Abstract
The aim of this research was to investigate the influence of modification with succinic acid/acetanhydride and azelaic acid/acetanhydride mixtures on chemical and physical properties of wheat starch. Starch was isolated from two wheat varieties and modified with mixtures of succinic acid and acetanhydride and azelaic acid and acetanhydride in 4, 6 and 8% (w/w). Total starch content, resistant starch content, degree of modification, changes in FT-IR spectra, colour, gel texture and freeze-thaw stability were determined. Results showed that resistant starch content increased by both investigated modifications, and degree of modification increased proportionally to amount of reagents used. FT-IR analysis of modified starches showed peak around 1,740 cm−1, characteristic for carbonyl group of ester. Total colour difference caused by modifications was detectable by trained people. Adhesiveness significantly increased, while freeze-thaw stability decreased by both investigated modifications.
Keywords: Wheat starch, Succinic acid/acetanhydride, Azelaic acid/acetanhydride, FT-IR, RS, Freeze-thaw stability
Introduction
Starch modification is important for the continued and increased use of starch as thickening, gelling, binding, adhesive and film forming agent. By different processes of modification (acid hydrolisys, estherification, etherification, oxidation, cross-linking, physical modification…) physico-chemical characteristics of native starch are altered to improve functional characteristics (Kaur et al. 2011; Balasubramanian et al. 2011).
Chemical and physical properties are very important for application of starch in food industry. Paste clarity is physical property of starch, influenced by many factors, such as concentration, pH, type and extent of modification. It is related to state of dispersion and retrogradation tendency of starch, and influences other technologically important qualities of starch (Bhandari and Singhal 2002). While transparent pastes are used in fruit juices and pie fillings, opacity is favourable in salad dressings, mayonnaises and instant deserts.
Gel texture is another physical property of starch that is crucial for its application. Textural and mechanical properties of starch gels depend on rheological properties of amylose phase, volume fraction of the granules, their deformability and interactions between dispersed and continuous phase (Choi and Kerr 2003; Puncha-arnon et al. 2008). These factors are dependent on amylose content and structure of amylopectin. Starches that form harder gels have higher amylose content and longer amylopectin chains (Sandhu and Singh 2007).
Amylose content is also related to resistant starch (RS) content. RS is fraction of starch and its degradation products that is not digested in the small intestine (of healthy individuals) and escapes to the large intestine, where is converted to short-chain fatty acids and other products of bacterial fermentation (Haralampu 2000; Jenkins and Kendall 2000; Deepa et al. 2010). Therefore, RS is nutritionally important carbohydrate, along with dietary fibers (Muthappa et al. 2003). It can be fractionated into four categories: physically inaccessible RS1, compact starch granules of RS2, retrograded starch RS3 (mainly retrograded amylose) and chemically modified RS4 (Cummings and Stephen 2007).
Esterification of starch is chemical reaction in which OH groups of anhydro-glucose molecules react with carboxylic groups of acids forming ester linkage. It can result in substitution, such as acetylation, or in cross-linking, such as reaction with adipic acid/acetanhydride mixture. Formation of new chemical bonds results in changes of thermal, chemical and physical properties of starch, and extension of these changes depends on degree of substitution (DS). DS is defined as average number of substituent per anhydro-glucose unit and is indication of the amount of substituent group formed on the starch molecule (Lawal et al. 2008).
Previous researches have shown that acetylation resulted in decreased gelatinisation and pasting temperature, increased paste viscosity, lower retrogradation, higher swelling power, higher paste clarity and lower freeze-thaw stability (Babić et al. 2007, 2009; Saartrat et al. 2005; Singh et al. 2004; Gonzales and Perez 2002; Bello-Perez et al. 2010).
Acetylated distarch adipate prepared from waxy potato starch exhibited excellent viscosity stability, acid resistance, salt tolerance and good breakdown (Luo et al. 2009). Pregelatinised acetylated distarch adipate prepared by extrusion of cassava starch had higher cold viscosity, water absorption index and gel hardness, reduced gel cohesiveness, paste clarity and retrogradation compared to native counterpart (Mali and Grossman 2001).
Starch succinates had reduced gelatinisation temperature and retrogradation, increased cold-water solubility, improved paste clarity and freeze-thaw stability, as well as stability in acidic and salt containing medium (Bhandari and Singhal 2002).
The aim of this research was to investigate influence of succinic acid/acetanhydride and adipic acid/acetanhydride on physical and chemical properties of wheat starch.
Materials and methods
Wheat varieties Golubica and Srpanjka (harvest 2008) were obtained from Agricultural Institute, Osijek, Croatia. According to the data provided with samples, Golubica variety contained 67.00% d.m. starch, 14.37% d.m. protein and 12.27% moisture, while Srpanjka variety contained 68.73% d.m. starch, 12.57% d.m. protein and 12.20% moisture. Wheat samples were stored in controlled conditions in silos until the starch isolation (app. 4 month). Starch was isolated and modified as previously described (Ačkar et al. 2010; Manuscript I). Briefly, wheat kernels were milled, sifted and the obtained flour was suspended in 0,25% NaOH by stirring for 1 h at room temperature. Suspension was centrifuged, protein layer was discarded and starch was washed with water, neutralized and air-dried. Isolated Golubica starch had 15.43% moisture, 0.29% ash, and proteins were not detected by the applied Kjeldahl method. Native Srpanjka starch contained 15.45% moisture, 0.20% ash and proteins were not detected.
Mixture of acetanhydride and succinic or azelaic acid was prepared by suspending:
0.1290 g acid in 3.8710 g acetanhydride for modification in 4% (w/w);
0.1935 g acid in 5.8065 g acetanhydride for modification in 6% (w/w);
0.2581 g acid in 7.7419 g acetanhydride for modification in 8% (w/w).
Starch (100 g d.m.) was suspended in distilled water (145 mL) and homogenised by magnetic stirrer (300 rpm/30 min). pH of starch suspension was adjusted to 9.0 with 1 M NaOH and mixture of acid and anhydride was drop-wise added with maintaining pH value close to 9. After addition of mixture of acid and anhydride, starch suspension was stirred for 30 min at room temperature. Overall reaction time was 2 h. Reaction was terminated by adjusting pH to 5.0 with drop-wise addition of 1 M HCl. Suspension was centrifuged (3,000 rpm/5 min) and starch pellet was washed with water, neutralised, centrifuged and obtained modified starch was air dried.
