ABSTRACT
The present study was carried out to analyze the prognosis of 163 cats with lymphoma classified anatomically and cytomorphologically. Anatomically, alimentary lymphoma was the most common form and showed significantly shorter survival than mediastinal and nasal lymphomas in cats. Cytomorphologically, there was no predominant subtype in feline lymphomas. Immunoblastic type (18%), centroblastic type (16%), globule leukocyte type (15%), lymphocytic type (12%), lymphoblastic type (12%), pleomorphic medium and large cell type (10%) and anaplastic large cell type (7%) were relatively common subtypes. Most of the cats with globule leukocyte lymphoma had the alimentary form. Comparing median survival time among classifications, cats with globule leukocyte lymphoma showed significantly shorter survival than those with high-grade and other low-grade lymphomas. Furthermore, cats with high-grade lymphomas showed significantly shorter survival than cats with other low-grade lymphomas. The present study indicated the clinical significance of anatomical and cytomorphological evaluation in feline lymphomas.
Keywords: feline, Kiel classification, lymphomas, prognosis
Lymphoma is one of the common neoplasms in cats. Recently, there are increased interest in evaluation of prognosis and choice of treatment. Also, lymphoma is known to have a great diversity of its morphology and biological behavior.
Of the various classification systems for non-Hodgkin’s lymphomas in humans [15], the Kiel classification was proposed in 1974, and then, the updated Kiel classification was created based on revision of the Kiel classification in 1988; both were used widely in Europe. At the same period, the WF (Working Formulation) classification was proposed by the National Cancer Institute (1982) and used mainly in North America. Since then, classification systems have continued to evolve, such as the updated Kiel classification and the REAL (Revised European-American classification of Lymphoid neoplasms, 1994) classification, with the updated WHO (World Health Organization) classification being established in 2001. Although most of the classifications require histopathology, it is not always possible to perform tissue biopsy at the time of diagnosis in small animal medicine. Thus, the Kiel classification is thought to be very convenient, because of its utility in determining subtypes by cellular morphology and the fact that it does not always require histopathological observation.
There are some studies about cytomorphology based on the Kiel classification in canine lymphoma [12, 13]. In those studies, cytomorphology was found to be of prognostic importance for overall survival time in dogs with lymphoma. However, there has been no study investigating the prognostic significance of cytomorphology of feline lymphoma, since it has been reported that morphological classification is applicable to feline lymphoma [1]. Moreover, studies comparing outcomes of each anatomical form of lymphoma have been poorly reported.
The aim of the present study was to evaluate whether the outcome of feline lymphoma is associated with anatomical status and cytomorphological grades according to the established classification of feline lymphoma [1]. The hypotheses of the present study were considered from practical experiences in cats and previous research of other species showing that alimentary lymphoma and high-grade lymphomas in cats could indicate poorer outcome.
MATERIALS AND METHODS
Cases: Cats with lymphoma and no concurrent severe disorder that were treated with chemotherapeutic agents at the Veterinary Medical Center of the University of Tokyo (UT-VMC) were included and analyzed in the present study. A total of 163 cats referred to the UT-VMC between 2000 and 2010 were diagnosed with lymphoma by cytopathological or histopathological examination. From these cases, samples for cytology and/or histopathology were obtained and evaluated. Of the cases, 76 cats described previously [1] were also included in the present prognostic study.
Treatment: Cats with high-grade lymphomas basically received an initial combination chemotherapy with L-asparaginase (400 IU/kg, S.C., week 1), vincristine (0.7 mg/m2, I.V., weeks 1, 3, 6, 8, 11, 15, 19 and 23), cyclophosphamide (250 mg/m2, I.V., weeks 2, 7, 13 and 21), doxorubicin (30 mg/m2, I.V., weeks 4, 9, 17 and 25) and prednisolone (2.0 mg/kg, q24h, P.O., tapered within 4 weeks). Cats with low-grade lymphomas including globule leukocyte lymphoma basically received an initial therapy with prednisolone (2.0 mg/kg, q24h, P.O., tapered within 12 weeks) as well as chlorambucil (2.0 mg/head, q3-7d, P.O., tapered in some cases) or melphalan (2.0 mg/head, q3-7d, P.O., tapered in some cases).
