Abstract
Yogurt-ice cream is a nutritious product with a refreshing taste and durability profoundly longer than that of yogurt. The probiotic Lactobacillus acidophilus (La-5) cells either in free or encapsulated form were incorporated into yog-ice cream and their survivability were studied. Fructooligosaccharide (FOS) as a prebiotic compound at three levels (0, 4 & 8 % w/w) was added to yogurt-ice cream mix and its effects on some chemical properties, overrun and firmness of product were evaluated. The higher the incorporated FOS concentration, the lower were the pH value and higher the total solid content of treatments. FOS incorporation (8 %) significantly increased the overrun of treatments and reduced their firmness. The viable counts of free probiotics decreased from ~9.55 to ~7.3 log cfu/g after 60 days of frozen storage while that of encapsulated cells merely decreased less than 1 log cycle. Encapsulation with alginate microbeads protected the probiotic cells against injuries in the freezing stage as well as, during frozen storage.
Keywords: Encapsulation, Lactobacillus acidophilus, Probiotic, Prebiotic, Yog-ice cream
Introduction
Yogurt-ice cream is a complex fermented frozen dairy dessert that combines the physical characteristics of ice cream with sensory and nutritional properties of fermented milk products. This elaboration results in a nutritious product with a refreshing taste and storage stability significantly longer than that of yogurt (Guven and Karaca 2002). Yog-ice cream can be produced by three basic techniques: (i) direct acidification or one stream method, (ii) indirect acidification or two streams method and (iii) blending method. When direct acidification technique is implemented, a typical ice cream mix is inoculated with a lactic acid starter, incubated for 12–18 h, cooled, frozen, hardened and stored under deep-freezing conditions. This is an untoward technique for industry due to the long time required for fermentation. Indirect acidification entails the production of two bases i.e. a typical ice cream mix and a plain stirred yogurt base. These streams are blended at different ratios in the range 5 %–70 % yogurt proportion, followed by freezing, hardening and storage. This is a feasible technique for industry due to the ease of process and complete fermentation. The blending method refers to the incorporation of lactic acid bacteria before the freezing step without any fermentation of the mix. The final product manufactured by this technique is not compatible with yogurt definition so reprobated by National Yogurt Association (NYA) and International Ice Cream Association (IICA) (Childs 1993; Soukoulis and Tzia 2008).
In recent years, there has been a worldwide increasing interest in incorporating intestinal bacterial species to fermented milks (Vinderola et al. 2000). Probiotics are live microbial supplements which if taken in adequate amounts (Lyer et al. 2011) beneficially affect the host by improving its intestinal microbial balance (Krasaekoopt et al. 2003). They impart therapeutic effects and promote health by preventing various diseases (Vanaja et al. 2011). The main effects attributed to these bacteria are enhancement of immunity against intestinal infections and improvement in lactose utilization, prevention of colon cancer and hypercholesterolaemia (Kailasapathy and Rybka 1997) as well as, improvement of calcium absorption, vitamin synthesis and pre-digestion of proteins (Nakasawa and Hosono 1992). The growth and/or activity of probiotics in colon are stimulated by the presence of non-digestible complex carbohydrates known as prebiotics (Donkor et al. 2007). A prebiotic product should resist the digestion in the upper gastrointestinal tract, be selectively metabolized in the colon by the beneficial microbes and consequently enhance the health. (Mohammadi and Mortazavian 2010). These compounds are consumed by probiotics in colon as a carbon or energy source which results in an increase in their count and a reduction in the number of pathogenic microorganisms (Homayouni et al. 2008).
Fructooligosaccharides (FOS) are linear polymers of fructosyl monomers linked through β(2 → 1) bounds (Azorin-ortuno et al. 2009) with a polymerization degree ≤10 and categorized as a subgroup of inulin (Akalin and Erisir 2008). Their structure is similar to corn sweeteners and is technically categorized as fat replacers. A food product that contains both probiotics and prebiotics is called synbiotic (Homayouni et al. 2007) which offer potential in the prophylactic management of gastrointestinal defects (Dhewa et al. 2012).
Since the therapeutic role of probiotics depends on the count of viable cells, International Dairy Federation (1991) has suggested that a minimum of 107 probiotic bacteria cells should be alive at the time of consumption per gram of the product. Hekmat and McMahon (1992) reported that during 17 weeks of frozen storage of probiotic ice cream, Lactobacillus acidophilus count decreased by two log cycles. Encapsulation of probiotic cells within microcarriers provides a relatively new approach to improve their survival. Encapsulation helps to protect the bacterial cells from the hostile environment of the food product, as well as the gastrointestinal tract, thus potentially preventing cell loss (Homayouni et al. 2008). It may cause an appropriate cell release in the intestinal medium (Mohammadi et al. 2011). Shah and Ravula (2000) reported that the survival of probiotic bacteria in fermented frozen desserts improved with encapsulation. They argued that microcapsules protected the bacterial cells against harsh environmental conditions such as freezing stress.
