Abstract
Horseradish peroxidase (HRP, EC 1.11.1.7) was applied to treat whole bovine milk in the presence or absence of ferulic acid (FA). The treated milk exhibited different rheological properties from the control milk, and was used to prepare set-yoghurt with commercial direct vat set starter. Some chemical, textural and rheological properties of the yoghurt prepared were measured and compared. Compared to that prepared with the control milk, the yoghurt prepared with the HRP- or HRP and FA-treated bovine milk exhibited an increased hardness and adhesiveness, lower syneresis extent, higher apparent viscosity, and higher storage modulus and viscous modulus. Observation of the microstructure of the yoghurt samples under scanning electron microscopy illustrated that HRP treatment of bovine milk led to the prepared yoghurt a more compact and uniform structure. The results in the present work stated that treatment of bovine milk with HRP in the presence of ferulic acid could be applied to improve the quality of set-yoghurt.
Keywords: Bovine milk, Set-yoghurt, Horseradish peroxidase, Ferulic acid
Introduction
Consumption of fermented dairy products including yoghurt in the world increased dramatically over the past decades mainly due to their contribution for nutrition and health. Texture of set-yoghurt is an important quality criteria (Lee and Lucey 2004; Lucey 2004), which is influenced by milk composition (Marafon et al. 2011), dry matter (Wu et al. 2009), type of starter culture (Amatayakul et al. 2006b), incubation temperature (Laligant et al. 2003), processing conditions (Rekha et al. 2011), storage time (Ünal et al. 2003) and other factors. Textural defects as roughness (irregularities in yoghurt matrix) and syneresis are undesirable to the consumers. Enzyme-induced protein interaction in yoghurt milk had been suggested as an applicable approach to improve textural and rheological properties of the yoghurt (Farnsworth et al. 2006; Gauche et al. 2010). Transglutaminase (TGase) can induce interaction through inter- and intra-molecular bonds in the protein molecules, resulting in formation of high molecular weight protein polymers and modification of the properties of the proteins and food products containing them (Nielsen et al. 1995; Motoki and Seguro 1998). TGase had been applied in some dairy products including yoghurt (Færgemand and Qvist 1997; Lorenzen, et al. 2002), but a need exists to study other enzyme systems capable of improving the quality of dairy products. Horseradish peroxidase (HRP, EC 1.11.1.7), a heme-containing enzyme from the tissue of edible horseradish (Yuan and Jiang 2003), can induce cross-linking of the proteins in the presence of H2O2 and a low molecular weight hydrogen donor (e.g. phenolic compounds) as a cross-linker (Stahmann et al. 1997). This is achieved through an oxidative phenolic coupling of adjacent cross-linkage of the proteins (Aeschbach et al. 1976). Ferulic acid (FA) is a ubiquitous phenolic acid of low toxicity in the plants. FA and its oxide, quinoid ferulic acid, can react with some amino acids such as tyrosine, lysine and cysteine in the proteins to cross-link the molecules (Cao et al. 2007). HRP and FA therefore have potential application to improve yoghurt quality.
The aim of this study was to evaluate the impact of HRP treatment of whole bovine milk in the presence or absence of FA on some chemical, textural, rheological indices and microstructure of the set-yoghurt samples prepared.
Materials and methods
Materials
Whole bovine milk was obtained from a local dairy producer in Harbin, Heilongjiang Province, China. Ferulic acid and horseradish peroxidase were purchased from Shanghai Guoyuan Biotech. Inc. (Shanghai, China). Direct vat set (DVS) starter applied in yoghurt preparation was purchased from Rhodia (Melle, France) and stored at −18 °C before use. Other chemicals used were of analytical grade. Highly purified water prepared with Milli-Q PLUS (Millipore Corporation, New York, NY, USA) was used to prepare all buffers and solutions.
Treatment of bovine milk with HRP
The milk was heated in a water bath to 90 °C for 5 min, cooled to 25 °C and adjusted to pH 9.5 by adding 1 molar NaOH solution. Cross-linking of the milk proteins was carried out as per the method of Li and Zhao (2009a, b) by adding 3 % (w/v) H2O2 (at a level of 1 mL L−1 milk) and HRP (at a level of 645 U g−1 proteins) to the milk, in the presence (7.8 mmol L−1 milk) or absence of FA. Control milk was also treated similarly but without the addition of H2O2, HRP and FA. The contents were mixed well and incubated at 37 °C for 4 h with gentle agitation. After reaction, the milk was adjusted to pH 6.6 with 1 molar HCl solution and heated at 90 °C for 5 min to inactivate the HRP. After the treatment, the prepared control, HRP-treated and HRP and FA-treated milk samples were subjected to rheological analysis and yoghurt preparation as mentioned below.
