Abstract
The effect of gamma irradiation (0.5 and 1.0 kGy) and/or cooking on the proximate composition, mineral content, tannin content, phytic acid content and the in vitro protein digestibility (IVPD) of two Sudanese faba bean cultivars (BB7-S1 and SH-S2) was investigated in the present study. The results obtained revealed that gamma irradiation and/or cooking treatments have slight effect in chemical composition and mineral content, while they caused significant (P ≤ 0.05) reduction on tannin content for both cultivars. Cooking of faba bean seeds also insignificantly (P ≤ 0.05) reduced phytic acid content for both cultivars, while irradiation process and/or cooking had fluctuated effect. For both cultivars, irradiation of seeds and/or cooking increased the in vitro protein digestibility (IVPD), with maximum value of IVPD (79.97%) obtained for cultivar BB7-S1. The results indicate that the treatments used in this study might improve the nutritive quality of faba bean seed due to reduction in antinutritional factors with a concomitant increase in IVPD
Keywords: Faba bean, Gamma irradiation, Cooking, Antinutritional factors, Protein digestibility
Introduction
Faba bean (Vicia faba L.) is an important cash crop in the Sudan. The crop contributes to human nutrition and it is the staple food for many people in Sudan, because of its high protein content as well as other essential nutrients. The high lysine content has encouraged the use of faba bean as a protein supplement for cereals. Besides their high nutritional value, food grain legumes contain naturally occurring compounds known as anti-nutritional factors, as phytic acid and tannins. These anti-nutrients negatively affect the nutritive value (protein quality and minerals availability) of the bean through direct and indirect reactions (Bressani 1993). Removal of such antinutritional factors is essential to improve the nutritive value of legumes. In order to inactivate or reduce the above mentioned antinutrients and improve the protein digestibility of legumes, various conventional, simple processing methods have been used such as dry heating, roasting, boiling, soaking in water, alkali and acid, solvent extraction, germination and fermentation (Liener 1994; Sathe and Salunkhe 1994; Siddhuraju and Becker 2001a; Siddhuraju and Becker 2001b). However, none of these methods is able to completely remove all the detected antinutrients that are present in seeds, grains or feed materials. Irradiation treatment as a method of preservation to enhance the shelf-life or to improve the hygienic qualities of raw and processed foods and agricultural commodities has been well established worldwide. Radiation processing has proved to be an effective means of disinfestations and decontamination of food and agricultural products (Anonymous 1991; Loaharanu 1994). Radiation treatment itself or in combination with other processing methods has been shown to reduce or eliminate some of the anti-nutrients in cereals and legumes (Farag 1989; Sattar et al. 1990; Siddhuraju et al. 2000). Ionizing radiations have also been proved effective in improving the overall nutritional attributes, including some desired changes in functional properties of seed flours (Rahma and Mostafa 1988; Dario and Salgado 1994; Dogbevi et al. 2000). Irradiated foods will be safe and beneficial to extend the shelf-life of consumables in regions lacking proper refrigeration facilities (FAO/IAEA 1997). A joint expert committee convened by the FAO/IAEA/WHO stated that irradiation of any food commodity up to 10 kGy presents no toxicological hazard (Anonymous 1981). Subsequently, a joint FAO/IAEA/WHO study group reviewed the toxicological, nutritional and radiation-induced chemical and physical aspects of irradiated foods above 10 kGy and concluded that application of ionizing radiation at 10 kGy or higher doses will be safe and nutritionally adequate (WHO 1999). Thus, effects of ionizing radiation on overall nutritional quality of food legumes up to the recommended dose and above assume importance. The objective of the present study was to investigate the effects of low doses of gamma irradiation (0.5 and 1.0 kGy) and/or cooking on some nutrients and antinutritional factors of faba bean cultivars.
Materials and methods
The seeds of two faba bean (Vicia faba) cultivars (BB7 –S1 and SH-S2) were obtained from Department of Agronomy, Faculty of Agriculture, University of Khartoum, Sudan. The seeds were carefully cleaned and freed from foreign materials and stored under ambient temperature during the study. All chemicals used in this study were of analytical grade.
