Abstract
Background
The gamma-aminobutyric acid (GABA) hypothesis in essential tremor (ET) implies a disturbance of the GABAergic system, especially involving the cerebellum. This review examines the evidence of the GABA hypothesis.
Methods
The review is based on published data about GABA dysfunction in ET, taking into account studies on cerebrospinal fluid, pathology, electrophysiology, genetics, neuroimaging, experimental animal models, and human drug therapies.
Results
Findings from several studies support the GABA hypothesis in ET. The hypothesis follows four steps: 1) cerebellar neurodegeneration with Purkinje cell loss; 2) a decrease in GABA system activity in deep cerebellar neurons; 3) disinhibition in output deep cerebellar neurons with pacemaker activity; and 4) an increase in rhythmic activity of the thalamus and thalamo-cortical circuit, contributing to the generation of tremor. Doubts have been cast on this hypothesis, however, by the fact that it is based on relatively few works, controversial post-mortem findings, and negative genetic studies on the GABA system. Furthermore, GABAergic drug efficacy is low and some GABAergic drugs do not have antitremoric efficacy.
Discussion
The GABA hypothesis continues to be the most robust pathophysiological hypothesis to explain ET. There is light in all GABA hypothesis steps, but a number of shadows cannot be overlooked. We need more studies to clarify the neurodegenerative nature of the disease, to confirm the decrease of GABA activity in the cerebellum, and to test more therapies that enhance the GABA transmission specifically in the cerebellum area.
Keywords: essential tremor, GABA, gamma-aminobutyric acid
Introduction
Essential tremor (ET) is a common movement disorder in adults, characterized by rhythmic shaking of the arms and possibly other parts of the body. It is often referred to as a benign disorder but moderate and advanced stages of ET can be physically and socially disabling.1 The few medications that have been used to treat ET have demonstrated only modest efficacy.2
The etiology and pathophysiology of ET are not yet well understood, thus limiting the rational development of new pharmacological therapies for ET. There is some consensus about the involvement of the cerebellum, but the exact neurochemical abnormalities underlying the disorder remain to be identified.
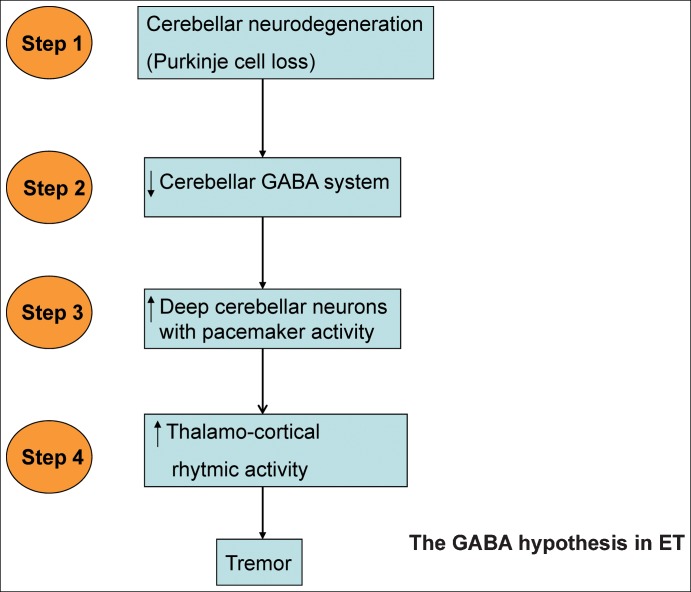
The major pathophysiological hypothesis of ET during the last two decades has been the GABA hypothesis.3 This hypothesis implies a disturbance of the gamma-aminobutyric acid (GABA)ergic system in ET, in particular involving the cerebellum. The hypothesis consists of four main steps (Figure 1). Step 1 involves cerebellar degeneration with Purkinje cell loss. In step 2, as a consequence of the previous step, activity of the GABA system decreases in deep cerebellar neurons. In step 3, pacemaker activity of deep cerebellar neurons is disinhibited. And in step 4, the rhythmic activity of the thalamus and thalamo-cortical circuit increases, causing tremor.
Figure 1. Schematic Representation of the Gamma-aminobutyric Acid Hypothesis in Essential Tremor.
This review considers the evidence for and against the GABA hypothesis with the objective of better understanding and defining the pathophysiology of ET. Table 1 summarizes all the studies considered herein concerning the GABA hypothesis in ET.