Supplementation of yog-ice cream with probiotic bacteria, such as L. acidophilus may provide additional health benefits. The acidic environment of this product is not however, optimum for survival of bacteria (Baig and Prasad 1995; Davidson et al. 1999). There was no report in literature on the yog-ice cream supplemented with microencapsulated probiotic cultures and FOS as the prebiotic fat replacer. The objective of the present study was therefore to evaluate the feasibility of incorporating FOS in yog-ice cream and monitor the viability of free and encapsulated L. acidophilus cells during storage of product at −18 °C for a period of 60 days.
Materials and methods
Materials
Pure freeze-dried probiotic culture of L. acidophilus (La-5) was purchased from Chr-Hansen (Horsholm, Denmark). Freeze-dried yogurt culture, LAT BY 1–63® (L. delbrueckii subsp. bulgaricus and Streptococcus thermophilus) was purchased from Lactina Ltd. (Sofia, Bulgaria). Skim milk powder (Dalone Multi industry, Mashhad, Iran), FOS (Orafti® P95, Sigma-Aldrich, Dorset, London, UK), ∝-monoglyceride (E471) (Sigma-Aldrich, London, UK) as emulsifier, panisol (monoglycerides of fatty acids emulsifiers, mainly stearic acid combined with natural gum stabilizers, mainly guar gum, Azma laban shargh Co., Mashhad, Iran), cream (Fat 30 % and Protein 2.5 %), and vanillin (obtained from Kalleh Dairy Co., Amol, Iran) were used for the preparation of yog-ice cream samples. Also, calcium alginate (medium viscosity and high mannuronic acid, Sigma-Aldrich, London, UK), Tween 80 (ICI America Inc. Bridgewater, NJ, USA), peptone (Merck KGaA, Darmstadt, Germany) and bile salts (mixture of sodium cholate and sodium deoxycholate, Sigma-Aldrich, London, UK) were used for the preparation of microcapsules.
Treatments
Nine treatments were made as follows: three samples with 0, 4 and 8 % FOS and without L. acidophilus coded as NC0, NC4 and NC8, respectively; three samples with 0, 4 and 8 % FOS and with free L. acidophilus cells coded as F0, F4 and F8, respectively; three samples with 0, 4 and 8 % FOS and with encapsulated L. acidophilus cells coded as E0, E4 and E8.
Preparation of probiotic culture and enumeration
Pure freeze-dried L. acidophilus (La-5) was activated by inoculating in MRS-Broth (de Man-Rogosa-Sharpe) medium at 37 °C and incubating for ~7 h under aerobic conditions. The cells were harvested by centrifuging the biomass at 4500 × g for 10 min at 4 °C and washed twice with sterile 0.1 % (w/w) peptone solution.
Enumeration of cells was carried out before and immediately after freezing of product as well as every 15 days until 60 days of storage at −18 °C. In order to enumerate the cells, MRS-bile agar was used as the selective media (Vinderola and Reinheimer 1999; Vinderola and Reinheimer 2000; Vinderola et al. 2000; Mortazavian et al. 2007a; Sohrabvandi et al. 2012). Bacterial count was done as previously described by Haynes and Playne (2002) and their averages are expressed as (cfu/g) of sample.
Encapsulated cells were released from capsules according to the method of Sheu and Marshall (1993). Ten grams of yog-ice cream were suspended in 100 mL sodium citrate buffer (0.01 g/mL, pH = 6), and gently shaken for 10 min. Then 1 mL aliquot dilutions were poured onto plates of the MRS-bile agar at 37 °C for 72 h under aerobic conditions in triplicate. A similar treatment was carried out for yog-ice cream containing free cells.
Encapsulation process
Calcium alginate microcapsules were made using an external gelation technique adapted from Sheu and Marshall (1993) as previously described by Allan-Wojtas et al. (2008). Initially 18 g sodium alginate solution (10 g/L) was mixed with 1 g washed bacteria suspension. The mixture was subsequently emulsified in 100 g vegetable oil containing 5 g/L Tween 80 using a magnetic stirrer at ~900 rpm for 20 min. Gelation was initiated by adding 32 mL of an emulsion containing Ca2+ ions (60 g vegetable oil, 5 g/L Tween 80 and 62.5 mM CaCl2). The alginate microcapsules were formed during continuous stirring for 20 min. The beads were allowed to stand for 30 min for gelification and then rinsed with and subsequently kept in sterile 0.1 % (w/w) peptone solution at 4 °C.