Cytopathological and histopathological evaluation: Cytological specimens stained with Wright-Giemsa stain and/or histological specimens stained with Hematoxylin-Eosin stain were reviewed to investigate morphological characteristics by at least two clinical pathologists (Fujino Y and Sato H and/or Chino J). By evaluating cytological or histological status, the subtype of the lymphoma was assigned according to the previous report based on the Kiel classification [1]. All cases with low-grade lymphomas were confirmed with histopathology by a pathologist (Uchida K).
Survival time: Overall survival time (OST) was defined according to the reported criteria [16]. OST was calculated as the time between the initiation of treatment and death resulting from any cause. Cats that were alive were censored.
Statistical analyses: OST curves were estimated by Kaplan-Meyer statistics for the different groups of cats. Univariate analyses on anatomical form, grades and other patient factors including immunophenotypes as well as WHO stages and substages for comparison of OST among different groups of cats with lymphoma were performed by using the log-rank test. Statistically significant factors by the univariate analysis were subjected to multivariate analysis by using the Cox proportional regression model. A value of P<0.05 was considered statistically significant.
RESULTS
Population: Of the 163 cats with lymphoma, 92 (56%) cats were male (80 were castrated), and 71 (44%) were female (59 were spayed). The median age was 10 years (range, 1–19 years). The median body weight was 3.7 kg (range, 2.0–8.8 kg). The breeds were as follows: mongrel (125 cats), American shorthair (14 cats), Persian (10 cats), Russian Blue (4 cats), Scottish Fold (3 cats), Abyssinian (2 cats), Ocicat (1 cat), Siamese (1 cat), Somali (1 cat), Singapura (1 cat) and Himalayan (1 cat). Of the 128 cats with lymphoma tested for infections with feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) at the time of diagnosis, 17 (13%) cats were positive for FeLV antigen, and 14 (11%) cats were positive for FIV antibody in the blood.
Anatomical classification of lymphoma cases: The anatomical distribution of lymphoma cases is presented in Table 1. Of all the cases, 85 (52%) cats were classified as having alimentary lymphoma, which was the most common form, followed by 25 (15%) cats classified as having nasal lymphoma, 19 (12%) cats classified as having mediastinal lymphoma, 14 (9%) cats classified as having multicentric lymphoma and 20 (12%) cats classified as having other forms (8 kidney, 5 cutaneous, 3 ocular, 3 pharyngolaryngeal and 1 skeletal muscle) of lymphoma.
Table 1. Cytomorphological classification and outcome in feline lymphomas.
| Classification | Total | Anatomical form |
MSTb) (days) | ||||
|---|---|---|---|---|---|---|---|
| Alimentarya) | Nasal | Mediastinal | Multicentric | Others | |||
| Low grade | 57 | 45 | 0 | 4 | 3 | 5 | 68 (315c)) |
| Lymphocytic | 20 | 13 | 0 | 3 | 3 | 1 | 315 |
| Small clear cell | 5 | 4 | 0 | 1 | 0 | 0 | All survived (>200 days) |
| Prolymphocytic | 4 | 2 | 0 | 0 | 0 | 2 | 64 |
| Centrocytic | 2 | 2 | 0 | 0 | 0 | 0 | 257 |
| Pleomorphic small | 1 | 1 | 0 | 0 | 0 | 0 | 25 |
| Mycosis fungoides | 1 | 0 | 0 | 0 | 0 | 1 | 521 |
| Globule leukocyted) | 24 | 23 | 0 | 0 | 0 | 1 | 29 |
| High grade | 106 | 40 | 25 | 15 | 11 | 15 | 86 |
| Immunoblastic | 30 | 5 | 10 | 6 | 3 | 6 | 109 |
| Centroblastic | 26 | 10 | 6 | 2 | 5 | 3 | 75 |
| Lymphoblastic | 19 | 5 | 5 | 6 | 1 | 2 | 119 |
| Pleomorphic medium/large | 16 | 11 | 3 | 0 | 0 | 2 | 48 |
| Anaplastic large cell | 11 | 7 | 0 | 1 | 2 | 1 | 75 |
| Burkitt like | 4 | 2 | 1 | 0 | 0 | 1 | 163 |
| Total | 163 | 85 | 25 | 19 | 14 | 20 | 84 |
| MSTb) (days) | 76 | 48 | 135 | 143 | 95 | 92 | |
a) Alimentary lymphomas, which include the liver form. b) MST: median survival time. c) MST of low-grade type lymphomas, which exclude globule leukocyte lymphoma. d) A subtype of feline lymphoma without human counterparts.
