Abstract
Qualitative analysis of hydrolysable extract from mango (Mangifera indica Linn. cultivar Chok-Anan) seed kernel was performed by means of reversed phase high-performance liquid chromatography coupled to diode array detection and electrospray ionization mass spectrometry (RPHPLC-DAD-ESI-MS). The main phenolic compound was identified as methyl gallate by comparing their retention time, UV–vis absorption spectra and mass spectra with a reference standard. Quantification of phenolic compounds was performed by HPLC-DAD, which revealed that the extract contained total phenolics at a concentration of 194.1 mg GAE/g dry weight of mango seed kernel (MSK), of which 85.7% was identified as methyl gallate. In addition, the antioxidant activities of the extract and the main compound were assessed by the 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) assays, by the ferric thiocyanate method and by an assay of metal chelating activity. Tyrosinase inhibition was also investigated. Furthermore, the antioxidant capacity and the total phenolic content of MSK extract stored in a plastic (polyethylene) PE bag decreased during storage at freezing (-20 °C), refrigerated (7 °C) and room (28–32 °C) temperature for 182 days. The loss of antioxidant capacity and total phenolic content increased at higher storage temperatures for more than 182 days.
Keywords: Antioxidant, Tyrosinase inhibitor, HPLC, Thai, Mango seed kernel, Stability
Introduction
Mango (Mangifera indica L.) is the most widely cultivated tropical fruit besides banana. The highest production of mango is in India, at 41% of the world’s production (10,800,000 MT), followed by China, Thailand, Mexico, Pakistan, Indonesia, the Philippines, Nigeria, and Brazil (Youngmok et al. 2009). Mangoes are consumed as fresh fruits and after processing into pickles, chutneys, canned or dried goods, juices, or nectars (Dube et al. 2004). During processing of mango, by products such as peel and kernel are generated. The kernel represents about 17–22% of the fruit (Soong and Barlow 2004). Detailed investigations on the management of mango seed waste in Thai factories revealed that the most common varieties of mango used in industry are Kaew and Choke Anan varieties which accounted for 29.5% and 27.9%, respectively. The mean rate of generation of Mango seed waste in Thailand was 1,063 kg per week (Maisuthisakul 2008a).
Extracts from kernels of eleven cultivars of Thai mango (Mangifera indica Linn.) seed showed antioxidant activity comparable to that of α-tocopherol. Extracts from the cultivar Chok-Anan showed the highest antioxidant efficiency. Most of the phenolic compounds of mango seed kernel were phenolic acids and flavonoids. The phenolic acids of mango seed kernels were in the form of free phenolic acids (42–56%) rather than insoluble bound phenolic acids (15–20%) and esterified phenolic acids (10–19%) (Maisuthisakul 2008b). The extracts from the mango seed kernel of the cultivar Chok-Anan also possessed tyrosinase inhibitory activity, which was greater than that of arbutin and they did not cause acute irritation of rabbit skins (Maisuthisakul and Gordon 2009). The control of tyrosinase activity is important in relation to browning control of food. Additionally, tyrosinase inhibitors are becoming important constituents of cosmetic products in relation to hyperpigmentation (Chen et al. 2005). Mango kernel was also shown to be a good source of phytosterols including campesterol, β-sitosterol, stigmasterol and it also contains tocopherols (Soong and Barlow 2004). Abdalla et al. (2007) has recently characterized the phenolic compounds in Egyptian mango seed kernels. Components present include tannins, gallic acid, coumarin, caffeic acid, vanillin, mangiferin, ferulic acid, cinammic acid and unknown compounds. 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose, methyl gallate and gallic acid have been identified as components of an ethanolic extract of Thai mango seed kernel cultivar Fahlun (Nithitanakool et al. 2009).
It is interesting and important to identify phenolic compounds and their biological activities in plant extracts. Determination of the content and composition of bioactive constituents in plant extracts is necessary to ensure their safety and for quality control in commercial usage. However, no information is available on the phenolic composition and active components in the extract from hydrolysed Thai mango seed kernel cultivar Chok-Anan.
The limitations of using plant extracts as Antioxidants include safety, availability, cost and shelf-life. The shelf life of extracts is defined as the time from manufacture to customer, where an extract retains the same quantity of phenolic constituents as stated on the label under recommended conditions (Piljac-Žegarac et al. 2009).
The experiment was undertaken with the aim of determining the composition of the phenolic constituents in Thai mango seed kernel of the cultivar Chok-Anan. The main phenolics in the extract were investigated and quantified. The antioxidant activity, chelating activity and ability to inhibit tyrosinase of the phenolic constituents were also compared and evaluated. Moreover, the stability of MSKE at refrigerated and frozen temperature was investigated to identify appropriate conditions for storage of MSKE.
Materials and methods
Materials
Three batches of sun dried seeds from ripened mango (M. indica cultivar Chok-Anan) obtained as by-products from fruit processed in Thailand from March to June 2008 were donated by Woraporn Co., Ltd., a mango processing company. The seeds were washed and again sun dried in the greenhouse for three days and the kernels were removed manually from the seeds for further extraction. The moisture content of dried mango seed kernel (MSK) on a dry weight basis determined according to the AOAC method (1990) was 9.81 ± 0.34%. The dried material was kept in a freezer at −20 °C for no longer than two months.
Folin Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), sodium carbonate, and methyl gallate were purchased from Sigma Chemical Co., Ltd (St. Louis, MO, USA). HPLC grade methanol and water were purchased from Fluka Co. (Buchs, Switzerland). The other chemicals and solvents used in this experiment were analytical grade, purchased from Sigma-Aldrich Co., Ltd (Steinheim, Germany).
