Abstract
Coriandrum sativum L. (coriander), an everyday spice in the Indian kitchen is known to add flavor to the cuisine. It is an annual herb belonging to the Apiaceae (Umbellifera) family. The hydro-alcohol extract of Coriandrum sativum L. at the dose of 1 mg/ml was subjected to a series of in vitro assays viz. 2, 2′- diphenyl-1-picrylhydrazyl, lipid peroxidation by thiobarbituric acid, reducing power and nitric oxide (NO) radical scavenging in order to study its antioxidant efficacy in detail. The amount of flavonoids in 70% ethanol extract was found to be 44.5 μg and that of the total phenols was 133.74 μg gallic acid equivalents per mg extract. The extracts of the leaves showed metal chelating power, with IC50 values, 368.12 μg/ml where as that of standard EDTA was 26.7 μg/ml. The IC50 values for 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid radical scavenging was 222 μg/ml where as that of standard ascorbic acid was 22.6 μg/ml. The NO scavenging activity of the extract of the leaves showed IC50 value of 815.6 μg/ml; at the same time the standard BHA had 49.1 μg/ml. All the plant extracts provided DNA damage protection; however, the protection provided at the dose of 8 μg/ml was comparable to that of standard gallic acid. The Coriandrum sativum leaf extract was able to prevent in vitro lipid peroxidation with IC50 values; 589.6 μg/ml where as that of standard BHA was 16.3 μg/ml. Our results also showed significant ferric reducing power indicating the hydrogen donating ability of the extract. This study indicated the potential of the leaf extract as a source of natural antioxidants or nutraceuticals that could be of use in food industry with potential application to reduce oxidative stress in living system.
Keywords: Coriandrum sativum, Antioxidant, DNA damage, DPPH, ABTS, Reactive oxygen species
Introduction
Free radicals are types of Reactive oxygen species (ROS), which include all highly reactive, oxygen-containing molecules. Types of ROS include the hydroxyl radical, the super oxide anion radical, hydrogen peroxide, singlet oxygen, nitric oxide radical, hypochlorite radical, and various lipid peroxides. All these are capable of reacting with membrane lipids, nucleic acids, proteins and enzymes and other small molecules, resulting in cellular damage (Shahidi and Wanasundara 1992; Buyukokuroglu et al. 2001).Cell damage caused by free radicals appears to be a major contributor to aging and degenerative diseases of aging such as cancer, cardiovascular disease (Halliwell and Gutteridge 1984; Ames et al. 1993; Harman 1995; Halliwell 1997), cataract, immune system decline, liver diseases, diabetes mellitus, inflammation, renal failure, brain dysfunction, etc. (Miller 1996; Halliwell 1997; Kowaltowski and Vercesi1999). Antioxidants are absolutely critical for maintaining optimal cellular and systemic health and well-being.
Coriandrum sativum L. (coriander) is an annual herb belonging to the Apiaceae (Umbellifera) family (Evans et al. 2002). Different parts of the plant, including the fruits and the green herbs, are used for medicinal purposes such as dyspeptic complaints and loss of appetite (Blumenthal et al. 2000). Pharmacological studies in animals have shown that coriander has anti-diabetic (Swanston-Flatt et al. 1990; Gray and Flatt 1999), hypolipidemic, (Chithra and Leelamma 1997; 1999) and anti-cancer effects (Chithra and Leelamma 2000). Sedative-hypnotic activity of coriander seeds have been evaluated in scientific studies in mice (Emamghoreishi et al. 2005). Linalool, the main monoterpenoids of coriander seeds is shown to have sedative and anticonvulsant activity in animal studies and anxiolytic and sedative activity in human studies (Karmakar et al. 2011). Report also states the in vivo antioxidant activities of coriander seed (Anilakumar et al. 2001; 2007; 2009; 2010). However, the studies on in vitro antioxidant and DNA damage protection properties of Coriandrum sativum leaves are sparse. Hence this work was taken up to evaluate the antioxidant activities of Coriandrum sativum leaves with a battery of in vitro assays.
Materials and methods
Plant material
Coriandrum sativum L. (coriander) was collected from local market of Mysore, India. The Coriandrum sativum leaves were washed and with clean distilled water, and allowed to dry in shade for three days in order to have the moisture content of 7.0%. This plant material was taken for further studies.
