ABSTRACT
The potential contamination of Toxoplasma gondii and Neospora caninum oocysts in the human environment is a concern from the public health viewpoint. However, estimation of their seroprevalences in humans cannot be performed in a manner that distinguishes between oocysts and tissue cysts as a source of infection. Rabbits are considered popular pet animals in Japan that can acquire natural infections by the aforementioned parasites only through the ingestion of oocysts. Therefore, this study was conducted to estimate the seroprevalences of T. gondii and N. caninum in pet rabbits in Japan as an indicator of the possible oocyst contamination in the environment surrounding human beings. Serum samples of 337 rabbits were examined by different serological methods. Enzyme-linked immunosorbent assays were performed to measure the titer of IgG and IgM antibodies. Samples revealed to be seropositive by ELISA were further analyzed by a latex agglutination test, Western blotting and an indirect immunofluorescence assay. The rates of seropositivity for T. gondii were 0.89% (3/337) and 0.29% (1/337) in IgG and IgM ELISA, respectively. SAG1 and SAG2 were detected as major antigens by the positive rabbit sera in Western blotting associated with strong staining observed by IFA in T. gondii tachyzoites. Regarding N. caninum, none of the serum samples showed a specific reaction in both Western blotting and the IFA. The results of this study indicate low seroprevalences of toxoplasmosis and neosporosis in pet rabbits in Japan, suggesting low oocyst contamination in the human environment.
Keywords: Neospora caninum, rabbit, seroprevalence, Toxoplasma gondii
Toxoplasma gondii (T. gondii) and Neospora caninum (N. caninum) are intracellular apicomplexan protozoa with worldwide distributions and are able to infect most warm blooded animals and humans, resulting in neuromuscular diseases and abortion in different species [37]. In humans, T. gondii infection can be life threatening for congenitally infected infants and immunocompromised patients as a result of either acute or reactivated infection [13]. Although T. gondii has been known as a causative agent of disease in human beings, there are only few reports about natural infection with N. caninum [16, 28, 38], but there is still concern about its zoonotic potential because it was found that it can infect nonhuman primates [4, 15].
Although toxoplasmosis and neosporosis are biologically two distinct diseases, their respective causative agents, T. gondii and N. caninum, are structurally, genetically and immunologically related parasites [18, 33]. Felids, particularly cats, are the only definitive hosts known to excrete the oocysts of T. gondii, while the definitive hosts of N. caninum are dogs [13, 15]. The cat or dog can deposit fecal oocysts in soil, grass, water or elsewhere. These oocysts are resistant to harsh environmental conditions, being an excellent reservoir for survival of the parasites. Humans can acquire the infections through ingestion of oocyst-contaminated soil and water and tissue cysts in undercooked meat, by transplantation, blood transfusion and laboratory accidents and congenitally [13]. Concerning the importance of oocyst contamination, Lass et al. [27] detected the presence of T. gondii oocysts on fresh fruits and vegetables from shops and gardens, suggesting environmental contamination in Poland. Also, toxoplasmosis outbreaks were reported previously and connected to water or soil contaminated with oocysts [8, 11]. The risk of acquiring T. gondii infection from environmental sources versus meat was measured by Munoz-Zanzi et al. [30], and the authors detected sporozoite-specific protein (SSP) antibodies in sera of 43% of recently infected pregnant women in Chile, implying the significant risk of the contaminated environment, which was almost equal to the hazard of meat containing the parasite cysts. In Japan, the seroprevalence of T. gondii antibodies was found to be 10.3% in pregnant women [34], while another report showed 5.4% IgG anti-T. gondii antibodies in HIV patients [31]. Moreover, ocular toxoplasmosis was diagnosed through detection of T. gondii DNA in ocular fluid taken from patients [36].
Rabbits can acquire T. gondii or N. caninum infections only through ingestion of sporulated oocysts of the aforementioned parasites, with the exception of possible congenital transmission from rabbit dams to their fetuses [40]. Contamination of the environment with oocysts shed by the definitive hosts is considered a potential risk for both humans and other animals [9, 12, 15, 37]. Although a few studies have been conducted to assess the level of oocyst contamination in Japan, some were conducted to estimate the seroprevalences of T. gondii or N. caninum in cats and dogs to evaluate such danger. The seroprevalence of T. gondii in domestic cats in Japan was previously reported to be 20.7% (40/193) [25] or 5.4% (78/1,447) [29]. The seroprevalence of N. caninum infection was also examined, and among 1,206 dogs, 126 (10.4%) were found to be positive for N. caninum infections [26].
Clinical toxoplasmosis in rabbits has been reported in various countries, such as Scandinavian countries [17, 24] and the U.S.A. [14]. The latter study reported fatal toxoplasmosis in three domestic rabbits in the U.S.A. Also, the prevalence of T. gondii in rabbits and its biological circumstances have been emphasized by many epidemiological studies, such as in Spain [1], Egypt [3], Mexico [2], Korea [35] and China [42].
In the present study, we are reporting the first seroprevalence study of T. gondii and N. caninum in pet rabbits in Japan. This investigation was performed with multiple tests based on the serological assay. IgG and IgM ELISA tests were performed using parasite lysates and recombinant proteins. Further analyses were done by latex agglutination test (LAT), Western blotting, immunoprecipitation and indirect immunofluorescence assay (IFA).