Determination of chemical properties of modified starches
Total starch was determined by Ewers method (ISO 10520:1997). Briefly, starch was cooked with HCl, cooled and phosphowolfram acid was added. After filtration, optical rotation of the suspension was measured by digital polarimeter (P3002RS, Krüss Optronic, Germany) and starch content was calculated as follows (Eq. 1):
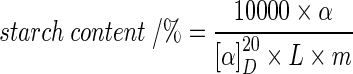 |
1 |
Where:
- α
rotation determined for the sample
- [α]20D
specific optical rotation of wheat starch (182,7)
- L
length of the polarimetric tube (2 dm)
- m
sample mass in grams.
Resistant starch content was determined by AOAC 2002.02 method, using Megazyme enzymatic kit.
Degree of substitution (DS) was determined titrimetically, following method of Ogawa et al. (1999). Five grams of modified starch and 50 ml of distilled water were dispersed in an Erlenmeyer flask with a stopper. Phenolphthalein was added to the suspension as an indicator, and then 0.1 M NaOH solution was added to get red colour. After the addition of 25 ml of 0.45 M NaOH solution, the mixture was stirred at 25 °C for 30 min. The flask was corked to prevent evaporation of produced acetate during saponification reaction. Finally, the excess alkali in the sample mixture was titrated with 0.2 M HCl solution. A blank test was also carried out with native starch using the same procedure. All samples were done in triplicate. DS is defined as the average number of sites per glucose unit that possess a substituent group. Since dicarboxylic acids were used in amounts significantly smaller than acetanhydride, DS is expressed through acetyl substituents and calculated as follows (Eq. 2):
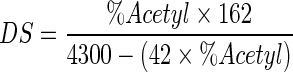 |
2 |
Where:
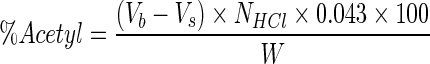 |
3 |
- Vb
ml 0.2 M HCl used to titrate the blank
- Vs
ml of 0.2 M HCl used to titrate the sample
- NHCl
the normality of HCl used for titration
- W
the sample weight (g).
FT-IR analyses were performed with FT-IR-8400S, Shimadzu, Japan between 4,000 and 400 cm−1 at a resolution of 4 cm−1. Samples were ground with KBr (1% w/w) and pressed into KBr pellets for FT-IR analyses.
Gel texture was determined using a TA-XT Plus (Stable Microsystem). A starch suspension (11% w/w) was prepared in a flask and then heated at 95 °C for 30 min in a temperature controlled shaking water bath with constant shaking. The cooked paste was cooled to 25 °C, weighed into plastic cans and allowed to gel in ClimaCell at 25 °C and 85% r. h. The gel formed in the can (45 mm in height and 35 mm in diameter) was compressed at the speed of 2.0 mm/s to the distance of 20 mm with a flat cylinder probe having 20 mm diameter. The peak height at 20 mm compression was termed hardness, and the negative area of the curve during retraction of the probe was termed adhesiveness (Saartrat et al. 2005). Five repeated measurements were performed for each sample.
Colour of starch (dry powder) was measured using Chroma Meter CR-300, Konika Minolta, Japan with granular materials attachment. The instrument was calibrated using white tile and colour was expressed in CIE-Lab parameters as L* (whiteness/darkness), a* (redness/greenness) and b* (yellowness/blueness) and in CIE-LCh parameters as C* (Chroma) and h* (hue). Five measurements were performed on each sample and mean value and standard deviations were calculated. Colour differences between starch samples were calculated according to Eq. 4.:
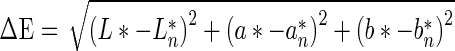 |
4 |
Where L*, a*, b* were parameters for modified starch and L*n, a*n, b*n were parameters for native starch.
Paste clarity (in triplicates) was determined by method described by Raina et al. (2006). 1% (d.w.b.) starch suspensions were heated for 30 min in boiling water bath with occasional shaking. After 1 h holding at room temperature% transmittance was read at 650 nm against distilled water as blank.
Freeze-thaw stability was measured by modified method of Lawal (2009). Starch suspension (5% w/w, d.w.b.) was heated in temperature controlled shaking water bath with constant shaking (200 rpm) for 1 h. The paste was weighed (10 g) in pre-weighed PP-centrifuge tubes and subjected to freeze-thaw cycles followed by centrifugation at 4,000 rpm for 30 min. Alternate freezing and thawing was performed by freezing for 22 h at −18 °C and thawing for 2 h at 30 °C. Seven freeze-thaw cycles were performed. The weight of water separated after each freeze-thaw cycle was measured and extent of syneresis was calculated following Eq. 5.:
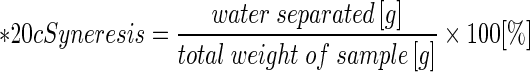 |
5 |
Experimental data were analysed by analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) with significance defined at P < 0.05. All statistical analyses were carried out using software program STATISTICA 7 (StatSoft, Inc, USA). All analyses were run in triplicates, with the exception of starch colour and texture measurements, which were run in five parallels.
Results and discussion
Total starch content is indicator of starch purity, and widely applied method for its determination is the acid hydrolysis method, applied in this research (Padmanabhan and Ramakrishna 1993). All investigated samples had high total starch content and exhibited high purity (Table 1), which was at level of commercial starches determined by McCleary et al. (1994).
Table 1.
