ABSTRACT
The anesthetic effect of a combination of medetomidine, midazolam and butorphanol (Me-Mi-Bu) was evaluated in healthy cynomolgus monkeys. The Me-Mi-Bu combination was intramuscularly administered as follows: Dose 1, Me 0.015 mg/kg-Mi 0.1 mg/kg-Bu 0.15 mg/kg; Dose 2, Me 0.02 mg/kg-Mi 0.15 mg/kg-Bu 0.2 mg/kg; and Dose 3, Me 0.04 mg/kg-Mi 0.3 mg/kg-Bu 0.4 mg/kg. The combination rapidly induced immobilization, and lateral recumbency was reached within 15 min. The duration of anesthesia for each dose administered was follows: Dose 1, 47 ± 27 min; Dose 2, 113 ± 31 min; and Dose 3, 190 ± 24 min. The anesthetic effect of the combination was abolished by the α2-adrenoceptor antagonist atipamezole. No marked changes in the levels of hematologic or serum biochemical parameters were noted in cynomolgus monkeys administered the combination plus atipamezole. Taken together, these results suggest that the Me-Mi-Bu combination exhibits reversible anesthetic effect and may be useful for studies involving cynomolgus monkeys.
Keywords: anesthetics, butorphanol, medetomidine, midazolam, monkey
Male cynomolgus monkeys (Macaca fascicularis) are used in research involving transplant surgery and pharmacokinetic studies during the development of new drugs [11]. However, monkeys present special hazards to handlers, such as biting and zoonotic infection, and often must be anesthetized for use in a study. Balanced anesthesia consisting of a combination of medetomidine, midazolam and butorphanol (Me-Mi-Bu) has been successfully used in mice (ICR, BALB/c and C57BL/6J) [10, 12], beagle dogs [8, 18], other species of monkeys (Erythrocebus patas and Lemur catta) [9, 20] and African lions (Panthera leo) [19]. Here, we investigated the use of the Me-Mi-Bu combination in cynomolgus monkeys (Macaca fascicularis) and evaluated the antagonistic effect of atipamezole on anesthesia induced by the Me-Mi-Bu combination. To prepare background data for safe use, we also evaluated the changes in blood parameters of monkeys following drug administration.
All animal experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc., which has been awarded Accreditation Status by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. Every effort was made to minimize the number of animals used and their degree of suffering.
Healthy male cynomolgus monkeys (4.9–6.0 kg, Japan Laboratory Animals, Tokyo, Japan) at the age of 5–6 years were used in this study. The monkeys were housed in stainless steel primate cages (650 × 680 × 1253 mm) with a 12:12 hr light-dark cycle (lights on from 07:00 to 19:00) in a controlled temperature (25 ± 1°C) and humidity (55 ± 5%) environment in compliance with the Guide for the Care and Use of Laboratory Animals [2]. HEPA filtered 100% fresh air was used with 15 to 20 changes per hr. Monkeys were fed standard laboratory food (approximately 100 g/animal/day, PS-A; Oriental Yeast, Tokyo, Japan), and tap water was available ad libitum before the experiment. An environmental enrichment program ensured that the monkeys were provided with toys, fresh fruit and other treats on a daily basis. Before the experiment, the monkeys were determined to be healthy on the basis of general appearance, activity and tuberculosis skin test. All animals were serologically negative for B virus.
Eight monkeys received seven different treatments at the rate of one treatment per week in a randomized order (Table 1). Medetomidine, midazolam and butorphanol were mixed in the same syringe just before use, and an intramuscular (i.m.) administration was made in the hind limb (the quadriceps muscle) of each animal. Dose 3 of the Me-Mi-Bu combination was determined by references to Kalema-Zikusoka et al. [9] and Williams et al. [20]. The i.m. administration of the Me-Mi-Bu combination was followed by atipamezole into the quadriceps muscles of monkeys after 30 min. The anesthetic effect of the drugs was evaluated based on their posture, which was evaluated according to the following criteria: Score 0, normal; Score 1, sedated but able to stand; Score 2, sternal recumbency; Score 3, lateral recumbency with apparent spontaneous movement (head and/or limb); Score 4, lateral recumbency with subtle spontaneous movement (twitching and/or blink); Score 5, lateral recumbency without spontaneous movement and unable get up [7]. Monkeys with Score 5 were regarded as showing a positive anesthetic effect.