Morphological subtypes of lymphoma: By microscopic observation of the cytopathological and/or histopathological specimens of lymphoma, morphological features of lymphoma from all of the 163 cases could be classified according to the previously established classification [1]. In all, 106 (65%) cases were classified as high-grade lymphomas, and 57 (35%) cases were classified as low-grade lymphomas (Table 1). Cytomorphologically, there was no predominant subtype in feline lymphomas. Relatively common lymphomas included the immunoblastic type (18%), centroblastic type (16%), globule leukocyte type (15%), lymphocytic type (12%), lymphoblastic type (12%), pleomorphic medium and large cell type (10%) and anaplastic large cell type (7%).
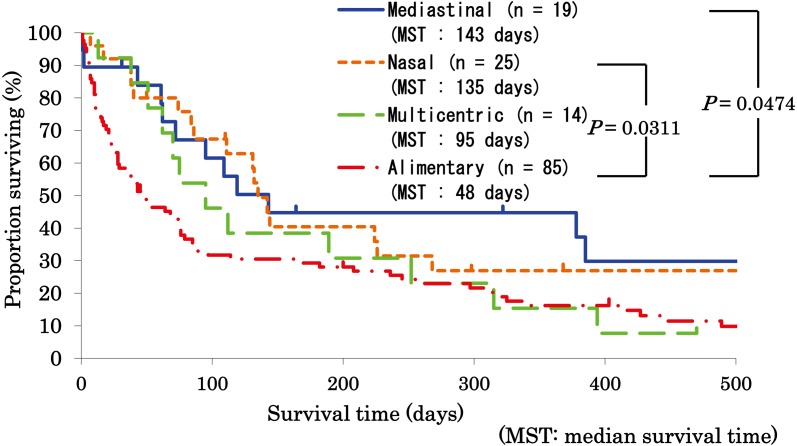
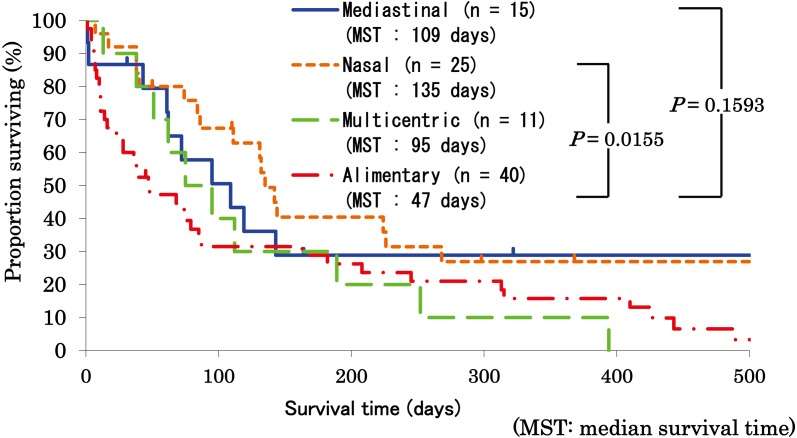
Survival analyses: At the end point of the study, 29 cats were alive, and 134 cats were dead. Comparing median survival time among anatomical forms, cats with alimentary lymphoma showed a significantly shorter median survival (48 days) than cats with nasal lymphoma (135 days; P=0.0311) and cats with mediastinal lymphoma (143 days; P=0.0474) (Fig. 1). To analyze the effect of lymphoma grading, high-grade lymphomas of each anatomical form were compared statistically, although most of the cats with low-grade lymphomas showed the alimentary form and there was a lack of statistically sufficient cases for comparison of each form of low-grade lymphoma. In the cases with high-grade lymphomas, cats with alimentary lymphoma also showed a significantly shorter median survival (47 days) than cats with nasal lymphoma (135 days; P=0.0155); however, there was no significant survival difference between cats with alimentary lymphoma and cats with mediastinal lymphoma (P=0.1593; Fig. 2).
Fig. 1.