Preparation of mango seed kernel extract
The frozen kernels (80 g) were ground for 1 min with 95% ethanol and refluxed with 1.2 M hydrochloric acid in ethanol for 3 h. The supernatant, after filtration through cheesecloth and Whatman No 4 filter paper, was evaporated under vacuum. The residue was dried in a freeze dryer and stored in aluminum foil after flushing with nitrogen at −20 °C until analysis. The dried extracts and methyl gallate at 0.3 mg/l were used to estimate the antioxidant properties by the DPPH, ABTS and the ferric thiocyanate methods. The mango seed kernel extracts (MSKE) were also used to evaluate metal chelating activity and tyrosinase inhibitory activity.
Sample preparation for chromatographic analysis
The chromatographic analysis of MSKE was performed as described in a previous report (Maisuthisakul et al. 2007). Briefly, MSKE was dissolved in methanol and passed through a Sep-Pak C18 cartridge (Waters, Milford, MA.). The cartridge bed was then rinsed with HCl (3%, 5 ml) and air-dried under vacuum for ~10 min. Phenolic compounds were eluted with HPLC grade methanol (2 ml). Samples were filtered through a 0.20 μm Millipore filter (type HA) into a 2 ml auto sampler vial for subsequent analysis by HPLC-DAD.
Determination of phenolics by HPLC-DAD and HPLC-ESI-MS
The HPLC-DAD analytical method was developed and validated on a Shimadzu LC-6 AD chromatograph with a diode array detector (SPD-M10Avp; Shimadzu), coupled with an auto injector (SIL-10AF; Shimadzu) and controlled by the software CLASS-VP 6.14. The solution (10 μL) was injected into the HPLC and analyzed with a Synergi Hydro RP column (150 × 4.6 mm id., 4 μm, Phenomenex), fitted with a Allsphere ODS-2 guard column (10 × 4.6 mm id., Alltech). Solvent A was 100% acetonitrile; solvent B was 1% formic acid in water. The program was isocratic at 10% A, 90% B for 15 min. The flow rate was 0.5 ml/min. The UV-DAD detector was set to record between 210 and 600 nm, and chromatograms were recorded at 210, 254 and 280 nm. The identification of peak 2 as methyl 3,4,5-trihydroxybenzoate (methyl gallate) was confirmed by co-injection of methyl gallate with the MSKE. The compounds corresponding to the main phenolic peaks were quantified and their concentration was expressed as gallic acid equivalents.
Each peak was identified by comparison of the retention time and UV spectrum with a standard and also by HPLC-ESI-MS chromatographic analysis. The HPLC system consisted of a Shimadzu HPLC as previously reported (Maisuthisakul et al. 2007). Nitrogen was used as nebulizing gas at a pressure of 6 bars, and the flow was adjusted to 1.5 l/min. The heated capillary and voltage were maintained at 230 °C and 1.7 kV, respectively. The full scan mass spectra of phenolic compounds were measured from m/z 50 up to m/z 1500. Mass spectrometry data were acquired in the positive and negative ionization modes. The HPLC conditions were the same as described above.
Storage stability determination of extract
All dried extracts were kept well-mixed and stored at room temperature (28–32 °C), 7 °C and −20 °C in polyethylene (PE) bag and aluminium foil (Al. foil) for 6 months. All samples were sampling out every 14 days by determining the antioxidant properties through using the DPPH and the thiocyanate methods. The MSK extracts were also used to evaluate total phenolic content and water activity.
Determination of total phenolic content
The total phenolic content of extracts was determined using Folin-Ciocalteu’s phenol reagent (Singleton and Rossi 1965). The concentration of total phenolic compounds in all plant extracts was expressed as mg of gallic acid equivalent per g dry weight of MSK. All determinations were performed in triplicate.
Determination of antioxidant properties
The total free radical-scavenging capacity of MSKE or reference samples was determined by the DPPH and ABTS methods. The antioxidant activity in a linoleic acid emulsion system was also determined.
The free radical scavenging activity of MSKE or reference samples was evaluated using the stable radical DPPH according to the method of Masuda et al. (1999). The DPPH radical scavenging activity of samples was calculated from 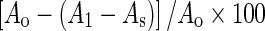 . Ao is the absorbance of the control solution (containing only DPPH); A1 is the absorbance of the DPPH solution containing plant extract; and As is the absorbance of the sample extract solution without DPPH. The radical scavenging activity (%) was plotted against the plant extract concentration (μg/ml) to determine the concentration of the extract that reduces activity by 50% (EC50). These values were changed to antiradical activity (AAR) defined as 1/EC50, since this parameter increases with antioxidant activity.
. Ao is the absorbance of the control solution (containing only DPPH); A1 is the absorbance of the DPPH solution containing plant extract; and As is the absorbance of the sample extract solution without DPPH. The radical scavenging activity (%) was plotted against the plant extract concentration (μg/ml) to determine the concentration of the extract that reduces activity by 50% (EC50). These values were changed to antiradical activity (AAR) defined as 1/EC50, since this parameter increases with antioxidant activity.
The ABTS radical scavenging activity was determined according to Re et al. (1999). The activity of each antioxidant was determined at three concentrations, within the range of the dose–response curve of Trolox, and the radical-scavenging activity was expressed as the Trolox equivalent antioxidant capacity (TEAC), defined as mMol of Trolox per gram of sample.