Encapsulation yield
Encapsulation yield (EY) i.e. the number of bacterial cells that survived the process and encapsulated inside the alginate particles was calculated as follows:
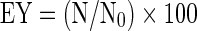 |
where N0 is the number of viable bacteria in cfu/g of biomass and N is the number of viable bacteria in cfu/g of capsules.
Preparation of yog-ice cream
To produce yog-ice cream, skim milk powder was reconstituted in distilled water (35 °C) to 12 % (w/w) total solids for yoghurt production. It was then pasteurized (80 °C, 20 min), cooled to 45 °C, inoculated with 2 % of a commercial freeze-dried lactic acid bacteria (mixed flora 1:1 of L. delbrueckii subsp. bulgaricus and Streptococcus thermophilus) and incubated at 45 °C for 4 h (until pH reached 4.7). For ice cream production, water, skim milk powder, 0.1 % (w/w) vanillin, 0.3 % (w/w) emulsifier (E471), 15 % (w/w) sugar, 9.43 % (w/w) cream (30 % fat), 0.2 % (w/w) panisol and FOS with various percentage (0, 4 and 8 % (w/w)) were heated to 45 °C and homogenized at 1000 rpm (Ultra-Turrax® T25, IKA®-Werke GmbH & Co. KG, Staufen, Germany) until no clumps were present. The mixture was pasteurized at 80 °C for 20 s and rapidly cooled to about 10 °C. Finally yoghurt was blended with the ice cream premix (3:2) and kept at 4 °C for 10 h. The yog-ice cream mixture was split into 300-gram batches, each of which supplemented with free or encapsulated L. acidophilus at a rate of 1 % w/v. The mixtures were then aerated and frozen by a self-contained freezing unit (Simac Il Gelato Junior, Treviso, Italy) and stored in 100 mL cups at −18 °C. Treatments were made in triplicate.
Chemical and physical measurements
The pH value of milk, yogurt and yog-ice cream was measured using a digital pH-meter (Jenway 3020, Spain). The titratable acidity of samples was measured according to IDF Standard (IDF 150:1991). Dry matter content of yog-ice cream was determined by drying samples at 105 ± 1 °C for 5 h using an air oven (Association of Official Analytical Chemists 2005; method number 990.19) and its fat content was determined by Gerber method. All chemical measurements were done in triplicate.
The overrun of the final product was determined using the following equation (Akin et al. 2007).
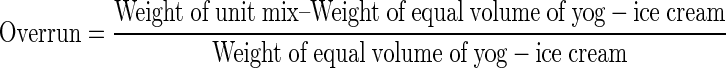 |
Morphology of the microcapsules was observed using a scanning electron microscope (LEO, 1450 VP, Germany) at an accelerating voltage of 10 kV. The encapsulated samples were mounted on the stub with the aid of double side tape and coated by sputter coater (SC 7620, England) for 2 min using Au-Pd coat.
Firmness of samples
The firmness of yog-ice cream samples was determined by the simplest fundamental test, uniaxial compression (Aziznia et al. 2008) using a Texture Analyser (Stable Microsystems Ltd., TA-XT2i, Surrey, UK) with a 500-N load cell. A cylindrical plunger with a flat base 34 mm in diameter was attached to the moving crosshead. For each sample, three measurements were carried out using a 5 mm stainless steel cylindrical probe attached to a 25 kg load cell. The penetration depth at the geometrical centre of the samples was 10 mm and the penetration speed was set at 2 mm/s. Firmness was defined as the peak force used in penetration (Aziznia et al. 2008; Soukoulis and Tzia 2008).
Statistical analysis
All statistical analyses were carried out using the SPSS statistical software program (version 16, SPSS Inc., Chicago, IL, USA). Multiple comparisons between means were analyzed with the Duncan’s multiple range method at (p < 0.05). All analyses were done in triplicate.