Total starch content (TS), resistant starch content (RS) and degree of substitution (DS) of starch isolated from wheat varieties Golubica (G) and Srpanjka (S) and modified with succinic acid/acetanhydride (SA) and azelaic acid/acetanhydride (AZA) mixtures in 4, 6 and 8%
| TS/% d. m. | RS/% d. m. | % Acet | DS | |
|---|---|---|---|---|
| G | 96.87 ± 0.16a | 0.48 ± 0.03a | ||
| GSA4 | 98.13 ± 0.06c | 0.60 ± 0.02b | 2.993 ± 0.000a | 0.116 ± 0.000a |
| GSA6 | 97.74 ± 0.09b | 1.19 ± 0.10c | 3.612 ± 0.000a | 0.141 ± 0.000a |
| GSA8 | 98.72 ± 0.07d | 0.69 ± 0.01b | 3.750 ± 0.000a | 0.147 ± 0.000a |
| GAZA4 | 94.65 ± 0.29a | 0.63 ± 0.05b | 2.804 ± 0.000a | 0.109 ± 0.000a |
| GAZA6 | 98.85 ± 0.22c | 0.49 ± 0.05a | 3.457 ± 0.000b | 0.135 ± 0.000b |
| GAZA8 | 98.62 ± 0.14c | 2.00 ± 0.05c | 3.629 ± 0.024c | 0.142 ± 0.001c |
| S | 96.11 ± 0.02b | 0.57 ± 0.11a | ||
| SSA4 | 98.72 ± 0.07d | 0.69 ± 0.01b | 2.769 ± 0.024a | 0.107 ± 0.001a |
| SSA6 | 96.11 ± 0.02b | 0.57 ± 0.11b | 3.320 ± 0.024b | 0.129 ± 0.001b |
| SSA8 | 96.28 ± 0.01c | 0.08 ± 0.01a | 3.853 ± 0.000c | 0.151 ± 0.000c |
| SAZA4 | 97.88 ± 0.02c | 14.02 ± 0.18c | 2.907 ± 0.000a | 0.113 ± 0.000a |
| SAZA6 | 97.44 ± 0.01b | 1.70 ± 0.04b | 3.320 ± 0.000b | 0.129 ± 0.000b |
| SAZA8 | 98.04 ± 0.01d | 0.37 ± 0.01a | 3.793 ± 0.012c | 0.148 ± 0.000c |
Values are means ± SD of triplicates. Values in the same column with different superscripts (a–d) are significantly different (p < 0.05) than native counterparts
Resistant starch (RS) content in starch isolated from wheat varieties Srpanjka and Golubica was influenced by both investigated modifications (Table 1). Modification with mixture of succinic acid and acetanhydride had more pronounced influence on RS content of Golubica starch. Namely, increase of RS content for this starch was from 25 to app. 148%. Srpanjka starch modified in 6% had exactly the same content of RS as native counterpart, while modification in 8% reduced RS content. Only starch modified in 4% had higher content of RS than native starch. Modification with azelaic acid/acetanhydride mixture resulted in increase of RS content in Golubica starch in all three investigated proportions, while it reduced RS content in Srpanjka starch modified in 8%. Resistant starch content is proportionally influenced by amylose content (Sajilata et al. 2006). Native Srpanjka starch had higher content of amylose (results shown in manuscript I), therefore higher content of RS. However, amylopectin reacts with modification reagents more easily (Singh et al. 2007), hence the higher influence of modification on increase of RS content in Golubica starch. In addition, amylose content (results shown in Manuscript I) was reduced after both modifications of Srpanjka starch, which contributed to reduction of RS content, while modification of Golubica starch with mixture of azelaic acid and acetanhydride resulted in increase of amylose content, as well as modification with mixture of succinic acid and acetanhydride in 4%.
However, highest RS content was determined in Srpanjka starch modified with mixtures of azelaic acid and acetanhydride in 4% (14.02% RS/d. m.), followed by same modification of Golubica starch in 8% (2.00% RS/d. m.) and Golubica starch modified by succinic acid/acetanhydride mixture in 6% (1.19% RS/d. m.). Kim et al. (2008) reported increase of RS content proportionally to increase of glutaric acid content used for adley starch modification. However, compared to raw adley starch, some conditions of modification resulted in decrease of RS content. John and Raja (1999) observed higher resistance of cassava starch–succinic acid complex towards α-amylase activity than native counterpart, but this resistance wasn’t observed with respect to ß-amylase.
Degree of substitution (DS) increased proportionally to increase of amount reagents used for both modifications, with somewhat higher values for Golubica starch (Table 1). This is confirmation of higher reactivity of Golubica starch towards modification. Obtained values are, however, significantly higher than values reported by Song et al. (2006) and Hui et al. (2009) for starch modified with octenyl succinic anhydride, and Van Hung and Morita (2005) for acetylated wheat starch.
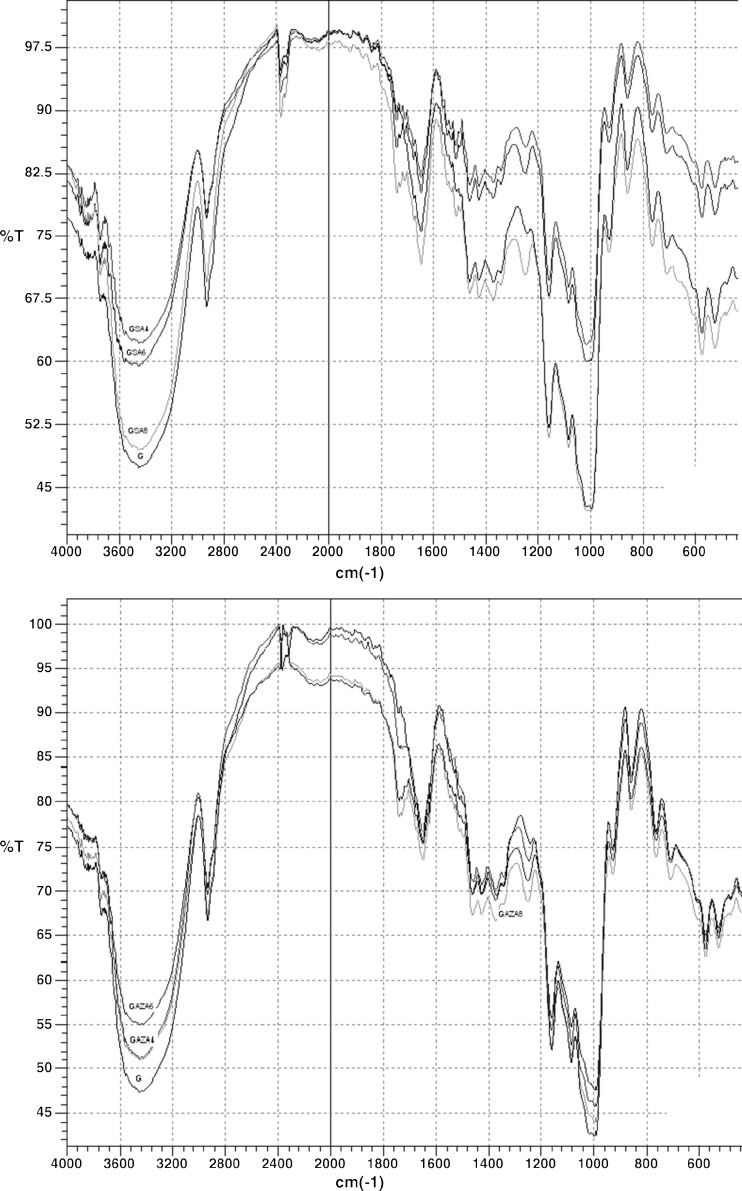
FT-IR spectra of starches bands originate mainly from the vibrational modes of amylose and amylopectin because these are the main components of starch (Amir et al. 2011).
