ABSTRACT
Trypanosoma congolense is a major livestock pathogen in Africa, causing large economic losses with serious effects on animal health. Reliable serodiagnostic tests are therefore urgently needed to control T. congolense infection. In this study, we have identified one T. congolense protein as a new candidate serodiagnostic antigen. The 46.4 kDa protein (TcP46, Gene ID: TcIL3000.0.25950) is expressed 5.36 times higher in metacyclic forms than epimastigote forms. The complete nucleotide sequences of TcP46 contained an open reading frame of 1,218 bp. Southern blot analysis indicated that at least two copies of the TcP46 gene were tandemly-arranged in the T. congolense genome. The recombinant TcP46 (rTcP46) was expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein. Western blot analysis and confocal laser scanning microscopy revealed that the native TcP46 protein is expressed in the cytoplasm during all life-cycle stages of the parasite. Moreover, an enzyme-linked immunosorbent assay (ELISA) based on rTcP46 detected the specific antibodies as early as 8 days post-infection from mice experimentally infected with T. congolense. No cross-reactivity was observed in the rTcP46-based ELISA against serum samples from cattle experimentally infected with Babesia bigemina, B. bovis and Anaplasma marginale. These results suggest that rTcP46 could be used as a serodiagnostic antigen for T. congolense infection.
Keywords: diagnosis, ELISA, nagana, TcP46, Trypanosoma.
Trypanosoma congolense is a major pathogen responsible for animal African trypanosomosis (nagana), which is transmitted by the tsetse fly (Glossina spp.) [5]. The disease can affect various species of mammals, and it is particularly important in cattle from the economic viewpoint [15]. The clinical manifestations of the acute stage after infection are fever, listlessness, emaciation, edema, anemia and eventually death [18]. The disease is the main constraint to livestock agriculture in large parts of the African continent, where it causes serious economic losses on an annual basis. The definitive diagnosis of acute animal African trypanosomosis (AAT) depends heavily on the direct detection of the parasite in blood by light-microscopy [12, 29]. However, microscopy has limitations in latent infections where the parasite is often difficult to detect in blood because of extremely low parasitemia. Polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) have recently been developed for detecting T. congolense infection with high sensitivity and specificity [6, 9, 17, 27]. Although these molecular tests potentially improve the specificity and sensitivity of AAT diagnosis, the field application of these state-of-the-art tests is hampered by the requirement for a specialized laboratory setup and skilled personnel. Alternatively, a variety of serodiagnostic tests have been developed for trypanosomosis [4, 13, 19]. In order to detect antibody responses against trypanosome infection, card agglutination test, antibody detection enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent antibody test are commonly utilized as recommended tests [21]. These tests, however, use trypanosome cell lysate or fixed parasite antigens, whose qualities often vary from batch to batch [10, 23]. In contrast, recombinant antigens can easily be prepared in large scale, are relatively stable and have higher specificity than parasite cell lysate antigens [28]. Thus, development of recombinant trypanosome antigen-based ELISA tests is urgently needed. T. congolense has at least four developmental stages in its life cycle, namely bloodstream form (BSF), procyclic form (PCF), epimastigote form (EMF) and metacyclic form (MCF) [22]. Among these forms, BSF and MCF are animal-infective stages, which are the major targets for diagnosis and treatment. Both BSF and MCF express variant surface glycoprotein (VSG), which allows antigenic variation to evade host antibody responses [7]. In general, VSGs are not suitable as diagnostic antigens, because of their antigenic variation. In contrast, the invariant antigens are likely to provoke protective immune responses including high antibody responses in the chronic phase of T. congolense infection [1]. Therefore, the invariant antigens are good candidates for diagnosis and vaccine development. The recombinant invariant surface glycoprotein 75 (ISG75) has been successfully expressed in E. coli, and rISG75-ELISA showed high specificity and sensitivity for T. evansi infection in camels [28]. In previous studies, we reported expressed sequence tags (EST) analysis and differential protein expression in each life cycle stage of T. congolense [8, 11]. The present study focused on identification of the proteins highly expressed in BSF and/or MCF stage from the EST and the proteome data sets and sought to evaluate novel invariable proteins as candidate serodiagnostic antigens for T. congolense infection.
MATERIALS AND METHODS
Parasites: T. congolense IL3000 strain is a savanna type parasite that was discovered near the Kenya/Tanzania border in 1966 (according to the records of the Biological Services Unit at the International Livestock Research Institute, Nairobi, Kenya). Samples of this parasite were stored in liquid nitrogen at the National Research Center for Protozoan Diseases in Japan. The PCF and BSF were cultured using TVM-1 and HMI-9 media, respectively [14]. The EMF and MCF of these parasites were produced from in vitro PCF culture [5, 14, 24]. PCF were routinely maintained by diluting 3 ml of log-phase parasite suspension with 7 ml of fresh medium every 2 days. Adherent EMF appeared in PCF cultures 1–2 months after the initiation of PCF cultures. EMF colonies became confluent within 2 months. The plastic-adherent EMF cultures were maintained by replacing the entire culture supernatant with fresh medium every 2 days. Live PCF were obtained from cultures by centrifugation at 1,500 × g for 10 min at 4°C. Live EMF were prepared from culture flasks by washing adherent cells three times with 10 ml of phosphate-buffered saline (PBS) containing 1% glucose (PSG) to remove non-adherent cells, and the remaining cells were removed with a rubber cell scraper followed by centrifugation at 1,500 × g for 10 min at 4oC. Since differentiation from EMF to MCF continuously occurs in EMF cultures, MCF accumulates in the culture supernatant. Hence, MCF was purified from EMF culture supernatants by DE 52 anion-exchange column chromatography (Whatman Plc., Buckinghamshire, U.K.) [16].