Comparison of survival among anatomical forms in feline lymphoma. Survival of cats with mediastinal (n=19), nasal (n=25), multicentric (n=14) and alimentary (n=85) lymphoma is statistically compared by the log-rank test. Median survival time (MST) of cats with each form of lymphoma is also described. Cats with alimentary lymphoma showed a significantly shorter median survival (48 days) than cats with nasal lymphoma (135 days) and cats with mediastinal lymphoma (143 days).
Fig. 2.
Comparison of survival among anatomical forms in feline high-grade lymphoma. Survival of cats with mediastinal (n=15), nasal (n=25), multicentric (n=11) and alimentary (n=40) high-grade lymphoma is statistically compared by the log-rank test. Median survival time (MST) of cats with each form of high-grade lymphoma is also described. Cats with alimentary high-grade lymphoma showed a significantly shorter median survival (47 days) than cats with nasal high-grade lymphoma (135 days). However, there was no significant survival difference between cats with alimentary high-grade lymphoma and cats with mediastinal high-grade lymphoma.
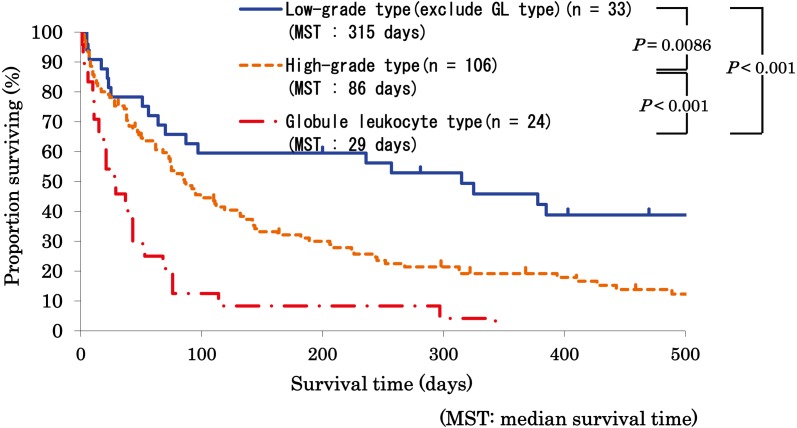
Comparing median survival time among cytomorphological grades, there was no significant difference between the high-grade type and the low-grade type. However, spinning off the globule leukocyte type from the low-grade type, which was the most common subtype in the low-grade type, cats with globule leukocyte lymphoma showed a significantly shorter median survival (29 days) than cats with high-grade lymphomas (86 days; P<0.001) and cats with other low-grade lymphomas (315 days; P<0.001). Furthermore, cats with high-grade lymphomas showed significantly shorter median survival than cats with other low-grade lymphomas (P=0.0086; Fig. 3).
Fig. 3.
Comparison of survival among cytomorphological classification in feline lymphoma. Survival of cats with low-grade excluding globule leukocyte (GL) type (n=33), high-grade (n=106) and GL (n=24) lymphoma is statistically compared by the log-rank test. Median survival time (MST) of cats with each lymphoma is also described. Cats with GL lymphoma showed a significantly shorter median survival (29 days) than cats with high-grade lymphomas (86 days) and cats with other low-grade lymphomas (315 days). Furthermore, cats with high-grade lymphomas showed a significantly shorter median survival than cats with other low-grade lymphomas.
Comparing median survival time among immunophenotypes or WHO stages, no significant difference was observed. Since most of the cases showed WHO substage b (presence of clinical signs associated with lymphoma), comparison of WHO substages lacked statistically sufficient cases. The Cox proportional regression model revealed that the anatomical form and grades showed statistical significance (P<0.05) as independent prognostic factors.