FT-IR analysis of modified starches (Figs. 1 and 2) showed changes in chemical structure of both investigated starches due to modifications.
Fig. 1.
FT-IR spectra of starch isolated from wheat variety Golubica (G) and modified with succinic acid/acetanhydride (SA) and azelaic acid/acetanhydride (AZA) mixtures in 4, 6 and 8%. Values are means of triplicates
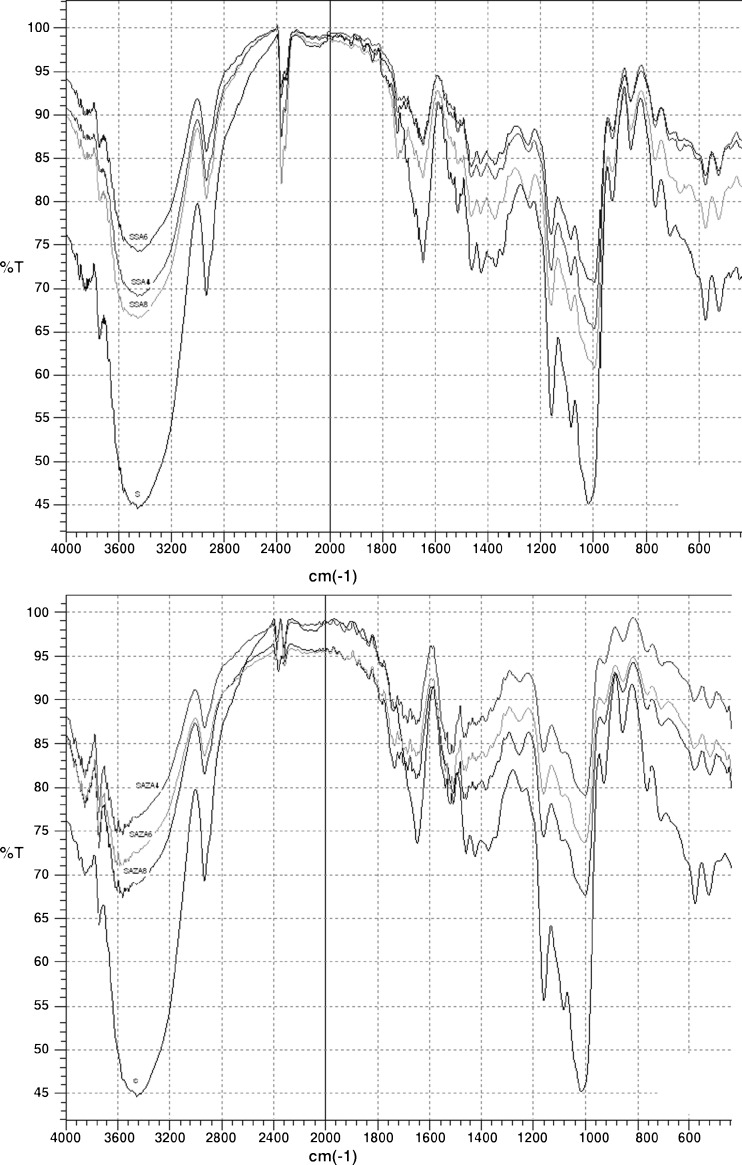
Fig. 2.
FT-IR spectra of starch isolated from wheat variety Srpanjka (S) and modified with succinic acid/acetanhydride (SA) and azelaic acid/acetanhydride (AZA) mixtures in 4, 6 and 8%. Values are means of triplicates
The band in the region 3,430–3,440 cm−1 is characteristic–OH stretching of starch molecules. Peak at 2,935 cm−1 is band of C–H stretching. Around 1,645 cm−1 are bands of–CH2 and–CH3 deformations and–OH bending. Vibrations of C–O and–OH are detected at 1,425–1,430 cm−1 and area at 1,157–1,165 cm−1 is attributed to stretching of C–O and–OH bending in C–OH group. Band at 1,085 cm−1 is the result of stretching of C–O glycosidic bond, and area of 1,010–1,020 cm−1 is CO/CC stretching and C–OH vibrations. Pyranose ring vibrations are characterised by bands at 929, 765 and 709 cm−1 and in areas of 574–578 and 522–526 cm−1 (Delval et al. 2004).
Band at 1,000 cm−1, which reflects crystalline order of starch (B-type granules) (Ispas-Szabo et al. 2000) and band in the region 3,430–3,440 cm−1 decreased after modifications of both investigated starches. Decrease of band characteristic for–OH stretching of starch molecules indicates that number of free–OH groups declined. New band around 1,740 cm−1 was detected for all modified starches. Its intensity increased proportionally to degree of modification. This band is characteristic for carbonyl group of esters which are formed by reaction of starch with carboxylic acids and acetanhydride. In addition, modified starches exhibited band in the region of 1,240–1,250 cm−1, characteristic for (C–COO)–vibrations.