MATERIALS AND METHODS
Samples: Serum samples were taken from 337 pet rabbits presented to 34 veterinary facilities in different 20 prefectures in Japan [22]. Healthy rabbits were brought to the veterinary clinics for either general health checks or other disease conditions. Among the rabbits, 54% were clinically healthy, and 29% were suffering from neurological disorders. The rest showed variable clinical conditions, such as embraced tumors or disorders of the digestive, respiratory or urinary systems. Ophthalmic and dermatological conditions were also involved. The examined rabbits ranged in age from 3 months to 10 years old, and the average age was 3.4 years. The breeds of the tested rabbits and their numbers were as following: 16 Holland Lop, 28 Netherland Dwarf, 3 Lion, 9 Angora, 3 Rex, 2 Dutch, 1 American Fuzzy Lop, 1 Jersey Wooly, 272 others and 2 unknown rabbits. All rabbit serum samples referred to our laboratory had been tested to assess the seroprevalences of T. gondii and N. caninum antibodies.
Parasites: T. gondii (RH and ME49 strains) and N. caninum (NC1 strain) tachyzoites were maintained in human foreskin fibroblast (HFF) cells cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO, Grand Island, NE, U.S.A.) supplemented with 7.5% heat-inactivated fetal bovine serum (FBS). For purification and lysis of tachyzoites, the infected cells were washed with cold phosphate-buffered saline (PBS). Cell pellets were resuspended in medium and passed through a 27-gauge needle and then through a 5.0 µm-pore filter (Millipore, Bedford, MA, U.S.A.). After centrifugation at 2,000 × g for 5 min at 4°C, the pellet was resuspended with RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.25 mM sodium deoxycholate, 0.1% Triton X-100 and 1% Nonidet P-40). The tachyzoite lysates were recovered after centrifugation at 2,000 × g for 5 min.
Recombinant protein production: Recombinant proteins of T. gondii surface antigen 2 (TgSAG2) and N. caninum surface antigen 1 (NcSAG1) were generated according to previous reports [20, 21].
Generation of polyclonal anti-TgSAG2 antisera: Polyclonal anti-TgSAG2 antisera were produced in BALB/c mice. Immunization of mice was performed as described previously [10]. In brief, mice were immunized subcutaneously at three different inoculation sites with 10 µg of recombinant TgSAG2 protein emulsified with an equal volume of Freund’s complete adjuvant. Two weeks later, mice were immunized with the same dose of antigen emulsified with Freund’s incomplete adjuvant. Another dose of the antigen with the incomplete adjuvant was given on day 28. Seven days later, mice were sacrificed under isoflurane anesthesia, and blood was collected for serum extraction. The reactivity of the sera collected was tested using Western blotting. The experiments were conducted according to the guidelines for the Care and Use of Laboratory Animals issued by Obihiro University of Agriculture and Veterinary Medicine.
ELISA: One hundred microliters of antigen (either tachyzoites lysate or recombinant protein) in a coating buffer (50 mM carbonate, pH 9.6) at concentration of 2 µg/ml were coated onto ELISA plates (Nunc, Roskilde, Denmark) and incubated overnight at 4°C. Plates were washed 5 times with PBS containing 0.05% Tween 20 (PBS-T) and blocked with PBS containing 1% skimmed milk (PBS-SM) for 1 hr at room temperature (RT). After washing with PBS-T, 100 µl of rabbit serum diluted at 1:500 with PBS-SM were added to duplicate wells and incubated for 1 hr at RT. After washing 5 times with PBS-T, the plates were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (GE Healthcare UK Limited, Buckinghamshire, U.K.) or IgM (SouthernBiotech, Birmingham, AL, U.S.A.) diluted at 1:2,000 with PBS-SM at RT for 1 hr. Plates were further washed 5 times before the substrate solution [0.1 M citrate buffer, pH 4, 0.003% H2O2 and 0.3 mg/ml 2, 2’-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); Sigma-Aldrich] was added to each well in 100 µl aliquots. The absorbance at 415 nm after 30 min of incubation at RT was measured using an ELISA reader (Corona microplate reader MTP-120; Corona, Tokyo, Japan). The cutoff point was determined as the average value of seronegative rabbit samples (n=24) plus 3 SD. All samples were examined at least twice to obtain reproducible data.
Latex agglutination test: The LAT was performed according to the kit manufacturer’s instructions (Toxocheck-MT, Eiken Chemical, Tokyo, Japan). Samples were considered positive when agglutination was observed at a dilution of 1:32 or greater.
Western blot analysis: Lysates of T. gondii (RH strain) and N. caninum were dissolved in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 140 mM 2-mercaptoethanol, 10% glycerol and 0.02% bromophenol blue) heated at 95°C for 5 min and separated on a 12% polyacrylamide gel. All separated proteins were electrically transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P Transfer Membrane, Millipore, Billerica, MA, U.S.A.) using a Western blot apparatus (HorizeBlot Type AE-6677, ATTO Bioscience & Biotechnology, Tokyo, Japan). After blocking for 1 hr in PBS-SM, membranes were probed with the rabbit serum diluted at 1:500 with PBS-SM for 1 hr. The membranes were washed 3 times for 10 min with PBS-T and then probed with HRP-conjugated anti-rabbit IgG diluted at 1:2,000 in PBS-SM for 1 hr at RT. After washing with PBS-T, the membranes were immersed in the detection solution (0.1 M Tris, pH 8.5, 1.25 mM luminol acid, 0.2 mM coumaric acid and 0.075% H2O2) and exposed to X ray film. Prestained molecular mass standards (GeneDireX, Biospeed, Las Vegas, NV, U.S.A.) were used.
