ABSTRACT
The causal relationship between severe allergic conditions and successful pregnancy remains unclear. We aimed to evaluate reproductive performance in an experimental mouse model of atopic disease (AD), and the appearance of uterine natural killer (uNK) cells that have crucial roles in placental formation was examined. In the NC/Nga pregnant mice with moderate skin allergic lesions and an 8.6-fold elevation of plasma IgE, significant differences were not detected in the reproductive indices of the number of normal fetuses, abortion rate and placental size. There were few uNK cells in the placenta of AD mice, and they showed a significant decrease regarding the immature subtype as compared with controls. These findings revealed that AD disturbs uNK cell differentiation and provides disadvantageous effects on placental formation, although it does not arrest the pregnancy process. It may be possible that specific immunological conditions behind AD operate favorably to recover the reproductive performance.
Keywords: atopic disease, NC/Nga mouse, placenta, uNK cell
Atopic dermatitis (AD) is a common allergic disease characterized by chronic inflammatory skin lesions and hyperproduction of IgE and Th-2-type cytokines, such as interleukin (IL)-4 and IL-13, in the blood [3, 12]. There are conflicting data from human studies regarding the relationship between AD and pregnancy. Hanzlikova et al. showed that blood levels of IL-4 and IgE were downregulated in patients with repeated pregnancy loss [2]. On the other hand, an epidemiological study has revealed that AD and allergic diseases were related to difficulty achieving a successful pregnancy [15].
In many mammals, specific lymphocytes appear in the uteri during pregnancy [4]. In rodents and humans, the dominant subset of lymphocytes in the pregnant uterus is natural killer (NK) cells, and they frequently appear in the decidua basalis region. The uterine NK (uNK) cells can be classified into subpopulations by surface markers. The mature population has potential functions in angiogenesis, vasodilation and local blood pressure control, suggesting that these cells contribute to vascular remodeling and placental circulation [1]. However, uNK cells have other cellular profiles to produce cytotoxic proteins, such as perforin and Fas ligand [6, 7]. Generally, NK cells express Fcγ receptors, which can bind to the IgE-immune complex and activate antibody-dependent cell cytotoxicity (ADCC). Therefore, abnormal immunological conditions have the possibility of transforming uNK cell profiles to unmask cytotoxicity against fetal tissue.
NC/NgaTndCrlj (NC/Nga) mice are a valuable experimental model of AD. In response to parasitic ticks or hapten stimulation, these mice develop severe skin lesions, enhanced blood levels of IgE and increased production of IL-4 and IL-13 [11]. In the present study, we evaluated the influence of AD on reproductive performance and uNK cellular profiles by using pregnant NC/Nga mice with AD.
Female NC/Nga mice were purchased from Charles River Japan (Yokohama, Japan). The experimental group was housed in a conventional breeding room and received first application of 5% picryl chloride dissolved in olive oil to the head and back, followed by repeated application of 0.8% picryl chloride for 1weak, according to the recommended protocol [8]. Following confirmation of moderate skin lesions and pruritic dermatitis, NC/Nga mice were mated with ICR male mice. Control NC/Nga female mice were received applications of olive oil in the same manner as the experimental group under specific pathogen free (SPF) condition and were mated with SPF-grade ICR male mice. The morning that a vaginal plug was detected was considered day 0 of pregnancy. On days 10 and 12 of pregnancy, blood and uteri were collected. Experiments were approved by the Ethics Committee for Animal Experiments at Yamaguchi University, and all animals were treated with humane care in keeping with the Yamaguchi University Experimental Guidelines.
The plasma IgE level was measured by using a Mouse IgE ELISA kit (Bethyl Labs, Montgomery, TX, U.S.A.). Each implantation site was visually checked regarding fetal viability and fixed with Bouin’s reagent. Tissue from the central region of the implantation site was cut transversally and prepared as 4 µm–thick paraffin-embedded sections. Some sections were used for periodic acid Schiff (PAS) staining, and the thickness of the placenta and each component region was measured by using a Biozero microscope system (Keyence, Osaka, Japan). Other tissue sections were used for staining with Dolichos biflorus agglutinin (DBA) lectin, which is a specific marker for uNK cell classification [10]. Briefly, deparaffinized sections were incubated with biotinylated-DBA lectin (Vector Labs., Burlingame, CA, U.S.A.) at 4°C overnight. Specific reactions were detected with Avidin-Biotin Complex solution (Vector Labs) and 3–3′diaminobenzidine tetrachloride. The number of uNK cells was counted according to the criteria of differentiation stages from subtypes I to IV [10]. For statistical evaluation, data were collected from at least 3 different mice, and more than 3 measurement fields were selected from at least 3 different sections prepared from different mice. P values<0.05 were considered to be significant following Fisher’s exact probability test (for abortion rate) or the Student’s t-test (for plasma IgE level, placental thickness and uNK cell number).