DISCUSSION
Anatomically, alimentary lymphoma, which was accounted for more than a half of the cases, was the most common form of feline lymphoma in the present study. Since wide implementation of tests and vaccination programs as well as indoor rearing for the control of FeLV infection began, FeLV-negative alimentary lymphoma has been the most common form [17]. FeLV has been associated with mediastinal (thymic) lymphomas other than alimentary lymphoma in cats [3]. Cats with alimentary lymphoma had a significantly shorter median survival than cats with nasal or mediastinal lymphoma in the present study. Alimentary lymphoma has previously been described to have unsatisfactory treatment results [9, 10, 14], and mediastinal lymphoma has also been reported to carry a poorer prognosis [5, 17]. In the present study, the median age of cats with mediastinal lymphoma was 10 years which was older than that described in previous reports, and only 10 (53%) of the 19 cats with mediastinal lymphoma were positive for FeLV antigen in the blood. These results indicated that the case populations varied compared with previous reports [5, 17]. To analyze the survival effect of lymphoma grading against anatomical form, high-grade lymphomas of each anatomical form were compared statistically (Fig. 2), although many cats with low-grade lymphomas showed the alimentary form and comparison of each form of low-grade lymphoma lacked statistical power. In the high-grade type, though there was no significant difference between cats with alimentary lymphoma and mediastinal lymphoma, a significant difference in survival was observed between cats with alimentary lymphoma and nasal lymphoma. It was noted in the present study that there was a significant difference in grading of feline lymphomas regardless of their anatomic forms (Fig. 3). Low-grade lymphomas other than globule leukocyte lymphoma showed a better outcome than high-grade lymphomas in cats. Immunophenotypes and WHO stages of the cases indicated no significance in survival of cats with lymphoma in the present study. The results suggest that not only anatomical statuses but also cytopathological grades are important for prognosis in feline lymphoma.
Based on the original Kiel classification, 139 of 163 feline cases could be classified into the subtypes observed in human non-Hodgkin’s lymphoma. The remaining 24 cases were classified as globule leukocyte lymphoma, which was not observed in humans [1]. Although the centroblastic type was the most dominant subtype in canine lymphomas [12], there was no predominant subtype in feline lymphomas, as observed previously [1].
The globule leukocyte neoplasm is a kind of large granular lymphocyte (LGL) lymphoma, which is distinct from other lymphomas based on its unique morphology and clinical aspects [1, 2, 4, 6, 11]. Globule leukocyte lymphoma has been considered to originate from cytotoxic T cells or natural killer cells according to the phenotype and the perforin-like activity [1, 2, 4, 6, 7, 11]. Although LGL lymphoma has been reported to be minimally responsive to chemotherapy and to be associated with grave outcome [8], the present study clarified the significant difference in survival between globule leukocyte lymphoma and the other lymphomas statistically for the first time. The unfortunate outcome of globule leukocyte lymphoma in cats might be caused by most cases of this lymphoma being alimentary malignancies and globule leukocytes initially retaining cytotoxicity, which could lead to gastrointestinal mucosal injury and perforation [1, 2, 4, 6, 7, 11]. Thus, a further clinical study employing more feline cases is required to compare outcomes among subtypes of alimentary lymphoma.
The present study included some limitations, such as retrospective analyses, referral cases, heterogenous population of anatomic form and use of treatment variations other than the initial therapy depending on the clinical case. Analyses of larger numbers of cases with each anatomical form of lymphoma as well as prospective case controlled studies should be performed to confirm the prognostic values of the factors.
Consequently, the present study was the first report indicating the clinical significance of both anatomical and cytomorphological classification in feline lymphomas. Lymphoid neoplasms show considerable variations in cellular origin and biological behavior. It is important in further studies for feline lymphomas to be classified into anatomical forms and cytomorphological subtypes to extend the clinical significance as well as scientific knowledge and to have a better understanding of their biological behavior.
Acknowledgments
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors thank all the veterinarians and stuff of Kariya Animal Hospital, Japan Small Animal Medical Center and Suto Animal Hospital for offering many specimens.