Processing techniques
Irradiation process
Faba bean seeds with initial moisture content of 2.98 & 2.73% for BB7–S1 and SH-S2 cultivars respectively were sealed in polythene bags of mass 500 g before and during irradiation process. Irradiation was carried out at room temperature (25°C) at Kaila irradiation processing unit, Sudanese Atomic Energy Corporation (SAEC). Samples were irradiated with a 3.89 KCi and 60Co source at 0.5 and 1.0 kGy with dose rate 3.2 kGy/h. To minimize the variations in radiation received by the samples gamma rays were exposure to the both sides through radiation process. A Fricke dosimetry system was used to measure the dose received by the batch based on the Gafchromic HD-810 film (International Specialty Products, NJ, USA; FAO/IAEA/USDA 2003). Non irradiated seeds served as control.
Cooking
Irradiated and non-irradiated seeds for both cultivars were subjected to cooking after soaking in water using water to grain ratio of 1:7 (w:v). Cooked seeds were dried at 50 °C for 24 h. (Kataria et al. 1989). The second part was left uncooked. The Seeds (cooked and uncooked) were milled to fine powder to pass a 0.4 mm mesh and kept in glass bottles at 4 °C for further analysis.
Proximate composition determination
Moisture and ash content of the samples were determined according to the methods of AOAC (1990). Fat content was assessed by soxhlet extraction method with petroleum ether (AOAC 1990). Protein was calculated from the percentage of nitrogen which was determined by using Kjeldahl method described by Pearson (1981). Crude fiber was determined by treating oil-free samples by sulphuric acid (0.26 N) and potassium hydroxide (0.23 N) solution in refluxing systems, followed by oven drying and muffle furnace incineration. (AOAC 1990). Müller and Tobin’s (1980) method was used to calculate total crude carbohydrates. The energy values of samples were calculated on Atwater factors (Sukker 1985), protein (4 kcal g−1), oil (9 kcal g−1) and carbohydrates (4 kcal g−1).
Total minerals determination
Minerals were extracted from the samples by the dry ashing method described by Chapman and Pratt (1982) About 2.0 g of sample was acid-digested in a digestion room. The digested samples were dissolved in distilled water and filtered (Whatman No. 42). The filtrate was made to 50 ml with distilled water and was used for determination of total calcium, Magnisum, phosphorus and iron. Calcium and Magnisum were determined by a titration method. Iron was determined by atomic absorption spectrophotometer (Perkin-Elmer 2380). Phosphorus was determined spectrophotometrically using molybdovanadate method.
In vitro protein digestibility determination
The in vitro protein digestibility was carried out using pepsin according to the method of Maliwal (1983) as described by Monjula and John (1991) with a minor modification. A known weight of the sample containing 16 mg nitrogen was taken in triplicate and digested with 1mg pepsin in 15 ml of 0.1 M HCl at 37 °C for 2 h. The reaction was stopped by addition of 15 ml of 10% trichloroacetic acid (TCA), the mixture was then filtered quantitatively through Whatman No. 1 filter paper. The TCA .soluble fraction was assayed for nitrogen using the micro-Kjeldahl method (Pearson 1981).
Calculations:
 |
Tannins content determination
Quantitative estimation of tannin for each sample was carried out using a modified vanillin–HCl in methanol method as described by Price et al. (1978). A 200 mg sample was extracted using 10 mL of 1% (v/v) concentrated HCl in methanol for 20 min in capped rotating test tubes. Vanillin reagent (0.5%, 5 ml) was added to the extract (1 ml) and the absorbance of the colour developed after 20 min at 30 °C was read at 500 nm. A standard curve was prepared expressing the results as catechin equivalents, i.e. amount of catechin (mg per ml) which gives a colour intensity equivalent to that given by tannins after correcting for blank.
Phytic acid determination
Phytic acid content was determined by the method described by Wheeler and Ferrel (1971) using 2.0 g of a dried sample. A standard curve was prepared expressing the results as Fe (NO3)3 equivalent. Phytate phosphorus was calculated from the standard curve assuming 4:6 iron to phosphorus molar ratio.
Statistical analysis
Each determination was carried out on three separate samples and analyzed in triplicate on dry weight basis; the figures were then averaged. Data were assessed by the analysis of variance (ANOVA) described by Snedecor and Cochran (1987) Comparisons of means for treatments were made using Duncan’s multiple range tests. Significance was accepted at P ≤ 0.05.