Table 1. Animal data used in this study.
| Animal No. | Age (years) | Sex | Body weight (kg) | |
|---|---|---|---|---|
| 1) The Me-Mi-Bu combination (dose response) | ||||
| Dose 1 | 0703657 | 5 | male | 5.5 |
| 0701719 | 6 | male | 5.4 | |
| 0704549 | 5 | male | 4.9 | |
| Dose 2 | 6001830806 | 5 | male | 5.8 |
| 0703657 | 5 | male | 5.3 | |
| 0701719 | 6 | male | 5.2 | |
| Dose 3 | 0704549 | 5 | male | 5.1 |
| 6001830806 | 5 | male | 6.0 | |
| 0701719 | 6 | male | 5.3 | |
| 2) Antagonisum by atipamezole | ||||
| Control (Dose 2) | 0704549 | 5 | male | 5.1 |
| 6001830806 | 5 | male | 5.8 | |
| 0703657 | 5 | male | 5.3 | |
| Atipamezole 0.1 | 6001830806 | 5 | male | 5.9 |
| 0703657 | 5 | male | 5.3 | |
| 0704549 | 5 | male | 5.0 | |
| Atipamezole 0.2 | 0703657 | 5 | male | 5.4 |
| 0704549 | 5 | male | 4.9 | |
| 6001830806 | 5 | male | 6.0 | |
| 3) Blood parameters | ||||
| Control (Dose 2) + Atipamezole 0.2 | 8650132066 | 5 | male | 5.1 |
| 0611747 | 6 | male | 5.5 | |
| 0704097 | 6 | male | 5.7 | |
| 0704385 | 6 | male | 5.3 | |
A total of 7 anesthetic treatments using 8 monkeys were evaluated.
The following drugs were used in this study: medetomidine hydrochloride and atipamezole hydrochloride (Nippon Zenyaku Kogyo, Fukushima, Japan), midazolam (Astellas Pharma, Tokyo, Japan) and butorphanol tartrate (Meiji Seika Pharma, Tokyo, Japan).
Blood was collected from the saphenous vein of the hind limb via a disposable syringe before dosing and 2 hr after the i.m. administration of the Me-Mi-Bu combination. Whole blood collected into K2EDTA-coated tubes was analyzed using the ADVIA 120 (Siemens Japan K.K., Tokyo, Japan) for hematologic parameters. For blood chemistry measurements, whole blood was collected in plastic tubes without anticoagulant, allowed to clot and then centrifuged at 3,000 rpm for 10 min at 4°C to separate serum. The resulting supernatant (e.g. the serum sample) was assayed using the Automatic Analyzer 7170S (Hitachi Ltd., Tokyo, Japan).
The results are expressed as mean ± standard error of the mean (SEM). Statistical significance was analyzed using the paired t-test for two groups. The difference between groups was considered statistically significant when P<0.05.
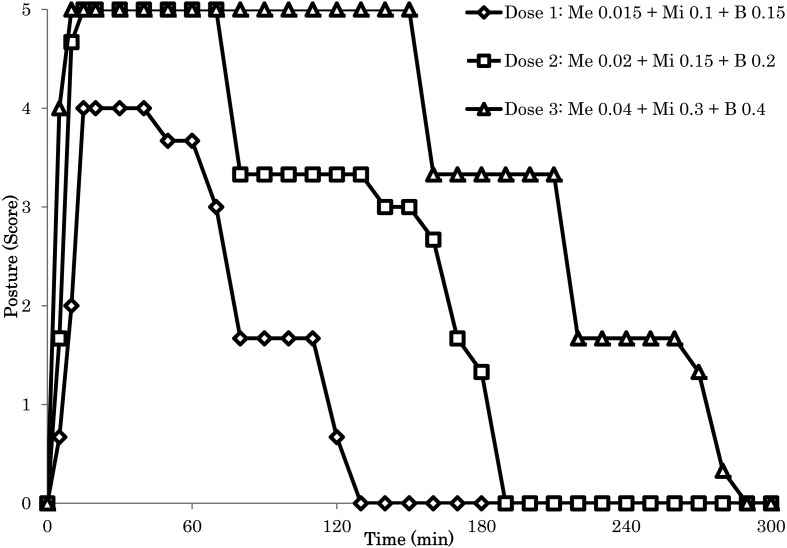
Figure 1 shows the anesthetic effect of i.m. administered the Me-Mi-Bu combination (Dose 1–3) in cynomolgus monkeys, as assessed by scoring posture. Drug administration was conducted at the following doses: Dose 1, Me 0.015 mg/kg-Mi 0.1 mg/kg-Bu 0.15 mg/kg; Dose 2, Me 0.02 mg/kg-Mi 0.15 mg/kg-Bu 0.2 mg/kg; and Dose 3, Me 0.04 mg/kg-Mi 0.3 mg/kg-Bu 0.4 mg/kg. The Me-Mi-Bu combination rapidly induced immobilization, and lateral recumbency appeared within 15 min. Duration of anesthesia for the Me-Mi-Bu combination for each dose was as follows: Dose 1 (47 ± 27 min), Dose 2 (113 ± 31 min) and Dose 3 (190 ± 24 min). However, 1 of the 3 monkeys that received the Me-Mi-Bu combination (Dose 1) did not exhibit lateral recumbency (posture score:>3) during the test. One of the reasons why a monkey showed insufficiant effect using Dose 1 is that level of medetomidine, midazolam and butophanol in the blood and the central nervous systems (CNS) including cerebrospinal fluid after administration of Dose 1 is lower than minimum effective blood concentration of these drugs.