Table 1. Studies Published Concerning the GABA Hypothesis in ET.
| CSF | Purkinje Cell Loss | Pathology GABA | Electrophysiology | Genetic | Animal Models | Imaging | Drug Therapies | |
|---|---|---|---|---|---|---|---|---|
| Pros | Mally et al.21 | Louis et al.15 | Mouginot and Gahwiler20 | Uusiaari and Knopfel24 | Kralic et al.34 | Boecket et al.35 | Theophylline, 199139 | |
| Axelrad et al.16 | Paris-Robidas et al.22 | Pinault and Deschenes28 | Gironell et al.36 | Muscimol, 199140 | ||||
| Louis et al.18 | Shill et al.23 | Gabapentin, 199941 | ||||||
| Gabapentin, 200043 | ||||||||
| Topiramate, 200245 | ||||||||
| Topiramate, 200646 | ||||||||
| Topiramate, 200848 | ||||||||
| Pregabaline, 200750 | ||||||||
| Cons | Rajput et al.61 | Deng et al.29 | Progabide, 198337 | |||||
| Thier et al.30 | Progabide, 198738 | |||||||
| Shill et al.62 | García-Martín et al.31 | Gabapentin, 199942 | ||||||
| Symanski et al.63 | García-Martín et al.32 | Topiramate, 200647 | ||||||
| Tiagabine, 200849 | ||||||||
| Pregabaline, 200951 | ||||||||
| Pregabaline, 201252 |
CSF, Cerebrospinal Fluid; ET, Essential Tremor; GABA, gamma-aminobutyric acid.
Cerebellum and GABA system
The cerebellum and the thalamus are the main regions involved in the genesis (cerebellum) and propagation (thalamus) of tremor in humans. Clinical case studies have shown that lesions of these structures can alleviate tremor.4,5 The ventral intermediate (VIM) nucleus is the most effective target for stereotactic interventions to treat tremor.6 Two studies using repetitive transcranial magnetic stimulation applied over the cerebellum found a tremorlytic action in ET patients.7,8 Furthermore, positron emission tomography (PET) activation studies show regional cerebral blood flow in the cerebellum is abnormally increased.9–11 Magnetic resonance imaging (MRI) studies including spectroscopy, voxel-based morphometry and functional MRI also support cerebellar involvement in ET.12–14 Furthermore, microscopic cerebellar pathology has been identified in these patients, with cerebellar Purkinje cell loss with torpedoes and Bergmann gliosis.15 In 2007, Louis and coworkers15 studied 33 ET brains and found that about 75% of cases exhibited cerebellar pathology with a loss of Purkinje cells and increased numbers of axonal torpedoes. Further studies by the same group confirmed these findings.16–18
Cerebellar Purkinje cells are large neurons that have many branching extensions. They are found in the cortex of the cerebellum and play a fundamental role in controlling motor movement.19 These cells are characterized by cell bodies that are flash-like in shape, the result of numerous branching dendrites, and by a single long axon. Most Purkinje cells release the neurotransmitter GABA, which exerts inhibitory actions on the dentate nucleus and certain deep cerebellar output neurons, thereby reducing the transmission of nerve impulses. These inhibitory functions enable Purkinje cells to regulate and coordinate motor movements.
GABA is the major neurotransmitter for fast inhibitory synaptic transmission.19 Because of its widespread presence and utilization, this neurotransmitter is involved in all functions of the central nervous system, including the cerebellum. In fact, the GABA system is the target of a wide range of drugs that act on the central nervous system, such as anxiolytics, sedative-hypnotics, general anesthetics, and anticonvulsants.
GABA is synthesized by a specific enzyme, l-glutamic acid decarboxilase (GAD) in one step from l-glutamate. Thus, glutamate must be available in certain nerve endings for biosynthesis of GABA. Much of the glutamate and GABA used as a neurotransmitter is derived from glial storage pools of glutamine. GABA is released from electrically stimulated inhibitory nerve cells. The application of GABA and structural analogues to cells innervated by GABAergic neurons produces effects on that target cell identical to those produced by stimulating the inhibitory innervation.
GABA binds to and mediates its effects via post-synaptic ionotropic GABAA receptors and pre-and post-synaptic metabotropic GABAB receptors.19,20 Whereas the GABAA receptors mediate fast responses, the GABAB receptors mediate slow responses. GABA is removed from the synaptic cleft by GABA transporters and metabolized. The GABAA receptors are the main players in central nervous system (CNS) function and relevance to psychopharmacology. These are the sites of action of benzodiazepines, barbiturates, alcohol, general anesthetics, and neurosteroids.
Cerebrospinal fluid studies
In 1996, Mally et al.21 compared the concentrations of amino acids in the cerebrospinal fluid (CSF) and serum in 20 patients with ET with those of a control group of 10 subjects. They found a slight increase in the concentration of glutamate and reduced levels of GABA, together with a decrease in glycine and serine and a slight increase in the concentration of glutamate.
This work suggested a GABAergic dysfunction in ET and was one of the first to provide evidence favoring the GABA hypothesis. Indeed, the pathophysiological basis of ET seemed simple and would be the result of a decrease in GABA, the main brain inhibitory neurotransmitter. However, this is the only study analyzing GABA levels in CSF and the data have not been replicated.
Pathologic studies
A neuropathological study in 199620 revealed that GABAB receptors are present on the presynaptic terminals of Purkinje cells in the deep cerebellar nuclei. These anatomical structures have since been considered involved in the probable genesis of ET.