Results and discussion
Effect of gamma irradiation and/or cooking on Chemical composition of faba bean seeds
Table 1 shows the values of proximate composition (%) of treated and untreated faba beans seeds for both cultivars. The results showed that there were no significant (P ≤ 0.05) differences in dry matter, ash and fiber contents between the treated and untreated seeds, however, the protein and fat contents were increased slightly for both cultivars after treatments. The results obtained are in agreement with those of previous work done by Bhattacharjeea et al. (2003) who found that irradiation with 0.25, 0.5, 0.75 and 1.0 kGy had no significant effect on the proximate composition of cashew nuts. Additionally, Inayatullah et al. (1987) found that irradiation of soybean with 0.25, 0.5, 1.0, 2.5 and 5 kGy had no significant effect on the water, fat, ash and carbohydrate contents.
Table 1.
Effects of gamma irradiation and/or cooking on chemical composition (%) of faba bean cultivars
| Cultivars | Irradiation Dose kGy | Dry matter | Ash | Protein | Fat | Crude fiber | Carbohydrates | Energy kcal | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | ||
| BB7- S1 | 0 | 97.0 ± 0.38 a | 97.9 ± 0.0 a | 3.9 ± 0.07 a | 4.3 ± 0.12 a | 32.2 ± 6.00 a | 37.7 ± 1.19 b | 1.4 ± 0.49 a | 2.4 ± 0.48 b | 7.6 ± 0.10 a | 11.8 ± 0.98 c | 58.4 ± 0.91 a | 43.8 ± 0.61 d | 360.8 ± 2.9 a | 347.6 ± 5.47 c |
| 0.5 | 97.4 ± 0.37 a | 97.9 ± 1.43 a | 3.9 ± 0.09 a | 3.9 ± 0.04 a | 35.9 ± 1.03 a | 30.8 ± 3.70 a | 2.8 ± 0.18 b | 1.6 ± 0.10 a | 7.6 ± 0.27 a | 8.1 ± 0.55 b | 49.6 ± 0.96 c | 53.6 ± 3.43 b | 367.7 ± 1.75 a | 360.2 ± 2.25 a | |
| 1.0 | 97.9 ± 0.14 a | 96.2 ± 0.34 a | 3.9 ± 0.30 a | 3.8 ± 0.11 b | 36.3 ± 0.60 b | 36.3 ± 0.60 b | 2.5 ± 0.31 b | 1.6 ± 0.10 b | 7.1 ± 1.04 a | 8.7 ± 0.08 b | 50.2 ± 0.66 bc | 52.0 ± 1.13 b | 368.8 ± 5.40 a | 357.8 ± 0.93 b | |
| SH- S2 | 0 | 97.3 ± 0.28 a | 97.9 ± 0.0.0 a | 3.7 ± 0.08 a | 3.5 ± 0.19 a | 32.2 ± 2.14 a | 36.4 ± 0.56 b | 1.9 ± 0.48 a | 1.6 ± 0.11 b | 5.6 ± 0.58 a | 8.5 ± 1.60 b | 56.7 ± 2.49 a | 49.2 ± 2.64 a | 372.5 ± 3.9 a | 364.2 ± 10.9 a |
| 0.5 | 97.9 ± 0.17 a | 96.6 ± 0.10 a | 3.7 ± 0.07 a | 3.6 ± 0.23 a | 33.6 ± 0.60 a | 35.6 ± 0.55 b | 2.7 ± 0.68 c | 1.3 ± 0.15 b | 8.5 ± 0.32 b | 7.7 ± 1.14 b | 51.5 ± 1.22 a | 50.2 ± 3.32 a | 366.3 ± 3.3 a | 368.7 ± 14.0 a | |
| 1.0 | 98.5 ± 0.27 a | 97.9 ± 1.19 a | 3.7 ± 0.04 a | 3.7 ± 0.12 a | 35.5 ± 0.54 b | 33.5 ± 0.77 a | 1.7 ± 0.23 b | 2.8 ± 0.20 c | 7.5 ± 0.65 b | 7.7 ± 0.40 b | 51.3 ± 0.39 a | 52.1 ± 1.02 a | 363.5 ± 3.6 a | 367.9 ± 1.6 a | |
Values are means ± SD (n = 3)
Values not sharing a common superscript in column significantly P ≤ 0.05 different
Table 2 shows the contents of calcium (Ca), magnesium (Mg), phosphorus (P) and iron (Fe) of faba bean seeds as affected by gamma irradiation and/or cooking. For both cultivars, the results indicated that gamma irradiation and/or cooking treatments of the seeds caused no significant (P ≤ 0.05) changes in Ca, Mg and Fe contents, while they caused slight fluctuations on the P contents (Table 2). These results revealed that gamma irradiation and/or cooking treatments of faba bean seeds have a minor effect on the total minerals contents. It was reported that during irradiation treatments at low doses up to 2 kGy the major and trace minerals do not suffer any significant (P ≤ 0.05) changes especially in legumes and cereals (Hassan et al. 2009). However, Al-Bachir and Laham (2002) reported that high doses of gamma irradiation 5, 10, 15 and 20 kGy caused a significant reduction on sodium, potassium and calcium contents of liquorice roots flour.