Rheological properties of bovine milk
A Bohlin Gemini II Rheometer (Malvern Instruments Ltd, Worcestershire, UK) was used to perform rheological analysis with the procedure described by Dickinson (1998). The apparent viscosity of the treated milk sample was determined at 25 °C by using a flat plate (60 mm diameter). A controlled shear rate ranging from 0.1 to 100 s−1 was applied during analysis. The sample was brought into lower plate by using a plastic spatula. The gap was filled up by lowering upper cone down to the designated gap (500 μm). Extra sample around the edge of the plate was wiped with tissue. Storage modulus (G′) and viscous modulus (G″) of the milk sample were also measured by using the flat plate with 60 mm diameter. During analysis, the applied frequency varied between 0.1 and 10 Hz at 5 % strain (determined from an amplitude sweep at 1 Hz) as per the method of Meza et al. (2009).
SDS-PAGE analysis
SDS-PAGE analysis of the treated milk sample was carried out under reducing condition by using separating and stacking gels containing 12 and 4 % (w/w) acrylamide, respectively, as described by Laemmli (1970). Protein content of all samples was fixed at 5 g L−1, and sample volume applied in each lane was 10 μL. Standard protein markers used and their molecular weights (kDa) were as follows: egg albumin lysozyme, 14.4; trypsin inhibitor, 20.1; bovine carbonic anhydrase, 31.0; ovalbumin, 43.0; bovine serum albumin, 66.2; phosphorylase b, 97.4. Protein content of all markers was fixed at 0.1 g L−1. The stacking gels were run at 120 V and the separating gels were run at 80 V. The gels were stained by using 2.5 % (w/v) Coomassie Brilliant Blue G250 in a 4.5:4.5:1 (v/v) mixture of deionized water, methanol and glacial acetic acid for 0.5 h, followed by repeated destaining in a mixture of 1:1:8 (v/v) methanol, glacial acetic acid and deionized water until the background of the gels became clear. Gel images were visualized and photographed by PhotoDoc-It Imaging System (UVP Inc., San Gabriel, CA, USA).
Yoghurt preparation
The set-yoghurt samples were prepared with the treated milk according to the procedure of Barrantes et al. (1994). After heat treatment at 90 °C for 5 min, the milk was cooled to 42 °C and inoculated with the DVS starter (containing S. thermophilus and L. delbrueckii subsp. bulgaricus) at a level of 0.5 g kg−1 milk as recommended by the supplier. Each container of 150 mL capacity was filled with 100 mL of the cultured milk. Samples were incubated at 42 °C for 4 h till a pH of 4.5 was reached. Samples were then stored at 4 °C for 36 h. Three yoghurt samples were selected randomly for further evaluation as mentioned below.
Chemical analysis of yoghurt samples
The prepared yoghurt samples were analyzed for their protein, fat and total solids contents by the Kjeldahl, Gerber and gravimetric methods as described in AOAC (1999). A pH meter (Model DELTA 320, Mettler-Toledo Instruments (Shanghai) Ltd., China) was used to monitor the pH of the yoghurt samples after storage. Titratable acidity of the yoghurt samples was measured with the method given in AOAC (1999), and was expressed as percentage of lactic acid. Approximately, 10.0 g of the yoghurt samples was diluted with 10 mL of distilled water before titration.
Syneresis measurement of yoghurt samples
Syneresis extent of the prepared yoghurt samples after storage was analyzed by using a centrifugation method (Amatayakul et al. 2006a) with slight modification in room temperature. About 20.0 g of the yoghurt sample was centrifuged (Model LG10-2.4A, Beijing, China) at 700 × g for 10 min. The supernatant was removed and its amount was measured. Syneresis extent of the yoghurt sample was related to its initial weight and expressed as percentage.
Texture profile analysis of yoghurt samples
Texture profile analysis of the yoghurt samples was carried out with a method of Sandoval-Castilla et al. (2004). The sample analyzed was carefully scooped into an acrylic cylindrical container (45 mm diameter × 80 mm height) with help of spatula to a depth of 50 mm. The analysis was carried out with a Stable Micro Systems Texturometer (Model TA-XT2, Stable Micro Systems, Surrey, England) with a 5 kg load cell.
A 35 mm diameter solid rod (A/BE35) was thrust into the container holding the sample. Two cycles were applied, at a constant crosshead velocity of 1 mm s−1, to a sample depth of 30 mm, with a surface trigger of 5.0 g. Three parameters viz., adhesiveness, cohesiveness and hardness, as defined by Hassan et al. (1996) were performed.
Apparent viscosity of yoghurt samples
The apparent viscosity of the prepared yoghurt samples was measured as per the method of Singh and Muthukumarappan (2008) using the Rheometer by using a flat plate of 60 mm diameter at 25 °C.
