Abstract
Purpose
We investigated sex-hormone receptor expression as predicting factor of recurrence and progression in patients with non-muscle invasive bladder cancer.
Materials and Methods
We retrospectively evaluated tumor specimens from patients treated for transitional cell carcinoma of the bladder at our institution between January 2006 and January 2011. Performing immunohistochemistry using a monoclonal androgen receptor antibody and monoclonal estrogen receptor-beta antibody on paraffin-embedded tissue sections, we assessed the relationship of immunohistochemistry results and prognostic factors such as recurrence and progression.
Results
A total of 169 patients with bladder cancer were evaluated in this study. Sixty-threepatients had expressed androgen receptors and 52 patients had estrogen receptor beta. On univariable analysis, androgen receptor expression was significant lower in recurrence rates (p=0.001), and estrogen receptor beta expression was significant higher in progression rates (p=0.004). On multivariable analysis, significant association was found between androgen receptor expression and lower recurrence rates (hazard ratio=0.500; 95% confidence interval, 0.294 to 0.852; p=0.011), but estrogen receptor beta expression was not significantly associated with progression rates.
Conclusion
We concluded that the possibility of recurrence was low when the androgen receptor was expressed in the bladder cancer specimen and it could be the predicting factor of the stage, number of tumors, carcinoma in situ lesion and recurrence.
Keywords: Androgen receptor, estrogen receptor beta, urinary bladder neoplasms
INTRODUCTION
Bladder cancer is one of the most common cancers, with a 3 times higher incidence in males than females.1,2 The origin of the differences in incidence between the genders is unknown. Tobacco smoking and some chemicals have been established as risk factors for bladder cancer, but the higher incidence in males cannot be fully explained by gender differences in smoking habits or occupational exposures.3,4 Hormonal differences have also been considered as a potential explanation for the gender disparity in the incidence of bladder cancer. Experiments on animal models with naturally occurring or chemically induced bladder cancer, demonstrated that hormonal manipulation changed the natural course of the tumors and that androgen suppression generally provided better survival and more benign courses,5,6 suggesting that androgens may have a role in the natural history of certain bladder tumors and that their presence may enhance tumor growth. However, in humans the role of sex steroid hormone receptors in the development of bladder cancer has been less well studied.
On the basis of prognostic factors, patients with non-muscle-invasive bladder cancer can be divided to subgroups. Subdivision by tumor stage and grade is most important, and we think that subdivision according to sex hormone receptor expression can play a role in treatment selection and strategy. Given the importance of androgen receptor and estrogen receptor roles in the initiation and progression of bladder cancer,7,8 we were interested in further investigating whether targeting the androgen receptor and estrogen receptor can have a therapeutic effect on bladder cancer. Herein, we report our efforts to determine whether or not the androgen receptor and estrogen receptor beta exist in transitional cell carcinoma of the bladder. We also investigated the sex-hormone receptor expression as a predicting factor of the recurrence and progression in patients with bladder cancer.