Immunoprecipitation: One hundred micrograms of T. gondii lysate were mixed with 2 µl of either seronegative or seropositive rabbit serum and kept on ice for 1 hr. Twenty microliters of protein G sepharose (GE Healthcare, Uppsala, Sweden) were added to each sample and rotated at 4°C for 1 hr. Beads were washed 5 times with PBS, and then protein samples were separated on 12% polyacrylamide gel and electrically transferred onto PVDF membranes. Mouse monoclonal anti-SAG1 antibodies (Anti-T. gondii clone TP3, Advanced Immunochemical Inc., Long Beach, CA, U.S.A.) or mouse polyclonal anti-SAG2 antibodies were used to probe the membranes at a dilution of 1:500 in PBS-SM. After washing 3 times with PBS-T, membranes were probed with HRP-conjugated anti-mouse IgG diluted at 1:2,000 in PBS-SM incubated for 1 hr at RT. Detection was performed as described above for Western blotting.
Indirect immunofluorescence assay (IFA): The indirect immunofluorescence was performed as described previously [39] with some modifications. Glass coverslips with confluent monolayers of HFF cells were infected with T. gondii (ME49 strain) and incubated in a CO2 incubator for 24 hr at 37°C. After washing 3 times with PBS, cells were fixed and permeabilized with 4% formaldehyde and 0.2% Triton X-100 in PBS (pH 7.2) for 15 min. Cells were then washed 3 times with PBS and blocked for 30 min in 3% BSA/PBS. Coverslips were incubated with rabbit serum samples diluted at 1:100, 1:500 and 1:1,000 in 3% BSA/PBS. Mouse anti-T. gondii inner membrane complex (IMC1) antibodies were used for counterstaining. After washing 3 times with PBS, coverslips were incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG and Alexa Fluor-488 conjugated goat anti-mouse IgG (Invitrogen) diluted at 1:1,000 in 3% BSA/PBS. Coverslips were washed twice in PBS and once in distilled water. Mowiol (4.8 g Mowiol, 12 g glycerol in 50 mM Tris HCl, pH 8.5) was used as a mounting medium. Samples were examined on a Leica TCS NT Confocal Laser Scanning Microscope (Leica Microsystems, Wetzlar, Germany) using the appropriate settings. Green and red fluorescence and differential interference contrast (DIC) images were recorded using the Leica PowerScan software.
Statistical analysis: The results of the different serological tests were estimated by the percentage of agreement, the sensitivity and specificity and the kappa values using (http://vassarstats.net/).
RESULTS
Detection of specific antibodies raised by natural infections of T. gondii or N. caninum: To assess the presence of toxoplasmosis or neosporosis in pet rabbits, IgG and IgM ELISA tests were carried out to screen all rabbit sera (337) using tachyzoites lysate of T. gondii or N. caninum and TgSAG2 or NcSAG1 recombinant protein as antigens. Seropositive samples were further examined by LAT, Western blotting and IFA. For T. gondii, IgG-ELISA identified 9 positive sera according to the calculated cutoff value (0.6), 6 samples scored an OD value below 1, 1 sample scored an OD value between 1 and 2, and the remaining 2 sera had OD values of more than 2. Only one sample was found to be positive in the IgM ELISA (Table 1). All rabbit sera that showed positive reactivity with lysate antigen were also reactive with the recombinant protein. Sample that scored OD values below 0.6 (328) were considered negative by ELISA test.
Table 1. Results of the serological tests performed on sera from pet rabbits.
| Tested parasite | No. of rabbits | ELISAa) IgG | ELISA IgM | LATc) | Western blot | IFAd) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abs (OD)b) |
Abs (OD) |
|||||||||
| 0.6–1 | 1–2 | ˃ 2 | 0.65–1 | 1–2 | ˃ 2 | |||||
| T. gondii | 337 | 6 | 1 | 2 | 0 | 0 | 1 | 3 | 3 | 3 |
| N. caninum | 337 | 9 | 1 | 0 | 0 | 0 | 0 | Not determined | 0 | |
a) ELISA: Enzyme-linked immunosorbent assay. b) OD: Optical density. c) LAT: Latex agglutination test. d) IFA: Indirect immunofluorescence antibody assay.
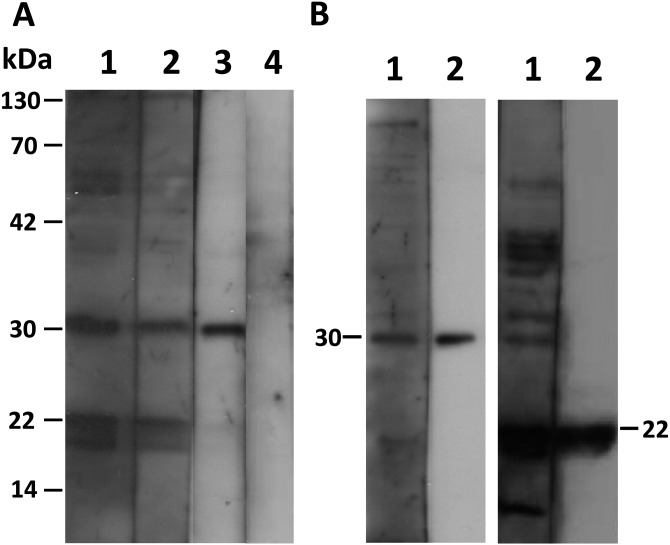
To include a wide range of samples, the latex agglutination test was applied to all samples that showed an OD value above 0.3 (35/337) using the recommended dilutions. Agglutination was observed only in 3 samples (OD value above 1) at dilutions of 1:2,048 and 1:128 (Table 1), while the remaining 32 samples did not show any agglutination and were therefore considered negative. In addition, all samples that showed OD values above the ELISA cutoff point (0.6) were analyzed by Western blotting and IFA. Both tests resulted in 3 positive sera that had shown positivity also in the LAT (Fig. 1A lanes 1–3 and Fig. 3 rows 1–3). We found that the only serum samples that scored ELISA OD values higher than 1.0 were detectable by LAT, Western blot and IFA (3/337).