Thixotropy of yoghurt samples
Thixotropy of the yoghurt samples was evaluated in the Rheometer with the method of Debon et al. (2010). The sample was placed on the inset plate and sheared at 500 s−1 for 1 min to diminish structural differences among the samples. This was followed by 5 min equilibration to allow for a structural rebuilding of the sample. Flow curves were generated by measuring shear stress as a function of shear rates from 0.1 to 100 s−1 (up and down sweeps). Flow behavior described by Ostwald de Waele model (τ = K·γn), where τ is shear stress (Pa), γ is shear rate (s−1), K and n are consistency factor (Pa·sn) and flow behavior index, respectively, was obtained from RheoWin Pro software of the Rheometer directly. Hysteresis loop area between the upward and downward flow curves of the sample was also obtained and calculated by using the software.
Storage modulus and viscous modulus of yoghurt samples
Storage modulus and viscous modulus of the yoghurt samples were also measured by using the plate of 60 mm diameter in the Rheometer. The applied frequency varied from 0.1 to 10 Hz at 5 % strain as described by Purwandari et al. (2007).
Microstructure of yoghurt samples
The analysis sample was separated from approximately 10 mm below the surface of the prepared yoghurt sample and fixed in a 2 % (w/w) glutaraldehyde solution in phosphate buffer (0.1 mM, pH 7.2) at room temperature for 1 h to cross-link milk proteins as per the method of Sandoval-Castilla et al. (2004). The fixed sample was washed with the phosphate buffer, and dehydrated for 15 min in a graded ethanol series consisting of 50, 70, 90 and 100 % ethanol. The sample was then frozen in liquid nitrogen. The dried sample was mounted on aluminum SEM stubs by using a carbon-based tape, and coated with gold in ES-1010 sputter coater (Hitachi, Tokyo, Japan). The prepared sample was observed under Hitachi S-5700 scanning microscope (Hitachi, Tokyo, Japan) at 5,000× magnification with 5 kV accelerating voltage.
Statistical analysis
All preparation experiments were carried out in triplicate (n = 3). Three analyses were carried out for each sample. All data were expressed as means or means ± standard deviations. Differences between the mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan’s multiple range tests. SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) and MS Excel 2003 software (Microsoft Corporation, Redmond, WA, USA) were used to analyze and report the data.
Results and discussion
Effect of HRP treatment on bovine milk
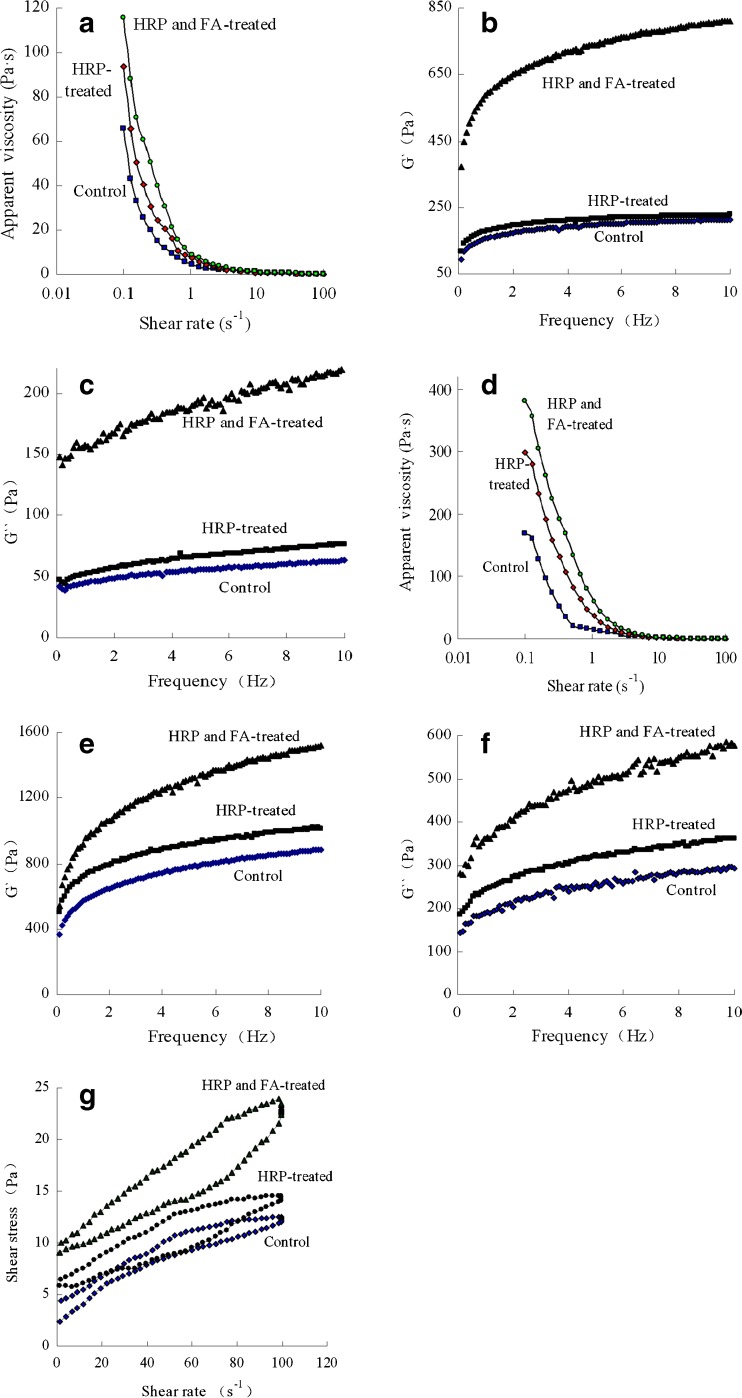
The apparent viscosity of the bovine milk with different treatments against shear rate is revealed in Fig. 1a. The data indicated that the apparent viscosity of the milk sample decreased as shear rate increased, as a result of shear-thinning behavior. The HRP and FA-treated milk had the highest value but the control milk had the lowest one, meaning that HRP treatment led to cross-linking of milk proteins and consequentially an enhanced apparent viscosity of the milk especially when cross-linker FA was added. Storage modulus (G′) and viscous modulus (G″) of the milk samples were also impacted significantly by HRP treatment (Fig. 1b and c). The HRP and FA-treated milk had the highest G′ and G″, but the control milk had the lowest one. Gauche et al. (2008) investigated the effect of TGase-induced cross-link of whey proteins on rheological properties of the samples, and found a marked increased apparent viscosity, G′ and G″. Their reported results support our findings.