Chemicals and reagents
Ethanol and distilled water were used as solvent for extraction of antioxidant compounds. H2SO4, NaOH, HCl, DPPH, BHA, gallic acid, Folin-Ciocalteu reagent, FeCl2, ferrozine, potassium ferricyanide, EDTA, ascorbic acid, TPTZ(2,4,6,-tripyridy-striazine), TCA, FeCl3, Na2CO3, catechin, thiobarbituric acid (TBA) were of analytical grade and were stored at prescribed conditions in the laboratory.
Extraction
150 g of crushed plant material was used for extraction. This sample was soaked overnight in 70% ethyl alcohol and repeated extraction was carried out by adding fresh solvent every time, total for 72 h. The pooled extract was subjected to filtration through normal filter paper followed by Whatman No.1. This filtered 70% alcohol extract was subjected to flash evaporator followed by lyophilization. The lyophilized samples were analyzed further for its antioxidant activities by various in vitro assays.
Estimation of total flavonoid content
Total flavonoid content was determined as described by Zou et al. (2004). 0.25 ml of various extracts was diluted with 1.25 ml of distilled water. 75 μl of a 5% NaNO2 solution was added followed by 150 μl of a 10% AlCl3.H2O after 6 min. After 5 min, 0.5 ml of 1 M NaOH was added to this mixture. The absorbance was measured immediately against the prepared blank at 510 nm. Catechin was used as a standard and the results were expressed as mg of catechin equivalents (CE) per mg of dry extract.
Determination of total phenols
Total phenol content was determined by the method adapted from Singleton and Rossi (1965) with some modifications using the Folin–Ciocalteu reagent. 1 ml of the extract was mixed with 1 ml of Folin–Ciocalteu’s phenol reagent. After 3 min, 1 ml of saturated Na2CO3 (35%) was added to the mixture and it was made up to 10 ml by adding deionised water. The mixture was kept for 90 min at room temperature in the dark. The absorbance was measured at 725 nm against the blank. The total phenolic content was expressed as mg of gallic acid equivalents (GAE) per mg of dry extract.
DPPH radical scavenging activity
The scavenging effect of leaf extracts on DPPH radicals was determined according to the method of Hatano et al. (1988). Various concentrations of sample (4 ml) were mixed with 1 ml of methanolic solution containing DPPH radicals, resulting in the final concentration of DPPH being 0.2 mM. The mixture was shaken vigorously and left to stand for 30 min, and the absorbance was measured at 517 nm.
Determination of reducing power
The reducing power of leaves extracts was determined according to the method of Oyaizu (1986). 2.5 ml of various concentrations of the extract, 2.5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide were mixed and incubated at 50 °C for 20 min and centrifuged for 10 min at 800 x g after addition of 2.5 ml of 10% trichloroacetic acid. 2.5 ml aliquot of supernatant was mixed with 2.5 ml of deionised water and 0.5 ml of 0.1% ferric chloride. After 10 min of incubation, the absorbance was measured at 700 nm against a blank.
Chelating effects on ferrous ions
The ability of the leaf extracts to chelate ferrous ions was estimated by the method of Dinis et al. (1994). Briefly, 2 ml of various concentrations of the extracts in methanol were added to a solution of 2 mM FeCl2 (0.05 ml). The reaction was initiated by the addition of 5 mM ferrozine (0.2 ml). The mixture was shaken vigorously and left at room temperature for 10 min. The absorbance of the solution was measured spectrophotometrically at 562 nm.
ABTS radical cation scavenging activity
The ABTS (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) cation radical scavenging activity was performed with slight modifications described by Roberta et al. (1999). The ABTS+ radicals were produced by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulphate, stored in the dark at room temperature for 12 h. Prior to use, the solution was diluted with ethanol to get an absorbance of 0.700 ± 0.025 at 734 nm. Free radical scavenging activity was assessed by mixing 10 μl of test sample with 1.0 ml of ABTS working standard in a microcuvette. The decrease in absorbance was measured exactly after 6 min.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was used to estimate the reducing capacity of leaves extracts, according to the method of Benzie and Strain (1996). The FRAP reagent contained 2.5 ml of a 10 mM TPTZ solution in 40 mM HCl, 2.5 ml of 20 mM FeCl3.6H2O and 25 ml of 300 mM acetate buffer (pH 3.6). It was freshly prepared and warmed at 37 °C. 900 μl FRAP reagent was mixed with 90 μl water and 30 μl of the extract. The reaction mixture was incubated at 37 °C for 30 min and the absorbance was measured at 593 nm.