Table 2.
Effects of gamma irradiation and/or cooking on mineral content (mg/g) of faba bean cultivars
| Cultivars | Irradiation Dose kGy | Ca | Mg | P | Fe | ||||
|---|---|---|---|---|---|---|---|---|---|
| irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | irradiated | Irradiated/cooked | ||
| BB7- S1 | 0 | 4.7 ± 0.23 a | 3.7 ± 0.13 a | 7.0 ± 0.26 a | 7.3 ± 0.15 a | 4.45 ± 0.21 a | 5.66 ± 0.2 c | 0.05 ± 0.005 a | 0.039 ± 0.003 a |
| 0.5 | 4.2 ± 0.19 a | 3.9 ± 0.09 a | 7.0 ± 0.22 a | 9.0 ± 0.36 a | 3.72 ± 0.09 b | 5.17 ± 0.69 c | 0.042 ± 0.04 a | 0.048 ± 0.001 a | |
| 1.0 | 4.0 ± 0.9 a | 2.9 ± 0.10 a | 5.1 ± 0.10 a | 8.0 ± 0.05 a | 4.41 ± 0.0 a | 5.17 ± 0.6 c | 0.044 ± 0.002 a | 0.048 ± 00.03 a | |
| SH- S2 | 0 | 2.5 ± 0.5 a | 2.0 ± 0.12 a | 8.8 ± 0.21 a | 7.2 ± 0.18 a | 6.56 ± 0.26 a | 3.45 ± 0.15 b | 0.044 ± 0.003 a | 0.038 ± 0.001 a |
| 0.5 | 3.5 ± 0.5 a | 3.0 ± 0.13 a | 5.4 ± 0.34 a | 7.7 ± 0.42 a | 6.06 ± 0.13 a | 3.12 ± 0.12 b | 0.043 ± 0.003 a | 0.035 ± 0.001 a | |
| 1.0 | 2.3 ± 0.02 a | 2.2 ± 0.07 a | 7.2 ± 0.20 a | 12.4 ± 0.11 a | 5.20 ± 0.33 a | 9.30 ± 0.48 c | 0.043 ± 0.005 a | 0.047 ± 0.001 a | |
Values are means ± SD (n = 3)
Values not sharing a common superscript in column significantly P ≤ 0.05 different
Effect of gamma irradiation and/or cooking on Tannin and Phytic acid contents of faba bean seeds
The contents of tannin and phytic acids of faba bean seeds as affected by gamma irradiation and/or cooking were described in Fig. 1a and b. As shown in Fig. 1a, tannin content of BB7-S1 cultivar was found 4.13 mg/g, while that of SH-S2 was 3.10 mg/g. For both cultivars, gamma irradiation treatments of the seeds gradually reduced the tannin content. Results obtained were agreed with those obtained by Villavicencio et al. (2000) who found that the amount of catechin in un-irradiated uncooked beans was 0.18% mEq, while it was 0.16%, 0.14% and 0.14% mEq when the samples were irradiated at 2.5, 5 and 10 kGy, respectively. Hassan et al. (2009) reported that dose of 2 kGy had no significant change in tannin content of two maize cultivars. Cooking of the faba bean seeds significantly (P ≤ 0.05) reduced tannin contents for both cultivars to 2.70 mg/g for SH-S2 cultivars (Fig. 1a). Elsheikh et al. (2000) reported that cooking decreased 50% of tannin content of faba bean. Cooking of irradiated seeds at doses 0.5 and 1 kGy insignificantly (P ≤ 0.05) reduced tannin content for both cultivars, it was found to be 2.09 & 2.02 mg/g for BB7- S1 and to 2.40 & 1.70 mg/g for SH-S2 cultivar (Fig. 1a). The same observation was reported by Brigide and Canniatti-Brazaca (2006) who concluded irradiation process with a 6 kGy dose showed a reduction in the antinutritional factors such as tannin in cooked beans.