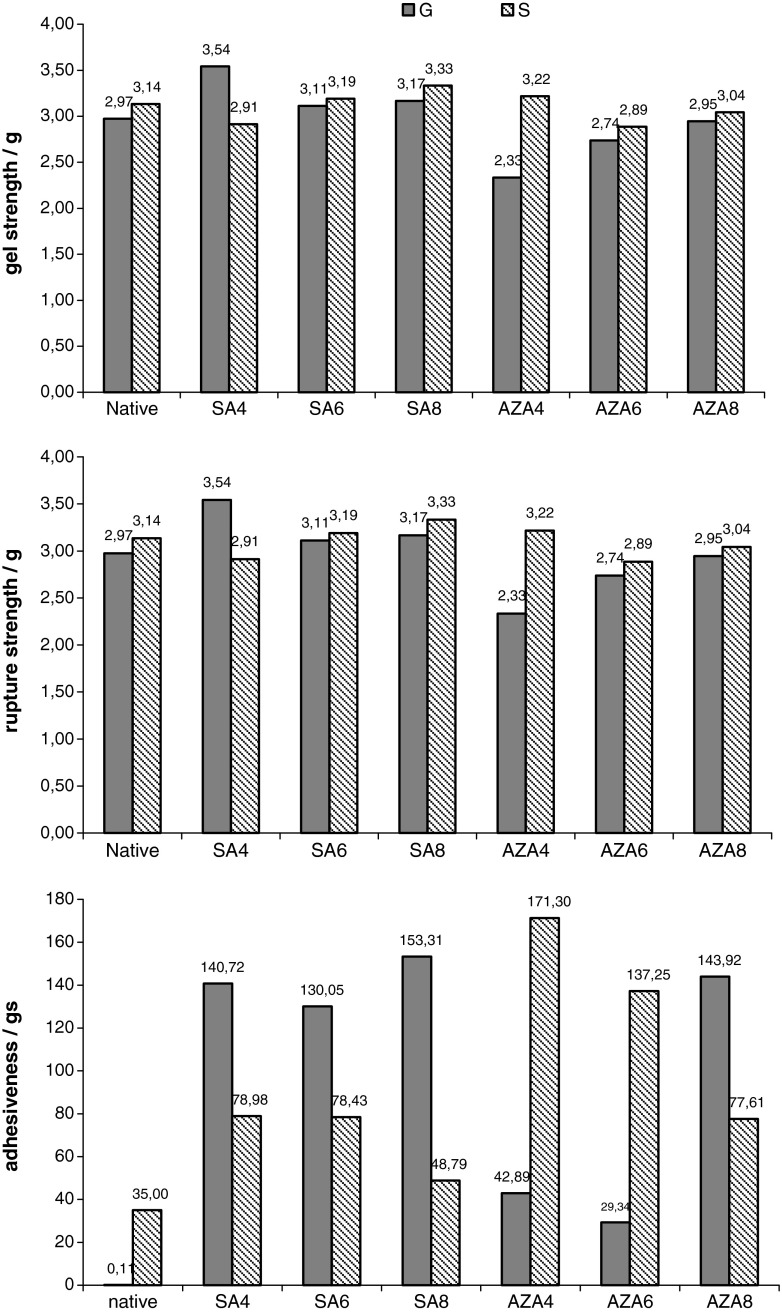
Gel texture properties of starch isolated from wheat varieties Golubica (G) and Srpanjka (S) and modified with succinic acid/acetanhydride (SA) and azelaic acid/acetanhydride (AZA) mixtures in 4, 6 and 8% are shown in Fig. 3. Gel strength of Golubica starch increased after modification with succinic acid/acetanhydride mixture, where highest increase was observed for starch modified in 4%. For Srpanjka starch, however, decrease of gel strength after modification in 4% was observed, and further increase of proportion of succinic acid/acetanhydride mixture resulted in increase of gel strength (Fig. 3). Modification with azelaic acid/acetanhydride mixture in 4% resulted in decrease of gel strength of Golubica starch. Further increase of proportion of reagent mixture proportionally increased gel strength. Gel strength of Srpanjka starch modified with azelaic acid/acetanhydride (SAZA) increased following order: SAZA6 < SAZA8 < native < SAZA4. Rupture strength had same trend as gel strength. Adhesiveness of both Golubica and Srpanjka starch increased after modifications with succinic acid/acetanhydride and azelaic acid/acetanhydride mixtures, with more pronounced effect of both modifications on Golubica starch. Increase of adhesiveness was also observed for starch dually modified with propylene oxide and mixture of adipic acid and acetanhydride and this phenomenon was attributed to the cross-linking effect (Raina et al. 2006). High adhesiveness and low hardness of food often contribute to good tasting (Huang et al. 2007).
Fig. 3.
Gel texture properties (gel strength, rupture strength and adhesiveness) of starch isolated from wheat varieties Golubica (G) and Srpanjka (S) and modified with succinic acid/acetanhydride (SA) and azelaic acid/acetanhydride (AZA) mixtures in 4, 6 and 8%. Values are means of five measurements
With the exception of Golubica starch modified with 4% succinic acid/acetanhydride mixture, all modifications improved starch whiteness, expressed as L* value (Table 2). In addition, a* and b* values approached closer to 0, which reduced proportion of green and yellow colour components and additionally improved perception of white. Chroma (C*) values, which represent saturation, decreased, thus contributed to perception of neutral, white colour. This perception is very important for consumer preference.
Table 2.