Fig. 1.
Assaying results for the apparent viscosity (a), storage modulus (G′) (b) and viscous modulus (G″) (c) of control, HRP-treated or HRP and FA-treated bovine milk, or for the apparent viscosity (d), storage modulus (G′) (e), viscous modulus (G″) (f) and thixotropy loops (g) of set-yoghurt samples prepared with control, HRP-treated or HRP and FA-treated bovine milk and stored at 4 °C for 36 h. All data are reported as means of three trials (n = 3)
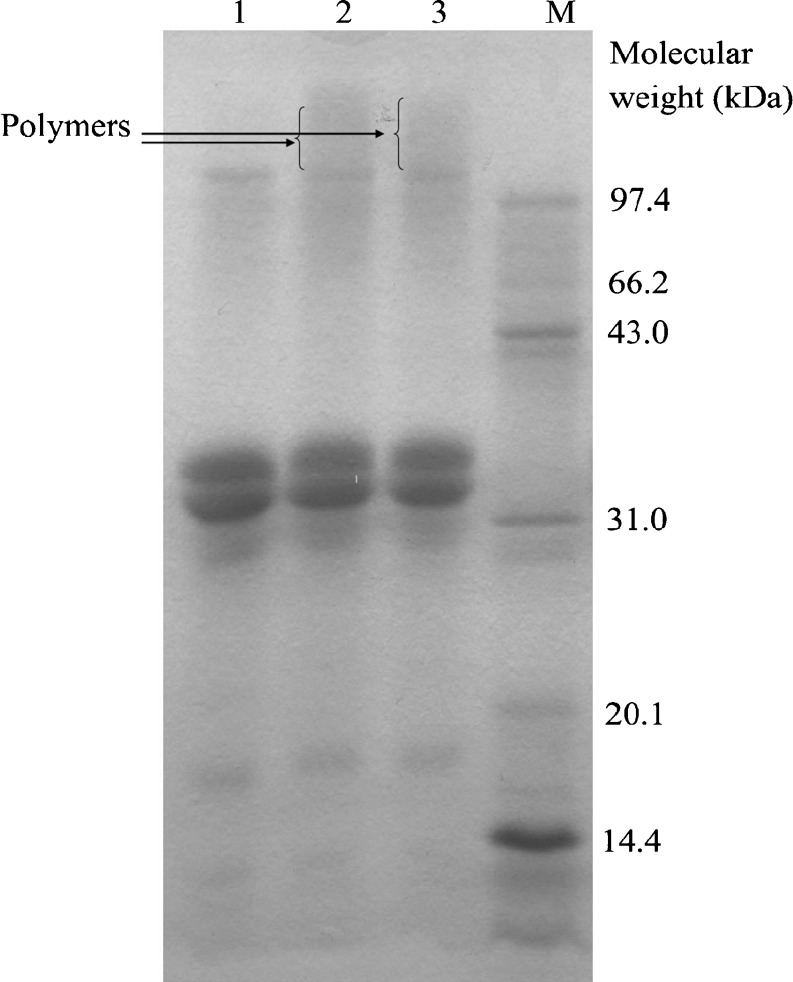
SDS-PAGE analysis shown in Fig. 2 indicated that some protein polymers existed in the HRP-treated or HRP and FA-treated bovine milk samples (lane 2 or lane 3) as result of cross-linking of the milk proteins although their content was low. These results shared similarity with those of Matheis and Whitaker (1984) who reported the formation of di- and ter-tyrosine due to cross-linking of casein and soybean proteins by peroxidase−H2O2.
Fig. 2.