Nitric oxide scavenging activity
Nitric oxide (NO) was generated from sodium nitroprusside (SNP) and was measured by the Griess reagent. SNP in aqueous solution at physiological pH spontaneously generates NO radical which interacts with oxygen to produce nitrite ions that can be estimated by the use of Griess Reagent. SNP (10 mM) in phosphate buffer saline (PBS) was mixed with different concentration of extract (1000 μg/ml) of the drug dissolved in ethanol and water and incubated at 25 °C for 180 min. The samples from the above were reacted with Griess reagent (1% sulphanilamide, 0.1% naphthylethylenediamine dichloride and 3% phosphoric acid). The absorbance of the chromophores formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with naphthylethylenediamine dichloride was read at 546 nm and referred to the absorbance of ascorbic acid, used as a positive control treated in the same way with Griess reagent (Marcoci et al. 1994).
DNA damage protective activity
DNA damage preventive property of Coriandrum sativum was checked using pBR322 plasmid. Plasmid was treated with 2, 2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH), the radical inducer in presence of the leaf extract and checked on 1% agarose according to Russo et al. (2003) with minor modifications. In brief, the experiment was performed using a micro centrifuge tube containing 200 ng of plasmid pBR322 DNA. AAPH was added at final concentration of 200 mM/ml with various concentrations of leaf extracts. Along with leaf extract, a known antioxidant such as gallic acid was also used at the concentration of 100 μM. The reactions were initiated by incubating the tubes for 60 min in incubator. After incubation the reaction mixture along with gel loading dye (6x) was loaded on to 1% agarose gel and run at 200v for 1 h. Untreated pBR322 plasmid DNA was used as a positive control in each run of gel electrophoresis.
In vitro anti lipid peroxidation
The anti lipid peroxidation assay was estimated by Liu et al. (1997) with modification. Normal male rats (250 g) were used for the preparation of liver homogenate. A 10% (w/v) rat liver sample homogenate was prepared with homogenizer at 0–4 °C with 0.15 M KCl. The homogenate was centrifuged at 900 x g for 15 min, and clear cell-free supernatant was used for the study with in vitro lipid peroxidation assay. Different concentrations (0–1000 μg/ml) of extract and standard BHA 0–100 μg/ml were added in test tubes and 1 ml of 0.15 M KCl followed by 0.5 ml of rat liver homogenates were added. Peroxidation was initiated by adding 1000 μl of 200 μM AAPH. After incubation at 37 °C for 30 min, the reaction was stopped by adding 15% TCA, 0.38% TBA, and 0.5% BHT. The reaction mixtures were heated at 80 °C for 60 min. The samples were cooled and centrifuged and the absorbance of the supernatant was measured at 532 nm.
Calculation
The percentage inhibition for all the above methods was calculated according to the formula:
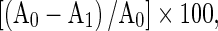 |
Where A0 was the absorbance of the control and A1 was the absorbance of the sample.
IC50 value was determined from the plotted graph of scavenging activity using the formula y = m x + c against the different concentrations of extracts of Coriandrum sativum L. which is defined as the total antioxidant necessary to inhibit radicals by 50%. The measurements were triplicated and their scavenging effect was calculated based on the percentage inhibition.
Statistical analysis
All assays were carried out in triplicates. IC50 values were calculated using regression equation in excel programme.
Results and discussion
Antioxidant methods and modifications have been proposed to evaluate antioxidant characteristics and to explain how antioxidants function. Of these, reducing power, metal chelation, free radical scavenging, lipid peroxidation and DNA damage protection activities are the most commonly used for the evaluation of the total antioxidant behavior of extracts (Amarowicz et al. 2000; Chang et al. 2002). Studies have shown that 70% ethanol extract of plant materials contain ethanol and water soluble bioactive compounds possessing antioxidant property (Kahkonen et al. 1999; Ivanova et al. 2005). Hence in this study, 70% ethanol was used for the extraction.IC50 values were calculated.