A more recent neuropathological study on the GABA hypothesis in ET found interesting results that agree with this hypothesis. In 2012 Paris-Robidas et al.22 reported a post-mortem decrease in GABAA (35% reduction) and GABAB (22–31% reduction) receptors in the dentate nucleus of the cerebellum in individuals with ET (n = 10) when compared to controls (n = 16) or individuals with Parkinson's disease (n = 10), as assessed by receptor-binding autoradiography. Furthermore, concentrations of GABAB receptors in the dentate nucleus were inversely correlated with the duration of ET symptoms, suggesting that the loss of GABAB receptors follows the progression of the disease. The authors proposed that a decrease in GABA receptors in the dentate nucleus disinhibits cerebellar pacemaker output activity, propagating along the cerebello-thalamo-cortical pathways to generate tremors.
In the same year, Shill et al.23 studied some biochemical changes in autopsied patients with ET comparing them with a control group. They found significant reduced levels of parvalbumin (a marker of GABAergic interneurons) in the pons and locus coeruleus with no difference in the cerebellum. The results of the study partly agree with the GABA hypothesis, suggesting a focal abnormality but not involving the cerebellum.
Electrophysiological studies
Basic electrophysiology has provided valuable data for the GABA hypothesis in ET. In 2008, Uusiaari and Knopfel24 demonstrated that all dentate nucleus cells are sensitive to GABAergic input from Purkinje cells. Using electrophysiological recordings of deep cerebellar nuclei neurons they showed that both GABAA and GABAB receptors mediate the inhibitory input coming from Purkinje cells.
Furthermore, several findings indicate that deep cerebellar nuclei neurons belong to the family of single cell oscillators, and have a pacemaker-like activity.20,25–28 Interestingly, in the case of deep cerebellar nuclei neurons, it has been shown that their pacemaker-like activity is under tonic control by the GABAergic input from Purkinje cells.20 Even more compelling is the demonstration that deep cerebellar nuclei neurons can impose their rhythmicity to their thalamic target neurons with highly regular patterns of activity.28
Genetic studies
After several lines of evidence supported the GABA hypothesis, a number of works in the last 10 years have analyzed the genetic bases of this theory. The first approximation was negative. In 2006, Deng et al.29 investigated the association between the GABAA receptor alpha-1 and ET in 76 familial ET patients. The association did not turn out to be significant. The authors concluded that missense, nonsense, or splice site mutations in the coding regions of the GABAA1 gene are not a major genetic cause of ET.
More recent studies have been also negative. In 2011, Thier et al.30 investigated an association between polymorphisms in 15 GABAA and four GABA transporter genes in ET. A total of 503 ET patients and 818 controls were analyzed. The authors did not find any evidence of an association between GABAA and GABA transporter genes in ET.
García-Martin et al.31 published two further negatives studies in 2011. In the first, they investigated the possible association between the GABA receptor subtypes rho1, rho2, and rho3 and allelic variants of the single nucleotide polymorphisms GABRR1-M26V, GABRR1-H27R, GABRR2-T455M, and GABRR3-Y205X and the risk of ET. The frequencies of GABBR1 genotypes and allelic variants of the studied polymorphisms did not differ significantly between patients with ET and controls and were unrelated to the age at onset of tremor, gender, localization of tremor, and response of tremor to ethanol. In a second work, the same authors studied the allelic and genotype frequencies for single nucleotide polymorphisms, such as GABRA4-L26M, GABRE-S102A, and GABRQ-I478F, in 200 patients with familial ET and 250 healthy controls using TaqMan genotyping.32 Genotype and allelic frequencies did not differ significantly between patients with ET and controls. The results of this study suggest that the single nucleotide polymorphisms studied in the GABRA4, GABRE, and GABRQ genes are not related to the risk for familial ET.
However, genetic studies in ET have several methodological shortcomings: a lack of stringent diagnostic criteria, small sample sizes, a lack of biomarkers, a high phenocopy rate, evidence of non-mendelian inheritance, and high locus heterogeneity in presumably monogenic ET.33
Experimental animal models
In 2005, Kralic et al.34 performed an interesting investigation using GABA(A) receptor alpha-1 subunit knockout mice. This mouse model exhibited postural and kinetic tremor and motor incoordination characteristic of ET. The mice were tested using current ET drug therapies such as primidone, propranolol, and gabapentin, and the amplitude of the pathologic tremor decreased. Non-sedative doses of ethanol eliminated tremor. No differences in abundance, gross morphology, or spontaneous synaptic activity were observed in cerebellar Purkinje cells. These cells exhibited a profound loss of all responses to synaptic or exogenous GABA.
The authors concluded that this genetic animal model elucidates a mechanism of GABAergic dysfunction in the major motor pathway and potential targets for pharmacotherapy of ET.
In vivo imaging studies
[11C]Flumazenil is a tracer that specifically binds to the central benzodiazepine receptor sites of the GABAA receptor complex. To date, there are two main controlled PET studies using this tracer in ET.