MATERIALS AND METHODS
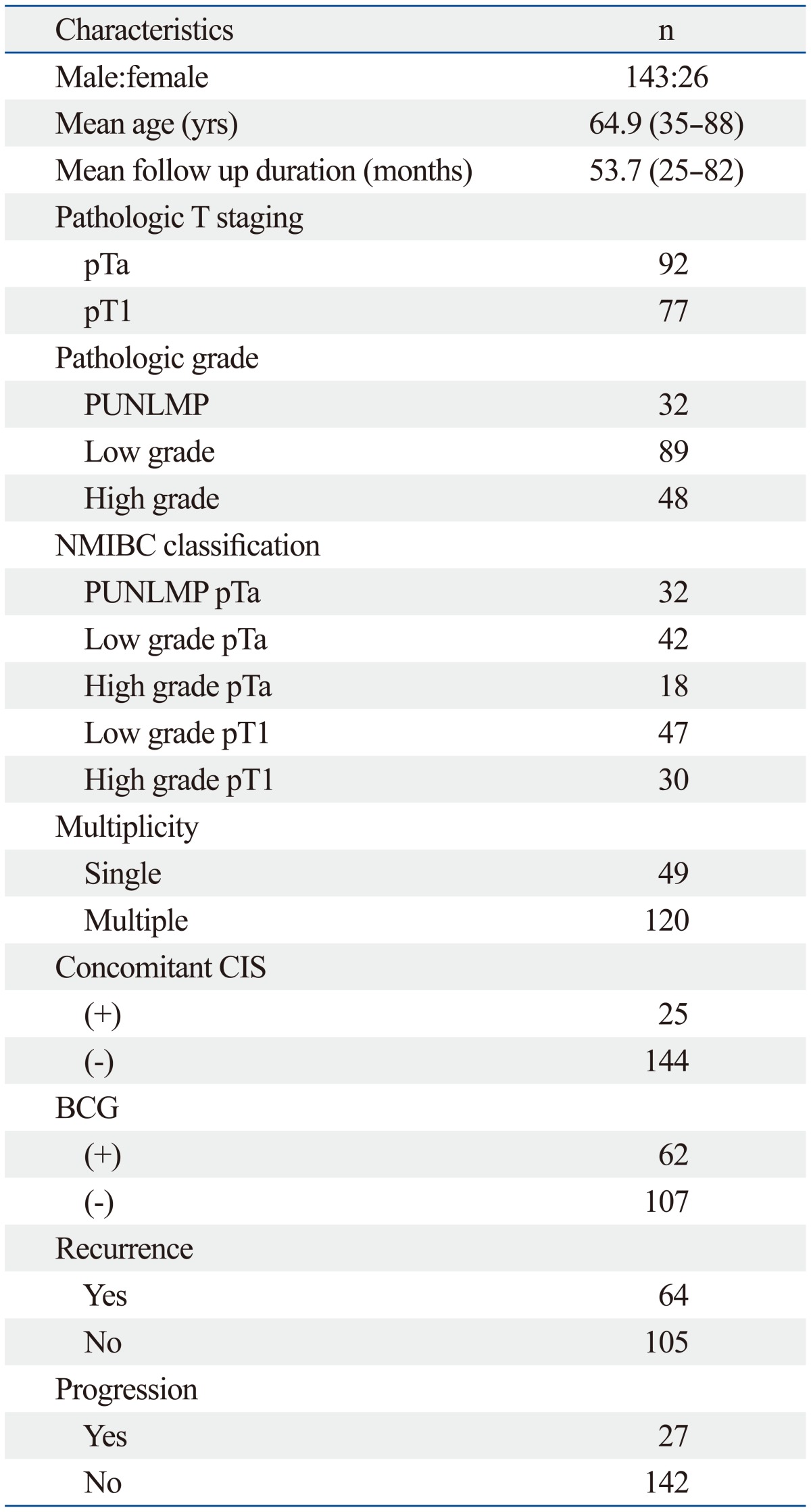
Tumor specimens from patients treated at our institution for bladder cancer between January 2006 and January 2011 were analyzed (Table 1). All cases were transitional cell carcinoma. Institutional Review Board approval was obtained for the study. After histopathologic evaluation, tumors were staged according to the 2002 American Joint Committee on Cancer staging system. Tumors were graded according to the 2004 World Health Organization/International Society of Urologic Pathology classification of urothelial neoplasia, and they were classified as papillary urothelial neoplasm of low malignant potential (PUNLMP), low grade and high grade urothelial carcinoma. Transurethral resection specimens were obtained from patients who had non-muscle invasive tumors after initial resection, excluding specimens of recurrent tumor cases. In addition, patients who received Bacillus Calmette-Guérin intravesical therapy were selected for the study to match the adjuvant treatment. The patients who were treated with chemotherapy or radiotherapy prior to surgery, who were incomplete resection of bladder tumor or second transurethral resection, who underwent radical cystectomy, and who were given hormonal replacement or deprivation for other disorders were excluded from the study. Formalin-fixed and paraffin embedded tissue sections made from TMA blocks were immunostained for estrogen receptor beta (Clone EMR02, Novocastra, Newcastle upon Tyne, UK) and androgen receptor (Clone 2F12, Novocastra) by using the streptavidin-biotin peroxidase method. For androgen receptor immnunostaining, antigen retrieval was performed by immersing slides in 10 mmol/L citrate buffer, pH 6 and heating in a 95℃ water bath for 20 minutes. After applying a hydrogen peroxide and blocking solution for 15 minutes, the slides were incubated overnight at 4℃ with primary antibody. The slides were then incubated in biotin and streptavidin for 20 minutes. Afterward, they were placed in a substrate-chromogen solution for 10 minutes and a then counterstained with hematoxylin. Estrogen receptor beta immunostaining was performed by using Ventana automated immunostainer (Ventana, Tucson, AZ, USA). Antigen retrieval was made using an Ethylene Diamine Triacetic Acid buffer for 90 minutes. The primary antibody incubation time was 60 minutes. Sections of the normal prostate gland and skin were used as positive controls for androgen receptor and estrogen receptor beta immunostaining, respectively.
Table 1.
Clinicopathologic Characteristics of Patients Included in the Study Sample
NMIBC, non-muscle-invasive bladder cancer; CIS, carcinoma in situ; PUNLMP, papillary urothelial neoplasm of low malignant potential; BCG, Bacillus Calmette-Guérin.
The expression levels of the androgen receptor and estrogen receptor beta were assessed by evaluating 500 tumor cells for each case, and the percentage of positively-stained nuclei with androgen receptor and estrogen receptor beta antibodies was recorded. Any distinct, brown nuclear staining was considered a positive staining. A cut-off value of 10% was used for androgen receptor and estrogen receptor beta expression based on another studies (Fig. 1).9,10
Fig. 1.
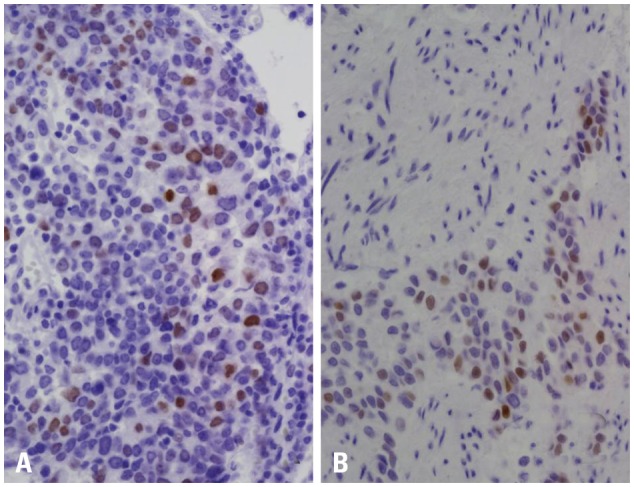
Immunohistochemical staining of androgen receptor (A) and estrogen receptor beta (B). (A) Diffuse, strong nuclear staining in low grade papillary tumor (×400). (B) Strong nuclear staining in high grade urothelial tumor (×400).