Results and discussion
Physical and chemical characteristics
The pH decreased slowly from 5.5 to 5.1–5.4 and acidity increased from 0.35 to 0.36–0.41 in all samples during storage at −18 °C for 60 days. Supplementation with FOS significantly influenced the pH, acidity and total solids of samples. The higher the FOS concentration, the lower was the pH and higher was the total solids (Table 1). This trend was observed for all treatments with or without free and encapsulated probiotic cells. Similar to our results, Ozer et al. (1998) and Akin et al. (2007) reported that the titratable acidity of yogurt and probiotic ice cream increased with increasing the inulin concentration. The increase in acidity of treatments is probably due to the stimulation of starter and/or probiotic cells by FOS. Supplementation with probiotics in the free or encapsulated form did not significantly (p > 0.05) influence the pH value of yog-ice cream.
Table 1.
Dry matter and fat content of freshly prepared yog-ice cream samples
| Sample | Dry matter (%) | Fat content |
|---|---|---|
| NC0 | 26.9c ± 0.20 | 5a ± 0.00 |
| NC4 | 27.1abc ± 0.05 | 3.6b ± 0.30 |
| NC8 | 27.5a ± 0.06 | 1.6c ± 0.30 |
| F0 | 26.8c ± 0.60 | 5a ± 0.00 |
| F4 | 27.1abc ± 0.01 | 3.5b ± 0.00 |
| F8 | 27.3ab ± 0.04 | 1.5c ± 0.00 |
| E0 | 27.0bc ± 0.18 | 5.0a ± 0.00 |
| E4 | 27.1abc ± 0.02 | 3.6b ± 0.30 |
| E8 | 27.5a ± 0.10 | 1.9c ± 0.30 |
Refer to text for samples NC0-E8
Means with different superscripts are statistically (p<0.05) different; (n = 3)
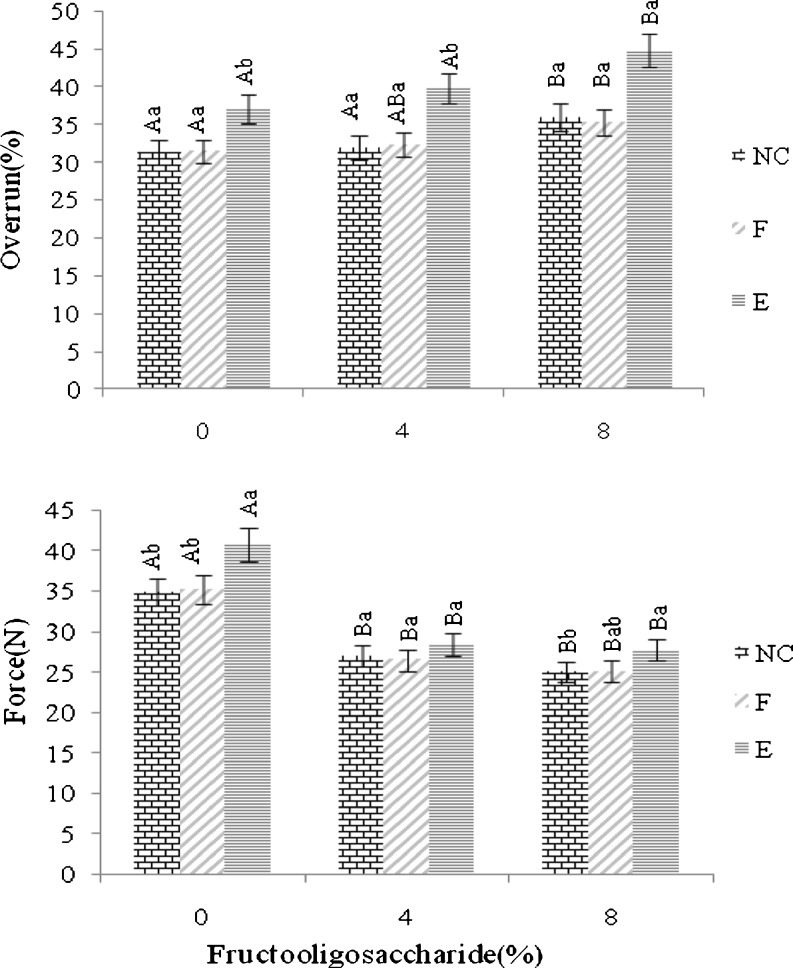
As shown in Fig. 1 incorporating FOS significantly increased the overrun of yog-ice cream treatments. The increase was more pronounced for samples with encapsulated L. acidophilus cells. Keeney and Maga (1965) similarly commented that higher levels of dry matter in ice cream mix result in higher overrun of the final product. During aeration, the air/serum interfaces formed due to air introduction are initially stabilized against immediate coalescence by adsorption of proteins from the aqueous phase to the interface (Goff 1997; Needs and Huitson 1991). As aeration continues, the bubbles are broken up into smaller ones (Emam-Djome et al. 2008); a number of those may depart the mixture. The increase in viscosity of the yog-ice cream mixture because of added FOS and/or sodium alginate used as the wall material of microcapsules, although probably reduced the incorporation rate of air to the mixture during aeration, may also decreased the departure rate of incorporated air bubbles from the mixture. As well, FOS molecules were probably adsorbed onto the air/serum interfaces along with proteins, increasing the stability of bubbles. These would lead to a higher proportion of air phase in the final product monitored as higher overrun.