Flavonoids
Flavonoids are a class of secondary plant phenolics with powerful antioxidant properties. Therefore it is valuable to determine the total flavonoids content of the extracts in the study. The amount of flavonoids in 70% ethanol extract was found to be 44.5 μg of catechin equivalent/mg extract. Several studies have shown that many flavonoids contribute significantly to the total antioxidant activity of plants (Zhonghong et al. 1999; Anilakumar et al. 2007; Vauzour, et al. 2008). There is abundant evidence that flavonoids are effective in blocking oxidant induced neuronal injury (Jeremy and Spencer 2009).
Total polyphenols
Phenolic constituents are very important in plants because of their scavenging ability due to their hydroxyl groups (Hatano et al. 1989). Phenolic compounds in plants constitute a major class of secondary plant metabolites with bioactive attributed to antioxidant and anti bacterial activities. The total phenols in the 70% ethanol extract of the leaf were 133.74 μg gallic acid equivalent/mg extract. These phenolic compounds may contribute directly to the antioxidative action. It has been suggested that up to 1.0 g polyphenolic compounds (from a diet rich in fruits and vegetables) ingested daily have inhibitory effects on mutagenesis and carcinogenesis in humans (Tanaka et al. 1998). In addition, it has been reported that phenolic compounds are associated with antioxidant activity and play an important role in stabilizing lipid peroxidation (Yen et al. 1993).
DPPH scavenging ability
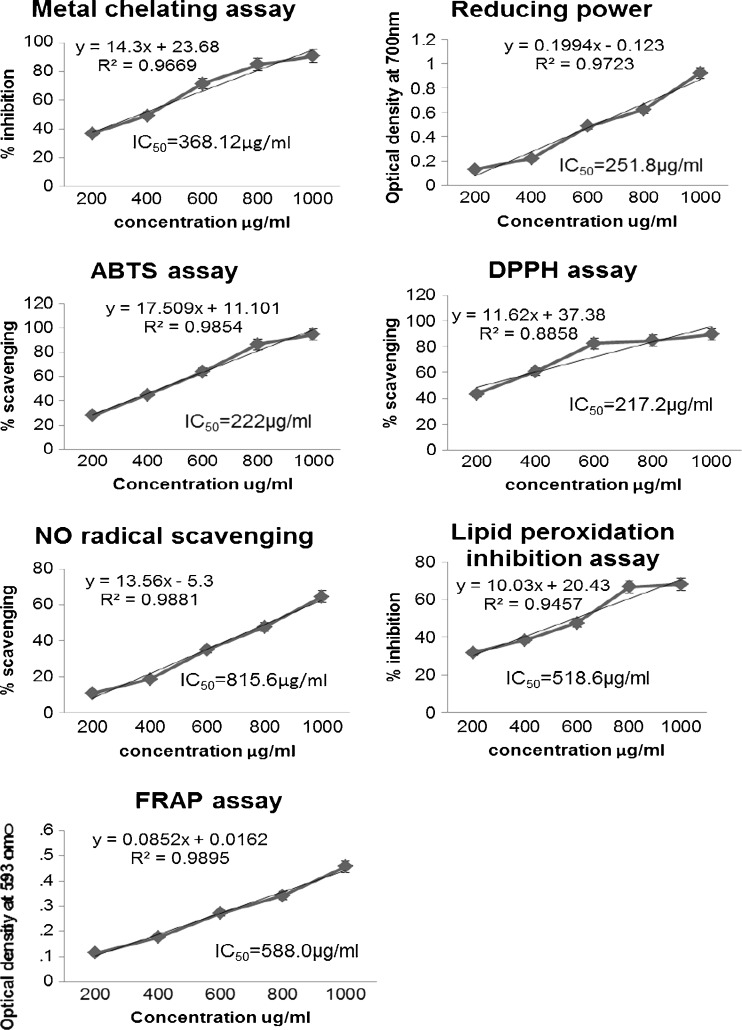
The stable DPPH radical model is a widely-used, relatively quick method for the evaluation of free radical scavenging activity. The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability (Baumann et al. 1979). DPPH· is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule (Soares et al. 1997). Because of its odd electron, DPPH. gives a strong absorption maximum at 517 nm by visible spectroscopy. As the odd electron of the radical becomes paired off in the presence of a hydrogen donor, that is, a free radical scavenging antioxidant, the absorption strength is decreased and the resulting decolorization is stoichiometric with respect to the number of electrons captured (Blios. 1958; Vani et al. 1997). Therefore when the above extracts were tested, the ethanolic extract of Coriandrum sativum leaves showed DPPH· scavenging activity, with IC50 values of 217.2 μg/ml which is more than that of standard BHA, with 4.5 μg/ml (Fig. 1).