Cloning of the TcP46: Total DNA was extracted from the parasite using a Puregene DNA Purification System Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions and stored at −30°C until used. The open reading frame of the TcP46 gene (Gene ID: TcIL3000.0.25950) was amplified by PCR from T. congolense total DNA using primers with the Eco RI and Sal I sites (underlined), namely P1 (5′-GCGAATTC ATG AAC GGA TCG GCT GT-3′) and P2 (5′-GCGGTCGAC TTA GTA ATT CGC CTC GC-3′). The PCR products were inserted into the pCR2.1-TOPO vector and sequenced with M13 forward and M13 reverse primers. The hydrophilic and antigenic characteristics of TcP46 were predicted using the DNASTAR analyzer program (Netwell, Tokyo, Japan). The putative N-terminal signal peptide was analyzed using the SignalP server (http://www.cbs.dtu.dk/services/SignalP/).
Southern blot analysis: Total DNA was prepared from T. congolense by the phenol-chloroform method [25]. For Southern blot analysis, total DNA was digested overnight with Hind III, Kpn I, Sal I, Xba I, Bcg I, Bsp MI, Msc I and Xho I restriction enzymes and electrophoresed on 1.0% (w/v) agarose gel. The electrophoresed DNA samples were transferred to a nylon membrane (Hybond-N+, GE Healthcare, Pittsburgh, PA, U.S.A.) as previously described [24]. Preparation of the labeled cDNA probe with the full-length TcP46 gene, DNA hybridization and labeling of the probe were performed using AlkPhos Direct Labeling Kit and Detection Systems (GE Healthcare). The result was visualized by using CDP-star (GE Healthcare) according to the manufacturer’s instructions. Imaging was performed using X-ray film (Eastern Kodak Co., Rochester, NY, U.S.A.).
Expression and purification of rTcP46: The open reading frame (ORF) of the TcP46 gene in the pCR2.1-TOPO vector was subcloned into a pGEX-4T-1 Escherichia coli expression vector (GE Healthcare). The correct orientation and sequence of the subcloned TcP46 gene was examined by sequence analysis and designated as the pGEX-4T-1/TcP46. rTcP46 was expressed as a glutathione S-transferase (GST)-fusion protein in the E. coli BL21 strain according to the manufacturer’s instructions (GE Healthcare). In order to purify rTcP46, E. coli was suspended in TNE buffer (50 mM Tris-HCl (pH 7.5), 100 mM NaCl and 2 mM EDTA), sonicated and then centrifuged at 9,000 × g for 10 min at 4°C. The rTcP46-GST fusion protein was affinity purified from the supernatant using glutathione-Sepharose 4B beads (GE Healthcare). Protein concentrations were measured using a modified Lowry protein assay kit (Thermo Scientific, Pittsburgh, PA, U.S.A.).
Preparation of mouse anti-rTcP46 immune sera: Five six-week-old ICR mice (Clea, Tokyo, Japan) were immunized intraperitoneally with 100 µg of purified rTcP46-GST in an equal volume of TiterMax® Gold (TiterMax USA Inc., Norcross, GA, U.S.A.) for the primary immunization. Two booster immunizations were given at 14 day intervals using the same amount of the antigen emulsified in TiterMax® Gold. Serum samples were collected 2 weeks after the last immunization. The experiment was conducted in accordance with the Guiding Principles for the Care and Use of Research Animals of the Obihiro University of Agriculture and Veterinary Medicine (No. 24-135).