Fig. 1.
Overrun and Firmness values of yog-ice cream treatments. NC, F and E refer to no treatment, treatment with free and encapsulated L. acidophilus, respectively. Capital letter superscripts compare a treatment in different FOS concentrations and small letter superscripts compare different treatments with the same FOS concentration. Means with different superscripts are statistically (p<0.05) different. Error bars represents standard error (n = 3)
Firmness of yog-ice cream samples supplemented with 4 and 8 % FOS was significantly lower than that of samples without FOS. The difference between samples entail 4 and 8 % FOS was not, however, statistically significant (Fig. 1). In agreement to our results, Nagar et al. (2002) observed that the addition of 5 % inulin to the low fat yog-ice cream base reduced the overall firmness of product. They attributed this subject to the formation of a viscous gel matrix. Karaca et al. (2009) argued that the decrease in the firmness of product because of added fat replacer is due to the modifications in the characteristics of fluid surrounding the air cells. Hydrocolloids act as cryoprotectants due to their ability to control water diffusion from and to the ice crystals by steric hindrance and water binding. The decrease in firmness with the increasing hydrocolloid content may be attributed to their freeze concentration in serum phase (Soukoulis et al. 2008). As well, the higher amount of air entailed within FOS-supplemented treatments could affect the textural properties of yog-ice cream. Similarly, Sofjan and Hartel (2004) and Wilbey et al. (1997) illustrated that the increased overrun led to a decrease in firmness of product.
Viability of free and encapsulated probiotic cells
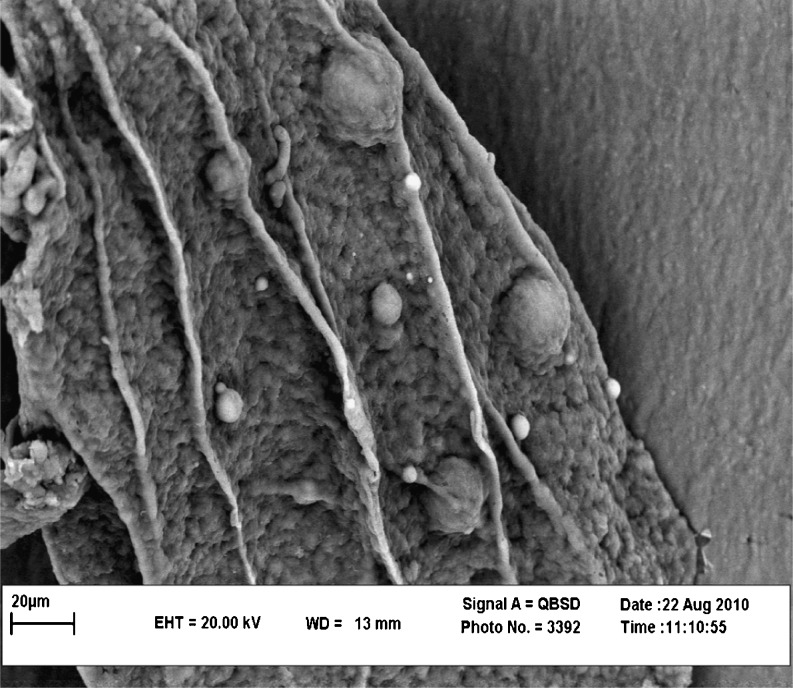
Viable cell count of L. acidophilus decreased from 11.82 to 11.78 log cfu/g due to encapsulation process indicating an entrapment efficiency of ~75 %. Figure 2 shows a scanning electron micrograph of capsules containing L. acidophilus cells. It is observed that capsules were spherical shaped particles with diameters less than 50 μm. Emulsion technique is suitable for encapsulating probiotic bacteria in the beads smaller than 1 mm, providing the possibility to control the size of alginate beads by using high stirring speed (Homayouni et al. 2008). It has been documented (Sheu and Marshall 1993) that microcapsules with a mean diameter of ~30 μm are desirable for use in frozen dairy desserts. Larger beads might cause the coarseness of texture, whereas smaller beads do not provide sufficient protection of the bacteria. Alginate capsules are biocompatible, cheap, easily prepared and properly resolved in the intestine to release the entrapped cells (Mortazavian et al. 2007b).