Fig. 1.
Western blot analysis. A) Lanes 1, 2 and 3 represent seropositive rabbit samples for T. gondii. Lane 4 represents a seronegative sample. The analysis was performed by using an equal volume of purified T. gondii tachyzoite lysate of the ME49 strain as the antigen. B) Specific bands of SAG1 can be observed at 30 kDa (left panel), and those of SAG2 can be observed at 22 kDa (right panel). Lane 1 shows seropositive rabbit sample naturally infected with T. gondii. Lane 2 shows mouse anti-SAG1 monoclonal (left panel) and mouse anti-SAG2 polyclonal (right panel) antibodies, respectively. Purified T. gondii lysate of the ME49 strain was used as the antigen.
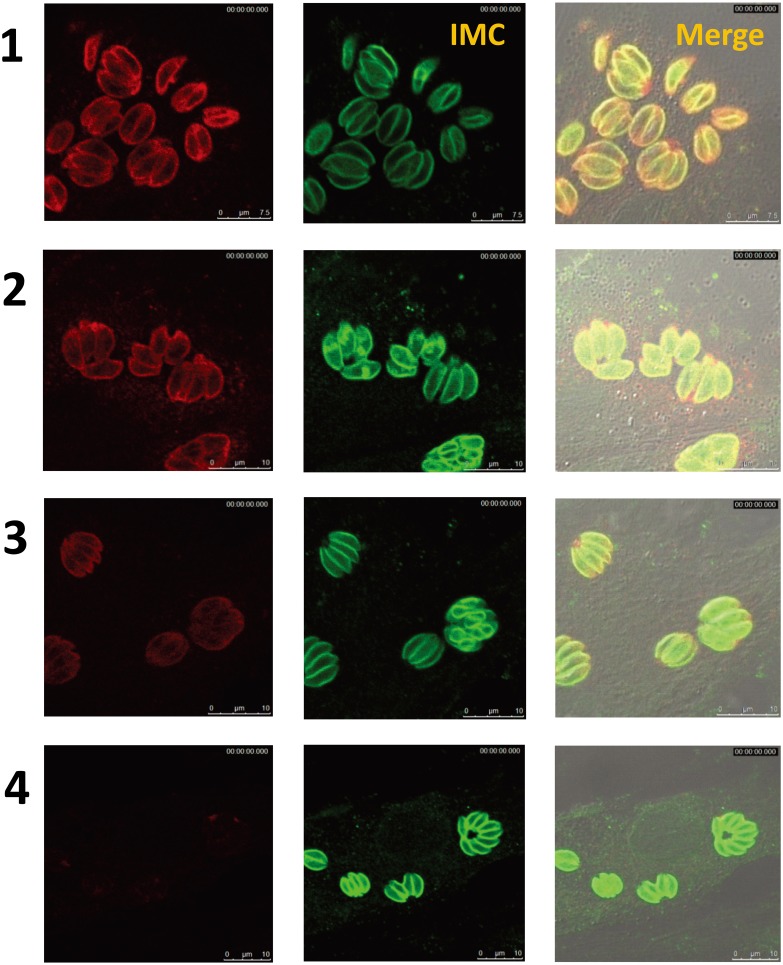
Fig. 3.
IFA. Detection of specific antibodies in RH strain tachyzoites of T. gondii. Rows 1, 2 and 3 represent T. gondii-seropositive samples (left panel, red). Row 4 represents a seronegative sample. The mouse anti-T. gondii IMC1 antibody (green) was used for counterstaining.
On the other hand, when rabbit samples tested for the presence of N. caninum antibodies, although IgG-ELISA showed 10 cases over the cutoff value (0.6), 9 of them had OD values below 1, and one case had an OD value between 1 and 2 (Table 1). No specific band or reactive staining was observed for N. caninum in both Western blot and the IFA.
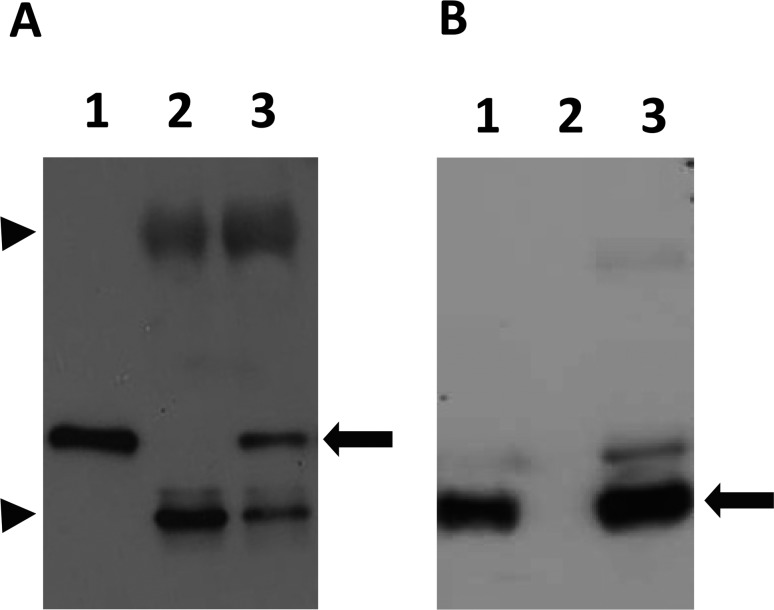
Detection of specific antigens in sera of naturally infected rabbits with T. gondii: In Western blotting, positive sera generated multiple distinct bands between 14 and 130 kDa (Fig. 1A). Certain protein bands were noticeably common in all positive sera at 30 and 22 kDa, the same molecular weights as the SAG1 [6] and SAG2 [32] antigens. To confirm this, Western blot analyses were performed to compare the sizes of identified bands in the sera of infected rabbits with the mouse anti-SAG1 monoclonal and anti-SAG2 polyclonal antibodies. Clearly equal bands were detected at 30 and 22 kDa (Fig. 1B). Further confirmation was performed by immunoprecipitation using seropositive and seronegative T. gondii rabbit serum samples that were tested previously by ELISA and LAT. Both SAG1 and SAG2 proteins could be precipitated by positive sera (Fig. 2). The staining pattern in the IFA of positive sera showed a strong signal on the surface of the parasites (Fig. 3).