Indirect fluorescent antibody test and confocal laser scanning microscopy: Blood smears of T. congolense PCF, EMF, MCF and BSF stages were fixed with 100% methanol for 30 min. Anti-rTcP46 mouse serum, diluted 1:100 with PBS containing 0.5% bovine serum albumin (PBS-BSA), was applied to the fixed smears as the primary antibody and incubated for 1 hr at 37°C. After three washings with PBS, Alexa-Fluor® 488 conjugated goat anti-mouse IgG secondary antibody (1:600 dilution in PBS-BSA, Molecular Probes, Eugene, OR, U.S.A.) was applied and incubated for 30 min at 37°C. The slides were washed four times with PBS and incubated with 6.25 µg/ml propidium iodide (PI) (Molecular Probes) containing 100 µg/ml RNase A (Qiagen) for 10 min at 37°C. After three washings with PBS, the glass slides were mounted by adding 50 µl of a 50% glycerol-PBS (v/v) solution and then covered with a cover glass. The slides were examined by confocal laser scanning microscopy (Leica, Solms, Germany).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis: To identify the molecular mass of native TcP46 throughout the life cycle stages of T. congolense, the mouse anti-rTcP46 serum was used to detect the native TcP46 from trypanosome cell lysates by Western blot analysis. Each life cycle of the parasite was harvested from in vitro culture and washed three times with PBS. The parasite pellets were treated with cell lysis buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM CaCl2, 10% glycerol and 1% Triton X-100) and incubated at 4°C for 1 hr. The cell lysates were sonicated and centrifuged at 7,000 × g for 20 min. The supernatants were collected, and BCA protein assay was used for protein quantification (BCA Protein Assay Kit, PIERCE Chemical Co., Rockford, IL, U.S.A.). The supernatant (50 µg/lane) was then subjected to SDS-PAGE. The cell lysates were mixed with SDS-PAGE sample buffer (125 mM Tris-HCl (pH 6.8), 4% (w/v) SDS, 20% (v/v) glycerol, 3% (v/v) 2-β-mercaptoethanol and 0.02% bromophenol blue). After incubating at 100°C for 5 min, the lysates were separated by SDS-PAGE with 10% gel. The separated parasite proteins were then transferred to an Immobilon-P transfer membrane (Millipore, Billerica, MA, U.S.A.). Western blot analysis was carried out as previously described [2]. To determine the antibody response against TcP46 in mice infected with T. congolense, the GST-rTcP46 (25 µg/lane) and GST protein (25 µg/lane) were subjected to SDS-PAGE, and transferred to a membrane, and then probed with 100 times diluted infected mouse sera and pre-infected mouse sera, respectively, by Western blot analysis.
Mice infections: After collection of blood to obtain pre-infection mouse sera, three ICR mice (female, 8 weeks old) were inoculated intraperitoneally with in vitro prepared BSF (103 parasites/mouse). The parasitemia of each mouse was examined every day for 78 days. Thereafter, it was examined weekly for another month. The level of parasitemia was estimated according to the matching method [12]. This experiment was conducted in accordance with the Guiding Principles for the Care and Use of Research Animals promulgated by Obihiro University of Agriculture and Veterinary Medicine (No. 24-135).
Enzyme-linked immunosorbent assay (ELISA): Individual wells of a microtiter plate (Thermo Scientific) were coated with the purified GST-rTcP46 protein (0.5 µg/well) or the control GST protein (0.5 µg/well) in an antigen coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6) overnight at 4°C. The plates were then blocked with PBS containing 3% (w/v) skim milk for 1 hr at 37°C. After washing, the plates were incubated with 200 times diluted mice serum samples or cattle serum samples. The plates were washed six times with PBST and then incubated with horseradish peroxidase (HRP) conjugated to goat anti-mouse IgG or HRP conjugated to goat anti-bovine IgG (Invitrogen, Carlsbad, CA, U.S.A.) diluted to 1:5,000 with the blocking solution for 1 hr at 37°C. Thereafter, the enzyme reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB, Kirkegaard & Perry Laboratories, Gaithersburg, MD, U.S.A.) at room temperature. Finally, 50 µl of stop solution (1 M phosphoric acid) was added, and the absorbance was read at 450 nm [20]. The cut-off value was defined as the mean value plus 3 standard deviations of the mean optical density (OD) obtained from 9 SPF mice serum samples and 26 normal cattle serum samples, respectively. At the same time, ELISA was also performed according to the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2012) using PCF cell lysate antigen. Each microplate well (Thermo Scientific) was coated with 160 ng of antigen and incubated overnight at 4°C. The subsequent protocols were performed as described above.
Serum samples: The serum samples used in this study include 6 serum samples from mice experimentally infected with T. congolense IL3000 strain, 9 samples from SPF mice, 26 samples from healthy cattle obtained in Japan, 9 samples from cattle experimentally infected with Babesia bovis, 15 samples from cattle experimentally infected with Babesia bigemina and 5 samples from cattle experimentally infected with Anaplasma marginale.
RESULTS
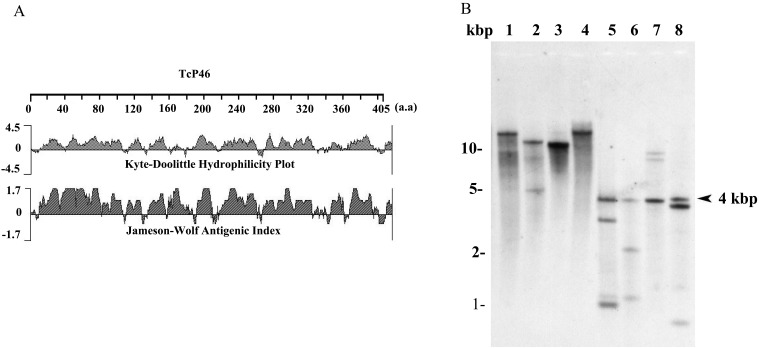
Identification and characterization of the TcP46 gene: Four proteins which were found to have greater expression in MCF and/or BSF stages were selected from the previously reported differential protein expression data set (Table 1) [8]. All of these proteins were successfully expressed by the bacterial expression system and purified for preliminary evaluation as diagnostic antigens. Western blot analysis of T. congolense-infected mouse sera was used for this evaluation. As a result, only the recombinant TcIL3000.0.25950 protein was recognized in the infected mouse sera (Data not shown). Analysis of the putative N-terminal signal peptide in the TcIL3000.0.25950 protein (TcP46) sequence using the SignalP server showed that this sequence had no signal peptide. The TcP46 consisted of highly hydrophilic amino acid residues, and its predicted antigenic index was high throughout the entire sequence (Fig. 1A).