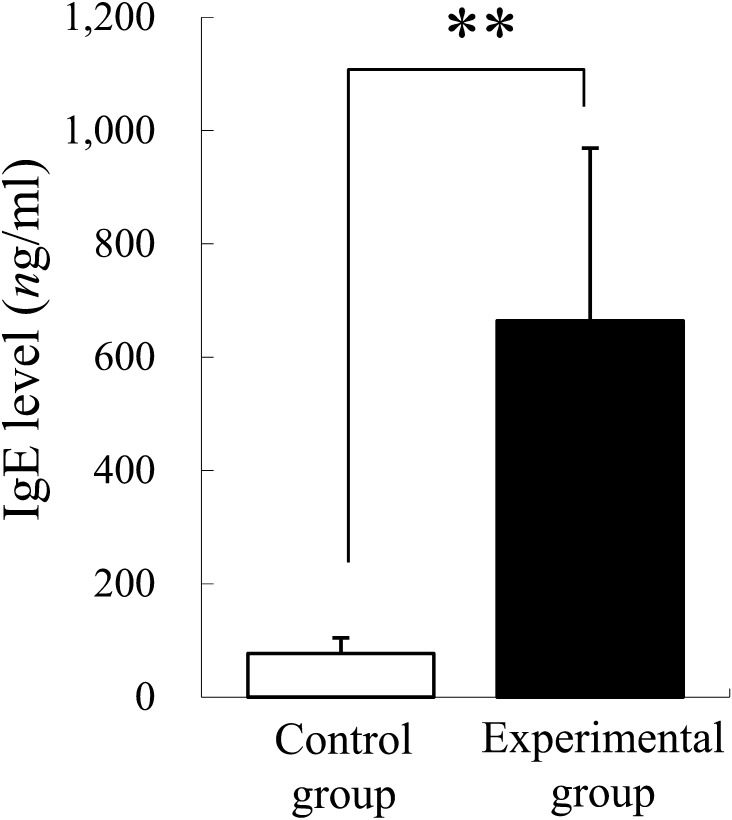
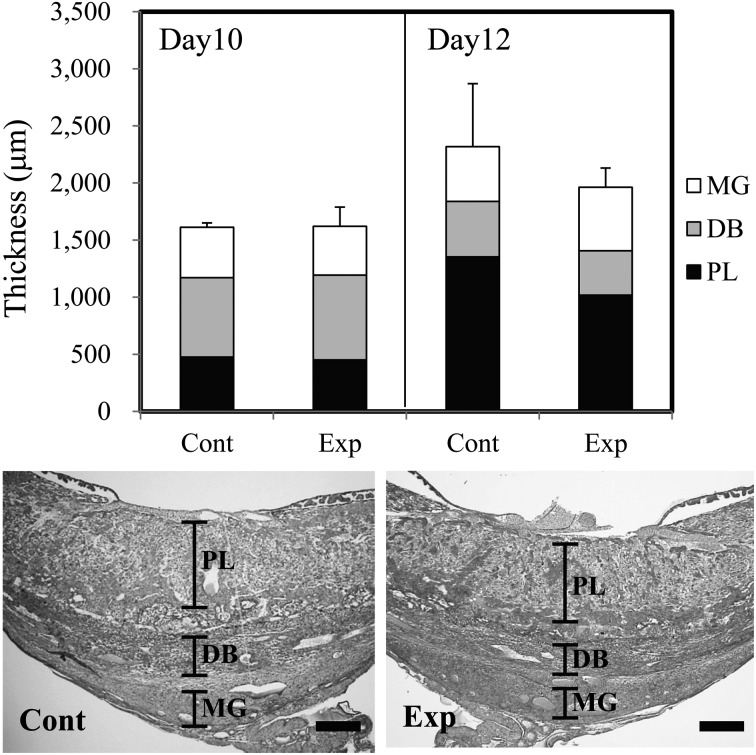
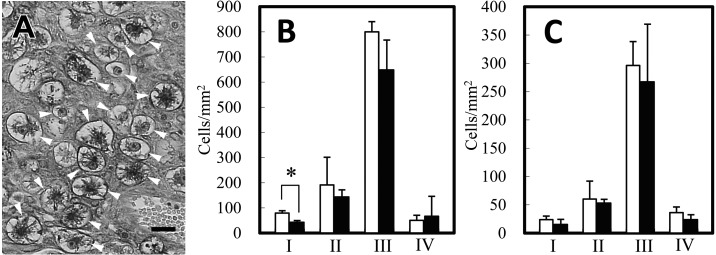
Mice in the experimental group showed an 8.6-fold elevation of plasma IgE levels (Fig. 1). Pregnant uteri contained spontaneously absorbed implantation sites that had shrunk in size and changed in color to dark-red. The abortion rate was higher in the experimental group at both days 10 and 12 (Table 1), although a statistically significant difference was not detected because of the large standard deviation (SD). The placentas of the experimental group showed a reduced thickness at day 12 as compared to control placentas, but the reduction was not statistically significant (Fig. 2). The ratios of each placental component area in the control and experimental groups, respectively, were as follows: (Day 10) metrial gland (MG): 27.4% and 26.3%; decidua basalis (DB): 43.1% and 45.8%; placental labyrinth (PL): 29.6% and 27.8%; (Day 12) MG: 20.7% and 28.3%; DB: 20.9% and 19.8%; PL: 58.4% and 51.9%. The DB and PL tended to be reduced in the experimental group. Apparent pathological changes were not observed in the experimental group placentas. The metrial gland and decidua basalis regions contained many uNK cells that were DBA-lectin positive (Fig. 3A). At most developmental stages, the number of uNK cells was smaller in the placentas of the experimental group as compared to the control group (Fig. 3B). In particular, a significantly lower density was observed on the immature type I uNK cells in the metrial gland region of experimental group placentas.