Fig. 1.
Anesthetic effect of the medetomidine-midazolam-butorphanol combination (Dose 1-3; i.m.) in cynomolgus monkeys, as assessed by scoring posture. Each symbol indicates the mean value (n=3).
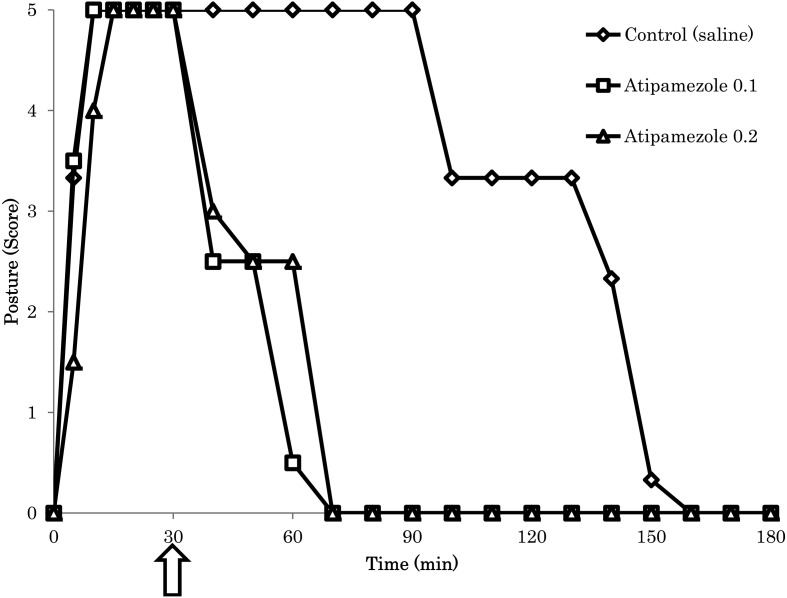
The anesthetic effect caused by the i.m. administration of the Me-Mi-Bu combination (Dose 2) was reversed by that of atipamezole at a dose of 0.1 or 0.2 mg/kg (Fig. 2). The duration of anesthesia for the Me-Mi-Bu combination (Dose 2-control) was 112 ± 16 min.
Fig. 2.
Effect of atipamezole on the medetomidine-midazolam-butorphanol combination (Dose 2; i.m.)-induced anesthesia in cynomolgus monkeys, as assessed by scoring posture. Atipamezole was administered intramuscularly 30 min after the medetomidine-midazolam-butorphanol combination injection. Each symbol indicates the mean value (n=3).
No marked changes were noted in the levels of hematologic and serum biochemical parameters in cynomolgus monkeys given the Me-Mi-Bu combination (Dose 2) plus atipamezole compared to those before dosing (Tables 2 and 3). These data were within normal ranges.