We retrospectively analyzed our patient data for age, gender, stage, grade, multifocality, concomitant carcinoma in situ (CIS), tumor size, recurrence and progression. Recurrence was defined as recurrent bladder cancer confirmed histologically after the initial resection. Progression was defined as recurrent cancer that invaded into the muscle layer. Recurrence-free survival and progression-free survival rates were calculated as time from the first transurethral resection to the date of recurrence and progression, respectively. Uni- and multivariate logistic regression analyses were performed to investigate the relationship between the immunohistochemistry results and age, gender, stage, grade, mutlifocality, concomitant CIS, tumor size, recurrence and progression.
In addition, relationships between the expression of androgen receptor or estrogen receptor beta and clinicopathologic variables were calculated using χ2 test and Fisher's exact test. Any p values lower than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS software, release 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
We evaluated 169 patients (143 males and 26 female) with bladder cancer who passed away more than 2 years from the time of operation. The mean age identified was 64.9 years (range 35-88). The mean follow-up duration was 53.7 months (range 25-82). Twenty four patients were not followed; 9 patients died from exacerbation of comorbidity, and 15 patients were lost to follow-up. The pathologic T stages were Ta (92 patients) and T1 (77 patients). The PUNLMP, low grade and high grade urothelial carcinoma were 32 patients, 89 patients and 48 patients, respectively. Of the 169 patients, 49 patients had single and 25 patients had concomitant CIS. Sixty-four (37.9%) patients had recurrence and 27 patients (16.0%) had progression at recurrence. All patients had no no lymph node metastasis and no distant metastasis (Table 1).
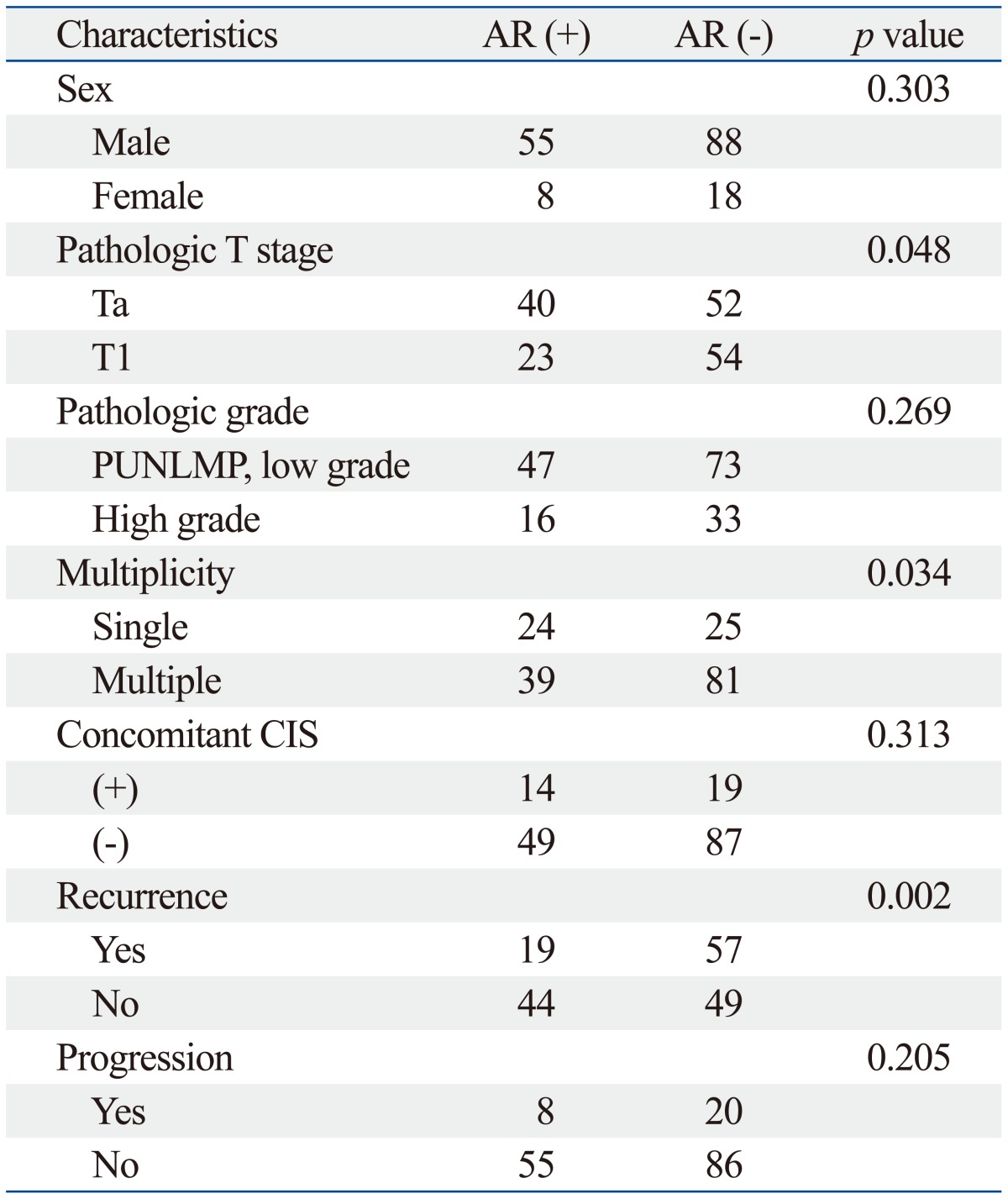
Of the 169 patients, 63 patients had immunohistochemical expression of androgen receptor and 52 patients had estrogen receptor beta. The distributions of androgen receptor and estrogen receptor beta according to patient gender were similar in both genders (Table 2 and 3). Androgen receptor expression was more frequently expressed in the pTa (p=0.048), single lesion (p=0.034) and a low recurrence rate (p=0.002). As shown in Table 3, the estrogen receptor beta expression was related to the pathologic stage (p=0.004), grade (p=0.043), recurrence (p=0.009), and disease progression (p=0.008).