SDS-PAGE profiles of control (lane 1), HRP-treated (lane 2) and HRP and FA-treated bovine milk (lane 3). Lane M, protein markers consisted of egg albumin lysozyme (14.4 kDa), trypsin inhibitor (20.1 kDa), bovine carbonic anhydrase (31.0 kDa), ovalbumin (43.0 kDa) bovine serum albumin (66.2 kDa) and phosphorylase b (97.4 kDa)
Influence of HRP treatment on some chemical and textural characteristics of yoghurt samples
Resolution chemical compositions of the prepared yoghurt samples are listed in Table 1. The data showed that HRP treatment in the presence or absence of FA did not exhibit significant (P > 0.05) impact on these evaluated indices of the prepared yoghurt.
Table 1.
Physico-chemical characteristics of set-yoghurt samples
| Yoghurt sample | Proteins (%, w/w) | Fat (%, w/w) | Total soilds (%, w/w) | pH | Titratable acidity (%, lactic acid) |
|---|---|---|---|---|---|
| 1 | 3.09 ± 0.23 A | 3.38 ± 0.22 A | 16.55 ± 0.29 A | 4.23 ± 0.04 A | 0.90 ± 0.02 A |
| 2 | 3.05 ± 0.18 A | 3.31 ± 0.13 A | 16.52 ± 0.13 A | 4.29 ± 0.06 A | 0.88 ± 0.01 A |
| 3 | 2.98 ± 0.10 A | 3.28 ± 0.15 A | 16.50 ± 0.08 A | 4.28 ± 0.05 A | 0.86 ± 0.01 A |
Yoghurt samples 1, 2, and 3 were set-yoghurt samples prepared with control, HRP-treated and HRP and FA-treated bovine milk, respectively, and stored at 4 °C for 36 h. All data are reported as means ± standard deviations of three trials (n = 3). The different capital letters after the values in the same column indicate that one-way ANOVA of the means is different significantly (P < 0.05)
Three textural parameters of the yoghurt samples were measured (Table 2). Two parameters, hardness and adhesiveness increased with the addition of HRP and FA to the milk (P < 0.05). Compared to that prepared with the control milk, the yoghurt prepared with the HRP-treated milk exhibited an increased hardness (from 76.7 to 91.8 g, about 20 % increase) or adhesiveness (from 98.7 to 126.7 g s, about 28 % increase); while the yoghurt prepared with the HRP and FA-treated milk had the higher hardness and adhesiveness values of 102.9 g and 139.1 g s, respectively (about 34 and 41 % increase). The cohesiveness of the yoghurt was not impacted significantly (P > 0.05) by HRP treatment of bovine milk. The yoghurt prepared with the HRP or/and FA-treated bovine milk had lower syneresis value than that prepared with the control milk (a decrease of 3.4 or 6.2 %). These results revealed that the quality of the set-yoghurt prepared from the HRP-treated milk was improved. Treatment of food proteins with H2O2 and peroxidase cause oxidative cross-linking of tyrosine residues and FA can increase the degree of cross-linking (Ou and Kwok 2004). The present results are consistent with this finding, and the set-yoghurt prepared from HRP and FA-treated milk showed the best quality indices.
Table 2.
Textural parameters and syneresis extent of set-yoghurt samples
| Yoghurt sample | Hardness (g) | Adhesiveness (g s) | Cohesiveness (g) | Syneresis (%) |
|---|---|---|---|---|
| 1 | 76.7 ± 4.97 A | 98.7 ± 7.59 A | 0.313 ± 0.002 A | 79.2 ± 3.68 C |
| 2 | 91.8 ± 5.48 B | 126.7 ± 7.04 B | 0.324 ± 0.003 A | 76.6 ± 5.69 B |
| 3 | 102.9 ± 6.49 C | 139.1 ± 8.45 C | 0.318 ± 0.005 A | 74.3 ± 3.45 A |
Yoghurt samples 1, 2, and 3 were set-yoghurt samples prepared with control, HRP-treated and HRP and FA-treated bovine milk, respectively, and stored at 4 °C for 36 h. All data are reported as means ± standard deviations of three trials (n = 3). The different capital letters after the values in the same column indicate that one-way ANOVA of the means is different significantly (P < 0.05)
Influence of HRP treatment on rheological properties of yoghurt samples
The apparent viscosity of the prepared yoghurt against shear rate is demonstrated in Fig. 1d, which indicates that HRP treatment of the milk had clear impact on the apparent viscosity of the prepared yoghurt. The yoghurt sample prepared with the HRP and FA-treated milk had the highest apparent viscosity, but that prepared with the control milk had the lowest one. This result was supported by the fact that the storage modulus (G′) or viscous modulus (G″) of the prepared yoghurt was also impacted by the HRP treatment as shown in Fig. 1e and f. The yoghurt prepared with the HRP and FA-treated milk had the highest G′ or G″ while that prepared with the control milk had the lowest one. Myllärinen et al. (2007) investigated the effect of TGase-induced cross-link of sodium caseinate on the rheological properties and also found a marked increased apparent viscosity, G′ and G″. The mentioned findings support the present results.