The antioxidant activity in a linoleic acid emulsion system of MSKE or reference samples was determined using the thiocyanate method (Kikuzaki and Nakatani 1993), with some modifications. Each sample dissolved in absolute ethanol (0.5 ml) was mixed with 0.5 ml of 5.21% linoleic acid, 1 ml of 0.05 M phosphate buffer (pH 7), and 0.5 ml of distilled water and placed in a screw capped tube. The reaction mixture was incubated in the dark at 40 °C in an oven. Aliquots of 0.1 ml were removed every 24 h during incubation and the degree of oxidation was measured by sequentially adding ethanol (9.7 ml, 75%), ammonium thiocyanate (0.1 ml, 30%) and ferrous chloride (0.1 ml, 0.02 M in 3.5% HCl). After the mixture was rested for 3 min, the peroxide value was determined by monitoring the absorbance at 500 nm until the absorbance of the control reached a maximum. The degree of linoleic acid peroxidation was calculated using the following formula: 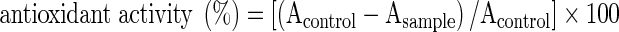 . The antioxidant activity was plotted against sample concentration in order to determine the concentration required to achieve a 50% inhibition of linoleic acid oxidation [AA50]. Antioxidant efficiency was calculated as 1/AA50.
. The antioxidant activity was plotted against sample concentration in order to determine the concentration required to achieve a 50% inhibition of linoleic acid oxidation [AA50]. Antioxidant efficiency was calculated as 1/AA50.
Determination of chelating activity
The ferrous ion chelating activity of samples was estimated based on the decrease in the maximal absorbance of the iron (Fe2+)-ferrozine complex according to previously reported methods (Dinis et al. 1994), with some modifications. Briefly, 1 ml of a solution of a test compound at various concentrations dissolved in ethanol was incubated with 0.5 ml of FeCl2•4H2O (1.0 mM). The reaction was initiated by the addition of 1 ml of ferrozine (5.0 mM), and then the total reaction volume was adjusted to 4 ml with ethanol. After the mixture had reached equilibrium (10 min), the absorbance at 562 nm was read. The control was prepared without the test compound. Fe2+ chelating activity of the test compound was calculated from the following formula: 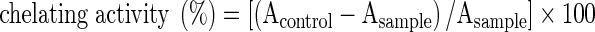 .
.
Determination of tyrosinase inhibitory activity
Mushroom tyrosinase (EC 1.14.18.1) was used for the bioassay because it is readily available. Since the mode of inhibition depends on the structure of both the substrate and inhibitor, L-DOPA was used as the substrate in this experiment, unless otherwise specified. Therefore, inhibitors discussed in this paper are inhibitors of diphenolase activity of mushroom tyrosinase, and their effect on the enzyme was determined by spectrophotometry based on dopachrome formation at 475 nm. All the samples were first dissolved in dimethyl sulfoxide (DMSO) and used for the experiment at 30 times dilution. The assay was performed as previously described (Nerya et al. 2003), with some modifications. L-DOPA solution (0.87 ml, 4.5 mM) was mixed with 0.9 ml of 0.1 M phosphate buffer (pH 6.8), and incubated at 30 °C for 5 min. Then, 0.9 ml of various concentrations of the sample solution and 0.03 ml of the aqueous solution of mushroom tyrosinase (4,000 units, added last) were added to the mixture and the enzyme reaction was monitored by measuring the change in absorbance at 475 nm (at 30 °C) corresponding to the formation of dopachrome for 25 min at 1 min intervals. Controls, without inhibitor but containing 3.3% DMSO, were routinely determined. The percent inhibition of the enzyme by the active compounds was calculated as follows: 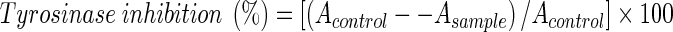 .
.
Determination of water activity
The dried extracts were determined the water activity by using Thermoconstanter NOVASINA instrument at 25 °C.
Statistical analysis
Each experiment, from sample preparation to analysis, was repeated in triplicate, and the data were analyzed by SPSS software program (SPSS Inc., Chicago, IL, USA). The general linear model procedure was applied and Duncan’s multiple range test was used to compare the mean values at p < 0.05. Mean values and pooled standard error of the mean (SEM) were then calculated.
Results and discussion
Identification of phenolic components in mango seed kernel extract
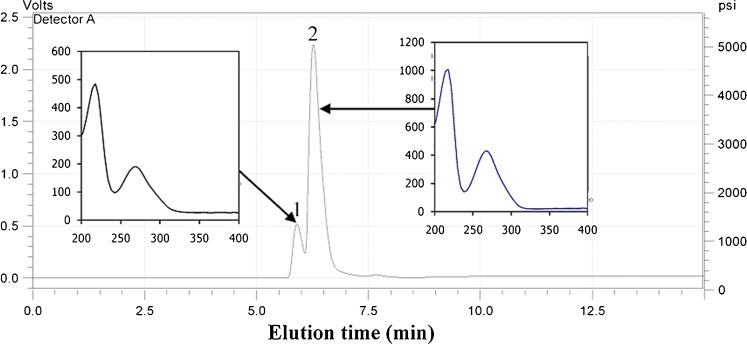
HPLC-DAD and HPLC-ESI-MS analysis of the hydrolysable extract of mango seed kernel was carried out with a reverse phase C-18 column. Figure 1 shows the chromatogram and UV–vis spectra of MSKE. The chromatogram of MSKE with detection at 210, 254 and 280 nm was similar, and therefore only the chromatogram with detection at 280 nm is presented. We found only two phenolic components in the MSKE. The components represented by peaks 1 and 2 eluted at 5.90 min and 6.27 min, respectively.
Fig. 1.