Fig. 1.
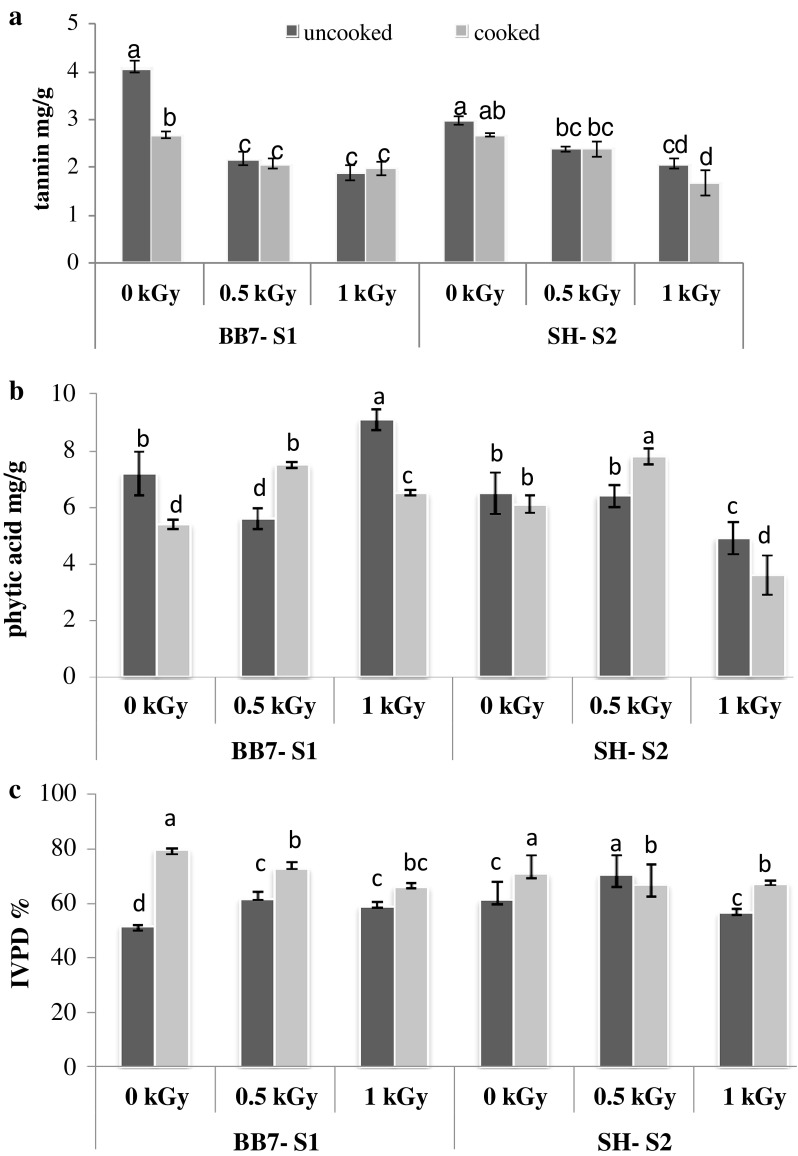
Effects of gamma irradiation (kGy) and/or cooking on (a) tannin content (mg/g), (b) phytic acid content (mg/g) and (c) in vitro protein digestibility (IVPD) of faba bean cultivars (BB7-S1 and SH-S2). Error bars indicate the standard deviation (n = 3). For each cultivar, values not sharing a common superscript are significantly (P ≤ 0.05) different
Phytic acid content (Fig. 1b) of faba bean seeds was found 7.2 and 6.5 mg/g for BB7-S1 and SH-S2, respectively. Gamma irradiation treatments with doses 0.5 and 1.0 kGy significantly (P ≤ 0.05) reduced the phytic acid contents for SH-S2 cultivars (Fig. 1b). For both faba bean cultivars BB7- S1 and SH-S2, cooking of the seeds reduced phytic acid content to 5.40 and 6.08, respectively. Gamma irradiated at dose 0.5 kGy followed by cooking slightly increased the phytic acid to 7.47 and 7.78 mg/g for BB7- S1 and SH-S2 cultivars, respectively, while gamma irradiated at dose 1.0 kGy followed by cooking decreased phytic acid for both cultivars (Fig. 1b). Duodu et al. (1999) reported that cooking and gamma irradiation treatments of sorghum grains caused significant reduction in phytic acid levels. Similarly, gamma irradiation alone or in combination with soaking of soybean reduced the level of phytate compared to untreated seeds (Sattar et al. 1990). This reduction is likely to be due to chemical degradation of phytate to lower inositol phosphates and inositol by the action of free radicals produced by radiation (De Boland et al. 1975). Moreover, a possible way of phytate reduction during irradiation may be due to cleavage of the phytate ring itself.