Thixotropic hysteresis loops of the yoghurt samples are given in Fig. 1g. The data showed that the yoghurt prepared with the HRP and FA-treated milk had the largest hysteresis loop area while that prepared with the control milk had the smallest one (Table 3). It is known that the hysteresis loops is assumed to be the difference between the energies required for structural breakdown and rebuilding, and stronger thixotropy led to greater hysteresis loops (Damin et al. 2009). In the present work, the yoghurt prepared with the HRP and FA-treated milk showed the largest thixotropy while that prepared with the control milk exhibited the smallest thixotropy. This result is in agreement with a study of Dickinson and Yamamoto (1996) in which milk proteins were cross-linked by TGase and the hysteresis loop area was also significantly larger for the TGase-treated yoghurt sample in comparison to a non-cross-linked control sample.
Table 3.
Flow behavior indices (n), consistency coefficient (K), regression coefficient (R 2) and hysteresis loop area of set-yoghurt samples
| Yoghurt sample | K (Pa·sn) | n | R 2 | Area of the loop (Unit) |
|---|---|---|---|---|
| 1 | 1.66 ± 0.007 A | 0.230 ± 0.010 A | 0.992 ± 0.005 A | 239.6 ± 10.5 A |
| 2 | 1.95 ± 0.004 B | 0.279 ± 0.020 A | 0.990 ± 0.004 A | 325.6 ± 16.8 B |
| 3 | 2.33 ± 0.004 C | 0.258 ± 0.020 A | 0.997 ± 0.003 A | 365.2 ± 18.9 C |
Yoghurt samples 1, 2, and 3 were set-yoghurt samples prepared with control, HRP-treated and HRP and FA-treated bovine milk, respectively, and stored at 4 °C for 36 h. All data are reported as means ± standard deviations of three trials (n = 3). The different capital letters after the values in the same column indicate that one-way ANOVA of the means is different significantly (P < 0.05)
The analysis results of flow behaviors of the yoghurt samples were fitted to the Ostwald de Waele model. HRP treatment of bovine milk enhanced consistency coefficient (K) significantly (P < 0.05), especially when FA was added, but had no influence on flow behavior indices (n) of the yoghurt (P > 0.05) (Table 3). The result is supported by the work of Lauber et al. (2000) because they also found that TGase enhanced consistency coefficient of the yoghurt significantly. It was therefore shown that treatment of bovine milk with HRP (especially when FA was added) indeed improved some quality indices of the set-yoghurt.
Influence of HRP treatment on microstructure of yoghurt samples
The microstructure of the yoghurt samples was observed under SEM (Fig. 3) as SEM study was widely used to study the microstructure of various semisolid gels including yoghurt and cheese (Ghosh et al. 2011). The network of the yoghurt prepared with the control milk was characterized as a loose structure with big and irregular holes (Fig. 3a). However, a more compact and small pores were formed in the yoghurt prepared with the HRP-treated milk (Fig. 3b). The yoghurt prepared with HRP and FA-treated milk had the densest, highest branched and uniform structure (Fig. 3c). Such microstructural improvement was due to the more inter- and intra-molecular protein interactions in the yoghurt. This result is same to the reported results of Li and Zhao (2009a & 2009b), in which the microstructure of the acidified casein gels was also improved by the carried out HRP or HRP and FA treatment.
Fig. 3.
Microstructure of set-yoghurt samples prepared with control (a), HRP-treated (b) and HRP and FA-treated bovine milk (c) and stored at 4 °C for 36 h. The observation was carried out under scanning electron microscopy at 5,000×
Conclusion
Treatment of whole bovine milk with horseradish peroxidase (HRP) in the presence or absence of ferulic acid (FA) gave the milk higher apparent viscosity, storage modulus and viscous modulus, and exhibited impacts on some quality criteria of the prepared set-yoghurt, especially when a cross-linker FA was added. The yoghurt prepared with HRP- or HRP and FA-treated milk showed increased hardness and adhesiveness, together with a slightly decreased syneresis. The apparent viscosity, viscoelastic (or thixotropy) behavior and microstructure of the yoghurt were also influenced by the HRP treatment of bovine milk. Treatment of bovine milk with HRP in the presence of FA might be capable of improving some quality indices of the set-yoghurt.
Acknowledgements
This work was funded by the Innovative Research Team of Higher Education of Heilongjiang Province (Project No. 2010td11). The authors thank the editors and the anonymous reviewers for their constructive and valuable works to the paper.