REFERENCES
- 1.Chino J., Fujino Y., Kobayashi T., Kariya K., Goto-Koshino Y., Ohno K., Nakayama H., Tsujimoto H.2013. Cytomorphological and Immunological Classification of Feline Lymphomas: Clinicopathological Features of 76 Cases. J. Vet. Med. Sci. 75: 701–707. doi: 10.1292/jvms.12-0246 [DOI] [PubMed] [Google Scholar]
- 2.Finn J. P., Schwartz L. W.1972. A neoplasm of globule leucocytes in the intestine of a cat. J. Comp. Pathol. 82: 323–326. doi: 10.1016/0021-9975(72)90012-6 [DOI] [PubMed] [Google Scholar]
- 3.Fujino Y., Ohno K., Tsujimoto H.2008. Molecular pathogenesis of feline leukemia virus-induced malignancies: insertional mutagenesis. Vet. Immunol. Immunopathol. 123: 138–143. doi: 10.1016/j.vetimm.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 4.Honor D. J., De Nicola D. B., Turek J. J., Render J. A., Serra D. A.1986. A neoplasm of globule leukocytes in a cat. Vet. Pathol. 23: 287–292 [DOI] [PubMed] [Google Scholar]
- 5.Jeglum K. A., Whereat A., Young K.1987. Chemotherapy of lymphoma in 75 cats. J. Am. Vet. Med. Assoc. 190: 174–178 [PubMed] [Google Scholar]
- 6.Kariya K., Konno A., Ishida T.1997. Perforin-like immunoreactivity in four cases of lymphoma of large granular lymphocytes in the cat. Vet. Pathol. 34: 156–159. doi: 10.1177/030098589703400210 [DOI] [PubMed] [Google Scholar]
- 7.Konno A., Hashimoto Y., Kon Y., Sugimura M.1994. Perforin-like immunoreactivity in feline globule leukocytes and their distribution. J. Vet. Med. Sci. 56: 1101–1105. doi: 10.1292/jvms.56.1101 [DOI] [PubMed] [Google Scholar]
- 8.Krick E. L., Little L., Patel R., Shofer F. S., Sorenmo K., Clifford C. A., Baez J. L.2008. Description of clinical and pathological findings, treatment and outcome of feline large granular lymphocyte lymphoma (1996–2004). Vet. Comp. Oncol. 6: 102–110. doi: 10.1111/j.1476-5829.2007.00146.x [DOI] [PubMed] [Google Scholar]
- 9.Kristal O., Lana S. E., Ogilvie G. K., Rand W. M., Cotter S. M., Moore A. S.2001. Single agent chemotherapy with doxorubicin for feline lymphoma: a retrospective study of 19 cases (1994–1997). J. Vet. Intern. Med. 15: 125–130 [DOI] [PubMed] [Google Scholar]
- 10.Mahony O. M., Moore A. S., Cotter S. M., Engler S. J., Brown D., Penninck D. G.1995. Alimentary lymphoma in cats: 28 cases (1988–1993). J. Am. Vet. Med. Assoc. 207: 1593–1598 [PubMed] [Google Scholar]
- 11.McPherron M. A., Chavkin M. J., Powers B. E., Seim H. B., 3rd1994. Globule leukocyte tumor involving the small intestine in a cat. J. Am. Vet. Med. Assoc. 204: 241–245 [PubMed] [Google Scholar]
- 12.Sözmen M., Tasca S., Carli E., De Lorenzi D., Furlanello T., Caldin M.2005. Use of fine needle aspirates and flow cytometry for the diagnosis, classification, and immunophenotyping of canine lymphomas. J. Vet. Diagn. Invest. 17: 323–330. doi: 10.1177/104063870501700404 [DOI] [PubMed] [Google Scholar]
- 13.Teske E., van Heerde P., Rutteman G. R., Kurzman I. D., Moore P. F., MacEwen E. G.1994. Prognostic factors for treatment of malignant lymphoma in dogs. J. Am. Vet. Med. Assoc. 205: 1722–1728 [PubMed] [Google Scholar]
- 14.Teske E., van Straten G., van Noort R., Rutteman G. R.2002. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J. Vet. Intern. Med. 16: 179–186. doi: 10.1111/j.1939-1676.2002.tb02352.x [DOI] [PubMed] [Google Scholar]
- 15.Uppenkamp M., Feller A. C.2002. Classification of malignant lymphoma. Onkologie 25: 563–570. doi: 10.1159/000068629 [DOI] [PubMed] [Google Scholar]
- 16.Vail D. M., Michels G. M., Khanna C., Selting K. A., London C. A.2010. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)–a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 8: 28–37. doi: 10.1111/j.1476-5829.2009.00200.x [DOI] [PubMed] [Google Scholar]
- 17.Vail D. M., Moore A. S., Ogilvie G. K., Volk L. M.1998. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J. Vet. Intern. Med. 12: 349–354. doi: 10.1111/j.1939-1676.1998.tb02134.x [DOI] [PubMed] [Google Scholar]