The first article was published in 2010.35 It was a comparative study of [11C]flumazenil PET in eight patients with bilateral ET, with 11 healthy controls. Parametric distribution volume images were calculated for focally altered [11C]flumazenil binding at the sites of tremor genesis, in particular at the level of the cerebellum and interconnected thalamo-cortical pathways. The authors found significant increases in binding of [11C]flumazenil at the benzodiazepine receptor site of the GABAA receptor in the cerebellum, the ventrolateral thalamus, and the lateral premotor cortex in the ET group.
The second paper appeared in 2012.36 The authors performed correlated clinical scale scores and parametric binding potential images of [11C]flumazenil PET in 10 ET patients at different stages of clinical severity. The severity of tremor statistically correlated with the abnormalities found in GABA receptor binding in the cerebellar vermis, bilateral posterior lobes, and right anterior lobe.
The results from both studies showed complete agreement with the GABA hypothesis. Both studies provide neuroimaging evidence of abnormally increased GABAA receptor binding in ET, especially in cerebello-thalamic output pathways. In fact, the binding changes were located in regions implicated in tremor genesis, such as the thalamus, the cerebellum, and the lateral premotor cortex. We can conclude from these findings that in vivo neuroimaging studies support the role of cerebellar GABAergic dysfunction as the main pathophysiological hypothesis of the disease.
Human drug therapies
One of the major premises of the GABA hypothesis in ET was the antitremoric effects observed in some GABAergic drugs, such as gabapentin, in the late 1990s.
Progabide
The first controlled trial of a GABA-agonist, progabide, in ET was performed in 1983. Mondrup et al.37 performed a study in 18 ET patients. They found no significant differences between progabide and placebo in tremor scores.
Four years later, Koller et al.38 performed another controlled trial with progabide in 10 ET patients. Again, there were no differences on tremor scores from placebo. The authors concluded that alterations in GABA neurotransmission do not appear to be involved in the pathogenesis of ET.
Theophylline
In 1991, Mally et al.39 studied the effects of theophylline in 20 ET patients in a double-blind crossover trial. Tremor improved significantly after 4 weeks of treatment. The authors hypothesized that theophylline-enhanced GABA explains the antitremoric effect.
Muscimol
In 1999, Pahapill et al.40 performed an interesting experiment injecting muscimol (GABAA agonist) into the ventralis intermedius thalamus in six patients undergoing stereotactic procedures for ET. The drug was administered in areas where tremor-synchronous cells were identified electrophysiologically with microelectrode recordings and where tremor reduction occurred with electrical microstimulation. Injections of muscimol but not saline solution consistently reduced tremor in each patient. The authors concluded that GABA-mediated thalamic neuronal inhibition may represent a mechanism underlying the effectiveness of surgery for tremor and that GABA analogues could potentially be used therapeutically.
Gabapentin
Gabapentin is structurally related to the neurotransmitter GABA. It penetrates the blood–brain barrier but it does not modify GABAA or GABAB radioligand binding, it is not converted metabolically into GABA or a GABA agonist, and it is not an inhibitor of GABA uptake or degradation. Its mechanism of action is not fully understood, but recent data indicate that it can increase human brain GABA levels and reduce intracortical excitability.
In 1999, Gironell et al.41 performed a randomized placebo-controlled comparative trial of gabapentin and propranolol in 16 ET patients. The patients were not taking antitremoric medication. On day 15, both gabapentin and propranolol demonstrated significant and comparable efficacy in reducing tremor from baseline in all tremor measures (clinic and accelerometric). Results of the study were in accordance with the central mechanism hypothesis that implicates the GABAergic system in the pathogenesis of ET. The authors concluded that their exploratory study suggested gabapentin deserved a role in the pharmacological treatment of ET.
In the same year, Pahwa et al.42 performed another controlled study of gabapentin in ET in 20 ET patients. Although some patient had benefits on tremor, the results were not statistically significant for total tremor scores.
One year later, Ondo et al.43 performed a multiple-dose, double-blind, placebo-controlled trial with gabapentin in 25 ET patients. Some end points of the study were positive (patient global assessment, water-pouring scores, observed tremor scores, and activities of daily living). However, accelerometry, spirographs, and investigator global impressions scores did not improve. The results were similar for high and low doses. The authors concluded that gabapentin may be effective in some cases of ET.
In the American Academy of Neurology (AAN) recent guidelines for ET, gabapentin appeared with a type B level of evidence.44
Topiramate
Topiramate is an anticonvulsant that blocks sodium channels and potentiates GABA activity. Following the GABA hypothesis in ET, several trials were done using this drug for ET. In 2002, Connor45 found significant antitremoric properties of topiramate in a controlled study in 24 ET patients. Topiramate improved all tremor clinical scores. In a multicenter-controlled study, Ondo et al.,46 revealed significant improvement in tremor in 208 ET patients. The mean percentage improvement in overall scores was 29%. In 2006, Frima and Grünewald,47 performed a controlled crossover study of topiramate in 16 ET patients. The study was negative, and provided no evidence of therapeutic benefit of topiramate in ET. And in 2008, Connor et al.48 performed a double-blind placebo controlled crossover study of topiramate in ET with 62 patients. The authors found a significant improvement (18–23%) in all tremor measures following treatment with topiramate. However, the drop-out rate was approximately 40% due to appetite suppression, weight loss, paresthesias, anorexia, and concentration difficulties.