References
- Aeschbach R, Amadò R, Neukom H. Formation of dityrosine cross-links in proteins by oxidation of tyrosine residue. Biochim Biophys Acta. 1976;439:292–301. doi: 10.1016/0005-2795(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Amatayakul T, Halmos AL, Sherkat F, Shah NP. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int Dairy J. 2006;16:40–51. doi: 10.1016/j.idairyj.2005.01.004. [DOI] [Google Scholar]
- Amatayakul T, Sherkat F, Shah NP. Physical characteristics of set yoghurt made with altered casein to whey protein ratios and EPS-producing starter cultures at 9 and 14 % total solids. Food Hydrocolloid. 2006;20:314–324. doi: 10.1016/j.foodhyd.2005.02.015. [DOI] [Google Scholar]
- Official methods of analysis (16th edn) Washington DC: Association of Official Analytical Chemists; 1999. [Google Scholar]
- Barrantes E, Tamime AY, Muir DD, Sword AM. The effect of substitution of fat by microparticulate whey protein on the quality of set-type, natural yogurt. J Soc Dairy Technol. 1994;47:61–68. doi: 10.1111/j.1471-0307.1994.tb01274.x. [DOI] [Google Scholar]
- Cao N, Fu Y, He J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocolloid. 2007;21:575–58. doi: 10.1016/j.foodhyd.2006.07.001. [DOI] [Google Scholar]
- Damin MR, Alcântara MR, Nunes AP, Oliveira MN. Effects of milk supplementation with skim milk power, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yoghurt. LWT-Food Sci Technol. 2009;42:1744–1750. doi: 10.1016/j.lwt.2009.03.019. [DOI] [Google Scholar]
- Debon J, Prudêncio ES, Petrus JCC. Rheological and physic-chemical characterization of prebiotic microfiltered fermented milk. J Food Eng. 2010;99:28–135. doi: 10.1016/j.jfoodeng.2010.02.008. [DOI] [Google Scholar]
- Dickinson E. Stability and rheological implications of electrostatic milk proteins- polysaccharide interactions. Trends Food Sci Technol. 1998;9:347–354. doi: 10.1016/S0924-2244(98)00057-0. [DOI] [Google Scholar]
- Dickinson E, Yamamoto Y. Rheology of milk protein gels and protein-stabilized emulsion gels cross-linked with transglutaminase. J Agr Food Chem. 1996;44:1371–1377. doi: 10.1021/jf950705y. [DOI] [Google Scholar]
- Færgemand M, Qvist KB. Transglutaminase: effect on rheological properties, microstructure and permeability of set style acid skim milk gel. Food Hydrocolloid. 1997;11:287–292. doi: 10.1016/S0268-005X(97)80058-6. [DOI] [Google Scholar]
- Farnsworth JP, Li J, Hendricks GM, Guo MR. Effects of transglutaminase treatment on functional properties and probiotic culture survivability of goat milk yogurt. Small Ruminant Res. 2006;65:113–121. doi: 10.1016/j.smallrumres.2005.05.036. [DOI] [Google Scholar]
- Gauche C, Vieira JTC, Ogliari PJ, Bordignon-Luiz MT. Crosslinking of milk whey proteins by transglutaminase. Process Biochem. 2008;43:788–794. doi: 10.1016/j.procbio.2008.04.004. [DOI] [Google Scholar]
- Gauche C, Barreto PLM, Bordignon-Luiz MT. Effect of thermal treatment on whey protein polymerization by transglutaminase: implications for functionality in processed dairy foods. LWT-Food Sci Technol. 2010;43:214–219. doi: 10.1016/j.lwt.2009.08.009. [DOI] [Google Scholar]
- Ghosh D, Chattoraj DK, Chattopadhyay P (2011) Studies on changes in microstructure and proteolysis in cow and soy milk curd during fermentation using lactic cultures for improving protein bioavailability. J Food Sci Technol. doi:10.1007/s13197-011-0421-1 [DOI] [PMC free article] [PubMed]
- Hassan AN, Frank JF, Schmidt KA, Shalabi SI. Textural properties of yoghurt made with encapsulated nonropy lactic cultures. J Dairy Sci. 1996;79:2098–2103. doi: 10.3168/jds.S0022-0302(96)76583-9. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laligant A, Famelart MH, Paqurt D, Brulé G. Fermentation by lactic bacteria at two temperatures of pre-heated reconstituted milk. II-Dynamic approach of the gel construction. Lait. 2003;83:307–320. doi: 10.1051/lait:2003018. [DOI] [Google Scholar]
- Lauber S, Henle T, Klostermeyer H. Relationship between the crosslinking of caseins by transglutaminase and the gel strength of yoghurt. Eur Food Res Technol. 2000;210:305–309. doi: 10.1007/s002170050554. [DOI] [Google Scholar]
- Lee WJ, Lucey JA. Structure and physical properties of yoghurt gels: effect of inoculation rate and incubation temperature. J Dairy Sci. 2004;87:3153–3164. doi: 10.3168/jds.S0022-0302(04)73450-5. [DOI] [PubMed] [Google Scholar]