Table 2.
The Association between the Clinical and Pathological Characteristics of Patients According to Androgen Receptor Expression Status
CIS, carcinoma in situ; PUNLMP, papillary urothelial neoplasm of low malignant potential; AR, androgen receptor.
Table 3.
The Association between the Clinical and Pathological Characteristics of Patients According to Estrogen Receptor Beta Expression Status
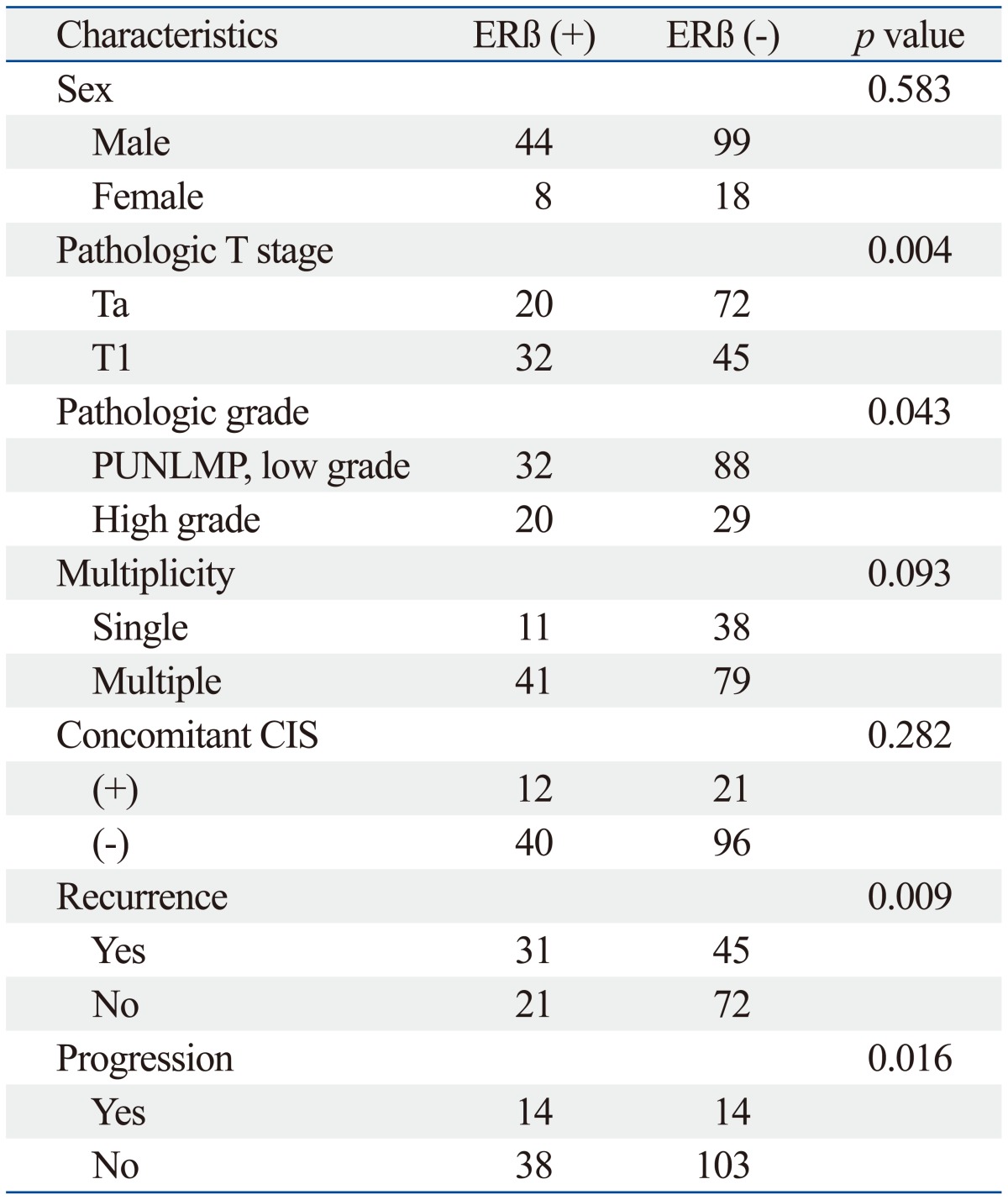
CIS, carcinoma in situ; PUNLMP, papillary urothelial neoplasm of low malignant potential; ERβ, estrogen receptor beta.