Colour measured in CIELab and CIELCh system and paste clarity (%T650 nm) of starch isolated from wheat varieties Golubica (G) and Srpanjka (S) and modified with succinic acid/acetanhydride (SA) and azelaic acid/acetanhydride (AZA) mixtures in 4, 6 and 8%
| L* | a* | b* | C* | h* (°) | ΔEnat | %T650 nm | |
|---|---|---|---|---|---|---|---|
| G | 75.1 ± 0.05b | −1.19 ± 0.01a | +1.35 ± 0.01c | 1.79 ± 0.01d | 131.5 ± 0.4a | 7.8 ± 0.14a | |
| GSA4 | 73.3 ± 0.01a | −1.21 ± 0.02a | +0.70 ± 0.01b | 1.37 ± 0.04c | 150.6 ± 0.5b | 1.96 | 8.9 ± 0.14b |
| GSA6 | 77.0 ± 0.05c | −1.16 ± 0.02a | +0.56 ± 0.01a | 1.30 ± 0.02a | 155.1 ± 0.3c | 2.00 | 14.6 ± 0.00c |
| GSA8 | 77.0 ± 0.05c | −1.04 ± 0.02a | +0.56 ± 0.01a | 1.19 ± 0.02b | 151.3 ± 0.4d | 2.02 | 16.1 ± 0.14d |
| GAZA4 | 77.1 ± 0.05b | −1.28 ± 0.02c | +0.54 ± 0.00b | 1.40 ± 0.02a | 156.5 ± 0.3b | 2.13 | 8.0 ± 0.07b |
| GAZA6 | 77.3 ± 0.02c | −1.18 ± 0.01a | +0.54 ± 0.01a | 1.32 ± 0.01b | 155.7 ± 0.3d | 2.29 | 7.9 ± 0.00b |
| GAZA8 | 75.4 ± 0.17d | −1.00 ± 0.01b | +0.81 ± 0.01a | 1.29 ± 0.03a | 140.9 ± 0.2c | 0.64 | 7.2 ± 0.14a |
| S | 76.4 ± 0.02a | −1.24 ± 0.02a | +1.22 ± 0.00d | 1.73 ± 0.03b | 136.0 ± 0.3a | 9.3 ± 0.00c | |
| SSA4 | 77.1 ± 0.09c | −0.90 ± 0.02b | +0.34 ± 0.02c | 0.96 ± 0.01a | 158.4 ± 0.4b | 1.12 | 8.9 ± 0.06b |
| SSA6 | 77.1 ± 0.04c | −0.91 ± 0.02b | +0.19 ± 0.01a | 0.95 ± 0.01a | 168.0 ± 0.6d | 1.26 | 10.1 ± 0.06d |
| SSA8 | 76.8 ± 0.07b | −0.91 ± 0.01b | +0.27 ± 0.01b | 0.96 ± 0.01a | 162.0 ± 0.6c | 1.08 | 8.0 ± 0.06a |
| SAZA4 | 76.7 ± 0.01b | −0.92 ± 0.01b | +0.23 ± 0.01c | 0.93 ± 0.02c | 165.9 ± 0.2b | 1.06 | 8.4 ± 0.00b |
| SAZA6 | 76.9 ± 0.05c | −0.89 ± 0.02b | +0.21 ± 0.01b | 0.89 ± 0.01b | 166.2 ± 0.3b | 1.17 | 6.4 ± 0.00a |
| SAZA8 | 76.7 ± 0.14b | −0.85 ± 0.01c | +0.12 ± 0.01a | 0.82 ± 0.01a | 172.2 ± 0.6c | 1.20 | 9.1 ± 0.06c |
Values are means ± SD of 5 measurements. Values in the same column with different superscripts (a–d) are significantly different (p < 0.05) than native counterparts. ΔEnat., colour difference between modified starch and native counterpart
Total colour difference (ΔEnat) for modified Golubica starch varied between 1.96 and 2.29 which indicated that it was detectable by trained people (Jukić et al. 2007). Only modification with 8% azelaic acid/acetanhydride mixture resulted in hardly detectable colour difference.
Colour change of Srpanjka starch was hardly detectable and ranged between 1.06 and 1.26. Modification of sweet potato starch with propylene oxide and adipic acid anhydride resulted in colour change detectable by trained people, with improvement of whiteness (Das et al. 2010), while banana starch whiteness decreased after substitution, cross-linking and pregelatinisation, respectively (Waliszewski et al. 2003).
Paste clarity of Golubica starch increased after both investigated modifications, with the exception of 8% modification with azelaic acid/acetanhydride mixture. On the contrary, paste clarity of Srpanjka starch decreased after both modifications, with the exception of modification with succinic acid/acetanhydride mixture in 6%. Paste clarity is influenced by starch source, amylose content, granule size distribution and degree of substitution upon modification (Singh et al. 2007). Acetylated starches have higher paste clarity compared to native counterparts due to higher retention of water within granules and restriction of formation of an ordered structure. On the other hand, highly cross-linked starches have reduced paste clarity due to incomplete gelatinization and reduced swelling (Singh et al. 2007). Das et al. (2010) observed increase of paste clarity for both acetylated and dual modified sweet potato starches, and Raina et al. (2006) reported that dual modification had greater impact on paste clarity increase than cross-linking or acetylation alone.
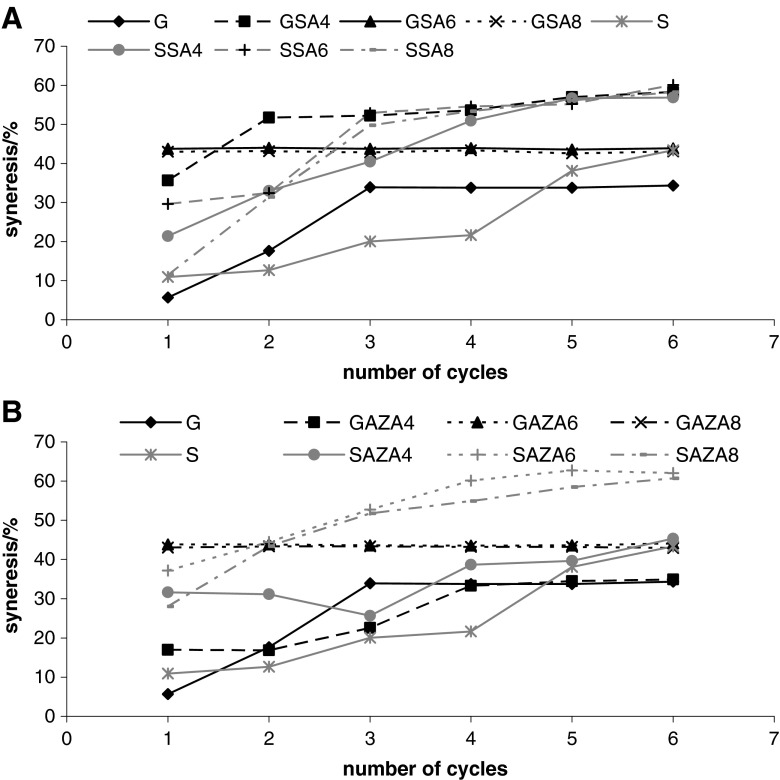
Freeze-thaw stability of both investigated starches decreased after both investigated modifications (Fig. 4). Woo and Seib (1990) reported freeze-thaw stability decrease after cross-linking of barley and maize starch, and Yeh and Yeh (1993) reported same trend for cross-linked rice starch. Although dual-modified rice starch had higher stability in the beginning of freeze-thaw, after 5th cycle it showed significantly higher syneresis than native counterpart (Deetae et al. 2008). Freeze-thaw induces starch retrogradation, hence the syneresis increases with increase of freeze-thaw cycle number. Possible causes of starch gel matrix disruption are thermal energy fluctuation and water phase change. When a starch gel is frozen, starch-rich regions with high solid concentration are created in the matrix. Starch chains associate and form thick filaments, whereas water molecules coagulate into ice crystals forming separate phase. Upon thawing, ice transforms to water, which can be readily released from polymeric network in the process known as syneresis (Lee et al. 2002).