- Li JW, Zhao XH. Hydrogen peroxide and ferulic acid-mediated oxidative cross-linking of casein catalyzed by horseradish peroxidase and the impacts on emulsifying property and microstructure of acidified gel. Afr J Biotechnol. 2009a;8:6993–6999. [Google Scholar]
- Li JW, Zhao XH. Oxidative cross-linking of casein by horseradish peroxidase and its impacts on emulsifying properties and the microstructure of acidified gel. Afr J Biotechnol. 2009b;8:5508–5515. [Google Scholar]
- Lorenzen PC, Neve H, Mautner A, Schlimme E. Effect of enzymatic cross-linking of milk proteins on functional properties of set-yoghurt. Int J Dairy Technol. 2002;55:152–157. doi: 10.1046/j.1471-0307.2002.00065.x. [DOI] [Google Scholar]
- Lucey JA. Cultured dairy products: an overview of their gelation and texture properties. Int J Dairy Technol. 2004;57:77–84. doi: 10.1111/j.1471-0307.2004.00142.x. [DOI] [Google Scholar]
- Marafon AP, Sumi A, Alcântara MR, Tamime AY, de Oliveira MN. Optimization of the rheological properties of probiotic yoghurts supplemented with milk proteins. LWT-Food Sci Technol. 2011;44:511–519. doi: 10.1016/j.lwt.2010.09.005. [DOI] [Google Scholar]
- Matheis G, Whitaker JR. Peroxidase-catalyzed cross linking of proteins. J Protein Chem. 1984;3:35–48. doi: 10.1007/BF01024835. [DOI] [Google Scholar]
- Meza BE, Verdini RA, Rubiolo AC. Viscoelastic behaviour of heat-treated whey protein concentrate suspensions. Food Hydrocolloid. 2009;23:661–666. doi: 10.1016/j.foodhyd.2008.03.015. [DOI] [Google Scholar]
- Motoki M, Seguro K. Transglutaminase and its use for food processing. Trends Food Sci Technol. 1998;9:204–210. doi: 10.1016/S0924-2244(98)00038-7. [DOI] [Google Scholar]
- Myllärinen P, Buchert J, Autio K. Effect of transglutaminase on rheological properties and microstructure of chemically acidified sodium caseinate gels. Int Dairy J. 2007;17:800–807. doi: 10.1016/j.idairyj.2005.10.031. [DOI] [Google Scholar]
- Nielsen GS, Petersen BR, Moller AJ. Impact of salt, phosphate and temperature on the effect of a transglutaminase (F XIIIA) on the texture of restructured meat. Meat Sci. 1995;41:293–299. doi: 10.1016/0309-1740(94)00002-O. [DOI] [PubMed] [Google Scholar]
- Ou SY, Kwok KC. Ferulic acid: pharmaceutical functions, preparation and application in foods. J Sci Food Agric. 2004;84:1261–1269. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- Purwandari U, Shah NP, Vasiljevic T. Effect of expolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-yoghurt. Int Dairy J. 2007;17:1344–1352. doi: 10.1016/j.idairyj.2007.01.018. [DOI] [Google Scholar]
- Rekha C, Girdhari RP, Patil AKS. High hydrostatic pressure technology in dairy processing: a review. J Food Sci Technol. 2011;48:260–268. doi: 10.1007/s13197-011-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Castilla O, Lobato-Calleros C, Aguirre-Mandujano E, Vermon-Carter EJ. Microstructure and texture of yoghurt as influenced by fat replacers. Int Dairy J. 2004;14:151–159. doi: 10.1016/S0958-6946(03)00166-3. [DOI] [Google Scholar]
- Singh G, Muthukumarappan K. Influence of calcium fortification on sensory, physical and rheological characteristics of fruit yogurt. LWT-Food Sci Technol. 2008;41:1145–1152. doi: 10.1016/j.lwt.2007.08.027. [DOI] [Google Scholar]
- Stahmann MA, Spencer AK, Honold GR. Cross linking of proteins in vitro by peroxidase. Biopolymers. 1997;16:1307–1318. doi: 10.1002/bip.1977.360160611. [DOI] [PubMed] [Google Scholar]
- Ünal B, Metin S, Işıklı ND. Use of response surface methodology to describe the combined effect of storage time, locust bean gum and dry matter of milk on the physical properties of low-fat set yoghurt. Int Dairy J. 2003;13:909–916. doi: 10.1016/S0958-6946(03)00118-3. [DOI] [Google Scholar]
- Wu S, Li D, Li SJ, Bhandari B, Yang BL, Chen XD, Mao ZH. Effects of incubation temperature, starter culture level and total solids content on the rheological properties of yogurt. Int J Food Eng. 2009;5:1–40. doi: 10.2202/1556-3758.1436. [DOI] [Google Scholar]
- Yuan ZY, Jiang TJ. Horseradish Peroxidase. In: Whitaker JR, Voragen AGJ, Wong DWS, editors. Handbook of food enzymology. New York: Marcel Dekker; 2003. pp. 403–411. [Google Scholar]