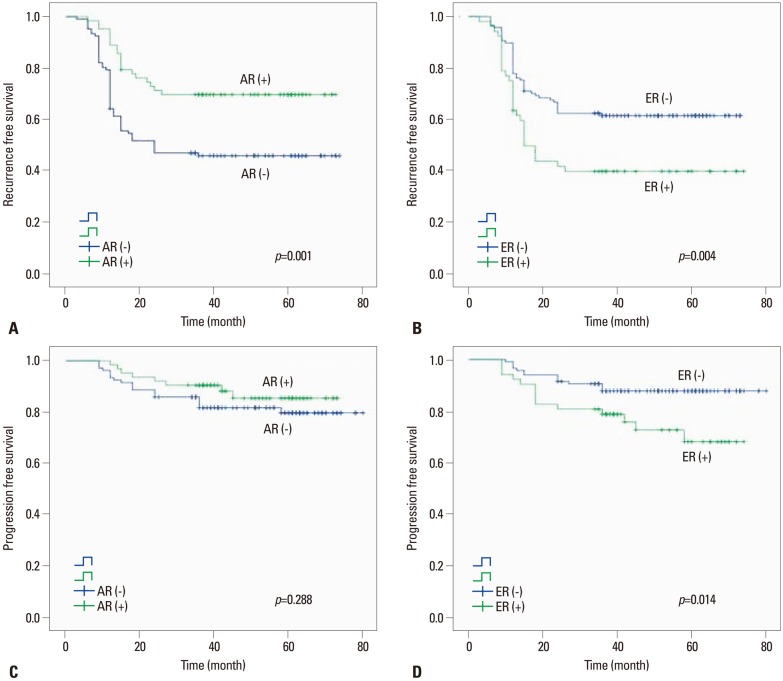
On univariable analysis, the recurrence-free survival probability of patients with androgen receptor positivity (p=0.001) or estrogen receptor beta negativity (p=0.004) were significantly higher than androgen receptor negativity or estrogen receptor beta positivity, respectively. Also, pathologic T stage (p<0.001), pathologic grade (p<0.001), multiplicity (p=0.001) and concomitant CIS (p<0.001) were significantly different (Table 4). The progression-free survival probability of patients with estrogen receptor beta negativity was higher than that of patients with estrogen receptor beta positivity (p=0.014) (Fig. 2). Furthermore, the pathologic T stage (p<0.001), pathologic grade (p<0.001), multiplicity (p=0.002), and concomitant CIS (p<0.001) were significantly different, however, the progression rate of the androgen receptor expression was not significantly different (Table 5).
Table 4.
Multivariate Analysis of Risk Factors for the Recurrence Free Survival and Progression Free Survival in Patients with Bladder Cancer
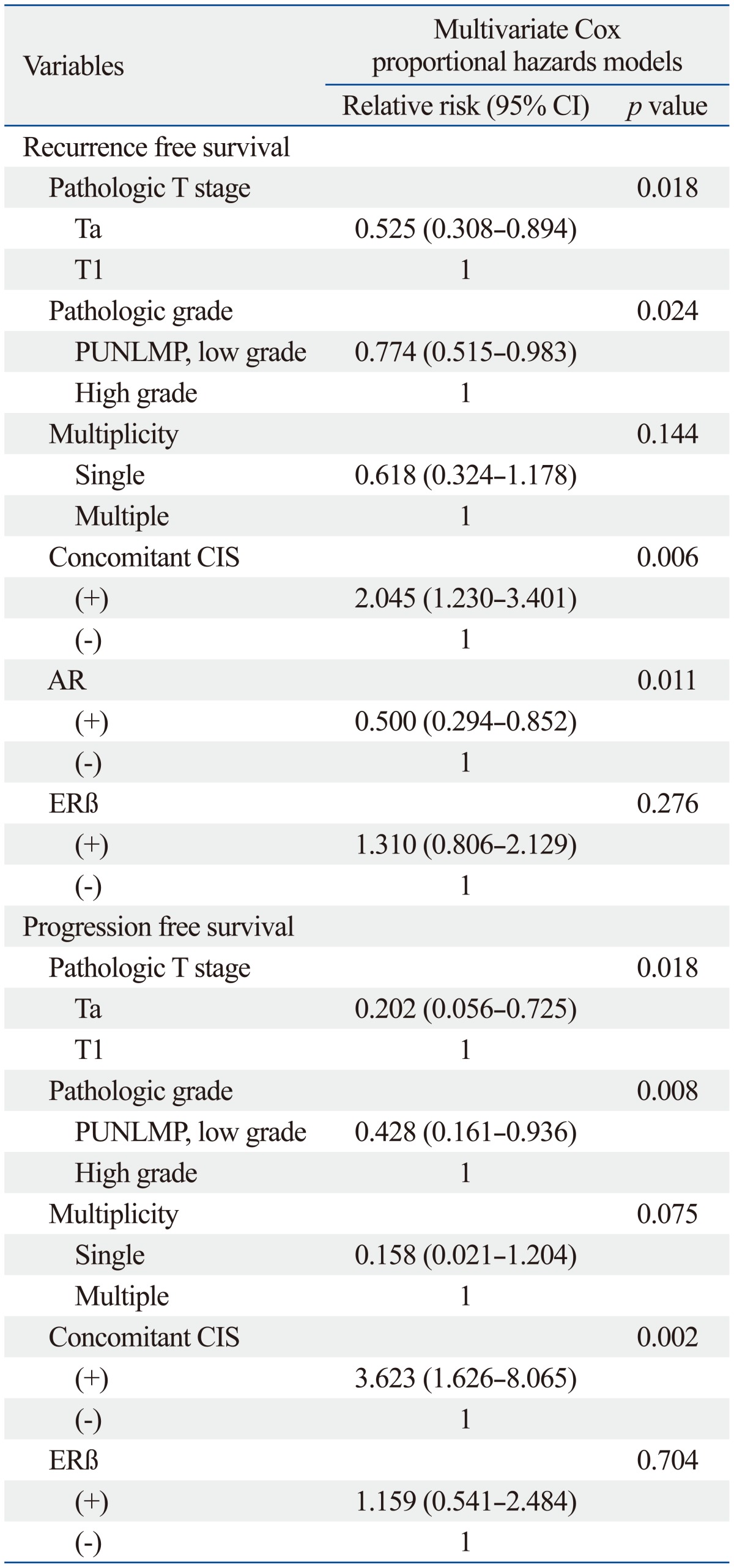
CIS, carcinoma in situ; PUNLMP, papillary urothelial neoplasm of low malignant potential; AR, androgen receptor; ERβ, estrogen receptor beta; CI, confidence interval.