Fig. 2.
Immunoprecipitates of either seronegative (lane 2) or seropositive (lane 3) samples reacted with either the mouse anti-SAG1 monoclonal antibody (left panel) or mouse anti-SAG2 polyclonal antibody (right panel). Lane 1, T. gondii lysate. The black arrow represents the specific band of SAG1 (left) and SAG2 (right) precipitated only by the positive sera. Arrowheads represent IgG heavy (upper) and light (lower) chains, respectively.
The results of ELISA, IFA and Western blot were statistically calculated to obtain the specificity and sensitivity and the kappa value to assess their ability to detect T. gondii antibodies in rabbit sera in comparison with the LAT as a reference test (Table 2).
Table 2. Specificity and sensitivity of the ELISA, IFA and Western blot in detecting T. gondii-specific antibodies in rabbit sera compared with the results in the reference LAT test.
| Parameter | Value of performed serological tests compared with
the LAT |
||
|---|---|---|---|
| ELISA | IFA | Western blot | |
| Sensitivity (%) | 100 (9/3) | 100 (3/3) | 100 (3/3) |
| Specificity (%) | 98 (328/334) | 100 (334/334) | 100 (334/334) |
| Concordance (%) | 98.2 | 100 | 100 |
| Kappa value | 0.49 | 1 | 1 |
DISCUSSION
Rabbits are especially popular as pets in Japan; dogs and cats may be the most frequent household animals, but the popularity of rabbits is increasing. Therefore, their health status is also important to consider. Moreover, questions often arise concerning the hazards in the surrounding environments for both pet rabbits and the people around them. T. gondii and N. caninum are protozoan parasites known to infect humans and animals [12, 37]. Human beings can be infected with T. gondii and possibly with N. caninum [16, 28, 38] by ingestion, the major route, of either oocysts in contaminated food and water or meat containing the parasite cysts. The ability to distinguish whether the original infection was by tissue cyst or oocysts and discover whether the outcomes are different is a great concern nowadays. Because cats and dogs are so abundant in the environment and indeed are increasing, contamination of the human environment with oocysts will continue to increase, and exposure will be unavoidable. Furthermore, recent studies reported that the majority of congenital infections and postnatal acute infections in the United States originated from oocysts [5], and this will increase the concern about the severity and outcomes of infections primarily induced by oocysts. Because the oocyst is the only source of infection in rabbits, pet rabbits are considered good indicators for assessment of the oocyst contamination in the human environment. Therefore, in view of the worldwide importance of these parasites and their infective stages, the seroprevalences of both T. gondii and N. caninum in pet rabbits were estimated in this study.
Serodiagnostic tools are important methods for detecting parasitic infections; one of these tools is the ELISA using tachyzoite-derived antigens or generated recombinant proteins [7, 19]. In this study, IgG and IgM ELISAs were used to analyze all rabbit sera by using the whole tachyzoites-derived antigen of either T. gondii or N. caninum. The use of such antigens may produce false positive results due to cross-reaction with other closely related parasites [23] or unknown reasons. To validate our results, ELISAs based on the recombinant proteins TgSAG2 and NcSAG1 were also carried out. Similar data were obtained, indicating that in this case, the antigenicity of the recombinant proteins did not show any difference compared with the lysates. Therefore, it was necessary to perform other tests, such as the LAT, Western blotting and IFA. These three tests identified only three positive sera that previously showed OD values >1 in the IgG ELISA.
Also, we identified SAG1 and SAG2 as major antigens detected by the positive rabbit sera. These findings were confirmed by Western blotting and immunoprecipitation, as shown in the results. Although the TgSAG2 recombinant proteins have already been established as specific antigens for diagnosis of T. gondii via ELISA [20], our results confirmed that SAG1 and SAG2 can be used as useful target antigens for specific testing of rabbit sera to detect T. gondii infection by other serological methods. Concerning N. caninum antibodies in rabbit sera, although ELISA detected 10/337 positive sera, Western blot and the IFA did not reveal any positivity.
Therefore, it could be plausibly accepted that toxoplasmosis was not common and that neosporosis was not prevalent among pet rabbits in Japan. Additionally, in this study, we compared the positivity of the results between the ELISA and the other serological methods. The rabbit sera tested by ELISA showed 2.67% positivity for T. gondii and 2.96% for N. caninum. However, the seropositivity was only 0.89% for T. gondii and 0% for N. caninum using Western blotting or the IFA. Therefore, it is reasonable to accept that using more than one method in serodiagnosis is recommended for accurate investigation.