Fig. 2.
SEM image of an alginate microcapsule entailing L. acidophilus (La-5)
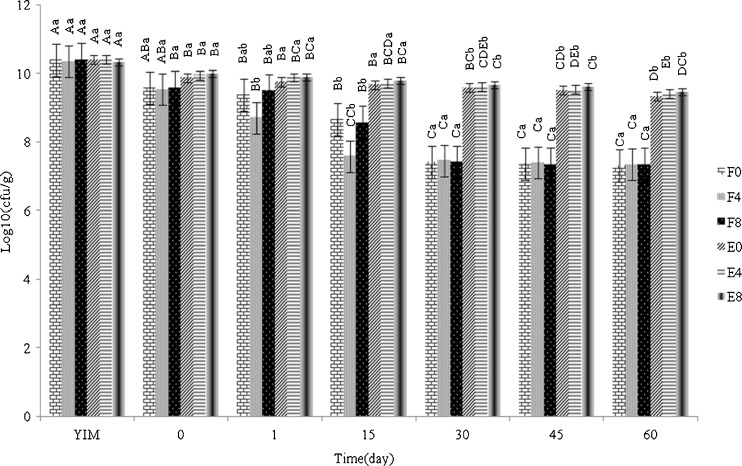
Changes in viable counts of L. acidophilus in yog-ice cream mix due to freezing and during frozen storage at −18 °C are presented in Fig. 3. Analysis of data showed that there was an approximately 0.5–1 log cycle decrease in the count of probiotic cells in the free form immediately after freezing. This indicates that freezing had destructive effects on probiotic cells most probably due to the freezing injury of cells. As well, the number of free cells decreased by an average of 1.5–2 log cycle at the end of 60 days frozen storage. Hekmat and McMahon (1992) reported that the viability of L. acidophilus in a standard ice cream mix decreased by 2 log cycles after storage for 17 weeks at −29 °C. Our results show that decrease in the number of free probiotic cells in all samples occurred at the first 30 days of frozen storage and after that, their viability did not significantly change (p < 0.05). A similar observation was reported by Akin et al. (2007) and Heydari et al. (2012). Encapsulation within alginate microbeads protected the probiotic cells against injuries in the freezing stage (Fig. 3) as well as, during frozen storage. Viable number of encapsulated L. acidophilus was merely decreased less than 1 log cycle after 60 days of frozen storage. Shah and Ravula (2000) illustrated that microencapsulation improved the viability of L. acidophilus MJLA1 and Bifidobacterium spp. BDBB2 in frozen fermented dairy desserts stored for 12 weeks.
Fig. 3.
Survival of free and encapsulated L. acidophilus (La-5) cells in synbiotic yog-ice cream with different percentages of fructooligosaccharide during 60 days of storage at −18 °C. YIM: Yog-ice cream mix; F0, F4, F8, E0, E4 and E8 refer to treatments with 0, 4 and 8 % fructooligosaccharide with free and encapsulated L. acidophilus, respectively. Capital letter superscripts compare a treatment in different days and small letter superscripts compare different treatments within a day. Means with different superscripts are statistically (p<0.05) different. Error bars represents standard error (n = 3)
Conclusion
Supplementation of yog-ice cream with FOS increased its overrun especially for treatments containing microencapsulated L. acidophilus probiotic cells. It also decreased the firmness of samples. Encapsulation of L. acidophilus cells via emulsion technique inside sodium alginate microbeads protects the cells against freezing injuries. The generated capsules are small enough to be incorporated within yog-ice cream without any undesirable affects on its sensory properties. The incorporation of microencapsulated L. acidophilus cells along with low concentrations of fructooligosaccharide in yog-ice cream mix is suitable to prepare a synbiotic product which meets the requirements by International Dairy Federation.
Acknowledgements
We express our appreciation to Dr. Reza Farahmandfar, (Department of Food Science and Technology, Faculty of Agriculture, Ferdowsi University, Mashhad, Iran) for his invaluable helps.
References
- Akalin AS, Erisir D. Effect of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low-fat probiotic ice cream. J Food Sci. 2008;73:184–188. doi: 10.1111/j.1750-3841.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- Akin MB, Akin MS, Kirmaci Z. Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice-cream. Food Chem. 2007;104:93–99. [Google Scholar]
- Allan-Wojtas P, Truelstrup HL, Paulson AT. Microstructural studies of probiotic bacteria-loaded alginate microcapsules using standard electron microscopy techniques and anhydrous fixation. LWT-Food Sci Technol. 2008;41:101–108. [Google Scholar]
- Association of Official Analytical Chemists (2005) Official methods of analysis of AOAC International (18th ed) Maryland, USA.