Table 1. Selected proteins with greater expression in BSF and/or MCF than in PCF and EMF.
| Gene ID | Length (bp) | BSF→PCF | PCF→EMF | EMF→MCF | MCF→BSF |
|---|---|---|---|---|---|
| TcIL3000.0.25950 | 1,218 | 0.26 | DNR | 5.36 | DNR |
| TcIL3000.10.3480 | 744 | 0.12 | DNR | 8.98 | DNR |
| TcIL3000.8.6290 | 318 | DNR | 0.37 | 6.94 | DNR |
| TcIL3000.7.1980 | 1,179 | DNR | DNR | DNR | 7.96 |
Number indicates fold expression level of each protein in the two life-cycle stages (Eyford et al., 2011). DNR: Data not reliable.
Fig. 1.
Genetic and molecular characterizations of TcIL3000.0.25950 protein (TcP46). (A) Software analysis of hydrophilicity and antigenicity of TcP46. (B) Southern blot analysis of TcP46. Genomic DNA was treated with Hind III (lane 1), Kpn I (lane 2), Sal I (lane 3), Xba I (lane 4), Bcg I (lane 5), Bsp MI (lane 6), Msc I (lane 7) and Xho I (lane 8). The restriction enzymes used for lanes 1–4 did not cut the TcP46 open reading frame (ORF), while the enzymes used for lanes 5–8 cut a single position within the ORF.
Southern blot analysis: Southern blot analysis was performed to determine the copy number of the TcP46 gene in the parasite genome. The genomic DNA probed with TcP46 cDNA showed a single hybridization band after DNA digestion by Hind III, Kpn I, Sal I and Xba I, which did not cut the TcP46 open reading frame (Fig. 1B, lanes 1–4). However, Bcg I, Bsp MI, Msc I and Xho I cut a single site within the TcP46 gene, yielding three bands with a common fragment at 4 kbp (Fig. 1B, lanes 5–8). These results suggest that genomic DNA of T. congolense contains at least 2 copies of tandemly arranged TcP46 genes. The distance between the tandemly arranged TcP46 genes is 2.8 kbp.
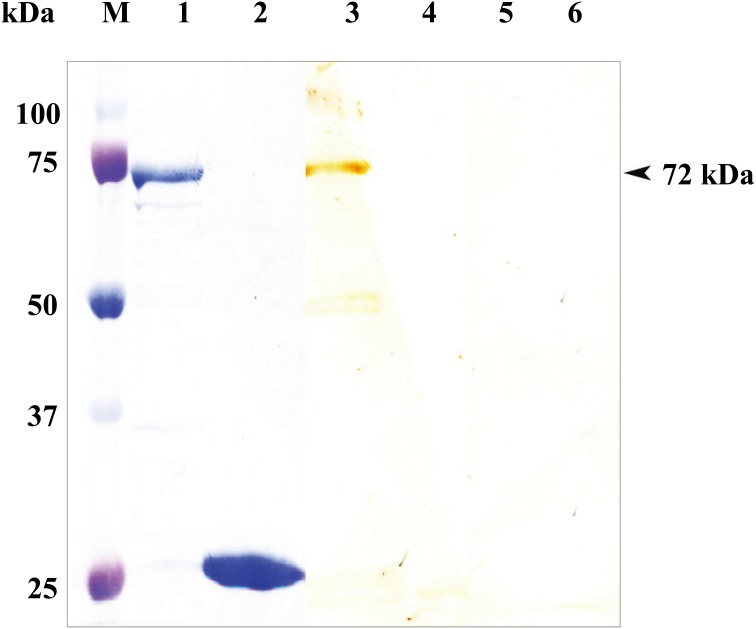
Detection of anti-rTcP46 antibody from T. congolense-infected mouse sera:The full-length TcP46 gene was cloned into prokaryotic expression vector pGEX-4T-1 and expressed in E. coli as a soluble GST-fusion protein with a molecular mass of approximately 72 kDa, including the 26 kDa GST tag (Fig. 2, lane 1). The rTcP46 protein was recognized in sera from mice experimentally infected with T. congolense by Western blot analysis (Fig. 2, lane 3), whereas there was no reaction with the GST protein (Fig. 2, lane 4). Neither GST-rTcP46 nor GST was recognized in pre-immune sera (Fig. 2, lanes 5 and 6).
Fig. 2.