Table 2. Levels of serum blood chemical parameters in peripheral blood before and after drug administration in male cynomolgus monkeys.
| Before | After | |
|---|---|---|
| Total protein (g/dl) | 7.1 ± 0.1 | 6.8 ± 0.3 |
| Albumin (g/dl) | 4.1 ± 0.3 | 4.0 ± 0.4 |
| Total bilirubin (mg/dl) | 0.11 ± 0.01 | 0.10 ± 0.01 |
| Cholestrol (mg/dl) | 109 ± 14 | 107 ± 14 |
| Triglycelides (mg/dl) | 28 ± 7 | 23 ± 5 |
| Alkaline phosphatase (mU/ml) | 613 ± 92 | 581 ± 89 |
| Aspartate aminotransferase (mU/ml) | 32 ± 7 | 50 ± 10 |
| Alanine aminotransferase (mU/ml) | 42 ± 13 | 42 ± 13 |
| Glucose (mg/dl) | 70 ± 3 | 65 ± 4 |
| Blood urea nitrogen (mg/dl) | 27 ± 4 | 26 ± 3 |
| Creatinine (mg/dl) | 1.0 ± 0.12 | 1.2 ± 0.14 |
| Inorganic P (mg/dl) | 5.2 ± 0.4 | 5.6 ± 0.5 |
| Ca2+ (mg/dl) | 10.0 ± 0.3 | 9.4 ± 0.3 |
| Na+ (mEq/l) | 149 ± 0.6 | 149 ± 0.5 |
| K+ (mEq/l) | 5.4 ± 0.2 | 5.4 ± 0.2 |
| Cl− (mEq/l) | 108 ± 1.2 | 110 ± 0.5 |
Me 0.02 mg/kg-Mi 0.15 mg/kg-Bu 0.2 mg/kg combination was administered intramuscularly, and 30 min after, atipamezole 0.2 mg/kg was administered. Blood sample was collected before dosing and 2 hr after i.m. administration of the Me-Mi-Bu combination. Values are means ± S.E.M. (n=4).
Table 3. Levels of hematological parameters in peripheral blood before and after drug administration in male cynomolgus monkeys.
| Before | After | |
|---|---|---|
| Red blood cell count (×106/μl) | 5.5 ± 0.2 | 5.2 ± 0.3 |
| Hemoglobin concentration (g/dl) | 13.7 ± 0.6 | 12.7 ± 0.9 |
| Hematocrit (%) | 45.3 ± 1.8 | 42.2 ± 3.1 |
| Mean corpuscular volume (fl) | 81.7 ± 1.3 | 81.1 ± 1.7 |
| Mean corpuscular hemoglobin (pg) | 24.6 ± 0.4 | 24.4 ± 0.4 |
| Mean corpuscular hemoglobin concentration (g/dl) | 30.1 ± 0.3 | 30.1 ± 0.2 |
| Red blood cell distribution width (%) | 13.0 ± 0.2 | 12.9 ± 0.2 |
| Hemoglobin concentration distribution width (g/dl) | 2.1 ± 0.1 | 2.2 ± 0.1 |
| Platelet count (×103/μl) | 448 ± 95 | 423 ± 76 |
| White blood cell count (×103/μl) | 10.9 ± 1.1 | 10.5 ± 2.4 |
Me 0.02 mg/kg-Mi 0.15 mg/kg-Bu 0.2 mg/kg combination was administered intramuscularly, and 30 min after, atipamezole 0.2 mg/kg was administered. Blood sample was collected before dosing and 2 hr after i.m. administration of the Me-Mi-Bu combination. Values are means ± S.E.M. (n=4).
Here, we examined the anesthetic effect of the Me-Mi-Bu combination in cynomolgus monkeys and observed that it was rapidly induced with lateral recumbency without spontaneous movement. After i.m. administration, the Me-Mi-Bu combination exerted a dose-dependent anesthetic effect that was completely reversed by α2-adrenocepter antagonist atipamezole.
Medetomidine is highly selective and specific as well as the most potent α2-adrenocepter agonist, producing deep sedation associated with muscle relaxation and analgesia via stimulation of the α2-adrenoceptor in the CNS [16]. Medetomidine is known to produce cardiovascular changes and decreases in respiratory rate [1]. The action of medetomidine is reversed by atipamezole, a specific antagonist of α2-adrenoceptors [17]. Midazolam, a benzodiazepine derivative, produces anxiolytic, sedative-hypnotic, muscle relaxant and anticonvulsant effects through the activation of γ-aminobutyric (GABAA) receptors when administered orally, intramuscularly or intravenously [15]. Midazolam has been widely used as a sedative and anticonvulsant in patients [21]. In healthy humans, midazolam produces significant reduction in blood pressure [15]. In addition, midazolam produces some respiratory depression [5]. Butorphanol, a synthetic agonist-antagonist of opioid receptors, is used as an analgesic drug to optimize well-being by reducing postoperative pain [6]. Butorphanol also induces respiratory depression in monkeys like above 2 sedative drugs [14].