Fig. 1.
Effect of Coriandrum sativum leaf extract (0–1000 μg/ml) on an array of in vitro antioxidant assays (n = 3)
Reducing power
This method is based on the principle of increase in the absorbance of the reaction mixture. The increase in the absorbance indicates increase in the antioxidant activity and in turn, the increase in absorbance of the reaction mixture indicates the reducing power of the samples (Jayaprakash et al. 2001). There is a direct correlation between antioxidant activity and reducing power of plant extract. The reducing properties are generally associated with the presence of reductones (Duh 1998), which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Gordon 1990). Our data on the reducing power tested extract suggest that it is likely to contribute significantly towards the observed antioxidant effect. There was also dose dependent increase in the absorbance with 70% ethanol extract of the leaves (Fig. 1).
Metal chelating power
The production of highly ROS such as superoxide anion radicals, hydrogen peroxide and hydroxyl radical is catalyzed by free iron through Haber-Weiss reaction. The ferrous ion chelating effect was shown by 70% ethanol extract and EDTA (Tab 1). The ferrous state of iron accelerates lipid oxidation by breaking down hydrogen and lipid peroxides to reactive free radicals via the Fenton reaction. Fe3+ ion also produces radicals from peroxides although the rate is 10-fold less than that of Fe2+ ion. Fe2+ ion is the most powerful pro-oxidant among the various species of metal ions. Ferrozine can quantitatively form complexes with Fe2+. It is reported that chelating agents, who form σ- bonds with metal, are effective as secondary antioxidants because they reduce the redox potential thereby stabilizing the oxidized form of metal ion (Gordon 1990). In the presence of chelating agents, the complex formation is disrupted, resulting in a decrease in the red colour complex. Therefore, measurement of colour reduction allows estimating the metal chelating activity of the co-existing chelator. The extracts of Coriandrum sativum leaves showed metal chelating power, with IC50 values, 368.12 μg/ml where as that of standard EDTA was 26.7 μg/ml (Fig. 1).
ABTS radical scavenging property
It permits the measurement of antioxidant activity of mixtures of substances and hence helps to distinguish between additive and synergistic effects. The assay is based on interaction between antioxidant and ABTS+ radical which has a characteristic color showing maxima at 645, 734 and 815 nm (Vinson and Hontz 1995; Simonetti et al. 1997). Coriandrum sativum leaves extract showed ABTS radical scavenging property, with IC50 values, 222 μg/ml where as that of standard ascorbic acid was 22.6 μg/ml (Fig. 1).
Ferric reducing antioxidant power
FRAP is one of the most rapid test and very useful for routine analysis. The FRAP assay measures the antioxidant effect of any substance in the reaction medium as reducing ability (Perumal and Becker 2007). The antioxidative activity is estimated by measuring the increase in absorbance caused by the formation of ferrous ions from FRAP reagent containing TPTZ and FeCl3.6H2O. Our results showed significant ferric reducing power indicating the hydrogen donating ability of the extract (Fig. 1).
Nitric oxide radical inhibition activity
Nitric oxide (NO) is a labile gaseous free radical with important roles in physiological and pathological conditions. Lui et al. (2001), was found that ischemia-reperfusion injury increased the production of NO and induced the expression of iNOS mRNA in the kidney. Molecular oxygen provided by the blood that flows during reperfusion afer ischemia is converted to the super oxide anion radical. Under these conditions, NO will react rapidly with superoxide anion to form ONOO. The relativities of the free radical NO and superoxide anion were found to be relatively low, but their metabolite ONOO- was extremely reactive and directly induced toxic reactions, including SH- group oxidation, protein tyrosine nitration, lipid peroxidation and DNA modifications (Yermilov et al. 1995; Babu et al. 2001). Coriandrum sativum showed antioxidant activity, with IC50 values of 815.6 μg/ml and standard BHA with 49.1 μg/ml. The coriander leaves extract also showed dose dependent increase in scavenging nitric oxide radical (Fig. 1).