Fig. 2.
Kaplan-Meier estimated recurrence-free survival probability of androgen receptor (A), estrogen receptor beta (B), and progression-free survival probability of androgen receptor (C), estrogen receptor beta (D). AR, androgen receptor; ER, estrogen receptor.
Table 5.
Univariate Analysis of Risk Factors for the Recurrence Free Survival and Progression Free Survival in Patients with Bladder Cancer
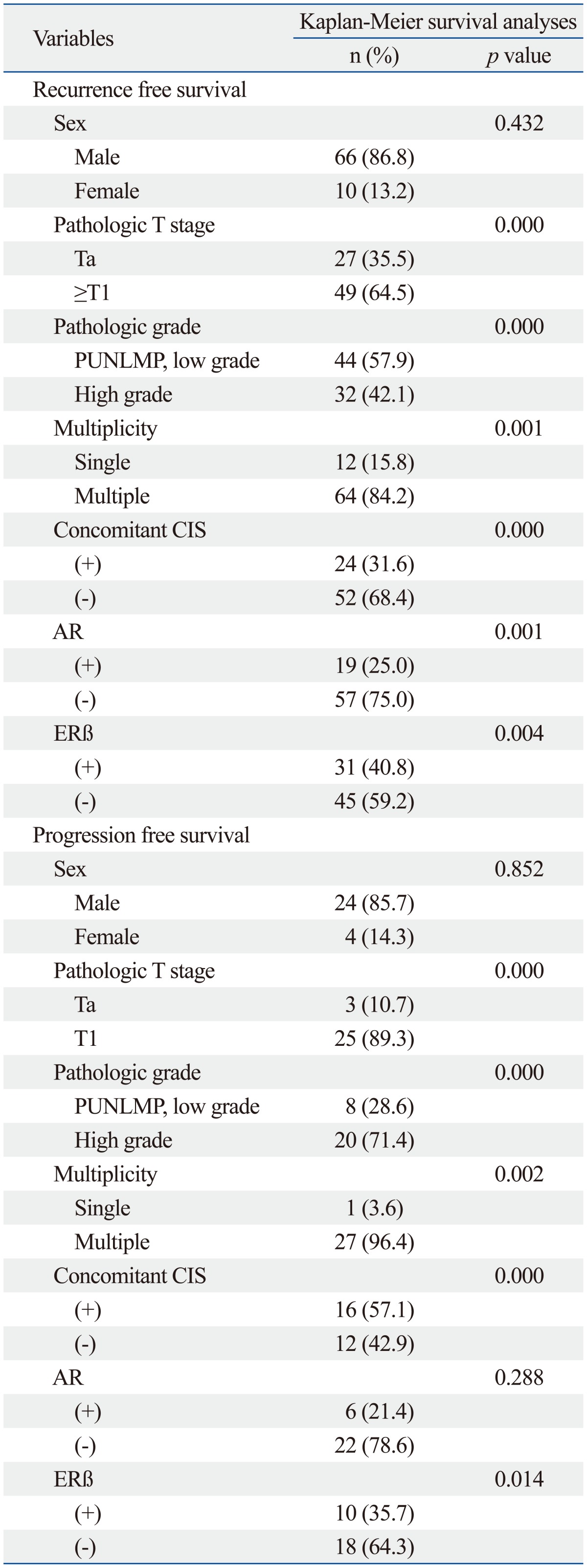
CIS, carcinoma in situ; PUNLMP, papillary urothelial neoplasm of low malignant potential; AR, androgen receptor; ERβ, estrogen receptor beta.
On multivariable analysis, significant association was found between androgen receptor expression and lower recurrence rates [hazard ratio (HR)=0.500; 95% confidence interval (CI), 0.294 to 0.852; p=0.011]. Moreover, pathologic T stage (HR=0.525; 95% CI, 0.308 to 0.894; p=0.018), pathologic grade (HR=0.774; 95% CI, 0.515 to 0.983; p=0.024) and concomitant CIS (HR=2.045; 95% CI, 1.230 to 3.401; p=0.006) were significantly associated with recurrence rates (Table 5). In the progression rate analysis, the pathologic T stage (HR=0.202; 95% CI, 0.056 to 0.725; p=0.018), pathologic grade (HR=0.428; 95% CI, 0.161 to 0.936; p=0.008), concomitant CIS (HR=3.623; 95% CI, 1.626 to 8.065; p=0.002) were significantly associated with risk factors of progression. However, estrogen receptor beta expression was not significantly associated with progression rates (Table 5).