Effect of gamma irradiation and/or cooking on In vitro protein digestibility of faba bean seeds
Figure 1c shows the effect of gamma radiation and/or cooking on pepsin in vitro protein digestibility (IVPD) of two faba bean cultivars. The IVPD was found to be 51.8 and 61.2% for BB7-S1 and SH-S1, respectively. Irradiation treatments of the seeds significantly (P ≤ 0.05) increased IVPD for both cultivars. Similar findings were observed by Mostafa (1987) who reported that the in vitro protein digestibility of groundnut flour treated with gamma irradiation of dose up to 3.5 kGy was higher than that of non-irradiated one. Additionally, the results show that cooking of the seeds caused a significant (P ≤ 0.05) increment in IVPD. It was found to be 79.97 and 70.92% for BB7-S1 and SH-S2, respectively. The results obtained indicated that cooking of legumes significantly affected the IVPD. This could be attributed to inactivation of antinutritional factors such as tannin and phytate. Abusin et al. (2009) reported that cooking caused about 10 and 20% increment in the IVPD of faba bean and white bean, respectively. Cooking of irradiated seeds at 0.5 and 1.0 kGy increased the IVPD to 72.9 and 66.0% for BB7-S1 and to 66.9 and 67.4% for SH-S2 (Fig. 1c). The improvement in protein digestibility after irradiation may be attributed to the decline of protein inhibitors activity (Joseph and Dikshit 1993). Moreover, it was reported that gamma irradiation can disrupts non-covalent bonds such as hydrogen and disulphide bonds that stabilize protein structure, by generating free radicals that attack protein molecules (Swallow 1991), breaking of these bonds may lead to a loss of conformational or structural integrity of protein that exposed additional peptide bonds and enhance proteolysis and hence improve its digestibility (Koppelman et al. 2005).
Conclusion
The results obtained in this study revealed that low doses of gamma irradiation (0.5 and 1.0 kGy) and/or cooking treatments of faba bean seeds caused no significant (P ≤ 0.05) changes in the chemical composition and mineral contents. However, at these low doses of gamma irradiation and/or cooking, the contents of antinutritional factors such as tannin and phytic acid were significantly reduced, whereas the in vitro protein digestibility significantly increased in faba bean seeds for both cultivars
References
- Abusin SAE, Hassan AB, Babiker EE. Nutritional evaluation of cooked Faba Bean (Vicia Faba L.) and White Bean (Phaseolus Vulgaris L.) Cultivars. Aust J Basic Appl Sci. 2009;3:2484–2490. [Google Scholar]
- Al-Bachir M, Laham G. The effect of gamma irradiation on the microbial load, mineral concentration and sensory characteristics of liquorice (Glycyrrhiza glabra L) J Sci Food Agric. 2002;83:170–175. [Google Scholar]
- Wholesomeness of irradiated food. Report of a Joint FAO/IAEA/WHO Expert committee. Technical report series 659. Geneva: World Health Organization; 1981. [PubMed] [Google Scholar]
- Analytical detection methods for irradiated foods. A review of the current literature. IAEA-TECDOC-587. Vienna: IAEA; 1991. [Google Scholar]
- Official methods of analysis of the Association of Official Analytical Chemists. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Bhattacharjeea P, Rekha S, Gholapb SA, Variyarb S, Dilip R. Compositional profiles of gamma-irradiated cashew nuts. Food Chem. 2003;80:159–163. doi: 10.1016/S0308-8146(02)00248-0. [DOI] [Google Scholar]