Fig. 4.
Freeze-thaw stability of starch isolated from wheat varieties Golubica (G) and Srpanjka (S) and modified with succinic acid/acetanhydride (SA) (Graph a) and azelaic acid/acetanhydride (AZA) (Graph b) mixtures in 4, 6 and 8%. Starch pastes (5% d.w.b.) were kept at −18 °C/22 h and thawed at 30 °C/2 h
Conclusions
Increase of RS content, expressed as percentage to the RS content of native starch indicates that modification of starch with mixtures of acetanhydride with succinic or azelaic acid has potential in production of RS. However, modification conditions should be adjusted to gain more product resistant to amylases.
Low paste clarity of modified starches makes it suitable for production of dressings and gravies, while low freeze-thaw stability indicates that these starches have low potential of application in frozen foods. However, since other food components (such as sugars, hydrocolloids etc.) influence freeze-thaw stability of starch, additional research should be conducted.
Acknowledgement
Results shown have outcome from scientific project “Development of new modified starches and their application in food industry” supported by the Ministry of Science, Education and Sports of the Republic of Croatia.
References
- Ačkar Đ, Babić J, Šubarić D, Kopjar M, Miličević B. Isolation of starch from two wheat varieties and their modification with epichlorohydrin. Carbohydr Polymer. 2010;81:76–82. doi: 10.1016/j.carbpol.2010.01.058. [DOI] [Google Scholar]
- Amir RM, Anjum FM, Khan MI, Khan MR, Pasha I, Nadeem M (2011) Appliaction of fourier transform infrared spectroscopy for the identification of wheat varieties. J Food Sci Technol-Mysore. doi:10.1007/s13197-011-0424-y [DOI] [PMC free article] [PubMed]
- AOAC official method 2002.02 Resistant starch in starch and plant materials. Official methods of analysis of the AOAC International (18th ed.). Gaithersburg, Maryland: AOAC International
- Babić J, Šubarić D, Ačkar Đ, Kovačević D, Piližota V, Kopjar M. Preparation and characterization of acetylated tapioca starch. DLR. 2007;103:580–585. [Google Scholar]
- Babić J, Šubarić D, Nedić Tiban N, Kopjar M. Acetylation and characterization of corn starch. J Food Sci Technol-Mysore. 2009;46:423–426. [Google Scholar]
- Balasubramanian S, Sharma R, Kaur J, Bhardwaj N (2011) Characterization of modified pearl millet (Pennisetum typhoides) starch. J Food Sci Technol-Mysore. doi:10.1007/s13197-011-0490-1 [DOI] [PMC free article] [PubMed]
- Bello-Perez LA, Agama-Acevedo E, Zamudio-Flores PB, Mendez-Montealvo G, Rodriguez-Ambriz SL. Effect of low and high acetylation degree in the morphological, physicochemical and structural characteristics of barley starch. LWT. 2010;43:1434–1440. doi: 10.1016/j.lwt.2010.04.003. [DOI] [Google Scholar]
- Bhandari PN, Singhal RS. Effect of succinylation on the corn and amaranth starch pastes. A review. Carbohydr Polymer. 2002;48:233–240. doi: 10.1016/S0144-8617(01)00310-1. [DOI] [Google Scholar]
- Choi SG, Kerr WL. Water mobility and textural properties of native and hydroxypropylated wheat starch gels. Carbohydr Polymer. 2003;51:1–8. doi: 10.1016/S0144-8617(02)00083-8. [DOI] [Google Scholar]
- Cummings JH, Stephen AM. Carbohydrate terminology and classification. Eur J Clin Nutr. 2007;61:S5–S18. doi: 10.1038/sj.ejcn.1602936. [DOI] [PubMed] [Google Scholar]
- Das AB, Singh G, Singh S, Rijar CS. Effect of acetylation and dual modification on physico-chemical, rheological and morphological characteristics of sweet potato (Ipomoea batatas) starch. Carbohydr Polymer. 2010;80:725–732. doi: 10.1016/j.carbpol.2009.12.018. [DOI] [Google Scholar]
- Deepa G, Singh V, Akhilender Naidu K. A comparative study on starch digestibility, glycemic index and resistant starch of pigmented (“Njavara” and “Jyothi”) and non-pigmented (“IR-64”) rice varieties. J Food Sci Technol-Mysore. 2010;47:644–649. doi: 10.1007/s13197-010-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deetae P, Shobsngob S, Varanyanond W, Chinachoti P, Naivikul O, Varavinit S. Preparation, pasting properties and freeze–thaw stability of dual modified crosslink-phosphorylated rice starch. Carbohydr Polymer. 2008;73:351–358. doi: 10.1016/j.carbpol.2007.12.004. [DOI] [Google Scholar]
- Delval F, Crini G, Bertini S, Morin-Crini N, Badot P-M, Vebrel J, Torri G. Characterization of crosslinked starch materials with spectroscopic techniques. J Appl Polym Sci. 2004;93:2663–3650. doi: 10.1002/app.20851. [DOI] [Google Scholar]
- Gonzales Z, Perez E. Effect of acetylation on some properties of rice starch. Starch/Staerke. 2002;54:148–154. doi: 10.1002/1521-379X(200204)54:3/4<148::AID-STAR148>3.0.CO;2-N. [DOI] [Google Scholar]
- Haralampu SG. Resistant starch—a review of the physical properties and biological impact of RS3. Carbohydr Polymer. 2000;41:285–292. doi: 10.1016/S0144-8617(99)00147-2. [DOI] [Google Scholar]
- Huang M, Kennedy JF, Li B, Xu X, Xie BJ. Characters of rice starch gel modified by gellan, carrageenan and glucomannan: a texture profile analysis study. Carbohydr Polymer. 2007;69:411–418. doi: 10.1016/j.carbpol.2006.12.025. [DOI] [Google Scholar]
- Hui R, Qui-He C, Ming-liang F, Quiong X, Guo-quing H. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009;114:81–86. doi: 10.1016/j.foodchem.2008.09.019. [DOI] [Google Scholar]
- ISO 10520:1997 Native starch—determination of starch content—Ewers polarimetric method