The Me-Mi-Bu combination induces cardiorespiratory effects, such as bradycardia, hypotension and respiratory depression, in two strains of monkeys [9, 20]. In addition, the authors noted that cynomolgus monkeys developed bradycardia, hypotension, respiratory depression (as determined by auscultation) and loss of thermoregulatory ability by injection of the Me-Mi-Bu combination (data not shown).
Ketamine (Ke) is the most widely used drug for the induction of anesthesia in monkeys. In the clinically effective dose at 10 mg/kg, ketamine has a rapid onset and relatively short duration of anesthetic effect after intramuscular injection [22]. If monkeys are treated with long-term surgery, it is, therefore, difficult to administrate ketamine alone at a dose of 10 mg/kg as anesthetic. The combination of medetomidine with ketamine for anesthetic induction has been used in several primate models [13]. However, Young et al. reported that a combination of i.m. Ke 2 mg/kg-Me 0.05 mg/kg has a relatively short duration of anesthetic action in cynomolgus monkeys and should only be used for procedures lasting less than 30 min [22]. The present study found that the Me-Mi-Bu combination had a longer duration of action than Ke alone or the Ke-Me combination, exerting its action via the synergy of three different receptor agonists working together to provide reversible CNS depression.
Anesthetics, such as pentobarbital or halothane, have been reported to cause abnormal changes in blood parameters [3, 4]. In contrast, treatment with the Me-Mi-Bu combination plus atipamezole in cynomolgus monkeys did not significantly affect the hematologic or serum biochemical parameters, suggesting that medication with the Me-Mi-Bu combination had no adverse effects on hematologic and serum biochemical parameters.
In conclusion, the Me-Mi-Bu combination, which provided a longer anesthetic effect and was fully reversible, is acceptable for safety use in cynomolgus monkeys.
Acknowledgments
The authors would like to thank Mr. H. Hashimoto, Mr. T. Nakamura, Mr. A. Yamada and Mr. M. Tanimoto (KAC Co., Ltd.) for their technical assistance.
REFERENCES
- 1.Capuano S. V., III, Lerche N. W., Valverde C. R.1999. Cardiovascular, respiratory, thermorgulatory, sedative, and analgesic effects of intravenous administration of medetomidine in rhesus macaques (Macaca mulatta). Lab. Anim. Sci. 49: 537–544 [PubMed] [Google Scholar]
- 2.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, and Institute for Laboratory Animal Research. 2010. Environment, housing, and management. pp.41–103. In: Guide for the Care and Use of Laboratory Animals, 8th ed. The National Academies Press, Washington, D.C. [Google Scholar]
- 3.D’Amour P., Rousseau L., Rocheleau B., Pomier-Layrargues G., Huet P. M.1996. Influence of Ca2+ concentration on the clearance and circulating levels of intact and carboxy-terminal iPTH in pentobarbital-anesthetized dogs. J. Bone Miner. Res. 11: 1075–1085. doi: 10.1002/jbmr.5650110806 [DOI] [PubMed] [Google Scholar]
- 4.Fink S., Bosia A., Hofmann J. G.1989. Eine Methode zur Erfassung halothaninduzierter Veränderungen der cytosolischen Calciumkonzentration in Blutplättchen. Z. Med. Lab. Diagn. 30: 207–218 [PubMed] [Google Scholar]
- 5.Forster A., Morel D., Bachmann M., Gemperle M.1983. Respiratory depressant effect of diferent doses of midazolam and lack of reversal with naloxone-A double-blind randomized study. Anesth. Analg. 62: 920–924. doi: 10.1213/00000539-198310000-00012 [DOI] [PubMed] [Google Scholar]