DISCUSSION
Despite co-localization of androgen receptors with estrogen receptors in the lower urinary tract, few studies have been done on the relationship between an urothelial tumor and sex hormone status. The reason for the differences in the incidence of transitional cell carcinoma in both genders and the different natural history of the disease are not well understood. It was originally hypothesized that a higher smoking rate and greater exposure to industrial carcinogens among men were the main driving forces responsible for the increased male incidence of bladder cancer.1,11 However, these epidemiological distinctions seem to exist no longer.3
The process of carcinogenesis is characterized by the loss of normal regulators by loss of suppressor genes and activation of oncogenes, which are usual elements in cell signaling pathways.12,13,14 Sex hormone receptors are found in bladder tissues, where they seem to function in regulating growth and differentiation. A possible explanation for the gender differences associated with bladder cancer incidence rate and outcome is differing hormone levels. Animal experiments have shown that bladder cancer is induced by chemical carcinogens more easily in males than females.6 Moreover, estrogens inhibit and androgens increase the growth and development of bladder malignancies in animal models.15,16,17 Thus, sex hormonal signals have been considered an explanation for the gender differences in bladder cancer incidence. In our study, however, we found that the levels of the androgen receptors and estrogen receptor beta expression in bladder cancer were similar in both genders (Table 2 and 3). Tuygun, et al.10 hypothesized that if an androgen receptor really had any effect on androgen-independent bladder carcinogenesis, the rate of the development of bladder cancer mediated by the androgen receptor might have been similar between men and women. Also, Kirkali, et al.18 concluded that the androgen receptor did not play any direct role in the malignant transformation of human transitional cell epithelium as they observed no androgen receptor expression in malignant bladder urothelium. Studies with estrogen receptor showed similar results.19,20
Data suggest that all or part of the human urinary bladder is hormone dependent.21 Embryonic development of the bladder is a doubled process. The dome the fundus and the lateral area are derived from the urogenital sinus, which in males also gives rise to the prostate, for which the role of androgens in both normal development and carcinogenesis is well established.22,23 Although the urinary bladder is not regarded as a sex organ, the connective tissue in the trigone is formed from the same embryonic bud as the anterior vagina that can be found in women, and remains responsive to steroid hormones.24 Also, sex hormone receptors were identified previously in patients with localized and advanced bladder cancer.24,25,26 However, it is not well understood yet, why bladder cancer is less frequent in women. This may be the result of a multifactorial process. The lower incidence of bladder cancer in women may be attributable to some interaction between tumors, sex hormones and some genetic and non-genetic factors.27
In our study, androgen receptor and estrogen receptor beta positivity were found in 63 bladder cancer patients (37.3%) and 52 patients (30.8%), respectively. Androgen receptor positivity recurred less frequently (Table 2), and estrogen receptor beta positivity was more frequent in high grade, stage, multifocality and progression (Table 3), like the finding in previous studies.10,28,29,30,31 However, we didn't find that androgen receptor expression within bladder cancers decreased with the advanced pathologic stage. Interestingly, the recurrence rates of the patients with androgen receptor positivity and estrogen receptor beta negativity were significantly lower, and the progression rates of the patients with estrogen receptor beta positivity were significantly higher (Fig. 2). In a multivariate analysis of risk factors for recurrence and progression, androgen receptor positivity was related to low recurrence rates only. Moreover, the estrogen receptor expression status was not found to affect the recurrence and progression-free survival (Table 5). These findings suggest that androgen receptor expression is helpful in predicting the recurrence of superficial bladder cancer, but the estrogen receptor does not have a direct role in the prognosis of bladder cancer patients.
Increasing evidence in many studies, indicates that the expression of sex hormone receptors are important for the development and progression of bladder cancer.32,33 Understanding the functional significance of androgen receptor in bladder cancer could possibly provide a new therapeutic strategy and prognostic factor. Our results suggested that the androgen receptor is a prognostic factor and a potential molecular target for the treatment of bladder cancer.
The reasons behind this difference in incidence between men and women are unknown. Differences in increased prevalence of smoking and exposure to environmental toxins have been brought up as a possible explanation. Interestingly, animal studies have shown that the incidence of chemically induced bladder cancer development has greater chance of urothelial cancer with male animals and a role for sex-hormone receptors has been hypothesized.6,17,32 However, data on sex-hormone receptors expression in human bladder cancer and its correlation with grade and stage are limited. In our study, sex-specific hormone receptors cannot be responsible for gender differences in bladder cancer rates because they were expressed at similar rates for both genders (Table 2 and 3). We conclude that the possibility of recurrence is low when the androgen receptor is expressed in a bladder cancer specimen. Although the estrogen receptor beta expression was not significantly associated with prognostic factors, we think that its negative expression may be associated with better progression rates.