SDS-PAGE and Western blot analysis of the recombinant TcP46. M: Molecular size marker. The rTcP46 fused with GST (lane 1) and purified rGST (lane 2) were stained by amide black. The GST-rTcP46 (lane 3) and the rGST (lane 4) were reacted with serum from mice infected with T. congolense. The rTcP46 (lane 5) and the rGST (lane 6) were reacted with pre-immune mouse sera.
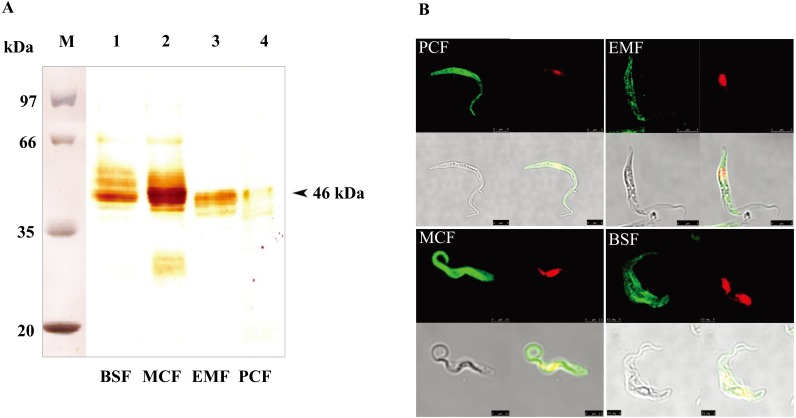
Characterization of the native TcP46: Mouse anti-rTcP46 sera were prepared and used to identify native TcP46 in all life cycle stages of T. congolense by means of Western blot analysis and confocal laser scanning microscopy. The anti-rTcP46 sera specifically reacted with the approximately 46 kDa protein in parasite lysates of all life cycle stages by Western blot analysis. Although the bands were broad, the molecular mass of native TcP46 was consistent with the expected mass (Fig. 3A). This indicates that TcP46 is an invariable protein constitutively-expressed throughout the life cycle stages. Meanwhile, specific and stronger reactions were detected in BSF and MCF-stage parasites in comparison with the weak reactions in the EMF and PCF-stage parasites (Fig. 3A). To determine the cellular localization of TcP46, all stages of T. congolense parasites were probed with the mouse anti-rTcP46 serum. Confocal laser scanning microscopy demonstrated that the expression of native TcP46 was mainly in the cytoplasm in all of the developmental stages (Fig. 3B).
Fig. 3.
Detection of the native TcP46 from all life cycle stages of the parasite. (A) Lane M: Molecular size marker. Western blot analysis of the native TcP46 was carried out using the cell lysate from BSF, MCF, EMF and PCF stages of T. congolense and anti-rTcP46 mouse serum. (B) Cellular localizations of the TcP46 in all four life cycle stages of T. congolense (PCF, EMF, MCF and BSF) were examined by immunofluorescence staining and confocal laser scanning microscopy. Green indicates immunofluorescence staining of TcP46, and red indicates nucleus and kinetoplast.
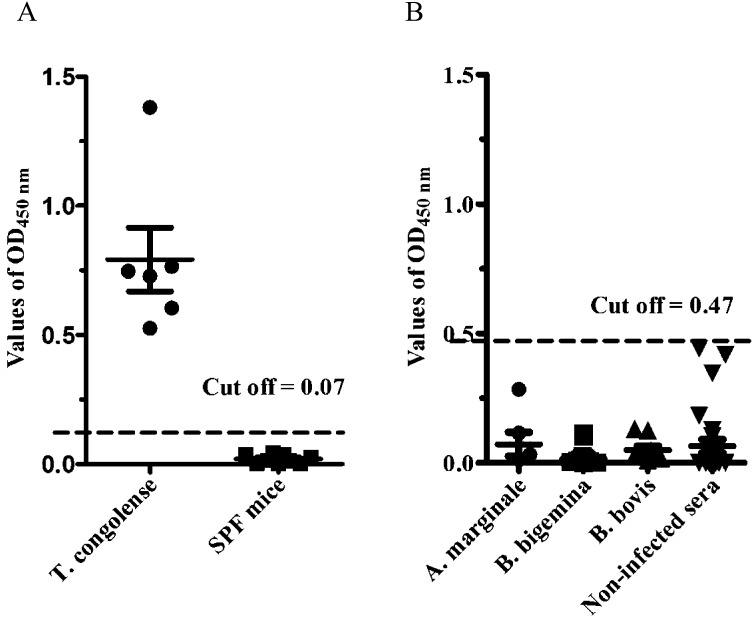
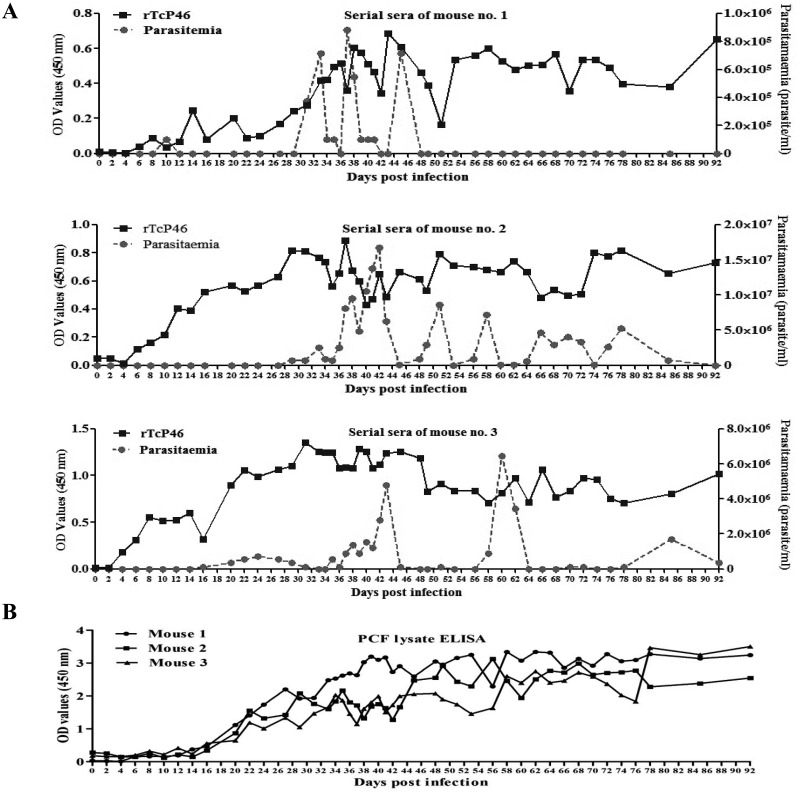
Specificity and sensitivity of the rTcP46-based ELISA: The specificity of the rTcP46-based ELISA (P46-ELISA) was evaluated using the sera of mice experimentally infected with T. congolense and the sera of non-infected SPF mice. The cut-off value of the P46-ELISA was 0.07. This was calculated by the OD value from the serum samples of 9 SPF mice. Furthermore, the 6 serum samples from mice experimentally infected with T. congolense showed a high absorbance value. There was no cross-reaction with 26 serum samples from healthy cattle, 9 samples from B. bovis-infected cattle, 15 samples from B. bigemina-infected cattle or 5 samples from A. marginale-infected cattle (Fig. 4). The cut-off value of the P46-ELISA for cattle serum samples was 0.46 (Fig. 4B). The sensitivity of P46-ELISA was examined using sera sequentially obtained from 3 mice infected with T. congolense IL3000. Specific antibodies against TcP46 were detected from sera of the 3 mice as early as 8 days post-infection. High antibody titers were maintained until the chronic stage of infection, which was characterized by undetectable levels of parasitemia (Fig. 5A); 6 days earlier than PCF cell lysate ELISA (Fig. 5B).
Fig. 4.
Evaluation of the specificity of rTcP46-based ELISA. (A) Evaluation of specificity with T. congolense experimentally infected mouse sera (n=6); SPF mouse sera (n=9), dashed line indicates the cutoff value (0.07). (B) Evaluation of specificity with Babesia bovis experimentally infected cattle sera (n=9); Babesia bigemina experimentally infected cattle sera (n=15); Anaplasma marginale experimentally infected cattle sera (n=5), dashed line indicates the cutoff value (0.46).