- Bressani R. Grain quality of common beans. Food Rev Int. 1993;9:217–297. doi: 10.1080/87559129309540960. [DOI] [Google Scholar]
- Brigide P, Canniatti-Brazaca SG. Antinutrients and “in vitro” availability of iron in irradiated common beans (Phaseolus vulgaris) Food Chem. 2006;98:85–89. doi: 10.1016/j.foodchem.2005.05.054. [DOI] [Google Scholar]
- Chapman HD, Pratt PF (1982) Method for the Analysis of Soil, Plant And Water 2nd ed California University Agricultural Division, California, pp. 170
- Dario AC, Salgado JM. Effect of thermal treatments on the chemical and biological value of irradiated and non-irradiated cowpea bean (Vigna unguiculata L.Walp.) flours. Plant Foods Hum Nutr. 1994;46:181–186. doi: 10.1007/BF01088771. [DOI] [PubMed] [Google Scholar]
- De Boland AR, Garner GB, O’Dell BL. Identification and properties of ‘phytate’ in cereal grains and oil-seed products. J Agri Food Chem. 1975;23:1186–1189. doi: 10.1021/jf60202a038. [DOI] [PubMed] [Google Scholar]
- Dogbevi MK, Vachon C, Lacroix M. Effect of gamma irradiation on the microbiological quality and on the functional properties of proteins in dry red kidney beans (Phaseolus vulgaris) Radiat Phys Chem. 2000;57:265–268. doi: 10.1016/S0969-806X(99)00442-9. [DOI] [Google Scholar]
- Duodu KG, Minnaar A, Taylor JRN. Effect of cooking and irradiation on the labile vitamins and antinutrient content of a traditional african sorghum porridge and spinach relish. Food Chem. 1999;66:21–27. doi: 10.1016/S0308-8146(98)00070-3. [DOI] [Google Scholar]
- Elsheikh EA, Fadul IA, El Tinay AH. Effect of cooking on anti-nutrition factors and in vitro protein digestibility (IVPD) of faba bean grown with different nutrional regimes. Food Chem. 2000;68:211–212. doi: 10.1016/S0308-8146(99)00153-3. [DOI] [Google Scholar]
- FAO/IAEA (1997) Technology transfer of food irradiation to reduce post-harvest food losses in Africa. Second FAO/IAEA Research Co-ordination Meeting on the Co-ordinated Research Programme, Tangier, Morocco
- Farag MDEH. Radiation deactivation of antinutritional factors: trypsin inhibitor and hemagglutinin in soybeans. Egypt J Radiat Sci Appl. 1989;6:207–215. [Google Scholar]
- Hassan AB, Osman GAM, Rushdi MA, Eltayeb MM, Diab EE. Effect of Gamma Irradiation on the Nutritional Quality of Maize Cultivars (Zea mays) and Sorghum (Sorghum bicolor) Grains. Pak J Nutr. 2009;8:167–171. doi: 10.3923/pjn.2009.167.171. [DOI] [Google Scholar]
- Inayatullah H, Zeb A, Ahmad M, Khan I. Effect of gamma irradiation on physico-chemical characteristics of soybean. Nucleus Karachi, Pakistan. 1987;24:31–34. [Google Scholar]
- Joseph A, Dikshit M. Effect of irradiation on the proteinase inhibitor activity and digestibility (in vitro) of safflower oil cake. J Am Oil Chem Soc. 1993;70:935–937. doi: 10.1007/BF02545359. [DOI] [Google Scholar]
- Kataria A, Chauhan BM, Puria D. Anti-nutrients and protein digestibility (in vitro) of Mung bean as affected by domestic processing and cooking. Food Chem. 1989;32:9–17. doi: 10.1016/0308-8146(89)90003-4. [DOI] [Google Scholar]
- Koppelman S, Nieuwenh WF, Gaspari M, Knippels LMJ, Pennincs AH, Knol EF, Hefle SL, De Jongh HJ. Revsrsible denaturation of Brazil nut 2S albumins and implications and destabilization on digesion by pepsin. J Agric Food Chem. 2005;53:132–132. doi: 10.1021/jf0491355. [DOI] [PubMed] [Google Scholar]
- Liener IE. Antinutritional factors related to proteins and amino acids. In: Hui YH, Gorham JR, Murrel KD, Cliver DO, editors. Foodborne disease handbook, (vol. 3) New York: Marcel Dekker Inc; 1994. pp. 261–309. [Google Scholar]