Since we used different tests here for serological detection, it was important to emphasize the capability of each test. Therefore, the results of ELISA, IFA and Western blot were statistically compared with those of the LAT as a reference test for detection of T. gondii antibodies (Table 2). Among the tests used, the ELISA showed a higher positive rate of detection of T. gondii-specific IgG antibodies than the IFA and Western blot (Table 1). Although ELISA achieved very high sensitivity in detection (100%), the six negative samples that were detectable by ELISA and not by LAT resulted in 98% specificity for ELISA. The kappa value of 0.49 could be interpreted as a moderate level of agreement. While, the sensitivity and specificity of the IFA and Western blot were both 100% when the LAT was used as a reference test associated with high agreement indicated by maximum kappa value (1) as summarized in Table 2. Therefore, we could conclude from this analysis that the LAT, IFA and Western blot had similar capabilities for detection of T. gondii antibodies in rabbit sera. However, the availability of these tests for rapid screening is not similar, because the IFA and Western blotting are more sophisticated techniques that might not be applicable to large number of samples or as a field test. Moreover, both tests were unable to detect the antibody titer, while the LAT is convenient, more applicable and provides the advantage of both qualitative and quantitative assessment of the infection status. Concerning N. caninum, the results of ELISA and Western blot showed 97% and 100% specificity, respectively, when the IFA was used as a standard test, while the sensitivity could not be calculated (data not shown).
In reference to the seroprevalence of T. gondii in rabbits in different countries, tested domestic rabbits reared on farms and in backyards and pet shops in northern Mexico showed that antibodies to T. gondii were found in 16.3% of 429 rabbits [2]. In Korea, a similar type of rabbits was surveyed from breeding farms, and 10.6% of them showed T. gondii-specific IgG antibodies [35]. In Egypt, high seroprevalences of T. gondii were also detected, 37.5% and 11.34%, in locally bred and commercial rabbits [3]. In the studies described above, the high seroprevalences of T. gondii infection could be correlated to factors, such as rearing outdoors or in a pet shop, feeding of possibly contaminated vegetables and cohabitation with cats or dogs.
Additionally, considerably high prevalences could also be found in free domestic or wild rabbits in other countries. For example, in China, the seroprevalence of T. gondii antibodies was 23.4% in free domestic rabbits from a rural area [42]. Also, a study in Spain [1] reported 14.2% T. gondii positivity among 456 wild rabbits captured or hunted from forests. On the other hand, a study on wild Cottontail rabbits in Northern Italy mentioned low prevalences of both T. gondii and N. caninum infections, 2.08% and 2.78%, respectively [41].
In the present study, the seroprevalence of T. gondii was 0.89% in pet rabbits of Japan. Referring to the previously mentioned studies performed on domestic or wild rabbits, results cannot be directly compared, although the susceptibilities were quite similar because the rabbits were from the same species. It is important to consider the surrounding environment and rearing conditions of the examined rabbits in this study. In general, wild rabbits or even domestic rabbits that are reared or have access to the outdoors might be more susceptible to such parasitic infections than pet rabbits confined indoors, bearing in mind the risk of direct contact with the contaminated environment. In Japan, rabbits are considered pets and are mostly kept indoors or in cages as in a zoo. Although they are sometimes given time to range freely outside, this is different to some extent from the case of domestic farm rabbits reared for meat purposes and to a great extent from wild rabbits. Given the unavailability of data concerning recovery of oocysts of the aforementioned parasites from the human environment, little is known about their potential hazards.
In conclusion, the present study indicates that there is probably a low level of oocyst contamination in the human environment according to estimation of the prevalences of toxoplasmosis and neosporosis in pet rabbits. We concluded that T. gondii was not common among healthy pet rabbits and those suffering from neurological disorders. Furthermore, as far as could be checked, there were no apparent N. caninum infections in pet rabbits. Assessment of contamination of the surrounding environment with oocysts of the parasites and its implications for humans and other animals requires further attention and investigation.
Acknowledgments
his study is supported in part by a Grant-in Aid for Scientific Research (C), No. 25450419 from the Japan Society for the Promotion of Science, and the Ministry of Higher Education and Scientific Research, Egypt. The authors also wish to thank the members of the Japanese Society of Exotic Pet Medicine who contributed greatly to this work by collecting the rabbit serum samples.