ACKNOWLEDGEMENTS
This work was supported by Pusan National University Yangsan Hospital Research Grant.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Negri E, La Vecchia C. Epidemiology and prevention of bladder cancer. Eur J Cancer Prev. 2001;10:7–14. doi: 10.1097/00008469-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 3.Scosyrev E, Trivedi D, Messing E. Female bladder cancer: incidence, treatment, and outcome. Curr Opin Urol. 2010;20:404–408. doi: 10.1097/MOU.0b013e32833c7a9b. [DOI] [PubMed] [Google Scholar]
- 4.Cantor KP, Lynch CF, Johnson D. Bladder cancer, parity, and age at first birth. Cancer Causes Control. 1992;3:57–62. doi: 10.1007/BF00051913. [DOI] [PubMed] [Google Scholar]
- 5.Okajima E, Hiramatsu T, Iriya K, Ijuin M, Matsushima S. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol Res. 1975;3:73–79. doi: 10.1007/BF00256185. [DOI] [PubMed] [Google Scholar]
- 6.Imada S, Akaza H, Ami Y, Koiso K, Ideyama Y, Takenaka T. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur Urol. 1997;31:360–364. doi: 10.1159/000474484. [DOI] [PubMed] [Google Scholar]
- 7.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 8.Celayir S, Ilçe Z, Dervisoglu S. The sex hormone receptors in the bladder in childhood - I: preliminary report in male subjects. Eur J Pediatr Surg. 2002;12:312–317. doi: 10.1055/s-2002-35951. [DOI] [PubMed] [Google Scholar]
- 9.Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106:2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 10.Tuygun C, Kankaya D, Imamoglu A, Sertcelik A, Zengin K, Oktay M, et al. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: a comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urol Oncol. 2011;29:43–51. doi: 10.1016/j.urolonc.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol. 2004;22:86–92. doi: 10.1016/S1078-1439(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 12.Borland RN, Brendler CB, Isaacs WB. Molecular biology of bladder cancer. Hematol Oncol Clin North Am. 1992;6:31–39. [PubMed] [Google Scholar]
- 13.Weinberg RA. Oncogenes and tumor suppressor genes. CA Cancer J Clin. 1994;44:160–170. doi: 10.3322/canjclin.44.3.160. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg RA. Prospects for cancer genetics. Cancer Surv. 1995;25:3–12. [PubMed] [Google Scholar]
- 15.Bertram JS, Craig AW. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer. 1972;8:587–594. doi: 10.1016/0014-2964(72)90137-5. [DOI] [PubMed] [Google Scholar]
- 16.Shirai T, Tsuda H, Ogiso T, Hirose M, Ito N. Organ specific modifying potential of ethinyl estradiol on carcinogenesis initiated with different carcinogens. Carcinogenesis. 1987;8:115–119. doi: 10.1093/carcin/8.1.115. [DOI] [PubMed] [Google Scholar]
- 17.Reid LM, Leav I, Kwan PW, Russell P, Merk FB. Characterizabetation of a human, sex steroid-responsive transitional cell carcinoma maintained as a tumor line (R198) in athymic nude mice. Cancer Res. 1984;44:4560–4573. [PubMed] [Google Scholar]
- 18.Kirkali Z, Cowan S, Leake RE. Androgen receptors in transitional cell carcinoma. Int Urol Nephrol. 1990;22:231–234. doi: 10.1007/BF02550398. [DOI] [PubMed] [Google Scholar]
- 19.Larocca LM, Giustacchini M, Maggiano N, Ranelletti FO, Piantelli M, Alcini E, et al. Growth-inhibitory effect of quercetin and presence of type II estrogen binding sites in primary human transitional cell carcinomas. J Urol. 1994;152:1029–1033. doi: 10.1016/s0022-5347(17)32649-6. [DOI] [PubMed] [Google Scholar]
- 20.Bødker A, Balslev E, Juul BR, Stimpel H, Meyhoff HH, Hedlund H, et al. Estrogen receptors in the human male bladder, prostatic urethra, and prostate. An immunohistochemical and biochemical study. Scand J Urol Nephrol. 1995;29:161–165. doi: 10.3109/00365599509180557. [DOI] [PubMed] [Google Scholar]
- 21.Saez S, Martin PM. Evidence of estrogen receptors in the trigone area of human urinary bladder. J Steroid Biochem. 1981;15:317–320. doi: 10.1016/0022-4731(81)90291-0. [DOI] [PubMed] [Google Scholar]
- 22.Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. Androgen receptors in prostate cancer. J Urol. 2003;170(4 Pt 1):1363–1369. doi: 10.1097/01.ju.0000075099.20662.7f. [DOI] [PubMed] [Google Scholar]
- 23.Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer. 1991;67:3057–3064. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Dunning WF, Curtis MR, Segaloff A. Strain differences in response to estrone and the induction of mammary gland, adrenal, and bladder cancer in rats. Cancer Res. 1953;13:147–152. [PubMed] [Google Scholar]
- 25.Noronha RF, Rao BR. Sex hormone receptors in localized and advanced transitional cell carcinoma of urinary tract in humans. Urology. 1986;28:401–403. doi: 10.1016/0090-4295(86)90073-7. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann O, Baume H, Dietel M. Detection of oestrogen recep tors in non-invasive and invasive transitional cell carcinomas of the urinary bladder using both conventional immunohistochemistry and the tyramide staining amplification (TSA) technique. J Pathol. 1998;186:165–168. doi: 10.1002/(SICI)1096-9896(1998100)186:2<165::AID-PATH155>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Kvist E, Albrectsen J. Oestrogen receptor investigations in bladder tumours. Scand J Urol Nephrol. 1994;28:369–370. doi: 10.3109/00365599409180515. [DOI] [PubMed] [Google Scholar]
- 28.Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64:383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Kontos S, Kominea A, Melachrinou M, Balampani E, Sotiropoulou-Bonikou G. Inverse expression of estrogen receptor-beta and nuclear factor-kappaB in urinary bladder carcinogenesis. Int J Urol. 2010;17:801–809. doi: 10.1111/j.1442-2042.2010.02603.x. [DOI] [PubMed] [Google Scholar]
- 30.Croft PR, Lathrop SL, Feddersen RM, Joste NE. Estrogen receptor expression in papillary urothelial carcinoma of the bladder and ovarian transitional cell carcinoma. Arch Pathol Lab Med. 2005;129:194–199. doi: 10.5858/2005-129-194-EREIPU. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–1726. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 33.Izumi K, Zheng Y, Hsu JW, Chang C, Miyamoto H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol Carcinog. 2013;52:94–102. doi: 10.1002/mc.21833. [DOI] [PubMed] [Google Scholar]