HPLC chromatogram detected at 280 nm and UV–vis spectrum of the extract from Mangifera indica Linn
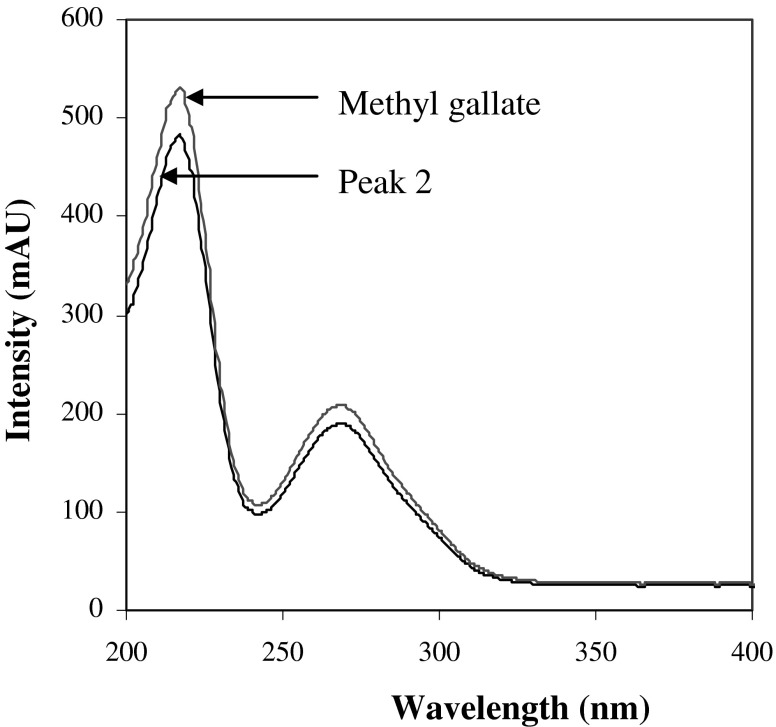
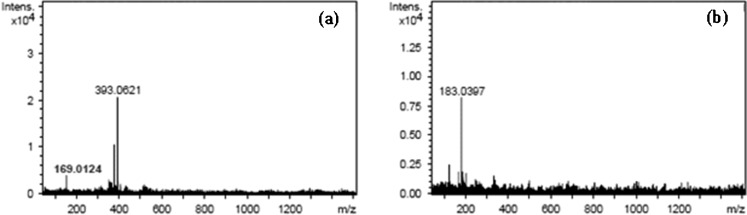
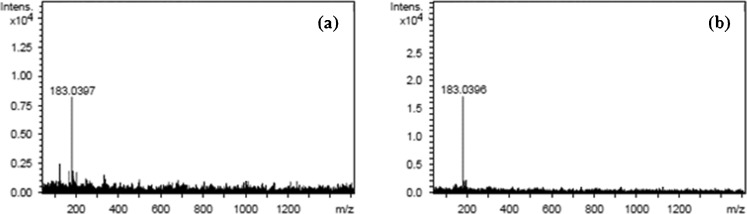
Peak identification was performed by comparing retention times (tR), UV–vis spectra and mass spectra with those of reference standards or literature data. Peak 2 corresponded to the main phenolic compound in MSKE (85.69% of total area). The peak had UV λmax at 218 and 274 nm (Fig. 2), and its ESI-MS spectrum showed a negative-ion mass spectrum at m/z 183 [M-H]- (Fig. 3b) indicating a molecular weight of 184. Based on comparison of the retention time, UV–vis spectrum and mass spectrum (Figs. 2 and 4) with those of a reference standard, the compound was identified as methyl gallate (Cheng et al. 2009; He and Xia 2007; Núñez Sellés et al. 2002). This compound was also found in a small quantity in the seed kernel cultivar Falun by Nithitanakool et al. (2009).
Fig. 2.
UV–vis spectra of peak 2 of Mangifera indica Linn. and methyl gallate
Fig. 3.
Negative ion LC-MS of a peak 1 b peak 2 of Mangifera indica Linn. extract
Fig. 4.
Negative ion LC-MS of a peak 2 of Mangifera indica Linn. and b methyl gallate
Methyl gallate is a highly potent antioxidant compound which shows a variety of biological activities. It was reported that methyl gallate is an excellent antioxidant, and it inhibits lipid oxidation effectively (Chen et al. 2005). Hsieh et al. (2004) showed that methyl gallate is a more effective scavenger of reactive oxygen species (ROS) than trolox. It has been found to be inhibitory to the growth of intestinal bacteria, for instance, Closridium perfringens, Esherichia coli, Salmonella typhimurium (Saxena et al. 1994; Chung et al. 1998). Moreover, it has been found to be an excellent tyrosinase inhibitor, and have a cytopathic effect on the human immunodeficiency virus (HIV) and the expression of HIV antigen in human lymphotropic virus type I (HTLV-I)-pos. MT-4 cells (Fiuza et al. 2004; Okuda 2005). Methyl gallate also showed strong antiviral activity (Kane et al. 1988).
Compound 1 was an unknown compound which showed UV λmax at 217 and 276 nm with a pseudomolecular ion at m/z 393 [M-H]- and the fragment ion at m/z 169 was also observed in the ESI-MS spectra of peak 1 (Fig. 3a). The UV spectrum is very similar to that of methyl gallate, but the exact structure needs further investigation and additional NMR data will be required to identify it.
Characterization of mango seed kernel extract
The hydrolysed ethanolic extract from the seed kernel of M. indica cultivar Chok-Anan had a high antioxidant capacity and a high phenolic content (Maisuthisakul 2008b; Maisuthisakul and Gordon 2009). Contents of phenolic compounds were determined by the HPLC-DAD method. The total content of phenolics was calculated as the sum of the individual phenolic compounds and estimated by using the Folin-Ciocalteu method. The results obtained are presented in Table 1. Methyl gallate was the major phenolic compound in MSKE and it accounted for 85.7% of the total phenolic content. Methyl gallate has also been found in other plant extracts (He and Xia 2007; Núñez Sellés et al. 2002).