Tremor efficacy of topiramate is similar to gabapentin, but titration is long and difficult, and tolerance is poor. In recent AAN guides for ET treatment, topiramate is considered a second-line therapy with a level of evidence B.44
Tiagabine
Tiagabine is a centrally acting GABA uptake inhibitor that allows more GABA to be available for post-synaptic receptor binding. Following the GABA hypothesis in ET, tiagabine may have antitremoric properties in ET patients.
Gironell et al.49 performed an open-label trial with seven ET patients. Results were negative, and at the dosage tested they did not lead to a significant improvement in tremor amplitudes in ET.
Pregabaline
Pregabaline is a structural derivative of the inhibitory neurotransmitter GABA. In a similar way to gabapentin, it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, does not increase GABA responses in cultured neurons, and does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons, prolonged application of pregabaline increases the density of GABA transporter protein and increases the rate of functional GABA transport.
In 2007, Zesievick et al.50 first published a double blind study of pregabaline in 22 ET patients. The authors found significant improvement in accelerometry and tremor clinical scores. However, 2 years later, Ferrara et al.51 performed another controlled study with pregabalin in 20 ET patients with a titration period of 6 weeks and found no improvement in any of the tremor measures.
Finally, in a multisite, double-blind study in 2012, Zesievick et al.52 published a study of pregabalin in 29 ET patients. The study was negative, and there were no improvements in any tremor measures.
Other drugs that enhance GABAergic transmission
Several classes of drugs that enhance GABAergic transmission by binding to sites on the GABAA receptors are effective in the treatment of ET. These include barbiturates (fenobarbital, primidone), and benzodiazepines (alprazolam and clonacepam). Primidone is a first-class antitremoric agent that has a similar efficacy to beta-blockers (level of evidence AAN: B).53,54 Alprazolam is probably efficacious in treating ET (level of evidence AAN: C).55
Ethanol, which is often highly effective in reducing the severity of tremor in ET, potentiates the activity of the GABAA receptor.56 Alcohol, in fact, is an indirect GABA agonist. It is believed to mimic GABA's effect on the brain, binding to GABA receptors and inhibiting neuronal signaling.
While propranolol is believed to have a therapeutic effect on ET by antagonistic binding to non-specific peripheral beta-adrenergic receptors, there is some evidence to suggest that it may also act by enhancing GABA receptivity in the central nervous system.57
GABA hypothesis in ET: light
Step 1: cerebellar neurodegeneration with cerebellar Purkinje cell loss.
Some pathological post-mortem studies agree with the GABA hypothesis, revealing Purkinje cell loss in ET.15–18 Furthermore, the neurodegenerative nature of ET is supported by the progressive course of the disease.58
Step 2: decrease in GABA system activity in deep cerebellar neurons
Several studies agree with this hypothesis:
CSF studies demonstrating a decrease in GABA levels.21
Pathological studies that found a decrease in GABA receptors in the dentate nucleus.22
In vivo imaging studies, with an increase of binding of flumazenil in GABA receptors in the cerebellar area, thus, suggesting a deficit in GABA in these areas.35,36
Animal model (knockout mice) of GABA dysfunction exhibit symptoms characteristic of ET.34
-Antitremoric efficacy of drugs that enhance GABA activity including, muscimol, theophylline, gabapentin, topiramate, tiagabine, pregabaline, primidone, benzodiazepines and alcohol.39,40,41,43,45,46,48,50,53–57
Step 3: disinhibition in output deep cerebellar neurons with pacemaker activity.
Electrophysiological studies indicating that dentate nucleus cells are sensitive to GABAergic input from Purkinje cells, and that deep cerebellar nuclei neurons have pacemaker-like activity.20,24–28
Evidence of this step is the increase in output cerebellar metabolism that appears in PET studies with 18Fludeoxyglucose (FDG).9–11
The antitremoric effect of inhibitory repetitive transcranial magnetic stimulation (rTMS) over the cerebellum also supports an increase in hypermetbolism and activity in this area.7,8
Step 4: Increase in rhythmic activity of thalamus and thalamo-cortical circuit.
Thalamic rhythmic or tremoric neurons, specifically in VIM nucleus, have been clearly seen in electrophysiological recordings in deep brain stimulation surgery.59,60
GABA hypothesis in ET: lights and shadows
There are a number of arguments against the GABA hypothesis in ET. The main one is that evidence of a decrease in cerebellar GABA activity is based on relatively few studies. Only one study has found a decrease in GABA in CSF and only one neuropathological study has revealed a decrease in GABA receptors. Besides, only two controlled studies have analyzed in vivo imaging data.
