Abstract
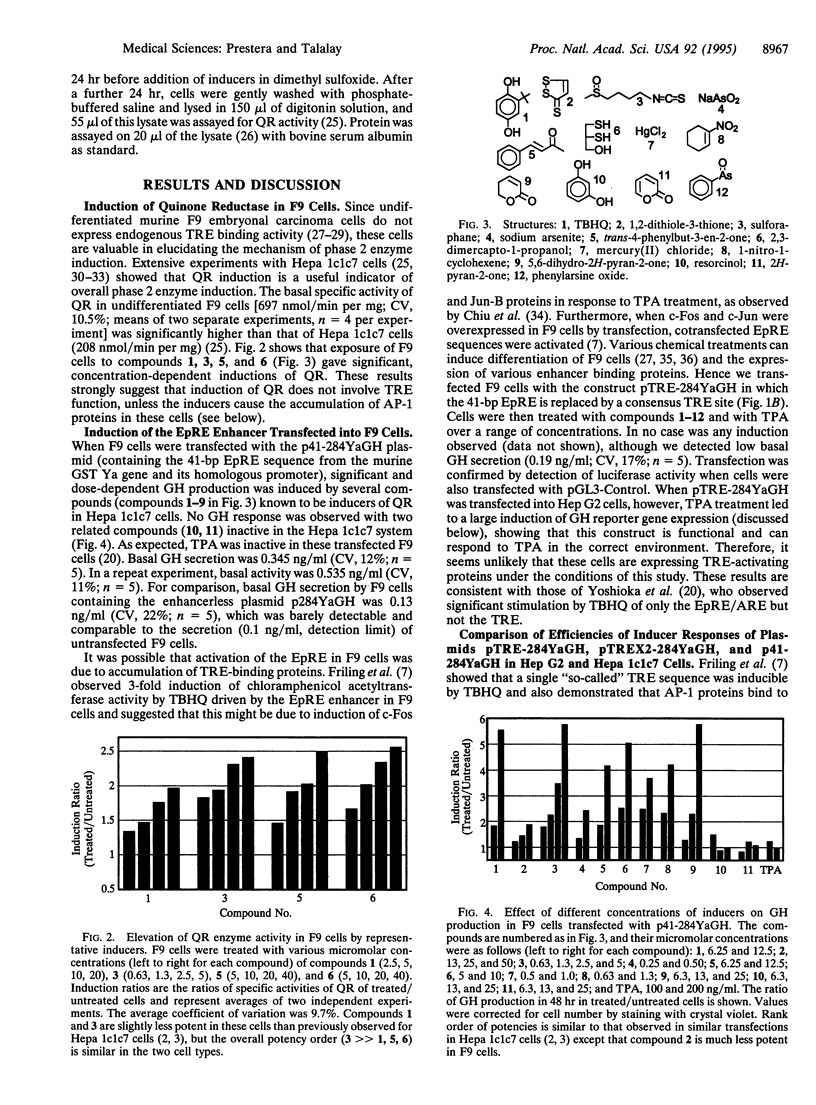
Detoxication (phase 2) enzymes, such as glutathione S-transferases (GSTs), NAD(P)H:(quinone-acceptor) oxidoreductase (QR), and UDP-glucuronsyltransferase, are induced in animal cells exposed to a variety of electrophilic compounds and phenolic antioxidants. Induction protects against the toxic and neoplastic effects of carcinogens and is mediated by activation of upstream electrophile-responsive/antioxidant-responsive elements (EpRE/ARE). The mechanism of activation of these enhancers was analyzed by transient gene expression of growth hormone reporter constructs containing a 41-bp region derived from the mouse GST Ya gene 5'-upstream region that contains the EpRE/ARE element and of constructs in which this element was replaced with either one or two consensus phorbol 12-tetradecanoate 13-acetate (TPA)-responsive elements (TREs). When these three constructs were compared in Hep G2 (human) and Hepa 1c1c7 (murine) hepatoma cells, the wild-type sequence was highly activated by diverse inducers, including tert-butylhydroquinone, Michael reaction acceptors, 1,2-dithiole-3-thione, sulforaphane,2,3-dimercapto-1-propanol, HgCl2, sodium arsenite, and phenylarsine oxide. In contrast, constructs with consensus TRE sites were not induced significantly. TPA in combination with these compounds led to additive or synergistic inductions of the EpRE/ARE construct, but induction of the TRE construct was similar to that induced by TPA alone. Transfection of the EpRE/ARE reporter construct into F9 cells, which lack endogenous TRE-binding proteins, produced large inductions by the same compounds, which also induced QR activity in these cells. We conclude that activation of the EpRE/ARE by electrophile and antioxidant inducers is mediated by EpRE/ARE-specific proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergelson S., Daniel V. Cooperative interaction between Ets and AP-1 transcription factors regulates induction of glutathione S-transferase Ya gene expression. Biochem Biophys Res Commun. 1994 Apr 15;200(1):290–297. doi: 10.1006/bbrc.1994.1447. [DOI] [PubMed] [Google Scholar]
- Bergelson S., Pinkus R., Daniel V. Induction of AP-1 (Fos/Jun) by chemical agents mediates activation of glutathione S-transferase and quinone reductase gene expression. Oncogene. 1994 Feb;9(2):565–571. [PubMed] [Google Scholar]
- Bergelson S., Pinkus R., Daniel V. Intracellular glutathione levels regulate Fos/Jun induction and activation of glutathione S-transferase gene expression. Cancer Res. 1994 Jan 1;54(1):36–40. [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Daniel V. Glutathione S-transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28(3):173–207. doi: 10.3109/10409239309086794. [DOI] [PubMed] [Google Scholar]
- Egner P. A., Kensler T. W., Prestera T., Talalay P., Libby A. H., Joyner H. H., Curphey T. J. Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis. 1994 Feb;15(2):177–181. doi: 10.1093/carcin/15.2.177. [DOI] [PubMed] [Google Scholar]
- Favreau L. V., Pickett C. B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991 Mar 5;266(7):4556–4561. [PubMed] [Google Scholar]