Table 1.
Content of phenolic compounds in mango seed kernel extract
| Phenolic compounds | Content (mg of GAE/g dry weight) |
|---|---|
| HHDP alkyl derivative | 27.8 a ± 1.11 |
| Methyl gallate | 166.3 b ± 0.68 |
| Total phenolicsa | 194.1 c ± 1.03 |
| Total phenolic contentb | 292.2 d ± 2.68 |
Means of three replications ± SD (standard deviation); Different superscript letters mean significant differences (P < 0.05) between conditions in each column
aSum of the individual phenolic compounds determined by HPLC
bDetermined by the Folin-Ciocalteu method (mg of GAE/g dry weight)
As shown in Table 1, the content of total phenolics, as calculated by the sum of the individual phenolic compounds determined by HPLC, was 194 mg GAE/g (db) while the value (292 mg GAE/g, db) obtained by the Folin-Ciocalteu method was over 1.5 times that of the former. The difference between the two analytical methods can be attributed to the interference of other reducing substances in the extract, which leads to an overestimation of the total phenolic content in the Folin-Ciocalteu colorimetric analysis (Schieber et al. 2001).
One approach to investigate antioxidant activity is to study the radical scavenging effect of plant extracts. In the present study, total antioxidant capacity was determined as the cumulative capacity of the compounds present in the sample to scavenge free radicals, using the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) cation radical (ABTS+•) reactions. The scavenging of DPPH• and ABTS+• by MSKE was compared to that of the major component, methyl gallate (Table 2). The DPPH radical scavenging activity of the MSKE was about 89.2% of that of methyl gallate, whereas the TEAC value of the MSKE determined by the ABTS method was about 89.1% of that of methyl gallate. The HPLC analysis showed that methyl gallate corresponded to 85.7% of the total phenolic content. The agreement between the radical scavenging activity and the HPLC phenolic content of the MSKE confirmed that methyl gallate is the main active ingredient in the extract.
Table 2.
The antioxidant capacity of mango seed kernel extract and methyl gallate
| Samples | DPPH radical scavenging activity (%) | ABTS activity (mMol of Trolox/g) | Antioxidant activity in emulsion (%) |
|---|---|---|---|
| MSKE | 62.51 a ± 1.11 | 1.41 a ± 0.01 | 54.12 a ± 0.19 |
| Methyl gallate | 70.11 b ± 0.36 | 1.60 b ± 0.06 | 59.63 b ± 0.67 |
Dry weight basis of mango seed kernel; Means of three replications ± SD (standard deviation); the concentration of samples was 0.3 mg/l for DPPH assay and 50 mg/l for ferric thiocyanate method. Different superscript letters mean significant differences (P < 0.05) between conditions in each column
The antioxidant capacity of MSKE was also determined using the ferric thiocyanate method (FTC), which measures increases in peroxide formation in an emulsion during incubation. MSKE exhibited good antioxidant activity in the emulsion as in the DPPH and ABTS assays (Table 2). These implied that the phenolic compounds found in MSKE were capable of scavenging free radicals and inhibiting lipid oxidation. Moreover, the DPPH radical scavenging activity of mango seed kernel cultivar Chok-Anan showed stronger activity than α-tocopherol from previous report (Maisuthisakul 2008b).
Metal chelating activity is an antioxidant mechanism, since it reduces the concentration of the catalyzing transition metal in lipid peroxidation. Among the transition metals, iron is known as an important prooxidant towards lipid oxidation due to its high reactivity and occurrence in biological samples. The ferrous state of iron accelerates lipid oxidation by catalyzing decomposition of hydrogen peroxide and lipid peroxides to reactive free radicals via the Fenton reaction. The Fe3+ ion also catalyzes radical formation from peroxides although the rate is less than that of Fe2+ ion. Thus, the Fe2+ ion is the most important prooxidant among the various species of metal ions (Liu et al. 2007). The metal chelating activity of the MSKE was about 89.6% of that of methyl gallate (Table 3) which was consistent with the results of antioxidant activity (90.76%). This indicated that the unknown compound was similar in activity to methyl gallate with respect to all the studied activities, when the effects were adjusted for concentration.
Table 3.
The chelating efficiency and tyrosinase inhibition of mango seed kernel extract and methyl gallate
| Samples | Chelating activity (%) | Tyrosinase inhibition (%) |
|---|---|---|
| MSKE | 62.4 a ± 0.11 | 61.1 a ± 0.68 |
| Methyl gallate | 69.6 b ± 0.28 | 67.5 b ± 1.06 |
Dry weight basis of mango seed kernel; Means of three replications ± SD (standard deviation); the concentration of samples was 35 mg/l for chelating activity and 4.5 mg/l for tyrosinase inhibition determination. Different superscript letters mean significant differences (P < 0.05) between conditions in each column
The hydrolysable extract of mango seed kernel was analyzed for tyrosinase inhibition activity using the modified dopachrome method with L-DOPA as the substrate. The tyrosinase inhibition of the MSKE was about 90.6% of that of methyl gallate (Table 3). This means that the antioxidant ability, high metal chelating and tyrosinase inhibition activity of the ethanolic extract of mango seed kernel were mainly contributed by methyl gallate.