Fig. 5.
(A) Detection of the specific antibody response against TcP46 in three mice experimentally infected with T. congolense by rTcP46-based ELISA. (B) Detection of the antibody responses in three mice experimentally infected with T. congolense by means of PCF cell lysate antigen ELISA.
DISCUSSION
T. congolense infection (nagana) causes significant losses in livestock production in Africa. To control this disease, it is important to develop sensitive and reliable serological tests for the detection of T. congolense infection in animals. So far, only a few recombinant antigens have been identified to develop serological diagnostic methods of trypanosome infection [2, 3, 26, 28]. Therefore, there is a need to seek more candidate diagnostic antigens in order to develop accurate and sensitive serodiagnostics for nagana. Since T. congolense undergoes a complex developmental cycle, each developmental stage of the parasite expresses both stage-specific and constitutive proteins. In this study, we focused on the proteins with high expression levels in the MCF and BSF stages, because of their importance in serodiagnosis. Four proteins (TcIL3000.0.25950, TcIL3000.0.10.3480, TcIL3000.8.629 and TcIL3000.7.1980) were selected from the data for differential protein expression in all life cycle stages of T. congolense (Table 1) [8]. All of these proteins were successfully expressed by the bacterial expression system and purified for preliminary evaluation as a diagnostic antigen by Western blot analysis using T. congolense-infected mouse sera (Data not shown). As a result, only the recombinant TcIL3000.0.25950 protein was recognized by T. congolense-infected mouse sera (Fig. 2). Thus, we decided to further investigate the TcIL3000.0.25950 protein as a candidate serodiagnostic antigen. The TcIL3000.0.25950 gene contained an ORF of 1,218 bp encoding a 46.4 kDa protein (TcP46). Southern blot analysis revealed that at least 2 copies of the TcP46 gene are tandemly arranged in the parasite genome (Fig. 1B). The TcP46 protein is expressed throughout the life cycle stages of the parasite as an approximately 46 kDa protein (Fig. 3A). As the TcP46 was predicted to be a soluble protein, TcP46 was localized in the parasite cytosol (Fig. 3B). Meanwhile, a strong reaction was observed in the BSF and MCF parasite stages compared with EMF and PCF. This result, in part, consists with the previously reported proteome analysis which revealed that TcP46 showed 5.36 times higher expression levels in MCF than EMF parasite levels [8]. The potential of rTcP46 as a serodiagnostic antigen was evaluated by ELISA with sequentially collected serum samples from T. congolense experimentally infected mice. The results showed that rTcP46-based ELISA was able to detect a specific antibody response from 8 days post-infection until the end of the experiment (92 days post-infection) (Fig. 5). This would imply that rTcP46-based ELISA may be an applicable diagnostic test of both the acute and chronic stages of the infection. In addition, the high antigenicity suggested that TcP46 may play an important role in the host immune response during T. congolense infection. No false-positive samples due to cross-reaction with sera derived from cattle infected with B. bovis, B. bigemina or A. marginale were detected by P46-ELISA. Since mixed infection of these protozoan parasites and T. congolense possibly occurs, the result indicates that ELISA could be a specific test.