DNA damage protective activity
Oxidative modification of DNA has been suggested to contribute to aging and various diseases including cancer and chronic inflammation. DNA damage protection studies were performed using 70% ethanol extract. All the plant extracts provided DNA damage protection; however, the protection provided at the dose of 8 μg was comparable to that of standard gallic acid.
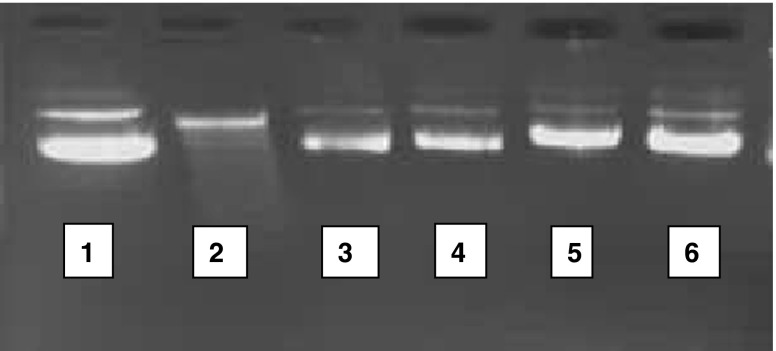
The super coiled form was the predominant band when the normal plasmid DNA pBR322 was run on an agarose gel. Exposure of the DNA to AAPH which induces generation of ROS especially hydroxyl radical and peroxyl radical resulted in DNA cleavage as evident from the loss of the super coiled form of the DNA in the control incubations. It is known that excessive stress can induce DNA damage in the form of oxidized nucleosides, strand breaks, or DNA cross-links (Atamaniuk et al.2004). The coriander extract was able to protect this damage to a large extent (Fig. 2).
Fig. 2.
DNA damage protection assay. 1. control: DNA; 2. + ve control: DNA+AAPH; 3. DNA+AAPH+ 4 μg 70% plant extract; 4. DNA+AAPH+ 8 μg 70% plant extract; 5. DNA+AAPH+ 12 μg 70% plant extract; 6. DNA+AAPH+ 4 μg gallic acid
Prevention of lipid peroxidation
Rat liver homogenate was used and induction of lipid peroxides by AAPH -induced generation of ROS, especially hydroxyl radical and peroxyl radical, which are capable of inducing lipid peroxidation leading to the production of malondialdehyde (MDA) was studied (Okhawa et al. 1979; Kimura et al. 1984; Gutteridge and Wilkins 1983). The Coriandrum sativum leaf extract was able to prevent lipid proxidation showing prevention of lipid peroxidation, with IC50 values, 589.6 μg/ml where as that of standard BHA was 16.3 μg/ml. The assays were conducted up to the concentration of 1 mg/ml because there was saturation in the values (Fig. 1).
Conclusion
In order to characterize antioxidant activity of a plant extract, it is desirable to subject it for the tests that evaluate the range of activities such as scavenging of the reactive oxygen species, metal ion chelation; inhibition of lipid peroxidation and DNA damage protection assay. This study demonstrated a concentration dependent free radical scavenging activity. As part of our ongoing research in to the phytochemistry and antioxidant properties of herbs, spices and plant-derived medicinal preparations, we have investigated a hydro-alcohol extract of Coriandrum sativum leaf of India. Linalool, a water soluble monoterpene may be one of the phytochemical, responsible for the antioxidant property. However, further studies are warranted to ascertain its significant role. This study indicated the potential of the leaf extract as a source of natural antioxidants or nutraceuticals that could be of use in food industry with potential application to reduce oxidative stress in living system with consequent health benefits.