Storage stability of mango seed kernel extract
The free radical scavenging activity of MSKE and its antioxidant activity in a linoleic acid micelle system during storage at −20 °C (freezing temperature), 7 °C (refrigerated temperature) and 28–32 °C (room temperature) in aluminum foil (Al. foil) and polyethylene (PE) bag were determined. In this study, storage temperature and packaging affected to antioxidant properties of MSKE. The antiradical activity of MSKE in Al. foil did not change during storage for 182 days at −20 °C (ranging from 4.70 and 4.71). However, sample stored in the PE bag changed slightly during storage at −20 °C (4.44–4.73). There is no previous report on the stability of MSKE, although there is information about the shelf life of mango fruit (Ueda et al. 2001).
When the extracts were stored at 7 °C, the antioxidant activity of MSKE decreased during storage (Table 4). This was due to the degradation of phenolic compounds in MSKE, which was dependent on storage time and the type of phenolic compound present. The degradation rate increased if the storage temperature was increased. The antioxidant activity of MSKE stored at 28–32 °C decreased very rapidly (Table 5) compared to the other temperature storage conditions. Julkunen-Tiito (1985) reported that prolonged exposure at 40 °C can also cause phenolic degradation in grape pomace. The storage conditions and packaging materials should be considered when the extract is kept for a long time. The appropriate temperature for extract storage was −20 °C. Under these conditions, the extract can be stored for longer than 182 days or more than six months with no significant change in antioxidant properties.
Table 4.
Antioxidant properties of mango seed kernel extract during 6-month storage at 7 °C
| Storage time (days) | DPPH• scavenging activity (AAR, 1/EC50) | Antioxidant efficiency (1/AA50) | ||
|---|---|---|---|---|
| Al.Foil | PE bag | Al. Foil | PE bag | |
| 0 | 4.70 a ± 0.02 | 4.70 a ± 0.02 | 0.018 a ± 0.002 | 0.019 a ± 0.002 |
| 14 | 4.68 ab ± 0.03 | 4.68 ab ± 0.03 | 0.018 a ± 0.004 | 0.018 a ± 0.001 |
| 28 | 4.68 ab ± 0.01 | 4.65 b ± 0.01 | 0.018 a ± 0.001 | 0.017 ab ± 0.002 |
| 42 | 4.66 ab ± 0.04 | 4.60 c ± 0.04 | 0.018 a ± 0.004 | 0.016 b ± 0.001 |
| 56 | 4.60 c ± 0.02 | 4.50 d ± 0.02 | 0.017 a ± 0.003 | 0.014 c ± 0.002 |
| 70 | 4.62 c ± 0.01 | 4.42 e ± 0.01 | 0.017 a ± 0.002 | 0.014 c ± 0.001 |
| 84 | 4.59 c ± 0.03 | 4.35 f ± 0.03 | 0.016 a ± 0.001 | 0.012 cd ± 0.002 |
| 98 | 4.56 cd ± 0.03 | 4.21 g ± 0.03 | 0.016 a ± 0.001 | 0.011 d ± 0.001 |
| 112 | 4.50 d ± 0.02 | 4.10 h ± 0.02 | 0.016 a ± 0.002 | 0.010 de ± 0.003 |
| 126 | 4.48 d ± 0.04 | 4.02 i ± 0.04 | 0.015 a ± 0.001 | 0.009 e ± 0.001 |
| 140 | 4.42 e ± 0.02 | 3.91 j ± 0.02 | 0.014 a ± 0.002 | 0.007 f ± 0.001 |
| 154 | 4.37 f ± 0.01 | 3.80 k ± 0.01 | 0.013 ab ± 0.002 | 0.007 f ± 0.001 |
| 168 | 4.32 g ± 0.03 | 3.65 l ± 0.03 | 0.012 b ± 0.002 | 0.006 f ± 0.002 |
| 182 | 4.26 h ± 0.01 | 3.41 m ± 0.01 | 0.011 b ± 0.002 | 0.005 f ± 0.002 |
Data followed by different letters within each column are significantly different according to Duncan’s New Multiple Range Test at P < 0.05. Data obtained from three replicates
Table 5.
Antioxidant properties of mango seed kernel extract during 6-month storage at room temperature (28–32 °C)
| Storage time (days) | DPPH• scavenging activity (AAR, 1/EC50) | Antioxidant efficiency (1/AA50) | ||
|---|---|---|---|---|