- 6.Gupta R., Kaur S., Singh S., Aujla K. S.2011. A comparison of epidural butorphanol and tramadol for postoperative analgesia using CSEA technique. J. Anaesthesiol.Clin. Pharmacol. 27: 35–38 [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi K., Nishimura R., Yamaki A., Kim H.Y., Matsunaga S., Sasaki N., Takeuchi A.1994. Comparison of sedative effects induced by medetomidine, medetomidine-midazolam and medetomidine-butorphanol in dogs. J. Vet. Med. Sci. 56: 951–956. doi: 10.1292/jvms.56.951 [DOI] [PubMed] [Google Scholar]
- 8.Itamoto K., Hikasa Y., Sakonjyu I., Itoh H., Kakuta T., Takase K.2000. Anaesthetic and cardiopulmonary effects of balanced anaesthesia with medetomidine-midazolam and butorphanol in dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 47: 411–420. doi: 10.1046/j.1439-0442.2000.00302.x [DOI] [PubMed] [Google Scholar]
- 9.Kalema-Zikusoka G., Horne W. A., Levine J., Loomis M. R.2003. Comparison of the cardiorespiratory effects of medetomidine-butorphanol-ketamine and medetomidine-butorphanol-midazolam in patas monkeys (Erythrocebus patas). J. Zoo Wildl. Med. 34: 47–52 [DOI] [PubMed] [Google Scholar]
- 10.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 11.Kinugasa F., Nagatomi I., Ishikawa H., Nakanishi T., Maeda M., Hirose J., Fukahori H., Ooshima S., Noto T., Higashi Y., Seki N., Mutoh S.2008. Efficacy of oral treatment with tacrolimus in the renal transplant model in cynomolgus monkeys. J. Pharmacol. Sci. 108: 529–534. doi: 10.1254/jphs.08142FP [DOI] [PubMed] [Google Scholar]
- 12.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee V. K., Flynt K. S., Haag L. M., Taylor D. K.2010. Comparison of the effects of ketamine, ketamine-medetomidine, and ketamine-midazolam on physilogic parameters and anesthesia-induced stress in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. J. Am. Assoc. Lab. Anim. Sci. 49: 57–63 [PMC free article] [PubMed] [Google Scholar]
- 14.Liguori A., Morse W. H., Bergman J.1996. Respiratory effects of opioid full and partial agonists in rhesus monkeys. J. Pharmacol. Exp. Ther. 277: 462–472 [PubMed] [Google Scholar]
- 15.Reves J. G., Fragen R. J., Ronald Vinik H., Greenblatt D. J.1985. Midazolam: phamacology and uses. Anesthesiology 62: 310–324. doi: 10.1097/00000542-198503000-00017 [DOI] [PubMed] [Google Scholar]
- 16.Sinclair M. D.2003. A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 44: 885–897 [PMC free article] [PubMed] [Google Scholar]
- 17.Vainio O., Vähä-Vahe T.1990. Reversal of medetomidine sedation by atipamezole in dogs. J. Vet. Pharmacol. Ther. 13: 15–22. doi: 10.1111/j.1365-2885.1990.tb00742.x [DOI] [PubMed] [Google Scholar]
- 18.Verstegen J., Petcho A.1993. Medetomizine-butorphanol-midazolam for anaesthesia in dogs and its reversal by atipamezole. Vet. Rec. 132: 353–357. doi: 10.1136/vr.132.14.353 [DOI] [PubMed] [Google Scholar]
- 19.Wenger S., Buss P., Joubert J., Steenkamp J., Shikwambana P., Hatt J.M.2010. Evaluation of butorphanol, medetomidine and midazolam as a reversible narcotic combination in free-ranging African lions (Panthera leo). Vet. Anaesth. Analg. 37: 491–500. doi: 10.1111/j.1467-2995.2010.00569.x [DOI] [PubMed] [Google Scholar]
- 20.Williams C. V., Glenn K. M., Levine J. F., Horne W. A.2003. Comparison of the efficacy and cardiorespiratory effects of medetomidine-based anesthetic protocols in ring-tailed lemurs (Lemur catta). J. Zoo Wildl. Med. 34: 163–170 [DOI] [PubMed] [Google Scholar]
- 21.Wilson C. M., Dundee J. W., Moore J., Howard P. J., Collier P. S.1987. A comparison of the early pharmacokinetics of midazolam in pregnant and nonpregnant women. Anaesthesia 42: 1057–1062. doi: 10.1111/j.1365-2044.1987.tb05168.x [DOI] [PubMed] [Google Scholar]
- 22.Young S. S., Schilling A. M., Skeans S., Ritacco G.1999. Short duration anaesthesia with medetomidine and ketamine in cynomolgus monkeys. Lab. Anim. 33: 162–168. doi: 10.1258/002367799780578363 [DOI] [PubMed] [Google Scholar]