antioxidant activity and phenolic content of raw and blanched Ameranthus species. Food Chem. 2006;94:47–52. doi: 10.1016/j.foodchem.2004.10.048. [DOI] [Google Scholar]
- Official methods of analysis. 16. Arlighton VA, USA: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Banik S, Nandi R. Effect of supplementation of rice straw with biogas residual slurry manure on the yield, protein and mineral contents of oyster mushroom. Ind Crop Prod. 2004;20:311–319. doi: 10.1016/j.indcrop.2003.11.003. [DOI] [Google Scholar]
- Barros L, Baptista P, Estevinho LM, Ferreira ICFR. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 2007;105:179–186. doi: 10.1016/j.foodchem.2007.03.063. [DOI] [Google Scholar]
- Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J Bioenergy Biomembr. 1994;26:349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- Caglarırmak N. The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus species) and an estimated approach to the volatile compounds. Food Chem. 2007;105:1188–1194. doi: 10.1016/j.foodchem.2007.02.021. [DOI] [Google Scholar]
- Chadha KL, Sharma SR. Advances in horticulture, volume 13, mushroom. New Delhi, India: M. P. H., Malhotra Publishing House; 1995. [Google Scholar]
- Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2007;100:1044–1048. doi: 10.1016/j.foodchem.2005.11.008. [DOI] [Google Scholar]
- Confortin FG, Marchetto R, Bettin F, Camassola M, Salvador M, Dillon AJP. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J Ind Microbiol Biotechnol. 2008;35:1149–1155. doi: 10.1007/s10295-008-0394-x. [DOI] [PubMed] [Google Scholar]
- Crisan EV, Sands A. Nutritional value. In: Chang ST, Hayes WA, editors. The biology and cultivation of edible mushrooms. New York: Academic; 1978. [Google Scholar]
- Findlay JA, Daljeet A, Murray PJ, Rej RN. Total synthesis of the ravidomycin aglycone (defucogilvocarcin V) Can J Chem. 1987;65:427–431. doi: 10.1139/v87-072. [DOI] [Google Scholar]
- Gasim AYAE, Mohammad MA, Baker AAA. Effect of soaking, sprouting and cooking on chemical composition, bioavalability of minerals and in vitro protein digestibility of Roselle (Hibiscus sabdariffa L.) seed. Pakistan J. Nutr. 2008;7(1):50–56. doi: 10.3923/pjn.2008.50.56. [DOI] [Google Scholar]
- Gursoy N, Sarikurkcu C, Cengiz M, Solak MH. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem Toxicol. 2009;47:2381–2388. doi: 10.1016/j.fct.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Jayakumar T, Ramesh E, Geraldine P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem Toxicol. 2006;44:1989–1996. doi: 10.1016/j.fct.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Jayakumar T, Thomas PA, Geraldine P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innovat Food Sci Emerg Tech. 2009;10:228–234. [Google Scholar]
- Kalac P. Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem. 2009;113:9–16. [Google Scholar]
- Kalac P, Svoboda L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000;69:273–281. [Google Scholar]
- Klein BP, Perry AK. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J Food Sci. 1982;47:941–945. [Google Scholar]
- Louli V, Ragoussis N, Magoulas K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour Technol. 2004;92:201–208. doi: 10.1016/j.biortech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Mallavadhani UV, Sudhakar AVS, Satyanarayana KVS, Mahapatra A, Li W, vanBreemen RB. Chemical and analytical screening of some edible mushrooms. Food Chem. 2006;95:58–64. [Google Scholar]
- Mattila P, Vaananen PS, Kongo K, Aro H, Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agr Food Chem. 2002;50:6419–6422. doi: 10.1021/jf020608m. [DOI] [PubMed] [Google Scholar]
- Mau JL, Chao GR, Wu KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J Agr Food Chem. 2001;49:5461–5467. doi: 10.1021/jf010637h. [DOI] [PubMed] [Google Scholar]