| Al.Foil | PE bag | Al. Foil | PE bag | |
| 0 | 4.71 a ± 0.03 | 4.71 a ± 0.02 | 0.018 a ± 0.002 | 0.019 a ± 0.002 |
| 14 | 4.66 b ± 0.02 | 4.62 b ± 0.04 | 0.018 a ± 0.004 | 0.018 a ± 0.001 |
| 28 | 4.64 c ± 0.02 | 4.60 b ± 0.03 | 0.017 a ± 0.001 | 0.017 ab ± 0.002 |
| 42 | 4.62 cd ± 0.04 | 4.51 c ± 0.04 | 0.017 a ± 0.004 | 0.016 b ± 0.001 |
| 56 | 4.60 d ± 0.01 | 4.41 d ± 0.04 | 0.017 a ± 0.003 | 0.014 c ± 0.002 |
| 70 | 4.56 e ± 0.02 | 4.34 e ± 0.02 | 0.016 a ± 0.002 | 0.013 c ± 0.001 |
| 84 | 4.51 f ± 0.01 | 4.22 f ± 0.03 | 0.016 a ± 0.001 | 0.012 cd ± 0.002 |
| 98 | 4.46 g ± 0.03 | 4.09 g ± 0.03 | 0.015 a ± 0.001 | 0.010 d ± 0.001 |
| 112 | 4.40 h ± 0.02 | 3.95 h ± 0.02 | 0.014 a ± 0.002 | 0.009 de ± 0.003 |
| 126 | 4.38 i ± 0.04 | 3.81 i ± 0.04 | 0.013 ab ± 0.001 | 0.008 ef ± 0.001 |
| 140 | 4.32 j ± 0.02 | 3.70 j ± 0.02 | 0.012 b ± 0.002 | 0.007 f ± 0.001 |
| 154 | 4.27 k ± 0.01 | 3.54 k ± 0.03 | 0.011 b ± 0.002 | 0.006 fg ± 0.001 |
| 168 | 4.22 l ± 0.01 | 3.38 l ± 0.03 | 0.011 bc ± 0.002 | 0.005 g ± 0.002 |
| 182 | 4.16 m ± 0.02 | 3.15 m ± 0.03 | 0.010 c ± 0.002 | 0.004 g ± 0.002 |
Data followed by different letters within each column are significantly different according to Duncan’s New Multiple Range Test at P < 0.05. Data obtained from three replicates
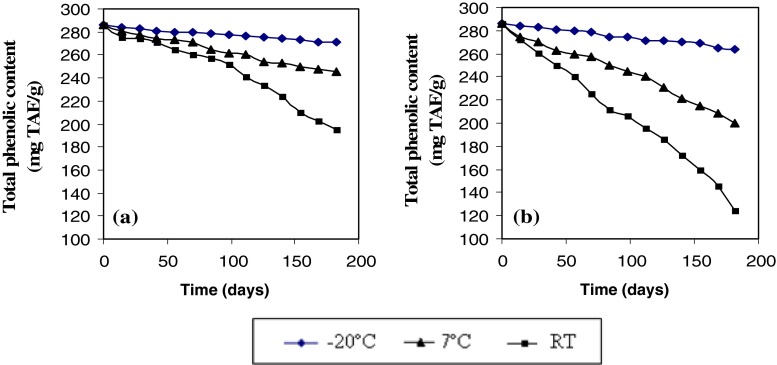
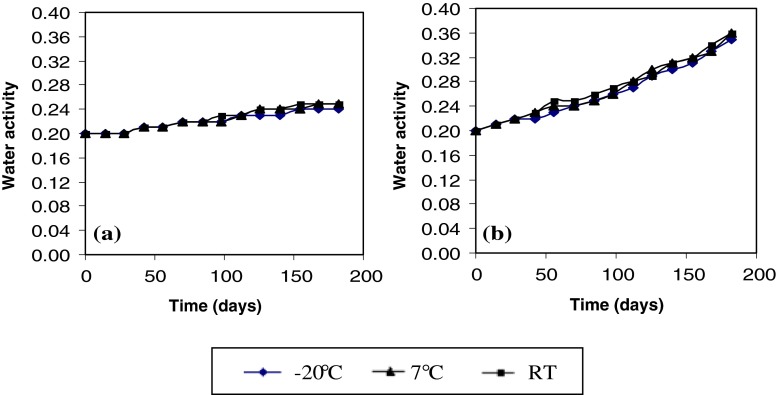
To better understand the effect of temperature storage and type of package on antioxidant activity of MSK, the total phenolic content and water activity of MSKE was evaluated. The total phenolic content of MSKE was retained better in Al. foil than in PE bag (Fig. 5). This explains why the antioxidant activity of MSKE in Al. foil showed higher values during storage at various temperatures. Moreover, the water activity of MSKE in Al. foil (Fig. 6a) changed less than that stored in a PE bag (Fig. 6b). The availability of oxygen and the higher water activity (aw) contribute to the increased reduction of total phenolics during storage of dried samples in the PE bag. These results showed that packaging the extract in Al. foil was appropriate for maintaining the extract quality during storage. The rate of water transmission and oxygen permeability of Al. foil is lower than that of the PE bag.
Fig. 5.
Total phenolic content of mango seed kernel extract in a Al. foil and b PE bag during storage at −20 °C, 7 °C and RT (28–32 °C)
Fig. 6.
Water activity of mango seed kernel extract in a Al. foil and b PE bag during storage at −20 °C, 7 °C and RT (28–32 °C)
Conclusions
In this study, the composition and properties of the hydrolysed extract from the seed kernel of M. indica cultivar Chok-Anan were studied. Methyl gallate consisted of 85.7% of the total phenolic content of the extract. The antioxidant, metal chelating activity and tyrosinase inhibition properties were mainly due to the presence of methyl gallate in the extract. Packaging and storage temperature affected the stability of phenolic antioxidants of mango seed kernel extracts. The appropriate condition for keeping the MSKE was packing in Al. foil and storage at −20 °C. This condition can maintain the quality of the extracts for more than 182 days.
Acknowledgements
This research was supported by the research fund of the University of the Thai Chamber of Commerce. We also thank Dr. Sirikarn Phasuk from Valaya Alongkorn Rajabhat University under Royal Patronage, Thailand for making equipment available for the experiment.