In conclusion, the TcP46 gene was identified and characterized as an immunodominant antigen that was a candidate serodiagnostic antigen of T. congolense infection. The GST-rTcP46-based ELISA had high specificity and was applicable for both the acute and chronic stages of infection. Overall, TcP46 might be a useful serodiagnostic antigen for T. congolense infection. A further study will require the use of a number of serum samples from T. congolense-infected cattle in order to evaluate its practical use in the field.
Acknowledgments
This study was financially supported by a grant from the Global COE Program and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan and Japan Society for the Promotion of Science (JSPS). This research was in part supported by JST/JICA, SATREPS to YS.
REFERENCES
- 1.Agur Z., Mehr R.1997. Modelling Trypanosoma congolense parasitaemia patterns during the chronic phase of infection in N’Dama cattle. Parasite Immunol. 19: 171–182. doi: 10.1046/j.1365-3024.1997.d01-195.x [DOI] [PubMed] [Google Scholar]
- 2.Bannai H., Sakurai T., Inoue N., Sugimoto C., Igarashi I.2003. Cloning and expression of mitochondrial heat shock protein 70 of Trypanosoma congolense and potential use as a diagnostic antigen. Clin. Diagn. Lab. Immunol. 10: 926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulangé A., Katende J., Authié E.2002. Trypanosoma congolense: expression of a heat shock protein 70 and initial evaluation as a diagnostic antigen for bovine trypanosomosis. Exp. Parasitol. 100: 6–11. doi: 10.1006/expr.2001.4667 [DOI] [PubMed] [Google Scholar]
- 4.Chappuis F., Loutan L., Simarro P., Lejon V., Büscher P.2005. Options for field diagnosis of human african trypanosomiasis. Clin. Microbiol. Rev. 18: 133–146. doi: 10.1128/CMR.18.1.133-146.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coustou V., Guegan F., Plazolles N., Baltz T.2010. Complete in vitro life cycle of Trypanosoma congolense: development of genetic tools. PLoS Negl. Trop. Dis. 4: e618. doi: 10.1371/journal.pntd.0000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox A. P., Tosas O., Tilley A., Picozzi K., Coleman P., Hide G., Welburn S. C.2010. Constraints to estimating the prevalence of trypanosome infections in East African zebu cattle. Parasit. Vectors 3: 82. doi: 10.1186/1756-3305-3-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donelson J. E., Hill K. L., El-Sayed N. M.1998. Multiple mechanisms of immune evasion by African trypanosomes. Mol. Biochem. Parasitol. 91: 51–66. doi: 10.1016/S0166-6851(97)00209-0 [DOI] [PubMed] [Google Scholar]
- 8.Eyford B. A., Sakurai T., Smith D., Loveless B., Hertz-Fowler C., Donelson J. E., Inoue N., Pearson T. W.2011. Differential protein expression throughout the life cycle of Trypanosoma congolense, a major parasite of cattle in Africa. Mol. Biochem. Parasitol. 177: 116–125. doi: 10.1016/j.molbiopara.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillingwater K., Mamabolo M. V., Majiwa P. A.2010. Prevalence of mixed Trypanosoma congolense infections in livestock and tsetse in KwaZulu-Natal, South Africa. J. S. Afr. Vet. Assoc. 81: 219–223. doi: 10.4102/jsava.v81i4.151 [DOI] [PubMed] [Google Scholar]
- 10.Goto Y., Duthie M. S., Nguyen T. T., Asada M., Kawazu S., Carter D., Inoue N.2011. Serological characterizations of tandem repeat proteins for detection of African trypanosome infection in cattle. Parasitol. Int. 60: 538–540. doi: 10.1016/j.parint.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helm J. R., Hertz-Fowler C., Aslett M., Berriman M., Sanders M., Quail M. A., Soares M. B., Bonaldo M. F., Sakurai T., Inoue N., Donelson J. E.2009. Analysis of expressed sequence tags from the four main developmental stages of Trypanosoma congolense. Mol. Biochem. Parasitol. 168: 34–42. doi: 10.1016/j.molbiopara.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert W. J., Lumsden W. H.1976. Trypanosoma brucei: A rapid matching method for estimating the hosts parasitemia. Exp. Parasitol. 40: 427–431. doi: 10.1016/0014-4894(76)90110-7 [DOI] [PubMed] [Google Scholar]