REFERENCES
- 1.Almería S., Calvete C., Pages A., Gauss C., Dubey J. P.2004. Factors affecting the seroprevalence of Toxoplasma gondii infection in wild rabbits (Oryctolagus cuniculus) 254 from Spain. Vet. Parasitol. 123: 265–270. doi: 10.1016/j.vetpar.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 2.Alvarado-Esquivel C., Alvarado-Esquivel D., Villena I., Dubey J. P.2013. Seroprevalence of Toxoplasma gondii infection in domestic rabbits in Durango State, Mexico. Prev. Vet. Med. 111: 325–328. doi: 10.1016/j.prevetmed.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 3.Ashmawy K. I., Abuakkada S. S., Awad A. M.2011. Seroprevalence of antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in farmed domestic rabbits in Egypt. Zoonoses Public Health 58: 357–364. doi: 10.1111/j.1863-2378.2010.01371.x [DOI] [PubMed] [Google Scholar]
- 4.Barr B. C., Conrad P. A., Sverlow K. W., Tarantal A. F., Hendrickx A. G.1994. Experimental fetal and transplacental Neospora infection in the nonhuman primate. Lab. Invest. 71: 236–242 [PubMed] [Google Scholar]
- 5.Boyer K., Hill D., Mui E., Wroblewski K., Karrison T., Dubey J. P., Sautter M., Noble A. G., Withers S., Swisher C., Heydemann P., Hosten T., Babiarz J., Lee D., Meier P., McLeod R.2011. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin. Infect. Dis. 53: 1081–1089. doi: 10.1093/cid/cir667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burg J. L., Perelman D., Kasperm L. H., Warem P. L., Boothroyd J. C.1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141: 3584–3591 [PubMed] [Google Scholar]
- 7.Chahan B., Gaturaga I., Huang X., Liao M., Fukumoto S., Hirata H., Nishikawa Y., Suzuki H., Sugimoto C., Nagasawa H., Fujizaki K., Igarashi I., Mikami T., Xuan X.2003. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 118: 177–185. doi: 10.1016/j.vetpar.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Coutinho S. G., Lobo R., Dutra G.1982. Isolation of Toxoplasma from the soil during an outbreak of toxoplasmosis in a rural area in Brazil. J. Parasitol. 68: 866–868. doi: 10.2307/3280995 [DOI] [PubMed] [Google Scholar]
- 9.Current W.L., Upton S.J., Long P.L.1990. Taxonomy and life cycles. pp.1–16. In: Coccidiosis of Man and Domestic Animals (Long, P. L. ed.), Boston, CRC Press. [Google Scholar]
- 10.Dautu G., Ueno A., Miranda A., Mwanyumba S., Munyaka B., Carmen G., Kariya T., Omata Y., Saito A., Xuan X., Igarashi M.2008. Toxoplasma gondii: detection of MIC10 antigen in sera of experimentally infected mice. Exp. Parasitol. 118: 362–371. doi: 10.1016/j.exppara.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 11.de Moura L., Bahia-Oliveira L. M. G., Wada M. Y., Jones J. L., Tuboi S. H., Carmo E. H., Ramalho W. M., Camargo N. J., Trevisan R., Graça R. M. T., da Silva A. J., Moura I., Dubey J. P., Garrett D. O.2006. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 12: 326–329. doi: 10.3201/eid1202.041115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey J. P.2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41: 1–16. doi: 10.3347/kjp.2003.41.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubey J. P., Jones J. L.2008. Review Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38: 1257–1278. doi: 10.1016/j.ijpara.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Dubey J. P., Brown C. A., Carpenter J. L., Moore J. J.1992. Fatal toxoplasmosis in domestic rabbits in the USA. Vet. Parasitol. 44: 305–309. doi: 10.1016/0304-4017(92)90127-U [DOI] [PubMed] [Google Scholar]
- 15.Dubey J. P., Carpenter J. L., Speer C. A., Topper M. J., Uggla A.1988. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 192: 1269–1285 [PubMed] [Google Scholar]
- 16.Dubey J. P., Schares G., Ortega-Mora L. M.2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20: 323–367. doi: 10.1128/CMR.00031-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafsson K., Uggla A., Svensson T., Sjoland L.1988. Detection of Toxoplasma gondii in liver tissue sections from brown Hares (Lepuseuropaeus) and mountain Hares (Lepusdimidus) using the peroxidase anti-peroxidase (PAP) technique as a complement to conventional histopathology. Zentralbl. Veterinarmed. B. 35: 402–407 [DOI] [PubMed] [Google Scholar]
- 18.Howe D. K., Sibley L. D.1999. Comparison of the major antigens of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 29: 1489–1496. doi: 10.1016/S0020-7519(99)00099-5 [DOI] [PubMed] [Google Scholar]
- 19.Huang X., Xuan X., Hirata H., Yokoyama N., Xu L., Suzuki N., Igarashi I.2004. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 42: 351–353. doi: 10.1128/JCM.42.1.351-353.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X., Xuan X., Kimbita E. N., Battur B., Miyazawa T., Fukumoto S., Mishima M., Makala L. H., Suzuki H., Sugimoto C., Nagasawa H., Fujisaki K., Mikami T., Igarashi I.2002. Development and evaluation of an enzyme-linked immunosorbent assay with recombinant SAG2 for diagnosis of Toxoplasma gondii infection in cats. J. Parasitol. 88: 804–807 [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim H. M., Huang P., Salem T. A., Talaat R. M., Nasr M. I., Xuan X., Nishikawa Y.2009. Short report: prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 80: 263–267 [PubMed] [Google Scholar]
- 22.Igarashi M., Oohashi E., Dauto G., Ueno A., Kariya T., Furuya K.2008. High Seroprevalence of Encephalitozoon cuniculi in Pet Rabbits in Japan. J. Vet. Med. Sci. 70: 1301–1304. doi: 10.1292/jvms.70.1301 [DOI] [PubMed] [Google Scholar]
- 23.Jenkins M., Baszler T., Bjorkman C., Schares G., Williams D.2002. Diagnosis and seroepidemiology of Neospora caninum –associated bovine abortion. Int. J. Parasitol. 32: 631–636. doi: 10.1016/S0020-7519(01)00363-0 [DOI] [PubMed] [Google Scholar]