References
- Abdalla AEM, Darwish SM, Ayad EHE, El-Hamahmy RM. Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem. 2007;103:1134–1140. doi: 10.1016/j.foodchem.2006.10.017. [DOI] [Google Scholar]
- Official methods of analysis. 15. Arlington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Chen Q, Song K, Qiu L, Liu X, Huang H, Guo H. Inhibitory effects on mushroom tyrosinase by p-alkoxybenzoic acids. Food Chem. 2005;91:269–274. doi: 10.1016/j.foodchem.2004.01.078. [DOI] [Google Scholar]
- Cheng K, Yang R, Tsou RCS, Lo CSC, Ho C, Lee T, Wang M. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. J Funct Foods. 2009;1:253–259. doi: 10.1016/j.jff.2009.01.013. [DOI] [Google Scholar]
- Chung KT, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem Toxicol. 1998;36:1053–1060. doi: 10.1016/S0278-6915(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Dube M, Zunker K, Neidhart S, Carle R, Steinhart H, PaschkeZunker A. Effect of technological processing on the allergenicity of mangoes (Mangifera indica L.) J Agric Food Chem. 2004;52:3938–3945. doi: 10.1021/jf030792r. [DOI] [PubMed] [Google Scholar]
- Fiuza SM, Gomes C, Teixeira LJ, Girão da Cruz MT, Cordeiro MNDS, Milhazes N, Borges F, Marques MPM. Phenolic acid derivatives with potential anticancer properties—a structure—activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorgan Med Chem. 2004;12:3581–3589. doi: 10.1016/j.bmc.2004.04.026. [DOI] [PubMed] [Google Scholar]
- He Z, Xia W. Analysis of phenolic compounds in Chinese olive (Canarium album L.) fruit by RPHPLC–DAD–ESI–MS. Food Chem. 2007;105:1307–1311. doi: 10.1016/j.foodchem.2007.04.049. [DOI] [Google Scholar]
- Hsieh T, Liu T, Chia Y, Chern C, Lu F, Chuang M. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem Toxic. 2004;42:843–850. doi: 10.1016/j.fct.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Julkunen-Tiito R. Phenolic constituents in the leaves of Northern willows, methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33:213–217. doi: 10.1021/jf00062a013. [DOI] [Google Scholar]
- Kane CJM, Menna JH, Sung C, Yeh Y. Methyl gallate, methyl-3,4,5 trihydroxybenzoate, is a potent and highly specific inhibitor of herbs simplex virus in vitro II, antiviral activity of methyl gallate and its derivatives. Bioscience Rep. 1988;8:96–102. doi: 10.1007/BF01128976. [DOI] [PubMed] [Google Scholar]
- Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. J Food Sci. 1993;58:1407–1410. doi: 10.1111/j.1365-2621.1993.tb06194.x. [DOI] [Google Scholar]
- Liu C, Wang C, Xu Z, Wang Y. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007;42:961–970. doi: 10.1016/j.procbio.2007.03.004. [DOI] [Google Scholar]
- Maisuthisakul P (2008a) Mango seed kernel waste material as potential use for natural antioxidant. In Proc NATPRO2. (pp. OR7). Payao: Naresuan University
- Maisuthisakul P. Antiradical scavenging activity and polyphenolic compounds extracted from Thai mango seed kernels. Asian J Food Ago-Ind. 2008;1:87–96. [Google Scholar]
- Maisuthisakul P, Gordon MH. Antioxidant and tyrosinase inhibitory activity of mango seed kernel by product. Food Chem. 2009;117:332–341. doi: 10.1016/j.foodchem.2009.04.010. [DOI] [Google Scholar]
- Maisuthisakul P, Pongsawatmanit R, Gordon MH. Characterization of the phytochemicals and antioxidant properties of extracts from Teaw (Cratoxylum formosum Dyer) Food Chem. 2007;100:1620–1629. doi: 10.1016/j.foodchem.2005.12.044. [DOI] [Google Scholar]
- Masuda T, Yonemori S, Oyama Y, Takeda Y, Tanaka T, Andoh T. Evaluation of the antioxidant activity of environmental plants: activity of the leaf extracts from seashore plants. J Agric Food Chem. 1999;47:1749–1754. doi: 10.1021/jf980864s. [DOI] [PubMed] [Google Scholar]
- Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S. Glabrene and isoliquiritigenin as tyrosinase inhibitore from licorice roots. J Agric Food Chem. 2003;51:1201–1207. doi: 10.1021/jf020935u. [DOI] [PubMed] [Google Scholar]
- Nithitanakool S, Pithayanukul P, Bavovada R. Antioxidant and hepatoprotective activities of Thai mango dees kernel extract. Planta Medica. 2009;75:1118–1123. doi: 10.1055/s-0029-1185507. [DOI] [PubMed] [Google Scholar]
- Núñez Sellés A, Vélez Castro HT, Agüero-Agüero J, González-González J, Naddeo F, De Simone F, Rastrelli L. Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from Mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J Agric Food Chem. 2002;50:762–766. doi: 10.1021/jf011064b. [DOI] [PubMed] [Google Scholar]
- Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochem. 2005;66:2012–2031. doi: 10.1016/j.phytochem.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Piljac-Žegarac J, Valek L, Martinez S, Belščak A. Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chem. 2009;113:394–400. doi: 10.1016/j.foodchem.2008.07.048. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proreggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Saxena G, McCutcheon AR, Farmer S, Towers GHN, Hancock REW. Antimicrobial constituents of Rhus glabra. J Ethnopharm. 1994;42:95–99. doi: 10.1016/0378-8741(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Schieber A, Keller P, Cale R. Determination of phenolic acids and flavonoids of apple and pear by high performance liquid chromatography. J Chromatogr A. 2001;910:265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Soong Y, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–417. doi: 10.1016/j.foodchem.2004.02.003. [DOI] [Google Scholar]
- Ueda M, Sasaki K, Utsunomiya N, Inaba K, Shimabayashi Y. Effect of temperature and time on some properties during storage of mango fruit (Mangifera indica L., ‘Irwin’) cultured in plastic house. J JPN Soc Food Sci. 2001;48:349–355. doi: 10.3136/nskkk.48.349. [DOI] [Google Scholar]
- Youngmok K, Lounds-Singleton AJ, Talcott ST. Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mangoes (Mangifera indica L.) Food Chem. 2009;115:989–993. doi: 10.1016/j.foodchem.2009.01.019. [DOI] [Google Scholar]