Fig. 1.
Plasma IgE levels in control and experimental groups. **: P<0.01.
Table 1. The number of fetus and the abortion rate.
| Day10 |
Day12 |
|||
|---|---|---|---|---|
| Control | Experimental | Control | Experimental | |
| Nomal fetuses | 7.00 ± 1.00 | 6.50 ± 3.54 | 7.33 ± 1.37 | 7.33 ± 1.53 |
| Absorbed fetuses | 0.67 ± 0.58 | 1.50 ± 0.71 | 1.00 ± 1.26 | 2.00 ± 2.00 |
| Abortion rate (%) | 8.47 ± 7.50 | 21.7 ± 16.5 | 11.8 ± 15.4 | 20.7 ± 20.0 |
Data represent Mean ± SD.
Fig. 2.
Upper column: Thickness of placenta and each placental area (MG: metrial gland, DB: decidua basalis, PL: placental labyrinth). Lower column: representative photo of a placenta from each group. Zonation of each placental area is indicated. Scale bar=500 µm
Fig. 3.
Lectin staining of placenta at day 12 (A). DBA lectin reacts on the surface and cytoplasmic granules of uNK cells (arrowheads). Subtypes for uNK cell maturation were determined by cell size and degree of granular development. Scale bar=20 µm. Cell density of uNK cells at the metrial gland (B) and the decidua basalis regions (C). Open bar: control group; closed bar: experimental group. *: P<0.05.
The present study showed a negative influence of AD and severe hyperimmunoglobulinemia on fetal survival, placental formation and appearance of uNK cells in placentas, but these effects seemed to be non-fatal. Insufficient uNK cell recruitment is considered to negatively affect reproductive performance in NC/Nga mice. Paffaro et al. suggested subtype I uNK cells as immature progenitor cells recruit from peripheral blood to pregnant uteri [10]. For vascular recruitment, plural adhesion molecules are required for uNK cell settlement in uteri [5]. Under allergic conditions, the microenvironment of adhesion molecules is modified by the IL-4 effect to elevate vascular cell adhesion molecule (VCAM)-1 and eotaxin, resulting in the activation of eosinophils and basophils [11]. Reactivity to eotaxin has been observed especially on the CD56dim NK cell subset, which is a cytotoxic subtype of human NK cells, while human CD56bright NK cells, which are analogous to mouse uNK cells, hardly show responsiveness to eotaxin [14]. Modification of the adhesion molecule pattern may induce the functional decline of uNK cells and inadequate vascular reconstitution [1, 9], resulting in a less efficient blood supply and reduced fetal survival. Additionally, since some atopic dams showed a high abortion rate, hyperimmunoglobulinemia itself could possibly affect the maternal reproductive system. However, a clear causal association between pregnancy loss and atopic disease could not be found in the present study.
In normal pregnancy, placental trophoblasts stably express IL-4, which stimulates the release of chorionic gonadotropin and subsequently promotes the production of ovarian progesterone [13]. Thus, IL-4 itself has a direct reproductive effect that contributes to normal pregnancy. It is also possible that vascular permeability, enhanced by allergic conditions through histamine and IgE-sensitized basophils, operates positively to enhance substance exchange and fetal nutrition. To protect an embryo from the maternal immune system as a semi-allograft, the development of immune privilege in the placenta is necessary. The induction of Th-2–type cytokine dominance (IL-4, 6, 10, etc.) is crucial for immune privilege in placentas. Th-2–type cytokine dominance inhibits lymphocyte cytotoxicity and promotes the release of pregnancy-essential cytokines and hormones [13]. Profiles of Th-2–type cytokines seem to be a key factor in the effect of AD on pregnancy physiology. Cytokine profiles of AD may coincidentally promote the recovery of normal reproductive ability. This idea is consistent with our finding that AD causes little damage to the normal reproductive process. The immunological specificity of AD may resemble that of the reproductive process, e.g. antigen commonality between allergens and embryonic tissue relating to the Th-2 cytokine-dominant condition. On the other hand, a study of the differences of immunological profiles between AD and pregnancy may be necessary to evaluate the pathogenesis of pregnancy disorder.