- 13.Hilali M., Abdel-Gawad A., Nassar A., Abdel-Wahab A., Magnus E., Büscher P.2004. Evaluation of the card agglutination test (CATT/T. evansi) for detection of Trypanosoma evansi infection in water buffaloes (Bubalus bubalis) in Egypt. Vet. Parasitol. 121: 45–51. doi: 10.1016/j.vetpar.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Hirumi H., Hirumi K.1991. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology 102: 225–236. doi: 10.1017/S0031182000062533 [DOI] [PubMed] [Google Scholar]
- 15.Kristjanson P. M., Swallow B. M., Rowlands G. J., Kruska R. L., de Leeuw P. N.1999. Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research. Agric. Syst. 59: 79–98. doi: 10.1016/S0308-521X(98)00086-9 [DOI] [Google Scholar]
- 16.Lanham S. M., Godfrey D. G.1970. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp. Parasitol. 28: 521–534. doi: 10.1016/0014-4894(70)90120-7 [DOI] [PubMed] [Google Scholar]
- 17.Laohasinnarong D., Thekisoe O. M., Malele I., Namangala B., Ishii A., Goto Y., Kawazu S., Sugimoto C., Inoue N.2011. Prevalence of Trypanosoma sp. in cattle from Tanzania estimated by conventional PCR and loop-mediated isothermal amplification (LAMP). Parasitol. Res. 109: 1735–1739. doi: 10.1007/s00436-011-2513-2 [DOI] [PubMed] [Google Scholar]
- 18.Mendoza-Palomares C., Biteau N., Giroud C., Coustou V., Coetzer T., Authié E., Boulangé A., Baltz T.2008. Molecular and biochemical characterization of a cathepsin B-like protease family unique to Trypanosoma congolense. Eukaryot. Cell 7: 684–697. doi: 10.1128/EC.00405-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nantulya V. M., Songa E. B., Hamers R.1989. Detection of circulating trypanosomal antigens in Trypanosoma evansi-infected animals using a T. brucei group-specific monoclonal-antibody. Trop. Med. Parasitol. 40: 263–266 [PubMed] [Google Scholar]
- 20.Nguyen T. T., Goto Y., Lun Z. R., Kawazu S., Inoue N.2012. Tandem repeat protein as potential diagnostic antigen for Trypanosoma evansi infection. Parasitol. Res. 110: 733–739. doi: 10.1007/s00436-011-2632-9 [DOI] [PubMed] [Google Scholar]
- 21.OIE, 2012. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th ed. OIE, Paris. [Google Scholar]
- 22.Peacock L., Cook S., Ferris V., Bailey M., Gibson W.2012. The life cycle of Trypanosoma (Nannomonas) congolense in the tsetse fly. Parasit. Vectors 5: 109. doi: 10.1186/1756-3305-5-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebeski D. E., Winger E. M., Okoro H., Kowalik S., Bürger H. J., Walters D. E., Robinson M. M., Dwinger R. H., Crowther J. R.2000. Detection of Trypanosoma congolense antibodies with indirect ELISAs using antigen-precoated microtitre plates. Vet. Parasitol. 89: 187–198. doi: 10.1016/S0304-4017(00)00194-1 [DOI] [PubMed] [Google Scholar]
- 24.Sakurai T., Sugimoto C., Inoue N.2008. Identification and molecular characterization of a novel stage-specific surface protein of Trypanosoma congolense epimastigotes. Mol. Biochem. Parasitol. 161: 1–11. doi: 10.1016/j.molbiopara.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J., Russell D.W., 2001. Preparation and analysis of eukaryotic genomic DNA, pp. 6.1–6.64. In: Molecular Cloning (Sambrook, J. and Russell, D.W. eds.), Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- 26.Sengupta P. P., Balumahendiran M., Balamurugan V., Rudramurthy G. R., Prabhudas K.2012. Expressed truncated N-terminal variable surface glycoprotein (VSG) of Trypanosoma evansi in E. coli exhibits immuno-reactivity. Vet. Parasitol. 187: 1–8. doi: 10.1016/j.vetpar.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 27.Thekisoe O. M., Kuboki N., Nambota A., Fujisaki K., Sugimoto C., Igarashi I., Yasuda J., Inoue N.2007. Species-specific loop-mediated isothermal amplification (LAMP) for diagnosis of trypanosomosis. Acta Trop. 102: 182–189. doi: 10.1016/j.actatropica.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Tran T., Claes F., Verloo D., De Greve H., Büscher P.2009. Towards a new reference test for surra in camels. Clin. Vaccine Immunol. 16: 999–1002. doi: 10.1128/CVI.00096-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo P. T.1970. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 27: 384–386 [PubMed] [Google Scholar]