- Aziznia S, Khosrowshahi A, Madadlou A, Rahimi J. Whey Protein concentrate and gum tragacanth as fat replacers in nonfat yogurt: chemical, physical, and microstructural properties. J Dairy Sci. 2008;91:2545–2552. doi: 10.3168/jds.2007-0875. [DOI] [PubMed] [Google Scholar]
- Azorin-ortuno M, Urban C, Ceron J, Tecles F, Allende A, Tomas-Barberan F, Espin J. Effect of low inulin doses with different polymerization degree on lipid metabolism, mineral absorption, and intestinal microbiota in rats with fat-supplemented diet. Food Chem. 2009;113:1058–1065. [Google Scholar]
- Baig MI, Prasad V. Biochemical characteristics and stability of Bifidobacterium bifidum in frozen yogurt supplemented with condensed cheese whey. J Dairy Foods Home Sci. 1995;15:99–108. [Google Scholar]
- Childs J. An identity for frozen yoghurt. Cultured Dairy Products J. 1993;29:23–24. [Google Scholar]
- Davidson RH, Duncan SE, Hackney CR, Eigel WN, Boling JW. Probiotic culture survival and implications in fermented frozen yogurt characteristics. J Dairy Sci. 1999;83:666–673. doi: 10.3168/jds.S0022-0302(00)74927-7. [DOI] [PubMed] [Google Scholar]
- Dhewa T, Pant S, Mishra V (2012) Development of freeze dried synbiotic formulation using a probiotic strain of Lactobacillus plantarum. J Food Sci Technol. doi:10.1007/s13197-011-0457-2 [DOI] [PMC free article] [PubMed]
- Donkor ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP. Survival and activity of selected probiotic organisms in set-Type yogurt during cold storage. Int Dairy J. 2007;17:657–665. [Google Scholar]
- Emam-Djome Z, Ebrahimzadeh-mousavi M, Gorbani AV, Madadlou A. Effect of whey protein concentrate addition on the physical properties of homogenized sweetened dairy creams. Int J Dairy Technol. 2008;61:183–191. [Google Scholar]
- Goff HD. Instability and Partial Coalescence in Whippable Dairy Emulsions. J Dairy Sci. 1997;80:2620–2630. [Google Scholar]
- Guven M, Karaca OB. The effects of varying sugar content and fruit concentration on the physical properties of vanilla and fruit ice cream type frozen yogurts. Int J Dairy Technol. 2002;55:27–31. [Google Scholar]
- Haynes IN, Playne MJ. Survival of probiotic cultures in low fat ice cream. Aust J Dairy Technol. 2002;57:10–14. [Google Scholar]
- Hekmat S, Mcmahon DJ. Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in ice cream for use as a probiotic food. J Dairy Sci. 1992;75:1415–1422. doi: 10.3168/jds.S0022-0302(92)77895-3. [DOI] [PubMed] [Google Scholar]
- Heydari S, Mortazavian AM, Ehsani MR, Mohammadifar MA, Ezzatpanah H, Sohrabvandi S. Biochemical, microbiological and sensory characteristics of probiotic yogurt containing various prebiotic or fiber compounds. Ital J Food Sci. 2012;23:153–163. [Google Scholar]
- Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. [Google Scholar]
- Homayouni A, Ehsani MR, Azizi A, Yarmand MS, Razavi SH. Effect of lecithin and calcium chloride solution on the microencapsulation process yield of calcium alginate beads. Iranian Polym J. 2007;16:597–606. [Google Scholar]
- IDF (1991). Yogurt: determination of titratable acidity- potentiometric method. International Dairy Federation Standard, 150. Brussels – Belgium.