In brief, the present study raised the possibility that AD inhibits uNK cell development at an early stage and adversely influences placental formation. On the other hand, cytokine profiles evoked by AD may potentially improve the immunological conditions for adapting to reproductive physiology.
Acknowledgments
This study was supported by JSPS KAKENHI, Grant No. 24380159 (to Y.K.) and No. 23580407 (to K-T.K.).
REFERENCES
- 1.Chen Z., Zhang J., Hatta K., Lima P. D. A., Yadi H., Colucci F., Yamada A. T., Croy B. A.2012. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol. Reprod. 87: 81. doi: 10.1095/biolreprod.112.102293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanzlikova J., Ulcova-Gallova Z., Malkusova I., Sefrna F., Panzner P.2009. TH1-TH2 response and the atopy risk in patients with reproduction failure. Am. J. Reprod. Immunol. 61: 213–220. doi: 10.1111/j.1600-0897.2009.00683.x [DOI] [PubMed] [Google Scholar]
- 3.Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q.1991. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J. Exp. Med. 173: 775–778. doi: 10.1084/jem.173.3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiso Y., Kusakabe K.1998. Some aspects of granulated metrial gland cells found at the feto-maternal interface during successful pregnancy. pp. 327–336. In: Reproductive Biology Update. Novel Tools for Assessment of Environmental Toxicity (Miyamoto, H. and Manabe, N. eds.), Shoukado, Kyoto. [Google Scholar]
- 5.Kruse A., Martens N., Fernekorn U., Hallmann R., Butcher E. C.2002. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol. Reprod. 66: 333–345. doi: 10.1095/biolreprod66.2.333 [DOI] [PubMed] [Google Scholar]
- 6.Kusakabe K., Otsuki Y., Kiso Y.2005. Involvement of Fas ligand and Fas system in apoptosis induction of mouse uterine natural killer cell. J. Reprod. Dev. 51: 333–340. doi: 10.1262/jrd.16086 [DOI] [PubMed] [Google Scholar]
- 7.Kusakabe K., Li Z. L., Kiso Y., Otsuki Y.2005. Perforin improves the morphogenesis of mouse placenta disturbed by IL-2 treatment. Immunobiology 209: 719–728. doi: 10.1016/j.imbio.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 8.Matsuda H., Tanaka A.1998. Effectiveness of NC/Nga mice as a model for atopic dermatitis. CRJ letters 11:1–8 (in Japanese). [Google Scholar]
- 9.Monk J. M., Leonard S., McBey B. A., Croy B. A.2005. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta 26: 835–838. doi: 10.1016/j.placenta.2004.10.016 [DOI] [PubMed] [Google Scholar]
- 10.Paffaro V. A., Bizinotto M. C., Joazeiro P. P., Yamada A. T.2003. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 24: 479–488. doi: 10.1053/plac.2002.0919 [DOI] [PubMed] [Google Scholar]
- 11.Prussin C., Metcalfe D. D.2003. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 111: S486–S494. doi: 10.1067/mai.2003.120 [DOI] [PubMed] [Google Scholar]
- 12.Reinhold U., Wehrmann W., Kukel S., Kreysel H. W.1990. Evidence that defective interferon-gamma production in atopic dermatitis patients is due to intrinsic abnormalities. Clin. Exp. Immunol. 79: 374–379. doi: 10.1111/j.1365-2249.1990.tb08098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito S.2000. Cytokine network at the feto-maternal interface. J. Reprod. Immunol. 47: 87–103. doi: 10.1016/S0165-0378(00)00060-7 [DOI] [PubMed] [Google Scholar]
- 14.Wilk E., Kalippke K., Buyny S., Schmidt R. E., Jacobs R.2008. New aspects of NK cell subset identification and inference of NK cells’ regulatory capacity by assessing functional and genomic profiles. Immunobiology 213: 271–283. doi: 10.1016/j.imbio.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 15.Zac R. I., Machado V. M., Alberti L. R., Petroianu A.2005. Association of allergy, infertility and abortion. Rev. Assoc. Med. Bras. 51: 177–180(in Portuguese). doi: 10.1590/S0104-42302005000300020 [DOI] [PubMed] [Google Scholar]