- Maynard AJ. Methods in food analysis. New York: Academic; 1970. [Google Scholar]
- Meda A, Lamien CE, Rmito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Misharina TA, Mukhutdinova SM, Zharikova GG, Terenina MB, Krikunova NI, Medvedeva IB. The composition of volatile components of dry cepe and oyster mushroom. Appl Biochem Microbiol. 2009;45(5):544–549. [PubMed] [Google Scholar]
- Misharina TA, Muhutdinova SM, Zharikova GG, Terenina MB, Krikunova NI. The composition of volatile components of Cepe (Boletus edulis) and oyster mushrooms (Pleurotus ostreatus) Appl Biochem Microbiol. 2009;45:187–193. [PubMed] [Google Scholar]
- Ouzouni PK, Petridis D, Koller W, Riganakos KA. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009;115:1575–1580. [Google Scholar]
- Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SMN, Somasundaram R. Antioxidant activity of indigenous edible mushrooms. J Agr Food Chem. 2006;54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- Ragunathan R, Swaminathan K. Nutritional status of Pleurotus spp. grown on various agro-wastes. Food Chem. 2003;80:371–375. [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable. New Delhi: Tata McGraw Hill Publishing Co. Ltd.; 2000. [Google Scholar]
- Salmi C, Loncle C, Vidal N, Letourneux Y, Fantini J, Maresca M, Taieb N, Pages JM, Brunel JM. Squalamine: an appropriate strategy against the emergence of multidrug resistant gram-negative bacteria? PLoS ONE. 2008;3(7):e2765. doi: 10.1371/journal.pone.0002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches NR, Cortez DAG, Schiavini MS, Nakamura CV, Filho BPD. An evaluation of antibacterial activities of Psidium guajava (L.) Braz Arch Biol Tech. 2005;48(3):429–436. [Google Scholar]
- Sani UM, Pateh UU. Isolation of 1, 2-benzenedicarboxylic acid bis (2-ethylhexyl) ester from methanol extract of the variety minor seeds of Ricinus communis Linn. (Euphorbiaceae) Nig J Pharm Sci. 2009;8(2):107–114. [Google Scholar]
- Schachter EN, Zuskin E, Goswami S, Castranova V, Arumugam U, Whitmer M, Siegel P, Chiarelli A, Fainberg J. Pharmacological study of oyster mushroom (Pleurotus Ostreatus) extract on isolated guinea pig trachea smooth muscle. Lung. 2005;183:63–71. doi: 10.1007/s00408-004-2527-y. [DOI] [PubMed] [Google Scholar]
- Shashirekha MN, Rajathnam S, Bano Z. Enhancement of bioconversion efficiency and chemistry of mushroom P. sajor caju (Berk and Br.) Sacc. produced on spent rice straw substrate supplemented with oil seed cakes. Food Chem. 2002;76:27–31. [Google Scholar]
- Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007;101:267–273. [Google Scholar]
- Venkateshwarlu G, Chandravadana MV, Tewari RP. Volatile flavour components of some edible mushrooms (Basidiomycetes) Flavour Fragr J. 1999;14:191–194. [Google Scholar]
- Wojtasiak R. Optical purity of (R)-())-1-octen-3-ol in the aroma of various species of edible mushrooms. Food Chem. 2004;86:113–118. [Google Scholar]
- Wong JY, Chye FY. Antioxidant properties of selected tropical wild edible mushrooms. J Food Compos Anal. 2009;22:269–277. [Google Scholar]
- Wong KH, Sabaratnam1 V, Abdullah1 N, Kuppusamy U, Naidu M, (2009). Effects of cultivation techniques and processing on antimicrobial and antioxidant activities of Hericium erinaceus (Bull.:Fr.) Pers. Extracts. Food Tech Biotechnol 47:47–55
- Yaltirak T, Aslim B, Ozturk S, Alli H. Antimicrobial and antioxidant activities of Russula delica Fr. Food Chem Toxicol. 2009;47:2052–2056. doi: 10.1016/j.fct.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Yang JH, Lin HC, Mau JL. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001;72:465–471. [Google Scholar]
- Yanga JH, Lin HC, Maub JL. Antioxidant properties of several commercial mushrooms. Food Chem. 2002;77:229–235. [Google Scholar]