- Loaharanu P. Food irradiation in developing courtiers: a practical alternative. IAEA Bull. 1994;36:30–35. [Google Scholar]
- Maliwal BP. In vitro method to assess the nutritive value of leaf concentrate. J Agric Food Chem. 1983;31:315–319. doi: 10.1021/jf00116a033. [DOI] [PubMed] [Google Scholar]
- Monjula S, John E. Biochemical changes and in vitro protein digestibility of endosperm of germinating Dolichos lablab. J Sci Food Agric. 1991;55:229–233. doi: 10.1002/jsfa.2740550208. [DOI] [Google Scholar]
- Mostafa MM. Nutritional aspects of thermal and irradiation processing of peanut kernels and their oil. Food Chem. 1987;26:31–45. doi: 10.1016/0308-8146(87)90165-8. [DOI] [Google Scholar]
- Müller HG, Tobin G. Nutrition and food processing. London: Croom Helm Ltd; 1980. p. 302. [Google Scholar]
- Pearson D (1981) Pearson’s chemical analysis of foods. In: Egan H, Kirk RS, Sawyer R (eds) 18th edn, London, New York
- Price ML, Van Socoyoc S, Butter LG. Acritical evaluation of the vanillin reaction as an assay for tannin in sorghumgrain. Agric Food Chem. 1978;26:1214–1218. doi: 10.1021/jf60219a031. [DOI] [Google Scholar]
- Rahma EH, Mostafa MM. Functional properties of peanut flour as affected by different heat treatments. J Food Sci Technol. 1988;25:11–15. [Google Scholar]
- Sathe SK, Salunkhe DK. Technology of removal of unwanted components of dry beans. Crit Rev Food Sci Nutr. 1994;21:263–287. doi: 10.1080/10408398409527402. [DOI] [PubMed] [Google Scholar]
- Sattar A, Neelofar X, Akhtar MA. Effect of radiation and soaking on phytate content of soybean. Acta Aliment. 1990;19:331–336. [Google Scholar]
- Siddhuraju P, Becker K. Effect of various indigenous processing methods on alpha-galactoside, mono-and disaccharide content of an Indian tribal pulse, Mucuna pruriens var. utilis. J Sci Food Agr. 2001;81:718–725. doi: 10.1002/jsfa.875. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Effect of various domestic processing methods on antinutrients and in vitro protein and starch digestibility of two indigenous varieties of Indian tribal pulse, Mucuna pruriens var. utilis. J Agr Food Chem. 2001;49:3058–3067. doi: 10.1021/jf001453q. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K, Makkar HPS. Studies on the nutritional composition and antinutritional factors of three different germplasm seed materials of an underutilized tropical legume, Mucuna pruriens var. utilis. J Agric Food Chem. 2000;48:6048–6060. doi: 10.1021/jf0006630. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG (1987) Statistical methods, 7th edn. The Iowa State University Press Ames, I. A., USA. 221–230
- Sukker MY. Human nutrition for medical studies and applied health science. London: Ithaca Press; 1985. [Google Scholar]
- Swallow AJ. Effect of irradiation on food protein. In: Hudson BJF, editor. Development in food protein 7. London: Elsevier; 1991. pp. 195–229. [Google Scholar]
- Villavicencio ALH, Mancini-Filho J, Delinceé H. Effect of irradiation on anti-nutrients (total phenolics, tannins and phytate) in Brazilian beans. Radiat Phys Chem. 2000;57:289–293. doi: 10.1016/S0969-806X(99)00393-X. [DOI] [Google Scholar]
- Wheeler EI, Ferrel RE. Methods for phytic acid determination in wheat and wheat fractions. Cereal Chem. 1971;48:312–320. [Google Scholar]
- WHO (1999) High-dose irradiation: Wholesomeness of food irradiated with doses above 10 kGy. Report of a Joint FAO/IAEA/WHO Study Group. WHO Tech Rep Ser 890: I-vi: 1–197 [PubMed]