There are several other important shadows hanging over the GABA hypothesis. The neuropathology of ET, in particular cerebellar pathology, is a matter of controversy. Several post-mortem studies have not revealed Purkinje cell loss in ET.61–63 However, the Rajput et al.61 study was underpowered to detect Purkinje cell loss, and the Shill et al.62 study is problematic for reasons of case definition. Based on these negative studies, some authors consider ET may not be a neurodegenerative disease, and point to an abnormal neuronal oscillation as the fundamental abnormality in this disorder.64,65 The controversial results of the various neuropathologic studies in ET are difficult to explain and may be related to three possibilities: 1) patient selection biases; 2) different methodologies and counting methods; and 3) differences in field sizes and lack of standardization across studies, restricting microscopy to linear segments.66 These biases have to be controlled in further standardized studies.
Moreover, all genetic studies involving several nucleotide GABA polymorphisms and GABAA receptor and GABA transporter genes have been negative.29–32
Also of concern is the therapeutic failure of several GABAergic drugs in controlled studies. These include progabide, gabapentin, topiramate, tiagabine, and pregabaline.37,38,42,47,49,51,52 Furthermore, efficacy of GABAergic drugs with antitremoric properties is relatively scarce, with maximum improvements of about 30–60%.2
However, simply enhancing GABA neurotransmission may be too gross a solution to the problem. Loss of GABA in ET may be a marker of the problem (Purkinje cell loss) but the problem produced by Purkinje cell loss may not simply be fixed by enhancing GABA transmission.
Final remarks
To sum up and conclude, we can state that the GABA hypothesis remains the most robust pathophysiological theory of ET to date. There is light in all the steps of the GABA hypothesis. However, the evident shadows and controversies cannot be overlooked. We clearly need to perform more studies to confirm the neurodegenerative nature of the disease and more studies about the decrease in GABA activity in the cerebellum, and we need to test more therapies that enhance GABA transmission specifically focused in the cerebellum area.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Louis ED. Essential tremor. Lancet Neurol. 2005;4:100–10. doi: 10.1016/S1474-4422(05)00991-9. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED. A new twist for stopping the shakes? Revisiting GABAergic therapy for essential tremor. Arch Neurol. 1999;56:807–808. doi: 10.1001/archneur.56.7.807. [DOI] [PubMed] [Google Scholar]
- 4.Dupuis MJ, Delwaide PJ, Boucquey D, Gonsette RE. Homolateral disappearance of essential tremor after cerebellar stroke. Mov Disord. 1989;4:183–187. doi: 10.1002/mds.870040210. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R, Bone I, Melville ID. Essential tremor cured by infarction adjacent to the thalamus. J Neurol Neurosurg Psychiatry. 1988;51:591–592. doi: 10.1136/jnnp.51.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pahwa R, Lyons KE, Wilkinson SB, et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104:506–512. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- 7.Gironell A, Kulisevsky J, Lorenzo J, Barbanoj M, Pascual-Sedano B, Otermin P. Transcranial magnetic stimulation of the cerebellum in essential tremor: A controlled study. Arch Neurol. 2002;59:413–417. doi: 10.1001/archneur.59.3.413. [DOI] [PubMed] [Google Scholar]
- 8.Popa T, Russo M, Vidailhet M, et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stimul. 2013;6:175–179. doi: 10.1016/j.brs.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Colebatch JG, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ. Preliminary report: Activation of the cerebellum in essential tremor. Lancet. 1990;336:1028–1030. doi: 10.1016/0140-6736(90)92489-5. [DOI] [PubMed] [Google Scholar]
- 10.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: A positron emission tomographic study. Ann Neurol. 1994;36:636–642. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- 11.Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. 1993;114:45–48. doi: 10.1016/0022-510X(93)90047-3. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: A proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333:17–20. doi: 10.1016/S0304-3940(02)00966-7. [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Shungu DC, Mao X, Chan S, Jurewicz EC. Cerebellar metabolic symmetry in essential tremor studied with 1H magnetic resonance spectroscopic imaging: Implications for disease pathology. Mov Disord. 2004;19:672–677. doi: 10.1002/mds.20019. [DOI] [PubMed] [Google Scholar]