- Ispas-Szabo P, Ravenelle F, Hassan I, Preda M, Mateescu MA. Structure–properties relationship in cross-linked high-amylose starch for use in controlled drug release. Carbohydr Res. 2000;323:163–175. doi: 10.1016/S0008-6215(99)00250-5. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC. Resistant starches. Curr Opin Gastroenterol. 2000;16:178–183. doi: 10.1097/00001574-200003000-00014. [DOI] [PubMed] [Google Scholar]
- John JK, Raja KCM. Properties of cassava starch-dicarboxylic acid complexes. Carbohydr Polymer. 1999;39:181–186. doi: 10.1016/S0144-8617(98)00164-7. [DOI] [Google Scholar]
- Jukić M, Ugarčić-Hardi Ž, Koceva Komlenić D. Colour changes of pasta produced with different supplements during drying and cooking. DLR. 2007;103:159–163. [Google Scholar]
- Kaur M, Oberoi DPS, Sogi DS, Gill BS. Physico-chemical, morphological and pasting properties of acid treated starches from different botanical sources. J Food Sci Technol-Mysore. 2011;48:460–465. doi: 10.1007/s13197-010-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Shoi SJ, Shin SI, Sohn MR, Lee CJ, Kim Y, Cho WI, Moon TW. Resistant glutarate starch from adley: preparation and properties. Carbohydr Polymer. 2008;74:787–796. doi: 10.1016/j.carbpol.2008.04.043. [DOI] [Google Scholar]
- Lawal OS. Starch hydroxyalkylation: physicochemical properties and enzymatic digestibility of native and hydroxypropylated finger millet (Eleusine coracana) starch. Food Hydroc. 2009;23:415–425. doi: 10.1016/j.foodhyd.2008.02.013. [DOI] [Google Scholar]
- Lawal OS, Lechner MD, Kulicke WM. The synthesis conditions, characterisations and thermal degradation studies of an etherified starch from an unconventional source. Polymer Degrad Stabil. 2008;93:1520–1528. doi: 10.1016/j.polymdegradstab.2008.05.010. [DOI] [Google Scholar]
- Lee MH, Baek MH, Cha DS, Park HJ, Lim ST. Freeze-thaw stabilization of sweet potato starch gel by polysaccharide gums. Food Hydroc. 2002;16:345–352. doi: 10.1016/S0268-005X(01)00107-2. [DOI] [Google Scholar]
- Luo FX, Huang Q, Fu X, Zhang LX, Yu SJ. Preparation and characterisation of cross-linked waxy potato starch. Food Chem. 2009;115:563–568. doi: 10.1016/j.foodchem.2008.12.052. [DOI] [Google Scholar]
- Mali S, Grossman MVE. Preparation of acetylated distarch adipates by extrusion. Lebensm Wiss Technol. 2001;34:384–389. doi: 10.1006/fstl.2001.0768. [DOI] [Google Scholar]
- McCleary BV, Gibson TS, Solah V, Mugford DC. Total starch measurement in cereal products: interlaboratory evaluation of a rapid enzymic test procedure. Cereal Chem. 1994;71:501–505. [Google Scholar]
- Muthappa SM, Puttaraj S, Prasad NN, Urooj A. Nutritionally important carbohydrates in ready-to-eat processed foods. J Food Sci Technol-Mysore. 2003;40:486–489. [Google Scholar]
- Ogawa K, Hirai I, Shimasaki C, Yoshimura T, Ono S, Rengakuji S, Nakamura Y, Yamazaki I. Simple determination method of degree of substitution for starch acetate. Bull Chem Soc Jpn. 1999;72:2785–2790. doi: 10.1246/bcsj.72.2785. [DOI] [Google Scholar]
- Padmanabhan S, Ramakrishna M. Methods for estimation of starch—a critical appraisal. J Food Sci Technol-Mysore. 1993;30:313–320. [Google Scholar]
- Puncha-arnon S, Pathipanawat W, Puttanlek C, Rungsardthong V, Uttapap D. Effects of relative granule size and gelatinisation temperature on paste and gel properties of starch blends. Food Res Int. 2008;41:552–561. doi: 10.1016/j.foodres.2008.03.012. [DOI] [Google Scholar]
- Raina CS, Singh S, Bawa AS, Saxena DC. Some characteristics of acetylated, cross-linked and dual-modified Indian rice starches. Eur Food Res Technol. 2006;223:561–570. doi: 10.1007/s00217-005-0239-z. [DOI] [Google Scholar]
- Saartrat S, Puttanlek C, Rungsardthong UD. Paste and gel properties of low-substituted acetylated canna starches. Carbohydr Polymer. 2005;61:211–221. doi: 10.1016/j.carbpol.2005.05.024. [DOI] [Google Scholar]
- Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch—a review. CRFSFS. 2006;5:1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Sandhu KS, Singh N. Some properties of corn starches II: physicochemical, gelatinisation, retrogradation, pasting and textural properties. Food Chem. 2007;101:1499–1507. doi: 10.1016/j.foodchem.2006.01.060. [DOI] [Google Scholar]
- Singh J, Kaur L, Singh N. Effect of acetylation on some properties of corn and potato starches. Starch-Starke. 2004;56:586–601. doi: 10.1002/star.200400293. [DOI] [Google Scholar]
- Singh J, Kaur L, McCarthy OJ. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—a review. Food Hydroc. 2007;21:1–22. doi: 10.1016/j.foodhyd.2006.02.006. [DOI] [Google Scholar]
- Song X, Xie G, Ruan H, Chen Q. Preparation and properties of octenyl succinic anhydride modified early Indica rice starch. Starch/Staerke. 2006;58:109–117. doi: 10.1002/star.200500444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hung P, Morita N. Effects of granule sizes on physico-chemical properties of cross-linked and acetylated wheat starches. Starch/Staerke. 2005;57:413–420. doi: 10.1002/star.200500417. [DOI] [Google Scholar]
- Waliszewski KN, Aparicio MA, Bello LA, Monroy JA. Changes of banana starch by chemical and physical modification. Carbohydr Polymer. 2003;52:237–242. doi: 10.1016/S0144-8617(02)00270-9. [DOI] [Google Scholar]
- Woo Y, Seib PA. Acetylated and hydroxypropylated distarch phosphates from waxy barley: paste properties and freeze-thaw stability. Cereal Chem. 1990;67:202–208. [Google Scholar]
- Yeh A-I, Yeh S-L. Some characteristics of hydroxypropylated and cross-linked rice starch. Cereal Chem. 1993;70:596–601. [Google Scholar]