- Friling R. S., Bensimon A., Tichauer Y., Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friling R. S., Bergelson S., Daniel V. Two adjacent AP-1-like binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K. Antioxidant response element. Biochem Pharmacol. 1994 Aug 3;48(3):439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991 Nov 5;30(44):10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Li Y., Jaiswal A. K. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1992 Jul 25;267(21):15097–15104. [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mulcahy R. T., Gipp J. J. Identification of a putative antioxidant response element in the 5'-flanking region of the human gamma-glutamylcysteine synthetase heavy subunit gene. Biochem Biophys Res Commun. 1995 Apr 6;209(1):227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Pickett C. B. Regulation of rat glutathione S-transferase Ya subunit gene expression. DNA-protein interaction at the antioxidant responsive element. J Biol Chem. 1992 Jul 5;267(19):13535–13539. [PubMed] [Google Scholar]
- Nguyen T., Rushmore T. H., Pickett C. B. Transcriptional regulation of a rat liver glutathione S-transferase Ya subunit gene. Analysis of the antioxidant response element and its activation by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. J Biol Chem. 1994 May 6;269(18):13656–13662. [PubMed] [Google Scholar]
- Pinkus R., Weiner L. M., Daniel V. Role of quinone-mediated generation of hydroxyl radicals in the induction of glutathione S-transferase gene expression. Biochemistry. 1995 Jan 10;34(1):81–88. doi: 10.1021/bi00001a010. [DOI] [PubMed] [Google Scholar]
- Prestera T., Holtzclaw W. D., Zhang Y., Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T., Zhang Y., Spencer S. R., Wilczak C. A., Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988 Mar;169(2):328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B., Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Putzer R. R., Zhang Y., Prestera T., Holtzclaw W. D., Wade K. L., Talalay P. Mercurials and dimercaptans: synergism in the induction of chemoprotective enzymes. Chem Res Toxicol. 1995 Jan-Feb;8(1):103–110. doi: 10.1021/tx00043a014. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R., Pickett C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991 Jun 25;266(18):11632–11639. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Talalay P., De Long M. J., Prochaska H. J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P. Mechanisms of induction of enzymes that protect against chemical carcinogenesis. Adv Enzyme Regul. 1989;28:237–250. doi: 10.1016/0065-2571(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Wang B., Williamson G. Detection of a nuclear protein which binds specifically to the antioxidant responsive element (ARE) of the human NAD(P) H:quinone oxidoreductase gene. Biochim Biophys Acta. 1994 Nov 22;1219(3):645–652. doi: 10.1016/0167-4781(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Xie T., Belinsky M., Xu Y., Jaiswal A. K. ARE- and TRE-mediated regulation of gene expression. Response to xenobiotics and antioxidants. J Biol Chem. 1995 Mar 24;270(12):6894–6900. doi: 10.1074/jbc.270.12.6894. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chiu R., Karin M. Elevation of AP1 activity during F9 cell differentiation is due to increased c-jun transcription. New Biol. 1990 Apr;2(4):351–361. [PubMed] [Google Scholar]
- Yoshioka K., Deng T., Cavigelli M., Karin M. Antitumor promotion by phenolic antioxidants: inhibition of AP-1 activity through induction of Fra expression. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4972–4976. doi: 10.1073/pnas.92.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]