- 14.Daniels C, Peller M, Wolff S, et al. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology. 2006;67:1452–1456. doi: 10.1212/01.wnl.0000240130.94408.99. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 16.Axelrad JE, Louis ED, Honing LS, et al. Reduced Purkinje cell number in essential tremor: A post-mortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–1004. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP. Quantification of cerebellar hemispheric Purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–9. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowery NG, Bettler B, Froestl W, et al. International union of pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: Structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 20.Mouginot D, Gahwiler BH. Presynaptic GABAB receptors modulate IPSPs evoked in neurons of deep cerebellar nuclei in vitro. J Neurophysiol. 1996;75:894–901. doi: 10.1152/jn.1996.75.2.894. [DOI] [PubMed] [Google Scholar]
- 21.Mally J, Baranyi M, Vizi ES. Change in the concentrations of amino acids in CSF and serum of patients with essential tremor. J Neural Transm. 1996;103:555–560. doi: 10.1007/BF01273153. [DOI] [PubMed] [Google Scholar]
- 22.Paris-Robidas A, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–116. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 23.Shill HA, Adler CH, Beach TG, et al. Brain biochemistry in autopsied patients with essential tremor. Mov Disord. 2012;27:113–117. doi: 10.1002/mds.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uusisaari M, Knopfel T. GABAergic synaptic communication in the GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Neuroscience. 2008;156:537–549. doi: 10.1016/j.neuroscience.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 25.Jahnsen H. Extracellular activation and membrane conductances of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:149–168. doi: 10.1113/jphysiol.1986.sp016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 27.Llinas R, Muhlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinault D, Deschenes M. The origin of rhythmic fast subthreshold depolarizations in thalamic relay cells of rats under urethane anaesthesia. Brain Res. 1992;595:295–300. doi: 10.1016/0006-8993(92)91063-K. [DOI] [PubMed] [Google Scholar]
- 29.Deng H, Xie WJ, Le WD, Huang MS, Jankovic J. Genetic analysis of the GABRA1 gene in patients with essential tremor. Neurosci Lett. 2006;401:16–19. doi: 10.1016/j.neulet.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 30.Thier S, Kuhlenbäumer G, Lorenz D, et al. GABA(A) receptor and GABA transporter polymorphisms and risk for essential tremor. Eur J Neurol. 2011;8:1098–1100. doi: 10.1111/j.1468-1331.2010.03308.x. [DOI] [PubMed] [Google Scholar]
- 31.García-Martin E, Martínez C, Alonso-Navarro H, et al. Gamma-aminobutyric acid GABRA4, GABRE, and GABRQ receptor polymorphisms and risk for essential tremor. Pahrmacogenet Genomics. 2011;7:436–439. doi: 10.1097/FPC.0b013e328345bec0. [DOI] [PubMed] [Google Scholar]
- 32.García-Martin E, Martínez C, Alonso-Navarro H, et al. Gamma-aminobutyric acid (GABA) receptor rho (GABRR) polymorphisms and risk for essential tremor. J Neurol. 2011;2:203–211. doi: 10.1007/s00415-010-5708-z. [DOI] [PubMed] [Google Scholar]
- 33.Kuhlenbäumer G, Hopfner F, Deuschl G. Genetics of essential tremor: Meta-analysis and review. Neurology. 2014;82:1000–1007. doi: 10.1212/WNL.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 34.Kralic JE, Criswell HE, Osterman JL, et al. Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice. J Clin Invest. 2005;115:774–779. doi: 10.1172/JCI200523625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boecker H, Weindl A, Brooks DJ, et al. GABAergic dysfunction in essential tremor: An 11C-Flumazenil PET study. J Nucl Med. 2010;51:1030–1035. doi: 10.2967/jnumed.109.074120. [DOI] [PubMed] [Google Scholar]
- 36.Gironell A, Figueiras FP, Pagonabarraga J, Herance JR, Pascual-Sedano B, Trampal C, Gispert JD. Gaba and serotonin molecular neuroimaging in essential tremor: A clinical correlation study. Parkinsonism Relat Disord. 2012;18:876–880. doi: 10.1016/j.parkreldis.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Mondrup K, Dupont E, Pederson E. The effect of the GABA-agonist, progabide, on benign essential tremor. Acta Neurol Scand. 1983;68:248–252. doi: 10.1111/j.1600-0404.1983.tb04833.x. [DOI] [PubMed] [Google Scholar]
- 38.Koller WC, Rubino F, Gupta S. Pharmacologic probe with progabide of GABA mechanisms in essential tremor. Arch Neurol. 1987;44:905–906. doi: 10.1001/archneur.1987.00520210007009. [DOI] [PubMed] [Google Scholar]
- 39.Mally J, Stone T. The effect of theophylline on essential tremor: The possible role of GABA. Pharmacol Biochem Behav. 1991;39:345–349. doi: 10.1016/0091-3057(91)90190-D. [DOI] [PubMed] [Google Scholar]
- 40.Pahapill PA, Levy R, Dostrovsky JO, et al. Tremor arrest with thalamic microinjections of muscimol in patients with essential tremor. Ann Neurol. 1999;46:249–252. doi: 10.1002/1531-8249(199908)46:2<249::AID-ANA15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 41.Gironell A, Kulisevsky J, Barbanoj M, López-Villegas D, Hernández G, Pascual-Sedano B. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol. 1999;56:475–480. doi: 10.1001/archneur.56.4.475. [DOI] [PubMed] [Google Scholar]