- Kailasapathy K, Rybka S. Lactobacillus acidophilus and Bifidobacterium spp. Their therapeutic potential and survival in yogurt. Aust J Dairy Technol. 1997;52:28–33. [Google Scholar]
- Karaca OB, Guven M, Yasar K, Kaya S, Kahyaoglu T. The functional, rheological and sensory characteristics of ice creams with various fat replacers. Int J Dairy Technol. 2009;62:93–99. [Google Scholar]
- Keeney PG, Maga JA. Factors affecting composition of a foam yield of a foam fraction recovered from ice cream. J Dairy Sci. 1965;48:1591–1596. [Google Scholar]
- Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yogurt. Int Dairy J. 2003;13:3–13. [Google Scholar]
- Lyer BK, Singhal RS, Ananthanaryan L (2011) Characterization and in vitro probiotic evaluation of lactic acid bacteria isolated from idli batter. J Food Sci Technol. doi:10.1007/s13197-011-0445-6 [DOI] [PMC free article] [PubMed]
- Mohammadi R, Mortazavian AM. Technological aspects of prebiotics in probiotic fermented milks. Food Rev Int. 2010;27:192–212. [Google Scholar]
- Mohammadi R, Mortazavian AM, Khosrokhavar R, da Cruz AG. Probiotic ice cream: viability of probiotic bacteria and sensory properties. Ann Microbiol. 2011;61(3):411–424. [Google Scholar]
- Mortazavian AM, Ehsani MR, Reinheimer J, Sohrabvandi S. MRS-bile agar: its suitability for enumeration of mixed probiotic cultures in cultured dairy products. Milchwissenschaft. 2007;62:270–272. [Google Scholar]
- Mortazavian AM, Razavi SH, Ehsani MR, Sohrabvandi S. A review: Principles and methods of microencapsulation of probiotics. Iran J Biotechnol. 2007;5:1–18. [Google Scholar]
- Nagar GE, Clowes G, Tudorica CM, Kuri V, Brennan CS. Rheological quality and stability of yog-ice cream with added inulin. Int J Dairy Technol. 2002;55:89–93. [Google Scholar]
- Nakasawa Y, Hosono A. Functions of fermented milk, challenges for the health science. London, UK: Elsevier Applied Sci; 1992. [Google Scholar]
- Needs EC, Huitson A. The contribution of milk serum proteins to the development of whipped cream structure. Food Struct. 1991;10:353–360. [Google Scholar]
- Ozer B, Robinson RK, Hrandison AS, Bell AE. Gelation properties of milk concentrated by different techniques. Int Dairy J. 1998;8:793–799. [Google Scholar]
- Shah NP, Ravula RR. Microencapsulation of probiotic bacteria and their survival in frozen fermented dairy desserts. Aust J Dairy Technol. 2000;55:139–144. [Google Scholar]
- Sheu TY, Marshall RT. Microencapsulation of lactobacilli in calcium alginate gels. J Food Sci. 1993;54:557–561. [Google Scholar]
- Sofjan RP, Hartel RW. Effects of overrun on structural and physical characteristics of ice cream. Int Dairy J. 2004;14:255–262. [Google Scholar]
- Sohrabvandi S, Mortazavian AM, Dolatkhah-nejad MR, Bhadori Monfared A. Suitability of MRS-bile agar for the selective enumeration of mixed probiotic bacteria in presence of mesophilic lactic acid culture and yogurt bacteria. Iran J Biotechnol. 2012;10:16–21. [Google Scholar]
- Soukoulis C, Chandrinos I, Tzia C. Study of the functionality of selected hydrocolloids and their blends with k-carrageenan on storage quality of vanilla ice cream. LWT-Food Sci Technol. 2008;41:1816–1827. [Google Scholar]
- Soukoulis C, Tzia C. Impact of the acidification process, hydrocolloids and protein fortifiers on physical and sensory properties of frozen yogurt. Int J Dairy Technol. 2008;61:170–177. [Google Scholar]
- Vanaja G, Gotcheva V, Angelov A, Agrawal R. Formation of volatiles and fattyacids of therapeutic importance in the probiotic Lactobacillus plantarum LPcfr adapted to resist GIT conditions. J Food Sci Technol. 2011;48:110–113. doi: 10.1007/s13197-010-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinderola CG, Bailo N, Reinheimer JA. Survival of probiotic microfelora in Argentinian yogurts during refrigerated storage. Food Res Int. 2000;33:97–102. [Google Scholar]
- Vinderola CG, Reinheimer JA. Enumeration of Lactobacillus casei in the presence of Lactobacillus acidophilus, Bifidobacteria and Lactic starter bacteria in fermented dairy products. Int Dairy J. 2000;10:271–275. [Google Scholar]
- Vinderola CG, Reinheimer JA. Culture media for the enumeration of Bifidobacterium bifidum and Lactobacillus acidophilus in the presence of yogurt bacteria. Int Dairy J. 1999;9:497–505. [Google Scholar]
- Wilbey RA, Cooke T, Dimos G. The effects of solute concentration, overrun and storage on the hardness of ice cream. In: Buchheim W, editor. Ice cream. Germany: International Dairy Federation; 1997. pp. 186–187. [Google Scholar]