- 24.Kapperud G.1978. Survey for toxoplasmosis in wild and domestic animals from Norway and Sweden. J. Wildl. Dis. 14: 157–162. doi: 10.7589/0090-3558-14.2.157 [DOI] [PubMed] [Google Scholar]
- 25.Kimbita E. N., Xuan X., Huang X., Miyazawa T., Fukumoto S., Mishima M., Suzuki H., Sugimoto C., Nagasawa H., Fujisaki K., Suzuki N., Mikami T., Igarashi I.2001. Serodiagnosis of Toxoplasma gondii infection in cats by enzyme- linked immunosorbent assay using recombinant SAG1. Vet. Parasitol. 102: 35–44. doi: 10.1016/S0304-4017(01)00522-2 [DOI] [PubMed] [Google Scholar]
- 26.Kubota N., Sakata Y., Miyazaki N., Itamoto K., Bannai H., Nishikawa Y., Xuan X., Inokuma H.2008. Serological survey of Neospora caninum infection among dogs in Japan through species-specific ELISA. J. Vet. Med. Sci. 70: 869–872. doi: 10.1292/jvms.70.869 [DOI] [PubMed] [Google Scholar]
- 27.Lass A., Pietkiewicz H., Szostakowska B., Myjak P.2012. The first detection of toxoplasma gondii DNA in environmental fruits and vegetables samples. Eur. J. Clin. Microbiol. Infect. Dis. 31: 1101–1108. doi: 10.1007/s10096-011-1414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobato J., Silva D. A., Mineo T. W., Amaral J. D., Segundo G. R., Costa-Cruz J. M., Ferreira M. S., Borges A. S., Mineo J. R.2006. Detection of immunoglobulin G antibodies to Neosporacaninum in humans: high seropositivity rates in patients who are infected by human immunodeficiency virus or have neurological disorders. Clin. Vaccine Immunol. 13: 84–89. doi: 10.1128/CVI.13.1.84-89.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama S., Kabeya H., Nakao R., Tanaka S., Sakai T., Xuan X., Katsube Y., Mikami T.2003. Seroprevalence of Bartonella henselae, Toxoplasma gondii, FIV and FeLV infections in domestic cats in Japan. Microbiol. Immunol. 47: 147–153. doi: 10.1111/j.1348-0421.2003.tb02798.x [DOI] [PubMed] [Google Scholar]
- 30.Munoz-Zanzi C. A., Fry P., Lesina B., Hill D.2010. Toxoplasma gondii oocyst-specific antibodies and source of infection. Emerg. Infect. Dis. 16: 1591–1593. doi: 10.3201/eid1610.091674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naito T., Inui A., Kudo N., Matsumoto N., Fukuda H., Isonuma H., Sekigawa I., Dambara T., Hayashida Y.2007. Seroprevalence of IgG anti-toxoplasma antibodies in asymptomatic patients infected with human immunodeficiency virus in Japan. Intern. Med. 46: 1149–1150. doi: 10.2169/internalmedicine.46.6402 [DOI] [PubMed] [Google Scholar]
- 32.Parmley S. F., Sgarlato G. D., Mark J., Prince J. B., Remington J. S.1992. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J. Clin. Microbiol. 30: 1127–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid A. J., Vermont S. J., Cotton J. A., Harris D., Hill-Cawthorne G. A., Könen-Waisman S., Latham S. M., Mourier T., Norton R., Quail M. A., Sanders M., Shanmugam D., Sohal A., Wasmuth J. D., Brunk B., Grigg M. E., Howard J. C., Parkinson J., Roos D. S., Trees A. J., Berriman M., Pain A., Wastling J. M.2012. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 8: e1002567. doi: 10.1371/journal.ppat.1002567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakikawa M., Noda S., Hanaoka M., Nakayama H., Hojo S., Kakinoki S., Nakata M., Yasuda T., Ikenoue T., Kojima T.2012. Anti Toxoplasma antibody prevalence, primary infection rate, and risk factor in a study of toxoplasmosis in 4,466 pregnant women in Japan. Clin. Vaccine Immunol. 19: 365–367. doi: 10.1128/CVI.05486-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin H. G., Lee S. E., Hong S. H., Kim S. M., Choi Y. G., Park H. J., Seo K. W., Song K. H.2013. Prevalence of Toxoplasma gondii infection in rabbits of Korea by serological tests and nested polymerase chain reaction. J. Vet. Med. Sci. 75: 1609–1613. doi: 10.1292/jvms.13-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugita S., Ogawa M., Inoue S., Shimizu N., Mochizuki M.2011. Diagnosis of ocular toxoplasmosis by two polymerase chain reaction (PCR) examinations: qualitative multiplex and quantitative real-time. Jpn. J. Ophthalmol. 55: 495–501. doi: 10.1007/s10384-011-0065-8 [DOI] [PubMed] [Google Scholar]
- 37.Tenter A. M., Heckeroth A. R., Weiss L. M.2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30: 1217–1258. doi: 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tranas J., Heinzen R. A., Weiss L. M., McAllister M. M.1999. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 6: 765–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueno A., Dautu G., Munyaka B., Carmen G., Kobayashi Y., Igarashi M.2009. Toxoplasma gondii: Identification and characterization of bradyzoite-specific deoxyribose phosphate aldolase-like gene (TgDPA). Exp. Parasitol. 121: 55–63. doi: 10.1016/j.exppara.2008.09.018 [DOI] [PubMed] [Google Scholar]
- 40.Uhlíková M., Hübner J.1973. Congenital transmission of toxoplasmosis in domestic rabbits. Folia Parasitol. (Praha). 20: 285–291 [PubMed] [Google Scholar]
- 41.Zanet S., Palese V., Trisciuoglio A., Cantón Alonso C., Ferroglio E.2013. Encephalitozoon cuniculi, Toxoplasma gondii and Neospora caninum infection in invasive Eastern Cottontail Rabbits Sylvilagus floridanus in Northwestern Italy. Vet. Parasitol. 197: 682–684. doi: 10.1016/j.vetpar.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Zhang H., Cao J., Gong H., Zhou J.2013. Isolation and genotyping of Toxoplasma gondii from domestic rabbits in China to reveal the prevalence of type III strains. Vet. Parasitol. 193: 270–276. doi: 10.1016/j.vetpar.2012.11.031 [DOI] [PubMed] [Google Scholar]