- 42.Pahwa R, Lyons K, Hubble JP, Busenbark K, Rienerth JD. Double-blind controlled trial of gabapentin in essential tremor. Mov Disord. 1998;13:465–467. doi: 10.1002/mds.870130315. [DOI] [PubMed] [Google Scholar]
- 43.Ondo W, Hunter C, Dat Wuong K, Scwartz K, Jankovic J. Gabapentin for essential tremor: A multiple-dose, double-blind placebo-controlled trial. Mov Disord. 2000;15:678–682. doi: 10.1002/1531-8257(200007)15:4<678::AID-MDS1012>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 44.Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence-based guideline update: Treatment of essential tremor: Report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 2011;17:1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connor GS. A double-blind placebo-controlled trial of topiramate treatment for essential tremor. Neurology. 2002;59:132–134. doi: 10.1212/WNL.59.1.132. [DOI] [PubMed] [Google Scholar]
- 46.Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: A double-blind, placebo-controlled trial. Neurology. 2006;66:672–677. doi: 10.1212/01.wnl.0000200779.03748.0f. [DOI] [PubMed] [Google Scholar]
- 47.Frima N, Grünewald RA. A double-blind, placebo-controlled, crossover trial of topiramate in essential tremor. Clin Neuropharmacol. 2006;2:94–96. doi: 10.1097/00002826-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Connor GS, Edwuards K, Tarsy D. Topiramate in essential tremor: Findings from double-blind, placebo-controlled, crossover trials. Clin Neuropharmacol. 2008;31:97–103. doi: 10.1097/WNF.0b013e3180d09969. [DOI] [PubMed] [Google Scholar]
- 49.Gironell A, Martínez-Corral M, Pagonabarraga X, Kulisevsky J. Tiagabine for essential tremor: An open-label trial. Mov Disord. 2008;23:1955–1956. doi: 10.1002/mds.22094. [DOI] [PubMed] [Google Scholar]
- 50.Zesiewicz TA, Ward CL, Hauser RA, et al. A pilot, double-blind, placebo-controlled trial of pregabalin in the treatment of essential tremor. Mov Disord. 2007;22:1660–1663. doi: 10.1002/mds.21629. [DOI] [PubMed] [Google Scholar]
- 51.Ferrara JM, Kenney C, Davidson AL, Shinawi L, Kissel AM, Jankovic J. Efficacy and tolerability of pregabalin in essential tremor: A randomized, double-blind, placebo-controlled, crossover trial. J Neurol Sci. 2009;285:195–197. doi: 10.1016/j.jns.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 52.Zesiewicz TA, Sullivan KL, Hinson V, et al. Multisite, double-blind, randomized, controlled study of pregabalin for essential tremor. Mov Disord. 2013;28:249–250. doi: 10.1002/mds.25264. [DOI] [PubMed] [Google Scholar]
- 53.Koller WC, Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology. 1989;39:1587–1588. doi: 10.1212/WNL.39.12.1587. [DOI] [PubMed] [Google Scholar]
- 54.Chakrabarti A, Pearce JMS. Essential tremor: Response to primidone. J Neurol Neurosurg Psychiatry. 1981;44:650. doi: 10.1136/jnnp.44.7.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huber SJ, Paulson GW. Efficacy of alprazolam for essential tremor. Neurology. 1988;38:241–243. doi: 10.1212/WNL.38.2.241. [DOI] [PubMed] [Google Scholar]
- 56.Manto M, Laute MA. A possible mechanism for the beneficial effect of ethanol in essential tremor. Eur J Neurol. 2008;15:697–705. doi: 10.1111/j.1468-1331.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 57.Cleeves L, Findley LJ. Propranolol and propranolol-LA in essential tremor: A double blind comparative study. J Neurol Neurosurg Psychiatry. 1988;51:379–384. doi: 10.1136/jnnp.51.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louis ED. Essential tremors: A family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hua SE, Lenz FA, Zirh TA, Reich SG, Dougherty PM. Thalamic neuronal activity correlated with essential tremor. J Neurol Neurosurg Psychiatry. 1998;64:273–276. doi: 10.1136/jnnp.64.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto AD, Lang AE, Chen R. The cerebellothalamocortical pathway in essential tremor. Neurology. 2003;60:1985–1987. doi: 10.1212/01.WNL.0000065890.75790.29. [DOI] [PubMed] [Google Scholar]
- 61.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: A clinicopathologic study of 20 cases. Neurology. 2004;62:932–936. doi: 10.1212/01.WNL.0000115145.18830.1A. [DOI] [PubMed] [Google Scholar]
- 62.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 63.Symanski C, Shill HA, Dugger B, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 64.Deuschl G, Elble R. Essential tremor: Neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;14:2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 65.Rajput AH, Adler CH, Shill HA, Rajput A. Essential tremor is not a neurodegenerative disease. Neurodegener Dis Manag. 2012;2:259–268. doi: 10.2217/nmt.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jellinger KA. Is there cerebellar pathology in essential tremor? Mov Disord. 2014;29:435–436. doi: 10.1002/mds.25852. [DOI] [PubMed] [Google Scholar]