References
- Amarowicz R, Naczk M, Shahidi F. Antioxidant activity of crude extracts of canola/rapeseed hulls. J Am Oil Chem Soc. 2000;77:957–961. doi: 10.1007/s11746-000-0151-0. [DOI] [Google Scholar]
- Ames BN, Shigena MK, Hegen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilakumar KR, Nagaraj NS, Santhanam K. Effect of Coriander seeds on hexachlorocyclohexane induced lipid peroxidation in rat liver. Nutr Res. 2001;21:1455–1462. doi: 10.1016/S0271-5317(01)00338-4. [DOI] [Google Scholar]
- Anilakumar KR, Saritha V, Khanum F, Bawa AS. Effect of cooking on total phenols, flavonoids and antioxidant activity in spices of India culinary. J Food Sci Technol. 2007;44(4):357–359. [Google Scholar]
- Anilakumar KR, Saritha V, Khanum F, Bawa AS. Ameliorative effect of ajwain extract on hexachlorocyclohexane induced lipid peroxidation in rat liver. Food Chem Toxicol. 2009;47:279–282. doi: 10.1016/j.fct.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Anilakumar KR, Farhath khanum, Bawa AS. Effect of Coriander seeds powder on 1, 2 dimethyl hydrazine induced changes in antioxidant enzymes system and lipid peroxidation formation in rats. J Diet Suppl. 2010;7(1):9–20. doi: 10.3109/19390210903534970. [DOI] [PubMed] [Google Scholar]
- Atamaniuk J, Vidotto C, Tschan H, Bachl N, Stuhlmeier KM, Muller MM. Increased concentrations of cell-free plasma DNA after exhaustive exercise. Clin Chem. 2004;50:1668–1670. doi: 10.1373/clinchem.2004.034553. [DOI] [PubMed] [Google Scholar]
- Babu BH, Shylesh BS, Padikkala Antioxidant and hepatoprotective effect of Alanthus icicifocus. Fitoterapia. 2001;72:272–277. doi: 10.1016/S0367-326X(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Baumann J, Wurn G, Bruchlausen FV. Prostaglandin synthetase inhibiting O2-radical scavenging properties of some flavonoids and related phenolic compounds. Naunyn-Schmiedebergs Arch Pharmacol. 1979;308:27–32. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Blios MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Blumenthal M, Goldberg A, Brinkmann J (2000) Coriander seed. In: Herbal Medicine-Expanded Commission E Monographs. 1st edition, Integrative Medicine Communications, Newton, MA, USA; 75–77.
- Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI. In-vitro antioxidant properties of dantrolene sodium. Pharmacol Res. 2001;44:491–495. doi: 10.1006/phrs.2001.0890. [DOI] [PubMed] [Google Scholar]
- Chang LW, Yen WJ, Huang SC, Duh PD. Antioxidant activity of sesame coat. Food Chem. 2002;78:347–354. doi: 10.1016/S0308-8146(02)00119-X. [DOI] [Google Scholar]
- Chithra V, Leelamma S. Hypolimedic effect of coriander seeds (Coriandrum sativum): mechanism of action. Plant Foods Human Nutr. 1997;51:167–172. doi: 10.1023/A:1007975430328. [DOI] [PubMed] [Google Scholar]
- Chithra V, Leelamma S. Coriandrum sativum-mechanism of hypoglycemic action. Food Chem. 1999;67:229–231. doi: 10.1016/S0308-8146(99)00113-2. [DOI] [Google Scholar]
- Chithra V, Leelamma S. Coriandrum sativum: effect on lipid metabolism in 1, 2-dimethylhydrazine induced colon cancer. J Ethnopharmacol. 2000;71:457–463. doi: 10.1016/S0378-8741(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetoaminophen, Salicylate and 5- aminosalicylate) as inhibitora of membrane lipid peroxidation and as proxy radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Duh PD. Antioxidants activity of burdock (Arctium lappa L) its scavenging effect on free radical and active oxygen. J Am oil chemist Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Emamghoreishi M, Khasaki M, Fath-Aazam M. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 2005;96:365–370. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Evans W.C, Trease and Evans (2002) Pharmacognocy. Fifteenth International edition, W.B. Saunders, Edinburgh, London, New York p. 262.
- Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London: Elsevier Applied science; 1990. pp. 1–18. [Google Scholar]
- Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of traditional anti-diabetic plant Coriandrum sativum. Br J Nutr. 1999;81:203–209. doi: 10.1017/S0007114599000392. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Wilkins S. Copper salt dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochem biophys Acta. 1983;759:38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidants and human disease: a general introduction. Nutr Rev. 1997;55:S44–S52. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;23:1396–1397. doi: 10.1016/S0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- Harman D. Role of antioxidant nutrients in aging: overview. Age. 1995;18:51–62. doi: 10.1007/BF02432519. [DOI] [Google Scholar]
- Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavanoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull. 1988;36:1010–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Hatano T, Edamatsu R, Mori A, Fujita Y, Yasuhara E. Chem Pharm Bull. 1989;37:2016. doi: 10.1248/cpb.37.2016. [DOI] [Google Scholar]
- Ivanova D, Gerova D, Chervenkov T, Yankova T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol. 2005;97(12):145–150. doi: 10.1016/j.jep.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Jayaprakash GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed extracts on peroxidation models in-vitro. J Agric Food Chem. 2001;55:1018–1022. [Google Scholar]
- Jeremy PE, Spencer Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4(4):243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47(10):3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Karmakar UK, Rahman MA, Roy DN, Sadhu SK, Ali ME. Chemical and biological investigations of Coriandrum sativum L. Int J Pharm Sci Res. 2011;2(4):999–1006. [Google Scholar]
- Kimura Y, Okuda H, Okuda T. Studies on activities of tannins and related compounds. Planta med. 1984;50:473–476. doi: 10.1055/s-2007-969776. [DOI] [PubMed] [Google Scholar]
- Kowaltowski JA, Vercesi EA (1999) Mitochondrial damage induced by conditions of oxidative stress. Free Radical biology and Medicine 26(3–4):463–471 [DOI] [PubMed]
- Liu J, Yeo HC, Doniger SJ, Ames BN. Assay of aldehydes from lipid peroxidation: gas chromatograph mass spectrometry compared to thiobarbituric acid. Anal Biochem. 1997;245:161–166. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- Lui SL, Chan LY, Zhang XH, Zhu W, Chan TM, Fung PC, Lai KN. Effect of mycophenolate mofetil on nitric oxide production and inducible nitric oxide synthase gene expression during renal izchaemia-reperfusin injury. Nephrol Dial Transplant. 2001;16:1577–1582. doi: 10.1093/ndt/16.8.1577. [DOI] [PubMed] [Google Scholar]
- Marcoci L, Maguire JJ, Droy-Lefaix MT. The NO scavenging properties of Gingko biloba extract EGb 761. Biochem Biophys Res Commun. 1994;15:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- Miller AL. Antioxidant flavonoids: structure, function and clinical usage. Alt Med Rev. 1996;1:103. [Google Scholar]
- Okhawa H, Ohishi W, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Roberta RN, Pellegrini A, Proteggente A, Pannala M, Yang, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation de-coloration assay. Free radical Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Russo A, Izzo AA, Borrelli F, Renis M, Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res. 2003;17:870–875. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara PD. Phenolic antioxidants. Cri Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Perumal S, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata L) Walp seed extract. Food Chem. 2007;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Simonetti P, Pietta P, Testolin G. Polyphenol content and total antioxidant potential selected Italian wines. J Agric Food Chem. 1997;45:1152–1155. doi: 10.1021/jf960705d. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic acid – phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Soares JR, Dins TCP, Cunha AP, Ameida LM. Antioxidant activity of some extracts of Thymus zygis. Free Radic Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes.Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–464. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kuei CW, Nagashima Y, Taguchi T, Gakk NS. Taguchi. Application of antioxidative maillrad reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkaishil. 1998;54:1409–1414. doi: 10.2331/suisan.54.1409. [DOI] [Google Scholar]
- Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the ayurvedic formulation triphala and its constituents. Inter J Pharmacognosy. 1997;35:313–317. doi: 10.1080/09251619708951274. [DOI] [Google Scholar]
- Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3(3–4):115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson JA, Hontz BA. Phenol antioxidant index: comparative antioxidant effectiveness of red and white wines. J Agri Food Chem. 1995;43:401–403. doi: 10.1021/jf00050a027. [DOI] [Google Scholar]
- Yen GC, Duh PD, Tsail CL. Relationship between antioxidant activity and maturity of peanut hulls. J Argic Food Chem. 1993;41:67–70. doi: 10.1021/jf00025a015. [DOI] [Google Scholar]
- Yermilov V, Rubio J, Friesen MD, Pignatelli B, Oshima H. Formation of 8-nitroguanine by the reaction of guanine with perozxynutite in vitro. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- Zhonghong G, Huang K, Yang X, Huibi Xu. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472(3):643–650. doi: 10.1016/S0304-4165(99)00152-X. [DOI] [PubMed] [Google Scholar]
- Zou YP, Lu YH, Wei DZ. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52:5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]